Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors
Abstract
:1. Introduction
- Metal oxide,
- Conducting polymer,
- Quartz crystal microbalance,
- Acoustic wave,
- Electro-chemical,
- Catalytic bead,
- Optical.
2. Materials and Methods
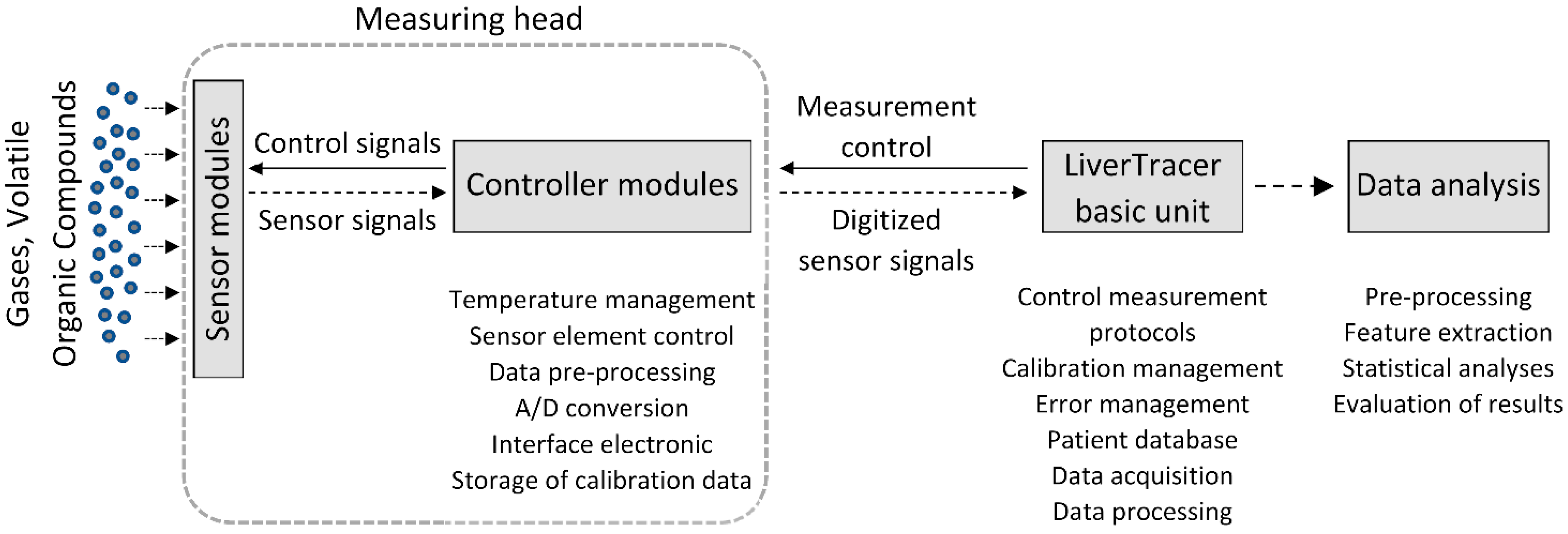
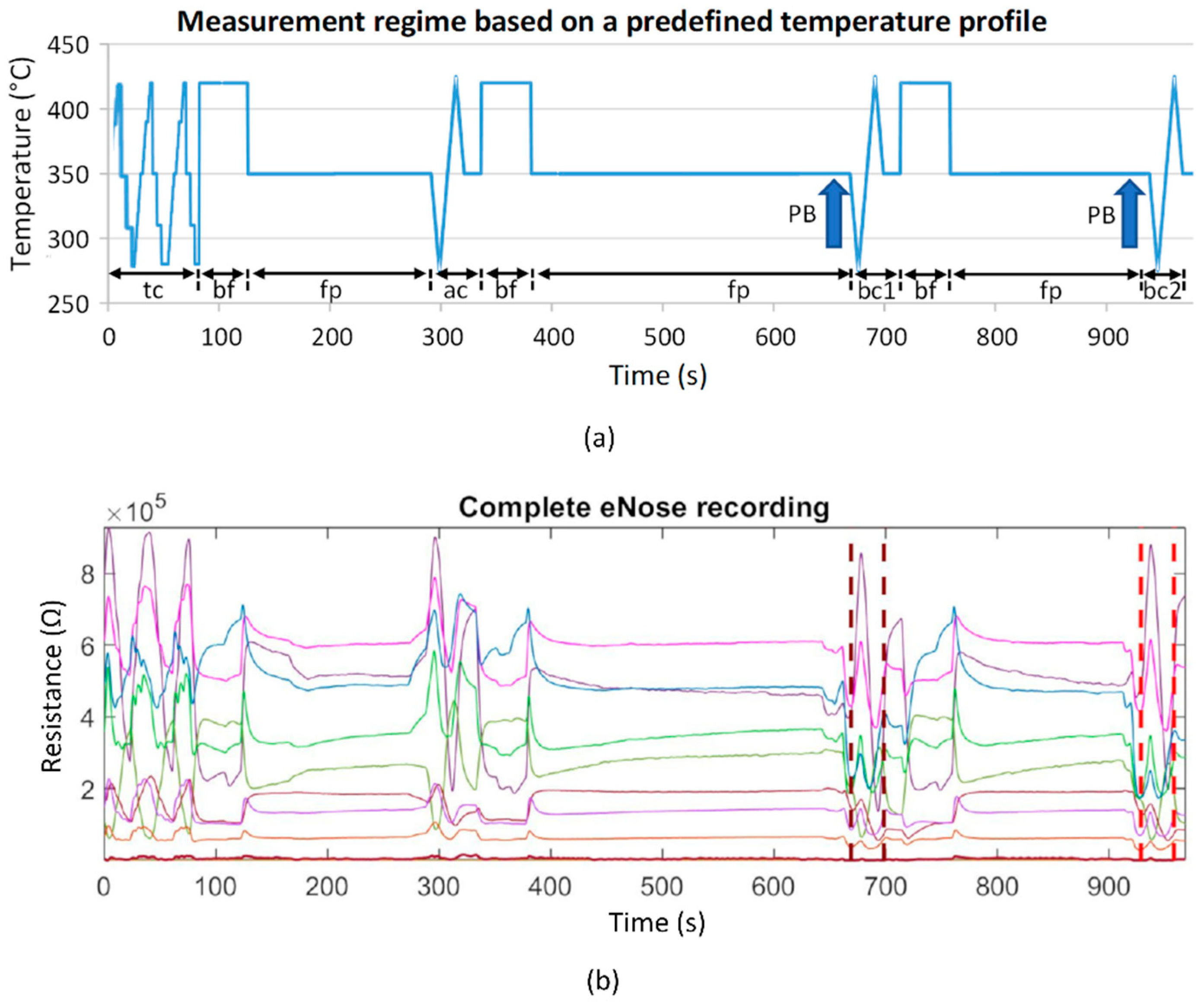
2.1. Electronic Nose and Signal Processing
- slope_startmax (Ω/s): slope from cycle start (Start) to absolute maximum (Max);
- s_slope_startmax (Ω/s): steepest slope of 1s duration from cycle start to Max;
- s_slope_startmax_pos (s): corresponding position of s_slope_startmax;
- s_slope_maxmin (Ω/s): steepest slope of 1s duration from Max to minimum (Min);
- area1 (Ω·s): area under the curve from cycle start to Max;
- area2 (Ω·s): area under the curve from cycle Max to midpoint;
- area3 (Ω·s): area under the curve from cycle midpoint to Min;
- area4 (Ω·s): area under the curve from cycle Min to cycle end;
- area3sec_9 (Ω·s): ninth subarea (24 s to 27 s); area under the curve is evaluated incrementally in 3 s subareas beginning from cycle start.
- p00—probability for the occurrence of the word type 00 within the resistance value time series.
2.2. Patients
2.3. Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartman, J. A possible objective method for the rapid estimation of flavors in vegetables. Proc. Amer. Soc. Hort. Sci 1954, 64, 335–342. [Google Scholar]
- Wilkens, W.F.; Hartman, J.D. An Electronic Analog for the Olfactory Processes. Ann. N. Y. Acad. Sci. 1964, 116, 608–612. [Google Scholar] [CrossRef]
- Dodd, G.H.; Bartlett, P.N.; Gardner, J.W. Odours—The stimulus for an electronic nose. In Proceedings of the NATO Advanced Research Workshop on Sensors and Sensory Systems for an Electronic Nose, Reykjavik, Iceland, 5–8 August 1991; pp. 1–11. [Google Scholar]
- Moncrieff, R.W. An instrument for measuring and classifying odors. J. Appl. Physiol. 1961, 16, 742–749. [Google Scholar] [CrossRef]
- Henderson, M.J.; Karger, B.A.; Wren Shall, G.A. Acetone in the breath: A study of acetone exhalation in diabetic and nondiabetic human subjects. Diabetes 1952, 1, 188–193. [Google Scholar] [CrossRef]
- Gardner, J.W.; Vincent, T.A. Electronic Noses for Well-Being: Breath Analysis and Energy Expenditure. Sensors 2016, 16, 947. [Google Scholar] [CrossRef] [Green Version]
- Farraia, M.V.; Cavaleiro Rufo, J.; Paciencia, I.; Mendes, F.; Delgado, L.; Moreira, A. The electronic nose technology in clinical diagnosis: A systematic review. Porto Biomed. J. 2019, 4, e42. [Google Scholar] [CrossRef]
- Wasilewski, T.; Migon, D.; Gebicki, J.; Kamysz, W. Critical review of electronic nose and tongue instruments prospects in pharmaceutical analysis. Anal. Chim. Acta 2019, 1077, 14–29. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Advances in electronic-nose technologies developed for biomedical applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic noses in classification and quality control of edible oils: A review. Food Chem. 2018, 246, 192–201. [Google Scholar] [CrossRef]
- Tan, J.; Xu, J. Applications of electronic nose (e-nose) and electronic tongue (e-tongue) in food quality-related properties determination: A review. Artif. Intell. Agric. 2020, 4, 104–115. [Google Scholar] [CrossRef]
- He, J.; Xu, L.; Wang, P.; Wang, Q. A high precise E-nose for daily indoor air quality monitoring in living environment. Integration 2017, 58, 286–294. [Google Scholar] [CrossRef]
- Karakaya, D.; Ulucan, O.; Turkan, M. Electronic Nose and Its Applications: A Survey. Int. J. Autom. Comput. 2020, 17, 179–209. [Google Scholar] [CrossRef] [Green Version]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B 1994, 18, 211–220. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, T.S.; Yadava, R.D.S. MEMS sensor array-based electronic nose for breath analysis-a simulation study. J. Breath Res. 2018, 13, 016003. [Google Scholar] [CrossRef]
- Wojnowski, W.; Dymerski, T.; Gebicki, J.; Namiesnik, J. Electronic Noses in Medical Diagnostics. Curr. Med. Chem. 2019, 26, 197–215. [Google Scholar] [CrossRef]
- Wilson, A.D. Advances in electronic-nose technologies for the detection of volatile biomarker metabolites in the human breath. Metabolites 2015, 5, 140–163. [Google Scholar] [CrossRef]
- Voss, A.; Witt, K.; Kaschowitz, T.; Poitz, W.; Ebert, A.; Roser, P.; Bar, K.J. Detecting cannabis use on the human skin surface via an electronic nose system. Sensors 2014, 14, 13256–13272. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Baier, V.; Reisch, R.; von Roda, K.; Elsner, P.; Ahlers, H.; Stein, G. Smelling renal dysfunction via electronic nose. Ann. Biomed. Eng. 2005, 33, 656–660. [Google Scholar] [CrossRef]
- Wang, P.; Tan, Y.; Xie, H.; Shen, F. A novel method for diabetes diagnosis based on electronic nose. Biosens. Bioelectron. 1997, 12, 1031–1036. [Google Scholar] [CrossRef]
- Dragonieri, S.; Pennazza, G.; Carratu, P.; Resta, O. Electronic Nose Technology in Respiratory Diseases. Lung 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Baldini, C.; Billeci, L.; Sansone, F.; Conte, R.; Domenici, C.; Tonacci, A. Electronic Nose as a Novel Method for Diagnosing Cancer: A Systematic Review. Biosensors 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Capelli, L.; Taverna, G.; Bellini, A.; Eusebio, L.; Buffi, N.; Lazzeri, M.; Guazzoni, G.; Bozzini, G.; Seveso, M.; Mandressi, A.; et al. Application and Uses of Electronic Noses for Clinical Diagnosis on Urine Samples: A Review. Sensors 2016, 16, 1708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voss, A.; Witt, K.; Fischer, C.; Reulecke, S.; Poitz, W.; Kechagias, V.; Surber, R.; Figulla, H.R. Smelling heart failure from human skin odor with an electronic nose. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA , 28 August–1 September 2012; pp. 4034–4037. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef]
- Millonig, G.; Friedrich, S.; Adolf, S.; Fonouni, H.; Golriz, M.; Mehrabi, A.; Stiefel, P.; Pöschl, G.; Büchler, M.W.; Seitz, H.K.; et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010, 52, 206–210. [Google Scholar] [CrossRef]
- Kamath, P.S.; Wiesner, R.H.; Malinchoc, M.; Kremers, W.; Therneau, T.M.; Kosberg, C.L.; D’Amico, G.; Dickson, E.R.; Kim, W.R. A model to predict survival in patients with end-stage liver disease. Hepatology 2001, 33, 464–470. [Google Scholar] [CrossRef]
- De Vincentis, A.; Pennazza, G.; Santonico, M.; Vespasiani-Gentilucci, U.; Galati, G.; Gallo, P.; Zompanti, A.; Pedone, C.; Antonelli Incalzi, R.; Picardi, A. Breath-print analysis by e-nose may refine risk stratification for adverse outcomes in cirrhotic patients. Liver Int. 2017, 37, 242–250. [Google Scholar] [CrossRef]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001. [Google Scholar] [CrossRef]
- Kiesewetter, O.; Ewert, A.; Melchert, V.; Kittelmann, S. Arrangement for the Detection of Air Constituents and Method for Operating the Arrangement. DE102004060101 B4, 13 December 2004. [Google Scholar]
- Kiesewetter, O.; Ewert, A.; Melchert, V.; Kittelmann, S. Assembly for Detecting Air Components. EP 1602924 B1, 2 June 2005. [Google Scholar]
- Kittelmann, S.; Ewert, A.; Kiesewetter, O. Air Content Detection Arrangement for Use in e.g., Building, HAS EVALUating Unit with Evaluating Resistance That Is Connected in Parallel with Individual or Combinations of Effective Resistances of Set of Operating Layers Connected with Unit. DE 102006033528 B3, 20 July 2006. [Google Scholar]
- Witt, K.; Fischer, C.; Reulecke, S.; Kechagias, V.; Surber, R.; Figulla, H.R.; Voss, A. Electronic nose detects heart failure from exhaled breath. Biomed. Tech. 2013, 58 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurths, J.; Voss, A.; Saparin, P.; Witt, A.; Kleiner, H.J.; Wessel, N. Quantitative analysis of heart rate variability. Chaos 1995, 5, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Voss, A.; Kurths, J.; Kleiner, H.J.; Witt, A.; Wessel, N.; Saparin, P.; Osterziel, K.J.; Schurath, R.; Dietz, R. The application of methods of non-linear dynamics for the improved and predictive recognition of patients threatened by sudden cardiac death. Cardiovasc. Res. 1996, 31, 419–433. [Google Scholar] [CrossRef]
- Sturges, H.A. The Choice of a Class Interval. J. Am. Stat. Assoc. 1926, 21, 65–66. [Google Scholar] [CrossRef]
- Hunt, S.A. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J. Am. Coll. Cardiol. 2005, 46, e1–e82. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. Global Initiative for Chronic Obstructive Lung Disease: The Changes Made. Cureus 2019, 11, e4985. [Google Scholar] [CrossRef] [Green Version]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar]
- Parikh, N.D.; Chang, Y.H.; Tapper, E.B.; Mathur, A.K. Outcomes of Patients With Cirrhosis Undergoing Orthopedic Procedures: An Analysis of the Nationwide Inpatient Sample. J. Clin. Gastroenterol. 2019, 53, e356–e361. [Google Scholar] [CrossRef]
- Wong, M.; Busuttil, R.W. Surgery in Patients with Portal Hypertension. Clin. Liver Dis. 2019, 23, 755–780. [Google Scholar] [CrossRef]
- Germanese, D.; Colantonio, S.; D’Acunto, M.; Romagnoli, V.; Salvati, A.; Brunetto, M. An E-Nose for the Monitoring of Severe Liver Impairment: A Preliminary Study. Sensors 2019, 19, 3656. [Google Scholar] [CrossRef] [Green Version]
- Arasaradnam, R.P.; McFarlane, M.; Ling, K.; Wurie, S.; O’Connell, N.; Nwokolo, C.U.; Bardhan, K.D.; Skinner, J.; Savage, R.S.; Covington, J.A. Breathomics--exhaled volatile organic compound analysis to detect hepatic encephalopathy: A pilot study. J. Breath Res. 2016, 10, 016012. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Liu, H.; Song, Q.; Song, G.; Wang, H.Z.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; Sun, G.P.; Chen, Z.D. The screening of volatile markers for hepatocellular carcinoma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2247–2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vincentis, A.; Vespasiani-Gentilucci, U.; Sabatini, A.; Antonelli-Incalzi, R.; Picardi, A. Exhaled breath analysis in hepatology: State-of-the-art and perspectives. World J. Gastroenterol. 2019, 25, 4043–4050. [Google Scholar] [CrossRef] [PubMed]
- De Vincentis, A.; Pennazza, G.; Santonico, M.; Vespasiani-Gentilucci, U.; Galati, G.; Gallo, P.; Vernile, C.; Pedone, C.; Antonelli Incalzi, R.; Picardi, A. Breath-print analysis by e-nose for classifying and monitoring chronic liver disease: A proof-of-concept study. Sci. Rep. 2016, 6, 25337. [Google Scholar] [CrossRef] [Green Version]
- Esfahani, S.; Tiele, A.; Agbroko, S.O.; Covington, J.A. Development of a Tuneable NDIR Optical Electronic Nose. Sensors 2020, 20, 6875. [Google Scholar] [CrossRef]
- Ruffer, D.; Hoehne, F.; Buhler, J. New Digital Metal-Oxide (MOx) Sensor Platform. Sensors 2018, 18, 1052. [Google Scholar] [CrossRef] [Green Version]
- Padilla, M.; Perera, A.; Montoliu, I.; Chaudry, A.; Persaud, K.; Marco, S. Drift compensation of gas sensor array data by Orthogonal Signal Correction. Chemom. Intell. Lab. Syst. 2010, 100, 28–35. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases (dagger). Sensors 2018, 18, 2613. [Google Scholar] [CrossRef] [Green Version]


| Control (n = 10) | Compensated Cirrhosis (n = 10) | Decompensated Cirrhosis (n = 10) | p-Value | |
|---|---|---|---|---|
| Sex (f/m) | 5/5 | 3/7 | 2/8 | 0.500 |
| Age (years) | 58 (51; 65) | 57 (52; 64) | 62 (56; 67) | 0.543 |
| Bodyweight (kg) | 81 (68; 96) | 94 (79; 101) | 80 (68; 97) | 0.136 |
| Height (cm) | 175 (167; 178) | 176 (167; 178) | 176 (169; 181) | 0.712 |
| Smoker (n,%) | 1 (10%) | 4 (40%) | 3 (30%) | 0.450 |
| Vital signs | ||||
| RR systolic (mmHg) | 135 (118; 161) | 126 (107; 155) | 122 (103; 136) | 0.266 |
| RR diastolic (mmHg) | 81 (76; 104) | 76 (61; 92) | 72 (63; 79) | 0.146 |
| Heart rate (pbm) | 78 (67; 102) | 85 (71; 88) | 92 (81; 104) | 0.212 |
| Temperature (°C) | 36.8 (3.4; 37.0) | 36.6 (36.1; 37.0) | 36.7 (36.4; 37.1) | 0.523 |
| Etiology of cirrhosis (n,%) | ||||
| Ethanol | N/A | 6 (60%) | 8 (80%) | 0.628 |
| Other | N/A | 4 (40%) | 2 (20%) | |
| Co-medication (n,%) | ||||
| Lactulose | 1 (10%) | 3 (30%) | 8 (80%) | 0.009 |
| Proton pump inhibitors | 5 (50%) | 7 (70%) | 9 (90%) | 0.262 |
| B-Blocker | 5 (50%) | 4 (40%) | 5 (50%) | 0.897 |
| Antibiotics | 1 (10%) | 3 (30%) | 7 (70%) | 0.016 |
| Rifaximin | 0 | 1 (10%) | 6 (60%) | |
| other | 1 (10%) | 2 (20%) | 1 (10%) |
| Group | Features | SENS | SPEC | ACC | AUC |
|---|---|---|---|---|---|
| CON—COMP | RS11_s_slope_maxmin (Ohm/s) RS32_area3sec_9 (Ohm·s) RS32_p00 | 1.00 | 1.00 | 1.00 | 1.00 |
| CON—DECOMP | RS31_slope_startmax (Ohm/s) RS32_s_slope_startmax_pos (s) RS33_p00 | 1.00 | 1.00 | 1.00 | 1.00 |
| COMP—DECOMP | RS32_Renyi4_entropy (bit) RS33_area2 (Ohm·s) | 0.90 | 1.00 | 0.95 | 0.97 |
| Group | CON | COMP | DECOMP | ||
|---|---|---|---|---|---|
| Test | Features | p | mv ± sd | mv ± sd | mv ± sd |
| CON vs. COMP | RS11_s_slope_maxmin (Ohm/s) | 0.046 | −86,258 ± 5225 | −81,023 ± 5676 | |
| RS32_area3sec_9 (Ohm·s) | 0.038 | 1,807,616 ± 207,540 | 2,071,884 ± 309,151 | ||
| RS32_p00 | 0.017 | 0.336 ± 0.050 | 0.276 ± 0.045 | ||
| CON vs. DECOMP | RS31_slope_startmax (Ohm/s) | 0.029 | 8901 ± 3207 | 6956 ± 1845 | |
| RS32_s_slope_startmax_pos (s) | 0.019 | 6.250 ± 1.161 | 6.900 ± 0.211 | ||
| RS33_p00 | 0.041 | 0.369 ± 0.045 | 0.319 ± 0.056 | ||
| COMP vs. DECOMP | RS32_Renyi4_entropy (bit) | 0.028 | 1.843 ± 0.386 | 2.179 ± 0.185 | |
| RS33_area2 (Ohm·s) | 0.131 | 48,252 ± 23,296 | 34,507 ± 14,547 |
| Categorized Bilirubin | Categorized INR | Ascites | Hepatic Encephalopathy | |
|---|---|---|---|---|
| CON | 100 | 86 | 100 | 100 |
| COMP | 10 | 40 | 70 | 100 |
| DECOMP | 90 | 60 | 50 | 50 |
| ACC | 63 | 59 | 73 | 83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voss, A.; Schroeder, R.; Schulz, S.; Haueisen, J.; Vogler, S.; Horn, P.; Stallmach, A.; Reuken, P. Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors. Biosensors 2022, 12, 70. https://doi.org/10.3390/bios12020070
Voss A, Schroeder R, Schulz S, Haueisen J, Vogler S, Horn P, Stallmach A, Reuken P. Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors. Biosensors. 2022; 12(2):70. https://doi.org/10.3390/bios12020070
Chicago/Turabian StyleVoss, Andreas, Rico Schroeder, Steffen Schulz, Jens Haueisen, Stefanie Vogler, Paul Horn, Andreas Stallmach, and Philipp Reuken. 2022. "Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors" Biosensors 12, no. 2: 70. https://doi.org/10.3390/bios12020070
APA StyleVoss, A., Schroeder, R., Schulz, S., Haueisen, J., Vogler, S., Horn, P., Stallmach, A., & Reuken, P. (2022). Detection of Liver Dysfunction Using a Wearable Electronic Nose System Based on Semiconductor Metal Oxide Sensors. Biosensors, 12(2), 70. https://doi.org/10.3390/bios12020070





