Current Status of Measurement Accuracy for Total Hemoglobin Concentration in the Clinical Context
Abstract
1. Background
2. Material and Methods
2.1. Measurement Systems and Application
2.2. Blood Gas Analyzer (BGA)
2.3. HemoCue 201+
2.4. Sysmex XN 9000
2.5. AHD Method
2.6. Preparation of Basic Blood Units
2.7. Data Acquisition
2.8. Definition of References
2.9. Bland and Altman Method (B and A) and Prediction Interval
2.10. Tolerance Level Analysis (TLA)
3. Results
4. Discussion
4.1. Limitations
4.1.1. Definition of the References
4.1.2. Manual Procedures from the Laboratory
4.1.3. Automatic Procedures
4.1.4. Causes of Variability in tHb Measurement
4.2. Representation According to B and A
4.3. Overlapping of B and A Definitions with the 95% Prediction Interval
4.4. Presentation as TLA
4.5. Clinical Consequences from the Results
4.5.1. For Patient Blood Management (PBM)
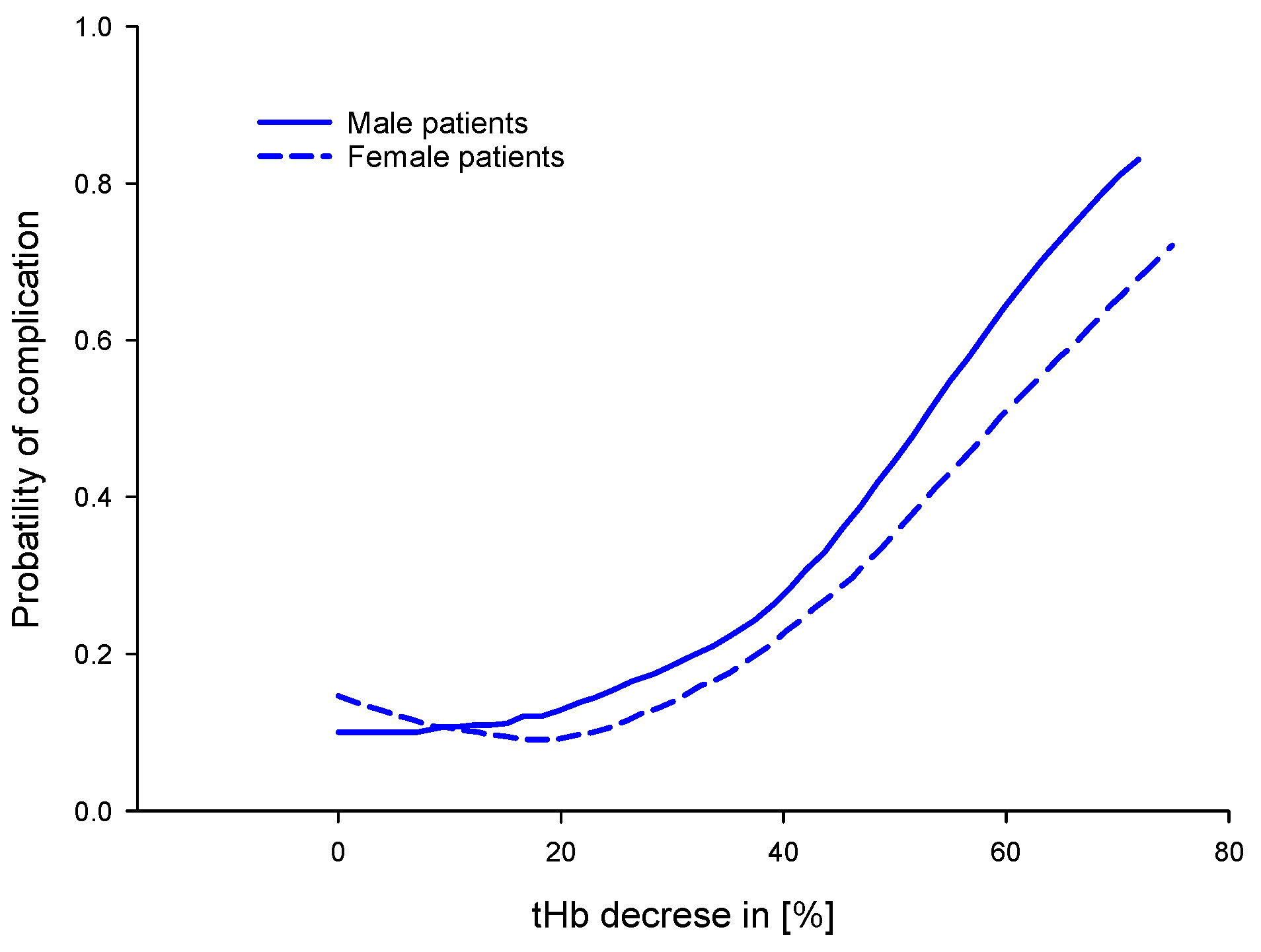
4.5.2. For the Identification of a Critical Threshold for Lowest tHb
4.5.3. For the Decision-Making Concerning Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AHA | Automatic hematology analyzer |
| AHD | Alkaline hematin detergent |
| B and A | Bland and Altman |
| BGA | Blood gas analyzer |
| DIN | German Institute for Standardization |
| HiCN | Cyanmethemoglobin method |
| PB | Passing–Bablok |
| PBM | Patient blood management |
| POCM | Point-of-care monitoring |
| POCT | Point-of-care testing |
| RBC | Red blood cell |
| REF | Reference |
| RMSE | Root mean square error |
| RSQ | R squared |
| SD | Standard deviation |
| SLS | Sodium lauryl sulfate |
| THb | Total hemoglobin concentration |
| TLA | Tolerance level analysis |
References
- Charpentier, E.; Looten, V.; Fahlgren, B.; Barna, A.; Guillevin, L. Meta-analytic estimation of measurement variability and assessment of its impact on decision-making: The case of perioperative haemoglobin concentration monitoring. BMC Med. Res. Methodol. 2016, 16, 7. [Google Scholar] [CrossRef]
- Habler, O.; Meier, J.; Pape, A.; Kertscho, H.; Zwissler, B. Tolerance to perioperative anemia. Mechanisms, influencing factors and limits. Der Anaesthesist 2006, 55, 1142–1156. [Google Scholar] [CrossRef]
- Karakochuk, C.D.; Hess, S.Y.; Moorthy, D.; Namaste, S.; Parker, M.E.; Rappaport, A.I.; Wegmüller, R.; Dary, O.; the HEmoglobin MEasurement (HEME). Working Group Measurement and interpretation of hemoglobin concentration in clinical and field settings: A narrative review. Ann. N. Y. Acad. Sci. 2019, 1450, 126–146. [Google Scholar] [CrossRef]
- WHO. The Urgent Need to Implement Patient Blood Management: Policy Brief; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Richter-Kuhlmann, E. Querschnitts-Leitlinien Hämotherapie: Aktualisiertes Kompendium. Dtsch Arztebl Int. 2020, 117, A-1857. [Google Scholar]
- Meybohm, P.; Froessler, B.; Goodnough, L.T.; Klein, A.; Muñoz, M.; Murphy, M.F.; Richards, T.; Shander, A.; Spahn, N.R.; Zacharowski, K. “Simplified International Recommendations for the Implementation of Patient Blood Management” (SIR4PBM). Perioper. Med. 2017, 6, 5. [Google Scholar] [CrossRef]
- Dietzel, F.; Dieterich, P.; Dörries, F.; Gehring, H.; Wegerich, P. Invasive and non-invasive point-of-care testing and point-of-care monitoring of the hemoglobin concentration in human blood–how accurate are the data? Biomed. Tech. 2019, 64, 495–506. [Google Scholar] [CrossRef]
- Mahecic, T.T.; Dünser, M.; Meier, J. RBC transfusion triggers: Is there anything new? Transfus. Med. Hemotherapy 2020, 47, 361–369. [Google Scholar]
- Gehring, H. Point of Care Monitoring of Blood: Invasive and Non-Invasive Monitoring. 2010. Available online: https://www.semanticscholar.org/paper/Point-of-care-monitoring-of-blood-%3A-invasive-and-Gehring/4c429a47c6638a1bfeb2306f836847b0721fe005 (accessed on 22 November 2022).
- Macknet, M.R.; Allard, M.; Applegate, R.L.; Rook, J. The Accuracy of Noninvasive and Continuous Total Hemoglobin Measurement by Pulse CO-Oximetry in Human Subjects Undergoing Hemodilution. Anesthesia Analg. 2010, 111, 1424–1426. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lilot, M.; Murphy, L.S.; Sidhu, K.S.; Yu, Z.; Rinehart, J.; Cannesson, M. Accuracy of continuous noninvasive hemoglobin monitoring: A systematic review and meta-analysis. Anesth. Analg. 2014, 119, 332–346. [Google Scholar] [CrossRef]
- Riou, B. Continuous measurement of hemoglobin: Methodological approach and lessons for the future. J. Am. Soc. Anesthesiol. 2013, 118, 497–499. [Google Scholar] [CrossRef]
- Hiscock, R.; Kumar, D.; Simmons, S.W. Systematic Review and Meta-Analysis of Method Comparison Studies of Masimo Pulse Co-Oximeters (Radical-7™ or Pronto-7™) and HemoCue® Absorption Spectrometers (B-Hemoglobin or 201+) with Laboratory Haemoglobin Estimation. Anaesth. Intensiv. Care 2015, 43, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.J.; Shander, A.; Ramsay, M.A. Continuous noninvasive hemoglobin monitoring: A measured response to a critical review. Anesth. Analg. 2016, 122, 565. [Google Scholar] [CrossRef] [PubMed]
- Lamhaut, L.; Apriotesei, R.; Combes, X.; Lejay, M.; Carli, P.; Vivien, B. Comparison of the Accuracy of Noninvasive Hemoglobin Monitoring by Spectrophotometry (SpHb) and HemoCue® with Automated Laboratory Hemoglobin Measurement. Anesthesiology 2011, 115, 548–554. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, R.C.; Romano, G.M.; Abbate, R.; Corcione, A.; De Robertis, E. Accuracy and trending ability of hemoglobin measurement by the Pulse CO-Oximeter during vascular surgery. Int. J. Clin. Monit. Comput. 2019, 34, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Shabaninejad, H.; Ghadimi, N.; Sayehmiri, K.; Hosseinifard, H.; Azarfarin, R.; Gorji, H.A. Comparison of invasive and noninvasive blood hemoglobin measurement in the operating room: A systematic review and meta-analysis. J. Anesthesia 2019, 33, 441–453. [Google Scholar] [CrossRef]
- Coquin, J.; Bertarrex, A.; Dewitte, A.; Lefèvre, L.; Joannes-Boyau, O.; Fleureau, C.; Winnock, S.; Leuillet, S.; Janvier, G.; Ouattara, A. Accuracy of Determining Hemoglobin Level Using Occlusion Spectroscopy in Patients with Severe Gastrointestinal Bleeding. Anesthesiology 2013, 118, 640–648. [Google Scholar] [CrossRef]
- Coquin, J.; Dewitte, A.; Le Manach, Y.; Caujolle, M.; Joannes-Boyau, O.; Fleureau, C.; Janvier, G.; Ouattara, A. Precision of noninvasive hemoglobin-level measurement by pulse co-oximetry in patients admitted to intensive care units for severe gastrointestinal bleeds*. Crit. Care Med. 2012, 40, 2576–2582. [Google Scholar] [CrossRef]
- Young, M.F.; Raines, K.; Jameel, F.; Sidi, M.; Oliveira-Streiff, S.; Nwajei, P.; McGlamry, K.; Ou, J.; Oladele, A.; Suchdev, P.S. Non-invasive hemoglobin measurement devices require refinement to match diagnostic performance with their high level of usability and acceptability. PLoS ONE 2021, 16, e0254629. [Google Scholar] [CrossRef]
- DIN 58931: 2010–08; Haematology–Determination of Haemoglobin Concentration in Blood–Reference Method. German Institute for Standardisation: Berlin, Germany, 2010.
- DIN 58931:2021-09; Haematology—Determination of Haemoglobin Concentration in Blood—Reference Method. German Institute for Standardisation: Berlin, Germany, 2021.
- Gehring, H.; Hornberger, C.; Dibbelt, L.; Roth-Isigkeit, A.; Gerlach, K.; Schumacher, J.; Schmucker, P. Accuracy of point-of-care-testing (POCT) for determining hemoglobin concentrations. Acta Anaesthesiol. Scand. 2002, 46, 980–986. [Google Scholar] [CrossRef]
- German Medical Association. Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen–Rili-BÄK. Dtsch Arztebl Int. 2019, 116, 2422. [Google Scholar]
- HemoCue Hb 201+ Operating Manual. Available online: www.hemocue.com (accessed on 22 November 2022).
- Sanchis-Gomar, F.; Cortell-Ballester, J.; Pareja-Galeano, H.; Banfi, G.; Lippi, G. Hemoglobin Point-of-Care Testing: The HemoCue System. SLAS Technol. Transl. Life Sci. Innov. 2012, 18, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sysmex NX-9000 Instructions for Use. 2012. Available online: https://www.sysmex-europe.com (accessed on 22 November 2022).
- Oshiro, I.; Takenaka, T.; Maeda, J. New method for hemoglobin determination by using sodium lauryl sulfate (SLS). Clin. Biochem. 1982, 15, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Hemoglobin, P. AHD 575. 2014. Available online: https://www.agdbio.com/alkaline-hematin-d-ahd-method-cyanide-free/ (accessed on 22 November 2022).
- Anchinmane, V.; Sankhe, S. Evaluation of hemoglobin estimation with non-cyanide alkaline haematin D-575 method. Int. J. Res. Med. Sci. 2016, 4, 4297–4299. [Google Scholar] [CrossRef]
- Gehring, H.; Duembgen, L.; Peterlein, M.; Hagelberg, S.; Dibbelt, L. Hemoximetry as the “Gold Standard”? Error Assessment Based on Differences Among Identical Blood Gas Analyzer Devices of Five Manufacturers. Obstet. Anesthesia Dig. 2007, 105, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Measurement in Medicine: The Analysis of Method Comparison Studies. Statistician 1983, 32, 307–317. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Measuring agreement in method comparison studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.; Cui, J.I. Using the Bland–Altman method to measure agreement with repeated measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef]
- Abu-Arafeh, A.; Jordan, H.; Drummond, G. Reporting of method comparison studies: A review of advice, an assessment of current practice, and specific suggestions for future reports. Br. J. Anaesth. 2016, 117, 569–575. [Google Scholar] [CrossRef]
- A Sadler, W. Using the variance function to generalize Bland–Altman analysis. Ann. Clin. Biochem. Int. J. Lab. Med. 2018, 56, 198–203. [Google Scholar] [CrossRef]
- Gerke, O. Reporting Standards for a Bland–Altman Agreement Analysis: A Review of Methodological Reviews. Diagnostics 2020, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.J.; Gravenstein, N.; Morey, T.E. Noninvasive hemoglobin monitoring: How accurate is enough? Anesth. Analg. 2013, 117, 902–907. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Candidate List of Substances of Very High Concern for Authorisation, in Evaluation, Authorisation and Restriction of Chemicals; European Chemicals Agency: Helsinki, Finland, 2019.
- Neufeld, L.M.; Larson, L.M.; Kurpad, A.; Mburu, S.; Martorell, R.; Brown, K.H. Hemoglobin concentration and anemia diagnosis in venous and capillary blood: Biological basis and policy implications. Ann. N. Y. Acad. Sci. 2019, 1450, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Rappaport, A.I.; Karakochuk, C.D.; Hess, S.Y.; Whitehead, J.R.D.; Namaste, S.M.L.; Dary, O.; Parker, M.E.; Neufeld, L.M.; Larson, L.M.; Newton, S.; et al. Variability in haemoglobin concentration by measurement tool and blood source: An analysis from seven countries. J. Clin. Pathol. 2020, 74, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.D., Jr.; Mei, Z.; Mapango, C.; Jefferds, M.E.D. Methods and analyzers for hemoglobin measurement in clinical laboratories and field settings. Ann. N. Y. Acad. Sci. 2019, 1450, 147–171. [Google Scholar] [CrossRef]
- Berkow, L. Factors affecting hemoglobin measurement. J. Clin. Monit. Comput. 2013, 27, 499–508. [Google Scholar] [CrossRef]
- Hayashi, K.; Hitosugi, T.; Kawakubo, Y.; Kitamoto, N.; Yokoyama, T. Influence of measurement principle on total hemoglobin value. BMC Anesthesiol. 2020, 20, 81–87. [Google Scholar] [CrossRef]
- Wittenmeier, E.; Lesmeister, L.; Pirlich, N.; Dette, F.; Schmidtmann, I.; Mildenberger, E. Assessment of haemoglobin measurement by several methods–blood gas analyser, capillary and venous HemoCue®, non-invasive spectrophotometry and laboratory assay–in term and preterm infants. Anaesthesia 2019, 74, 197–202. [Google Scholar] [CrossRef]
- Zatloukal, J.; Pouska, J.; Kletecka, J.; Pradl, R.; Benes, J. Comparison of the accuracy of hemoglobin point of care testing using HemoCue and GEM Premier 3000 with automated hematology analyzer in emergency room. Int. J. Clin. Monit. Comput. 2015, 30, 949–956. [Google Scholar] [CrossRef]
- Avcioglu, G.; Nural, C.; Yilmaz, F.M.; Baran, P.; Erel, Ö.; Yilmaz, G. Comparison of noninvasive and invasive point-of-care testing methods with reference method for hemoglobin measurement. J. Clin. Lab. Anal. 2017, 32, e22309. [Google Scholar] [CrossRef]
- John, B.M.; Jamal, A.Z. Efficacy of noninvasive hemoglobin measurement by pulse co-oximetry in neonates. J. Clin. Neonatol. 2020, 9, 57. [Google Scholar] [CrossRef]
- Khalafallah, A.; Chilvers, C.; Thomas, M.; Sexton, M.; Vialle, M.; Robertson, I. Usefulness of non-invasive spectrophotometric haemoglobin estimation for detecting low haemoglobin levels when compared with a standard laboratory assay for preoperative assessment. Br. J. Anaesth. 2015, 114, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.T.; Ebrahim, A. Adequacy of physician documentation of red blood cell transfusion and correlation with assessment of transfusion appropriateness. Arch. Pathol. Lab. Med. 2006, 130, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, E.; Prajapati, S.; Avadhani, V.; Annen, K.; Friedman, M.T. Adequacy of physician documentation and correlation with assessment of transfusion appropriateness: A follow-up study in the setting of prospective audits and patient blood management. Transfusion 2016, 57, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists Task Force on Perioperative Blood Management. Practice Guidelines for Perioperative Blood Management: An Updated Report by the American Society of Anesthesiologists Task Force on Perioperative Blood Management*. Anesthesiology 2015, 122, 241–275. [Google Scholar] [CrossRef]
- Meybohm, P.; Richards, T.; Isbister, J.; Hofmann, A.; Shander, A.; Goodnough, L.T.; Muñoz, M.; Gombotz, H.; Weber, C.F.; Choorapoikayil, S.; et al. Patient Blood Management Bundles to Facilitate Implementation. Transfus. Med. Rev. 2016, 31, 62–71. [Google Scholar] [CrossRef]
- Tang, B.; Yu, X.; Xu, L.; Zhu, A.; Zhang, Y.; Huang, Y. Continuous noninvasive hemoglobin monitoring estimates timing for detecting anemia better than clinicians: A randomized controlled trial. BMC Anesthesiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Fakhry, S.M.; Fata, P. How low is too low? Cardiac risks with anemia. Crit. Care 2004, 8, S11–S14. [Google Scholar] [CrossRef][Green Version]
- Spolverato, G.; Kim, Y.; Ejaz, A.; Frank, S.M.; Pawlik, T.M. Effect of Relative Decrease in Blood Hemoglobin Concentrations on Postoperative Morbidity in Patients Who Undergo Major Gastrointestinal Surgery. JAMA Surg. 2015, 150, 949–956. [Google Scholar] [CrossRef]
- Shander, A.; Javidroozi, M.; Naqvi, S.; Aregbeyen, O.; Çaylan, M.; Demir, S.; Juhl, A. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion 2014, 54, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.; Park, L.; Gilbert, R.W.D.; Ahn, H. Intraoperative red blood cell transfusion decision-making: A systematic review of guidelines. Ann. Surg. 2021, 274, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Carson, J.L.; Stanworth, S.J.; A Dennis, J.; Trivella, M.; Roubinian, N.; A Fergusson, D.; Triulzi, D.; Dorée, C.; Hébert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst. Rev. 2021, 12, CD002042. [Google Scholar] [CrossRef]
- Man, J.; Zielinski, M.D.; Das, D.; Sir, M.Y.; Wutthisirisart, P.; Camazine, M.; Pasupathy, K.S. Non-invasive Hemoglobin Measurement Predictive Analytics with Missing Data and Accuracy Improvement Using Gaussian Process and Functional Regression Model. J. Med. Syst. 2022, 46, 72. [Google Scholar] [CrossRef]
- Wegerich, P.; Gehring, H. An In vitro Laboratory Investigation on Layer Thickness-Independent Prediction of the Hemoglobin Concentration. Curr. Dir. Biomed. Eng. 2018, 4, 351–354. [Google Scholar] [CrossRef]
- Gehring, H.; Wegerich, P. Validation of A Laboratory Setup for Generating A High Rate of Data Sets as Training Unit of Machine Learning Concerning the Optical Properties of Hemoglobin under Clinical Conditions: Preliminary Results. ASA 2018. Available online: https://www.abstractsonline.com/pp8/#!/4593/presentation/5908 (accessed on 22 November 2022).
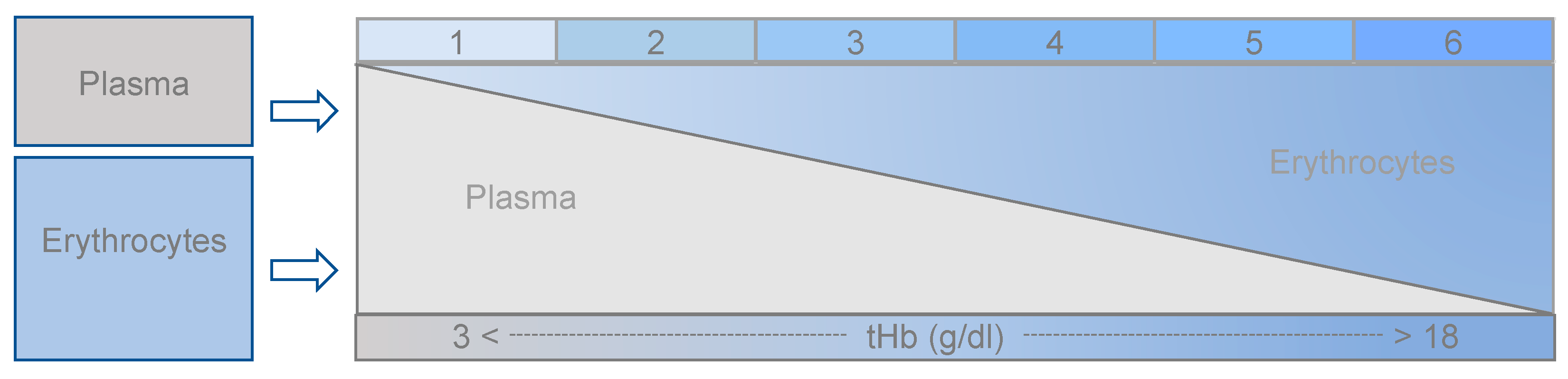
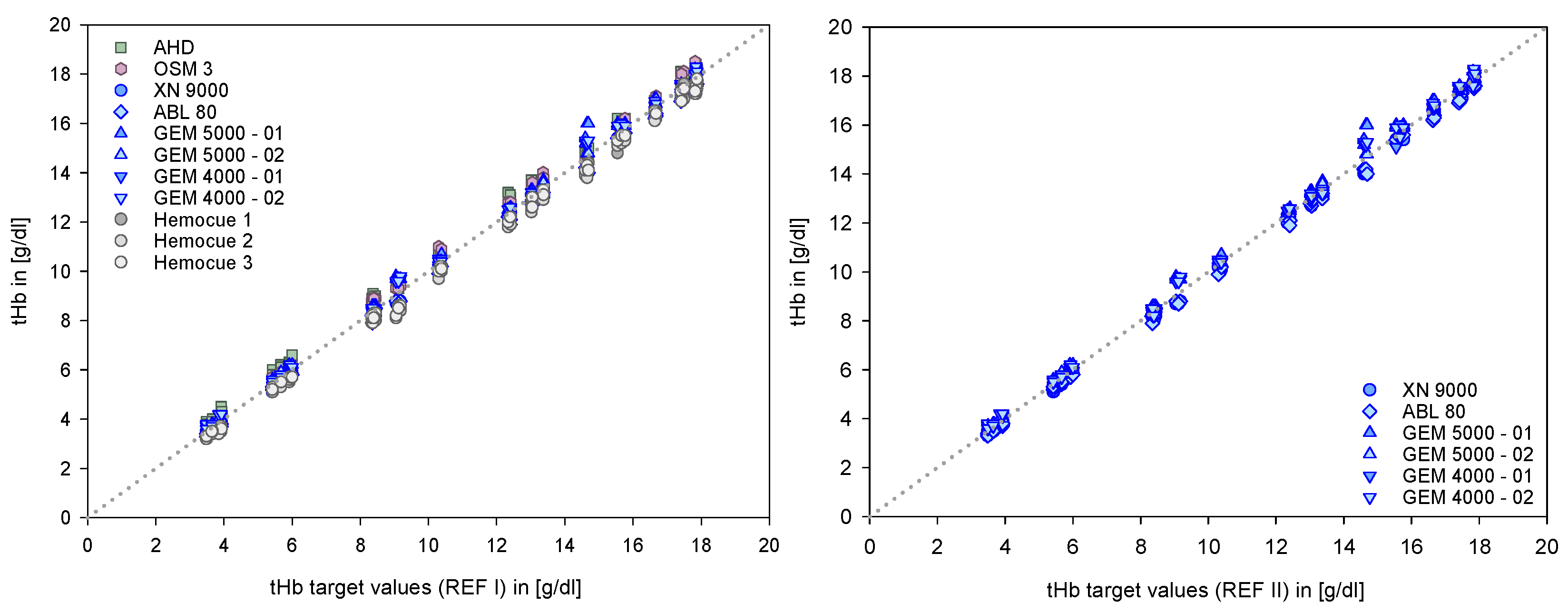
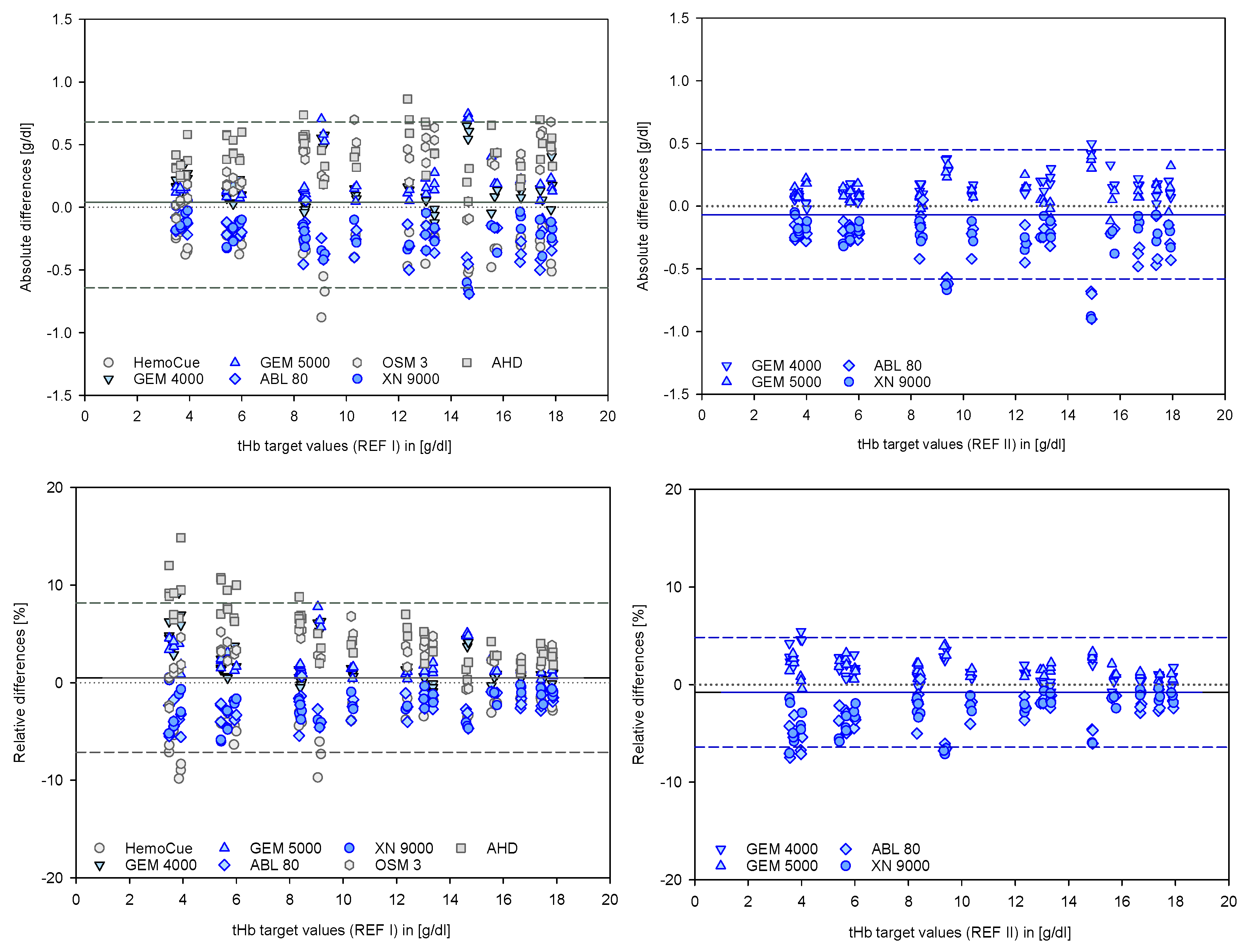




| Device | Manufacturer | n | Serial Number | N | System | Method | Measurement |
|---|---|---|---|---|---|---|---|
| GEM 4000 | Instrumentation Laboratory * | 2 | 10033403 | 3 | BGA | CO-oximeter | optical |
| 10043637 | 3 | BGA | CO-oximeter | optical | |||
| GEM 5000 | Instrumentation Laboratory * | 2 | 16080371 | 3 | BGA | CO-oximeter | optical |
| 18122404 | 3 | BGA | CO-oximeter | optical | |||
| ABL 80 COOX | Radiometer | 1 | 305040 | 3 | Stand-alone | CO-oximeter | optical |
| OSM 3 | Radiometer | 1 | 89R62N03 | 3 | Stand-alone | CO-oximeter | optical |
| Sysmex XN 9000 | Sysmex Europe GmbH | 1 | 12784 + 15591 | 3 | Analyzer | SLS reagent | optical |
| HemoCue 201+ | HemoCue AB, Sweden | 3 | 945013284 | 3 | POCT | Microcuvette | optical |
| 619012266 | 3 | POCT | Microcuvette | optical | |||
| 619012265 | 3 | POCT | Microcuvette | optical | |||
| Norm | DIN 58931:2010-08 | 1 | 3 | Laboratory | AHD reagent | optical |
| All Data | Mean of the Identical Devices | ||||
|---|---|---|---|---|---|
| Devices & Method | Device Nr. | n = | Assigned to | n = | Assigned to |
| GEM 4000 | 01 | 54 | REF I | 54 | REF II |
| 02 | 54 | REF I | |||
| GEM 5000 | 01 | 54 | REF I | 54 | REF II |
| 02 | 54 | REF I | |||
| ABL 80 CO-OX | 01 | 54 | REF I | 54 | REF II |
| OSM 3 | 01 | 54 | REF I | ||
| XN 9000 | 01 | 54 | REF I | 54 | REF II |
| HemCue 201+ | 01 | 54 | REF I | ||
| 02 | 54 | REF I | |||
| 03 | 54 | REF I | |||
| AHD | 01 | 54 | REF I | ||
| Total n = | 594 | 216 |
| Devices | Deming Slope | Deming Intercept | PB Slope | PB Intercept | RMSE | RSQ |
|---|---|---|---|---|---|---|
| Hemocue 1 | 1.002 | −0.321 | 1 | −0.295 | 0.327 | 0.999 |
| Hemocue 2 | 0.983 | −0.232 | 0.983 | −0.196 | 0.434 | 0.999 |
| Hemocue 3 | 0.997 | −0.246 | 0.996 | −0.2 | 0.3 | 0.999 |
| ABL 80 | 0.987 | −0.109 | 0.986 | −0.104 | 0.266 | 1 |
| XN 9000 | 0.992 | −0.149 | 0.993 | −0.123 | 0.264 | 0.999 |
| GEM 5000 1 | 1.018 | 0.066 | 1.011 | 0.082 | 0.357 | 0.998 |
| GEM 5000 2 | 1 | 0.151 | 0.999 | 0.115 | 0.193 | 0.999 |
| GEM 4000 1 | 1 | 0.162 | 0.996 | 0.157 | 0.23 | 0.999 |
| GEM 4000 2 | 1.001 | 0.139 | 0.998 | 0.151 | 0.231 | 0.999 |
| AHD | 1.002 | 0.423 | 1.004 | 0.408 | 0.46 | 0.999 |
| OSM 3 | 1.023 | 0.064 | 1.027 | 0.023 | 0.357 | 0.999 |
| Absolute Differences in [g/dl] | Relative Differences in [%] | |||||
|---|---|---|---|---|---|---|
| All Device in Test | Mean | SD | 1.96 SD | Mean | SD | 1.96 SD |
| Hemocue—REF I | −0.33 | 0.14 | 0.28 | −3.93 | 2.21 | 4.43 |
| GEM 4000—REF I | 0.16 | 0.18 | 0.35 | 2.07 | 2.29 | 4.59 |
| GEM 5000—REF I | 0.20 | 0.18 | 0.36 | 2.22 | 1.69 | 3.38 |
| ABL 80—REF I | −0.24 | 0.14 | 0.28 | −2.69 | 1.38 | 2.77 |
| XN 9000—REF I | −0.23 | 0.15 | 0.30 | −2.52 | 1.67 | 3.34 |
| OSM 3—REF I | 0.30 | 0.21 | 0.42 | 2.88 | 1.97 | 3.93 |
| AHD—REF I | 0.44 | 0.16 | 0.33 | 5.50 | 3.17 | 6.35 |
| Clinically relevant devices | ||||||
| GEM 4000—REF II | 0.12 | 0.11 | 0.21 | 1.48 | 1.37 | 2.74 |
| GEM 5000—REF II | 0.16 | 0.11 | 0.23 | 1.66 | 0.99 | 1.98 |
| ABL 80—REF II | −0.28 | 0.18 | 0.36 | −3.22 | 1.98 | 3.97 |
| XN 9000—REF II | −0.27 | 0.20 | 0.40 | −3.06 | 1.99 | 3.98 |
| References | ||
|---|---|---|
| Origin of the blood sample | ||
| Capillary vs. arterial vs. venous | [15,41,42,43] | |
| Population dependent | [3,20,41,42] | |
| Blood collection technique | ||
| Preanalytical failure | [9,23,42,44] | |
| Choice of anticoagulants | [23] | |
| Methods of Measurements | ||
| Standard and reference methods | [36,43,45,46,47] | |
| Invasive and non-invasive measurements (POCT, POCM) | [1,11,20,46,48,49,50] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawschenko, E.; Schaller, T.; Kern, B.; Bode, B.; Dörries, F.; Kusche-Vihrog, K.; Gehring, H.; Wegerich, P. Current Status of Measurement Accuracy for Total Hemoglobin Concentration in the Clinical Context. Biosensors 2022, 12, 1147. https://doi.org/10.3390/bios12121147
Stawschenko E, Schaller T, Kern B, Bode B, Dörries F, Kusche-Vihrog K, Gehring H, Wegerich P. Current Status of Measurement Accuracy for Total Hemoglobin Concentration in the Clinical Context. Biosensors. 2022; 12(12):1147. https://doi.org/10.3390/bios12121147
Chicago/Turabian StyleStawschenko, Elena, Tim Schaller, Benjamin Kern, Berit Bode, Frank Dörries, Kristina Kusche-Vihrog, Hartmut Gehring, and Philipp Wegerich. 2022. "Current Status of Measurement Accuracy for Total Hemoglobin Concentration in the Clinical Context" Biosensors 12, no. 12: 1147. https://doi.org/10.3390/bios12121147
APA StyleStawschenko, E., Schaller, T., Kern, B., Bode, B., Dörries, F., Kusche-Vihrog, K., Gehring, H., & Wegerich, P. (2022). Current Status of Measurement Accuracy for Total Hemoglobin Concentration in the Clinical Context. Biosensors, 12(12), 1147. https://doi.org/10.3390/bios12121147





