Current Status and Future Perspectives of Lactate Dehydrogenase Detection and Medical Implications: A Review
Abstract
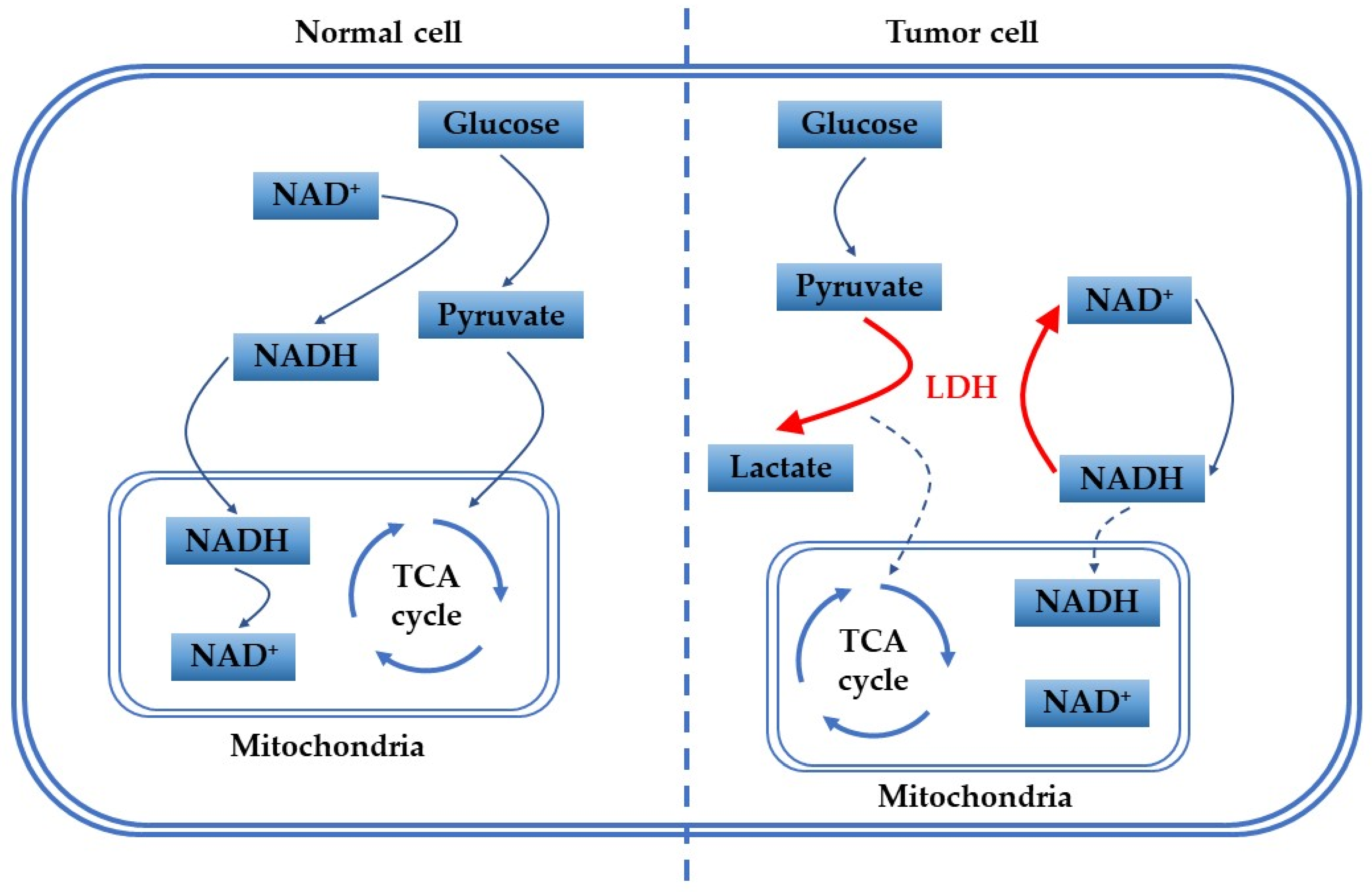
1. Introduction
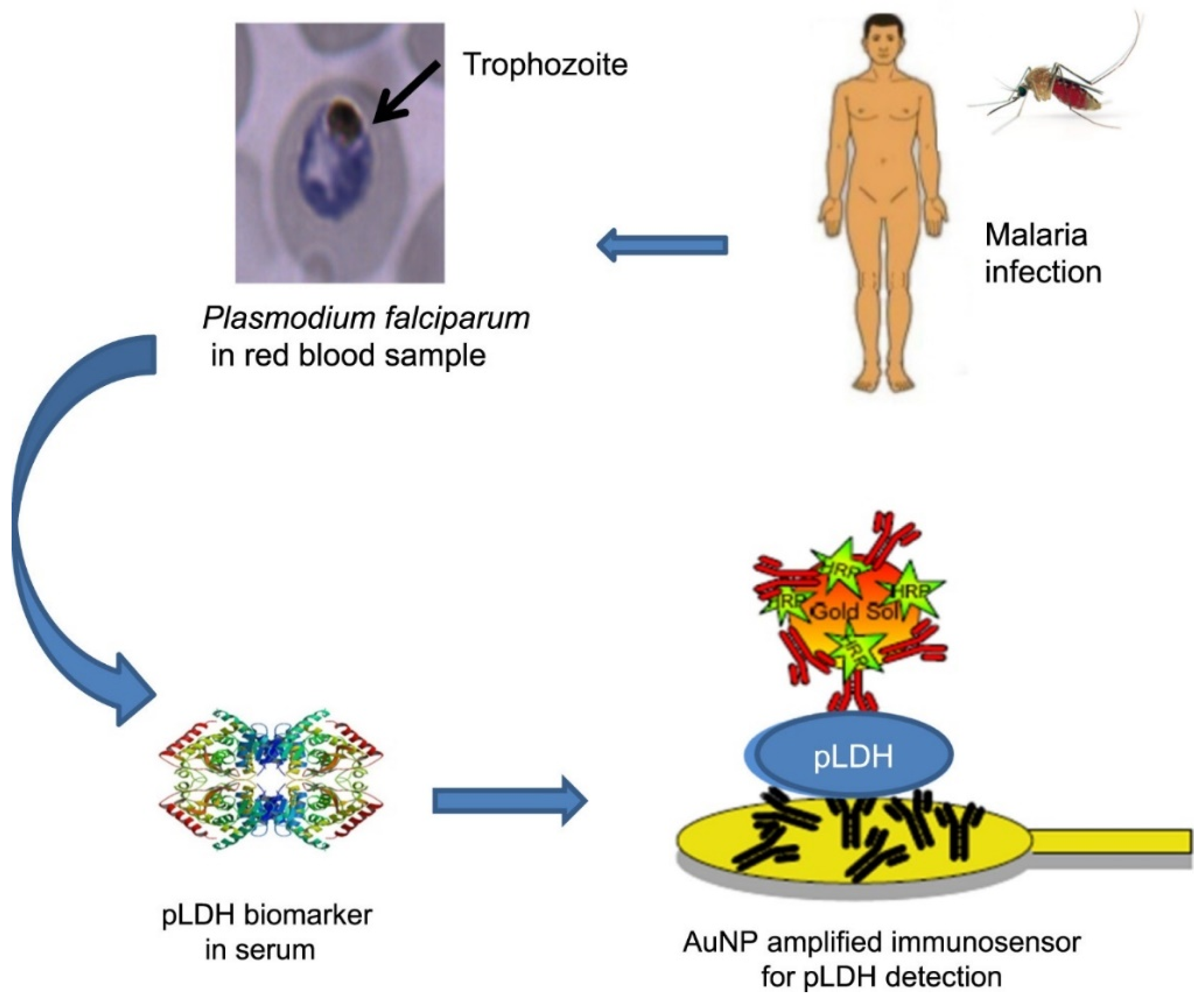
2. Various Substances Involved in LDH Testing
3. LDH Detection Methods
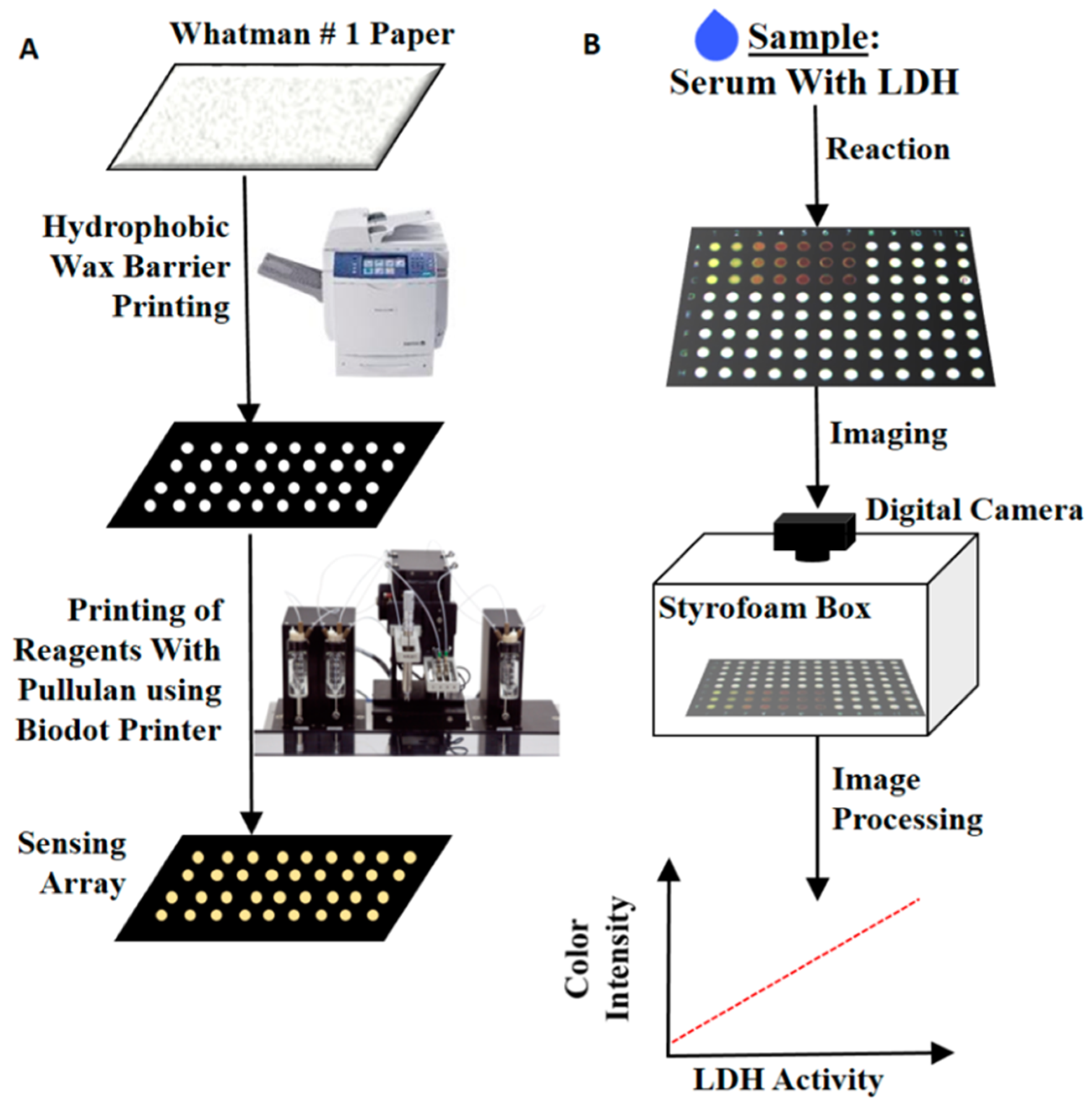
3.1. Colorimetric Method
3.2. Spectrophotometric Method
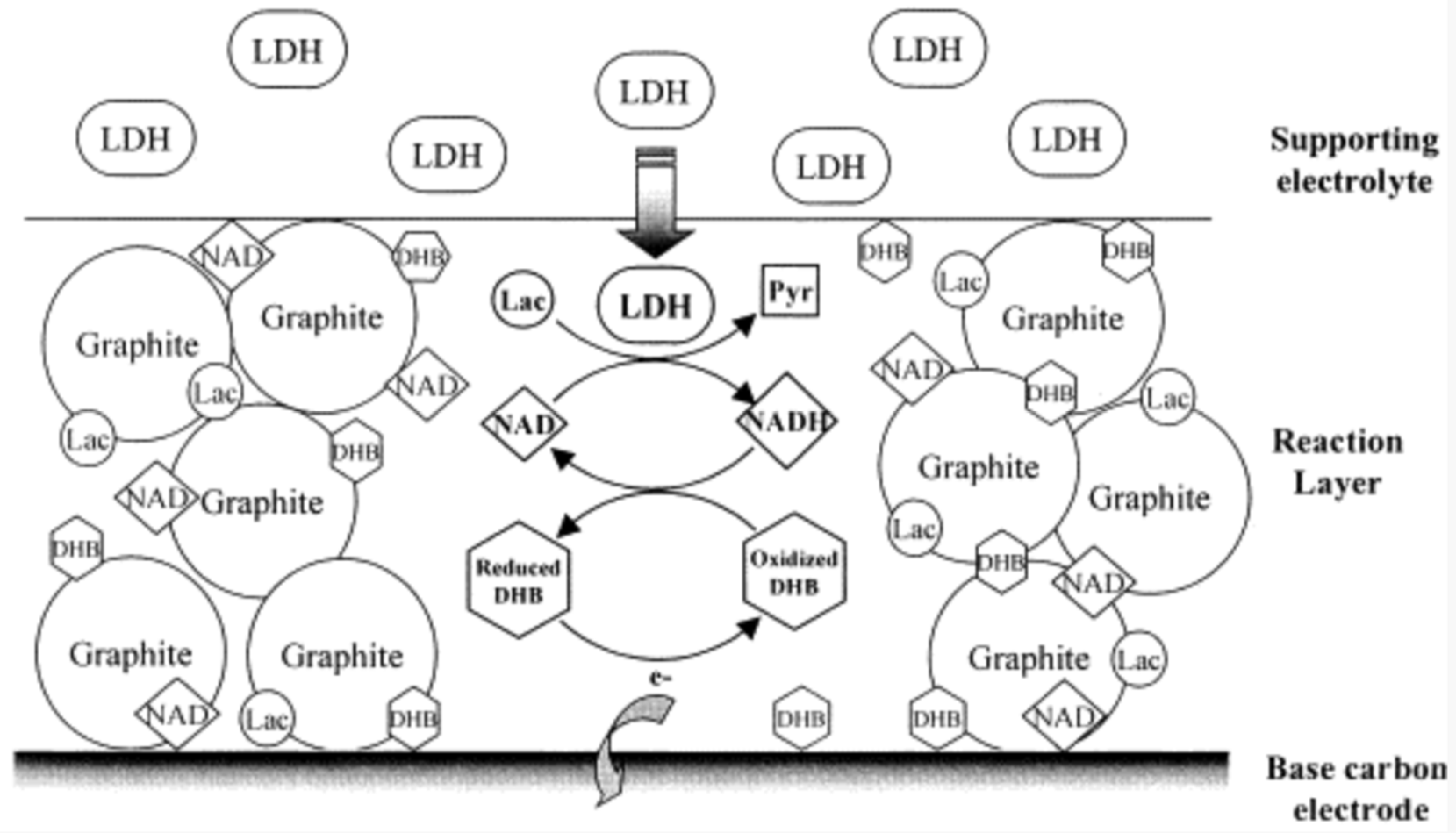
3.3. Electrochemical Measurement
3.4. Fluorometric Measurement
4. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef] [PubMed]
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate detection sensors for food, clinical and biological applications: A review. Environ. Chem. Lett. 2021, 19, 1135–1152. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V. Recent Update on Human Lactate Dehydrogenase Enzyme 5 (hLDH5) Inhibitors: A Promising Approach for Cancer Chemotherapy. J. Med. Chem. 2016, 59, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Allemailem, K.S.; Alhumaydhi, F.A.; Gowder, S.J.T.; Rahmani, A.H. The Biochemical and Clinical Perspectives of Lactate Dehydrogenase: An Enzyme of Active Metabolism. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 855–868. [Google Scholar] [CrossRef]
- Mishra, D.; Banerjee, D. Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvironment. Cancers 2019, 11, 750. [Google Scholar] [CrossRef]
- Donnelly, R.P.; Finlay, D.K. Glucose, glycolysis and lymphocyte responses. Mol. Immunol. 2015, 68, 513–519. [Google Scholar] [CrossRef]
- Alam, F.; RoyChoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the biosensors for lactate and pyruvate detection for medical applications: A review. TrAC Trends Anal. Chem. 2018, 110, 160–172. [Google Scholar] [CrossRef]
- Urbańska, K.; Orzechowski, A. Unappreciated Role of LDHA and LDHB to Control Apoptosis and Autophagy in Tumor Cells. Int. J. Mol. Sci. 2019, 20, 2085. [Google Scholar] [CrossRef]
- Kolappan, S.; Shen, D.L.; Mosi, R.; Sun, J.; McEachern, E.J.; Vocadlo, D.J.; Craig, L. Structures of lactate dehydrogenase A (LDHA) in apo, ternary and inhibitor-bound forms. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 185–195. [Google Scholar] [CrossRef]
- Patel, C.H.; Powell, J.D. Warburg meets epigenetics. Science 2016, 354, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qi, M.; Yang, M. Fluorescence determination of lactate dehydrogenase activity based on silicon quantum dots. Acta Part A Mol. Biomol. Spectrosc. 2021, 268, 120697. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tan, M.; Cai, Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015, 356, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.H.; Letts, J.A.; Maldonado, M. Structural insights into the assembly and the function of the plant oxidative phosphorylation system. New Phytol. 2022, 235, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Sukonina, V.; Ma, H.X.; Zhang, W.; Bartesaghi, S.; Subhash, S.; Heglind, M.; Foyn, H.; Betz, M.J.; Nilsson, D.; Lidell, M.E.; et al. FOXK1 and FOXK2 regulate aerobic glycolysis. Nature 2019, 566, 279–283. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Gentric, G.; Mieulet, V.; Mechta-Grigoriou, F. Heterogeneity in Cancer Metabolism: New Concepts in an Old Field. Antioxid Redox Signal 2017, 26, 462–485. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Warburg Effect and Redox Balance. Science 2011, 334, 1219–1220. [Google Scholar] [CrossRef]
- Wortley, C. Warburg: Father of the metabolic approach to cancer. Lancet Gastroenterol. Hepatol. 2022, 7, 911. [Google Scholar] [CrossRef]
- Sanderson, S.M.; Locasale, J.W. Revisiting the Warburg Effect: Some Tumors Hold Their Breath. Cell Metab. 2018, 28, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Hönigova, K.; Navratil, J.; Peltanova, B.; Polanska, H.H.; Raudenska, M.; Masarik, M. Metabolic tricks of cancer cells. Biochim Biophys Acta Rev Cancer 2022, 1877, 188705. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yuan, M.; Dai, J.D.; Lin, Q.Y.; Lin, Y.H.; Wang, W.L.; Jiang, Y.F.; Wang, H.H.; Zhao, F.; Wu, J.Y.; et al. Dual inhibition of glycolysis and oxidative phosphorylation by aptamer-based artificial enzyme for synergistic cancer therapy. Nano Res. 2022, 15, 6278–6287. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Sun, Y.T.; Protopopova, M.; Gera, S.; Bandi, M.; Bristow, C.; McAfoos, T.; Morlacchi, P.; Ackroyd, J.; Agip, A.N.A.; et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat. Med. 2018, 24, 1036–1046. [Google Scholar] [CrossRef]
- Xue, D.; Xu, Y.; Kyani, A.; Roy, J.; Dai, L.; Sun, D.; Neamati, N. Multiparameter Optimization of Oxidative Phosphorylation Inhibitors for the Treatment of Pancreatic Cancer. J. Med. Chem. 2022, 65, 3404–3419. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, J.; Wang, W.; Liu, Z.; Chen, M.; Hu, X.; Zeng, L.; Zheng, C.; Song, H.; Zhang, Q. Drug self-delivery nanorods enhance photodynamic therapy of triple-negative breast cancer by inhibiting oxidative phosphorylation. Int. J. Pharm. 2022, 621, 121775. [Google Scholar] [CrossRef] [PubMed]
- Boreel, D.F.; Span, P.N.; Heskamp, S.; Adema, G.J.; Bussink, J. Targeting Oxidative Phosphorylation to Increase the Efficacy of Radio- and Immune-Combination Therapy. Clin. Cancer. Res. 2021, 27, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Comandatore, A.; Franczak, M.; Smolenski, R.T.; Morelli, L.; Peters, G.J.; Giovannetti, E. Lactate Dehydrogenase and its clinical significance in pancreatic and thoracic cancers. Semin. Cancer Biol. 2022, 86, 93–100. [Google Scholar] [CrossRef]
- Dang, C.V. Links between metabolism and cancer. Genes Dev. 2012, 26, 877–890. [Google Scholar] [CrossRef]
- Kopperschlager, G.; Kirchberger, J. Methods for the separation of lactate dehydrogenases and clinical significance of the enzyme. J. Chromatogr. B 1996, 684, 25–49. [Google Scholar] [CrossRef]
- Schwartz, M.K. Lactic dehydrogenase. An old enzyme reborn as a cancer marker? Am. J. Clin. Pathol. 1991, 96, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Shi, L.L.; Lu, P.; Yao, S.; Xu, H.L.; Hu, J.J.; Liang, X.; Liang, X.J.; Wei, S.Z. Creation of a Novel Nomogram Based on the Direct Bilirubin-To-Indirect Bilirubin Ratio and Lactate Dehydrogenase Levels in Resectable Colorectal Cancer. Front. Mol. Biosci. 2021, 8, 751506. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.F.; Lu, P.; Liang, X.J.; Wei, S.Z. Prognostic role of apolipoprotein and lactate dehydrogenase levels in resectable colorectal cancer. J. Clin. Oncol. 2021, 39, e15541. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Li, Y.; Yan, X.; Song, Q.; Wang, G.Q.; Hu, Y.; Jiao, S.C.; Wang, J.L. Pretreatment lactate dehydrogenase may predict outcome of advanced non small-cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Cancer Med. 2019, 8, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Tjokrowidjaja, A.; Lord, S.J.; John, T.; Lewis, C.R.; Kok, P.S.; Marschner, I.C.; Lee, C.K. Pre- and on-treatment lactate dehydrogenase as a prognostic and predictive biomarker in advanced non-small cell lung cancer. Cancer 2022, 128, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Agarwala, S.S.; Keilholz, U.; Gilles, E.; Bedikian, A.Y.; Wu, J.; Kay, R.; Stein, C.A.; Itri, L.M.; Suciu, S.; Eggermont, A.M.M. LDH correlation with survival in advanced melanoma from two large, randomised trials (Oblimersen GM301 and EORTC 18951). Eur. J. Cancer 2009, 45, 1807–1814. [Google Scholar] [CrossRef]
- Majithia, N.; Manana, A.V.; Yan, Y.; Kottschade, L.A.; Dronca, R.S.; Block, M.S.; Nevala, W.K.; Markovic, S. The prognostic role of preoperative serum lactate dehydrogenase (LDH) in patients with resected advanced melanoma. J. Clin. Oncol. 2017, 35, e21054. [Google Scholar] [CrossRef]
- Girgis, H.; Masui, O.; White, N.M.A.; Scorilas, A.; Rotondo, F.; Seivwright, A.; Gabril, M.; Filter, E.R.; Girgis, A.H.A.; Bjarnason, G.A.; et al. Lactate Dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol. Cancer 2014, 13, 101. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Zhu, D.; JiRiGaLa; Yu, D.; Wang, C.; WuYunBiLiGe; Amin.; ZhiHong; Yu, H.; et al. Prognostic role of pretreatment lactate dehydrogenase in patients with metastatic renal cell carcinoma: A systematic review and meta-analysis. Int. J. Surg. 2020, 79, 66–73. [Google Scholar] [CrossRef]
- Cui, Z.L.; Li, Y.; Gao, Y.N.; Kong, L.Y.; Lin, Y.F.; Chen, Y. Cancer-testis antigen lactate dehydrogenase C4 in hepatocellular carcinoma: A promising biomarker for early diagnosis, efficacy evaluation and prognosis prediction. Aging 2020, 12, 19455–19467. [Google Scholar] [CrossRef]
- Wu, S.J.; Lin, Y.X.; Ye, H.; Xiong, X.Z.; Li, F.Y.; Cheng, N.S. Prognostic value of alkaline phosphatase, gamma-glutamyl transpeptidase and lactate dehydrogenase in hepatocellular carcinoma patients treated with liver resection. Int. J. Surg. 2016, 36, 143–151. [Google Scholar] [CrossRef] [PubMed]
- de la Serna, E.; Arias-Alpizar, K.; Borgheti-Cardoso, L.N.; Sanchez-Cano, A.; Sulleiro, E.; Zarzuela, F.; Bosch-Nicolau, P.; Salvador, F.; Molina, I.; Ramirez, M.; et al. Detection of Plasmodium falciparum malaria in 1 h using a simplified enzyme-linked immunosorbent assay. Anal. Chim. Acta 2021, 1152, 338254. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kang, T.; Kwak, K.J.; Park, K.; Yi, S.Y.; Lee, U.J.; Shin, Y.B.; Jeong, J. Simple, rapid, and accurate malaria diagnostic platform using microfluidic-based immunoassay of Plasmodium falciparum lactate dehydrogenase. Nano Converg. 2020, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, K.M.; Jeon, W.; Jo, H.; Shim, Y.B.; Ban, C. A highly sensitive aptasensor towards Plasmodium lactate dehydrogenase for the diagnosis of malaria. Biosens. Bioelectron. 2012, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, L.J.; Wang, J.J.; Chen, J.; Chen, J.; Wang, Y.M. Clinical value of jointly detection serum lactate dehydrogenase/pleural fluid adenosine deaminase and pleural fluid carcinoembryonic antigen in the identification of malignant pleural effusion. J. Clin. Lab. Anal. 2017, 31, e22106. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oak, C.H.; Jung, M.H.; Jang, T.W.; Je, H.; Choi, J. High Level of ADA in Pleural Effusion, Diagnostic Value of CEA, NSE, Amylase, Glucose, and Lactic Dehydrogenase for the Differential Diagnosis of Pleural Effusion. Chest 2016, 150, 558A. [Google Scholar] [CrossRef]
- Kelderman, S.; Heemskerk, B.; van Tinteren, H.; van den Brom, R.R.H.; Hospers, G.A.P.; van den Eertwegh, A.J.M.; Kapiteijn, E.W.; de Groot, J.W.B.; Soetekouw, P.; Jansen, R.L.; et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer. Immunol. Immun. 2014, 63, 449–458. [Google Scholar] [CrossRef]
- Diem, S.; Kasenda, B.; Spain, L.; Martin-Liberal, J.; Marconcini, R.; Gore, M.; Larkin, J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br. J. Cancer 2016, 114, 256–261. [Google Scholar] [CrossRef]
- Chen, X.Y.; Huang, M.Y.; Xiao, Z.W.; Yang, S.; Chen, X.Q. Lactate dehydrogenase elevations is associated with severity of COVID-19: A meta-analysis. Crit. Care 2020, 24, 459. [Google Scholar] [CrossRef]
- Stoeckle, K.; Witting, B.; Kapadia, S.; An, A.; Marks, K. Elevated inflammatory markers are associated with poor outcomes in COVID-19 patients treated with remdesivir. J. Med. Virol. 2022, 94, 384–387. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, M.F.; Duan, L.M.; Wu, F.; Hu, G.R.; Wang, Z.H.; Huang, Q.; Liao, T.T.; Xu, J.J.; Ma, Y.L.; et al. Development and validation of a risk factor-based system to predict short-term survival in adult hospitalized patients with COVID-19: A multicenter, retrospective, cohort study. Crit. Care 2020, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Gibellini, L.; De Biasi, S.; Meschiari, M.; Gozzi, L.; Paolini, A.; Borella, R.; Mattioli, M.; Lo Tartaro, D.; Fidanza, L.; Neroni, A.; et al. Plasma Cytokine Atlas Reveals the Importance of TH2 Polarization and Interferons in Predicting COVID-19 Severity and Survival. Front. Immunol. 2022, 13, 842150. [Google Scholar] [CrossRef] [PubMed]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R.M. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef]
- Jin, Z.M.; Zheng, M.; Shi, J.C.; Ye, X.C.; Cheng, F.; Chen, Q.L.; Huang, J.P.; Jiang, X.G. Correlation Analysis Between Serum Uric Acid, Prealbumin Level, Lactate Dehydrogenase, and Severity of COVID-19. Front. Mol. Biosci. 2021, 8, 615837. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yao, L.; Wang, Y.; Zhu, X.Y.; Wang, X.F.; Tang, P.J.; Chen, C. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir. Res. 2020, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, H.D.; Mu, S.C.; Wei, W.; Jin, C.Y.; Tong, C.Y.; Song, Z.J.; Zha, Y.F.; Xue, Y.; Gu, G.R. Lactate dehydrogenase, an independent risk factor of severe COVID-19 patients: A retrospective and observational study. Aging 2020, 12, 11245–11258. [Google Scholar] [CrossRef]
- Poggiali, E.; Zaino, D.; Immovilli, P.; Rovero, L.; Losi, G.; Dacrema, A.; Nuccetelli, M.; Vadacca, G.B.; Guidetti, D.; Vercelli, A.; et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin. Chim. Acta 2020, 509, 135–138. [Google Scholar] [CrossRef]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19—A systematic review. Life. Sci. 2020, 254, 117788. [Google Scholar] [CrossRef]
- Neto, F.L.; Salzstein, G.A.; Cortez, A.L.; Bastos, T.L.; Baptista, F.V.D.; Moreira, J.A.; Lauterbach, G.P.; de Oliveira, J.C.; de Assis, F.C.; Aguiar, M.R.A.; et al. Comparative assessment of mortality risk factors between admission and follow-up models among patients hospitalized with COVID-19. Int. J. Infect. Dis. 2021, 105, 723–729. [Google Scholar] [CrossRef]
- Chamchoy, K.; Pakotiprapha, D.; Pumirat, P.; Leartsakulpanich, U.; Boonyuen, U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019, 20, 4. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Yoon, J. Fluorescent and colorimetric chemosensors for detection of nucleotides, FAD and NADH: Highlighted research during 2004–2010. Chem. Soc. Rev. 2011, 40, 2222–2235. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, T.; Salari, R.; Amjadi, M. Morphology transition of Ag nanoprisms as a platform to design a dual sensor for NADH sensitive assay. J. Photochem. Photobiol. A: Chem. 2022, 431, 114043. [Google Scholar] [CrossRef]
- Monosik, R.; dos Santos, V.B.; Angnes, L. A simple paper-strip colorimetric method utilizing dehydrogenase enzymes for analysis of food components. Anal. Methods 2015, 7, 8177–8184. [Google Scholar] [CrossRef]
- Kjeld, M. An automated colorimetric method for the estimation of lactate dehydrogenase activity in serum. Scand. J. Clin. Lab. Invest. 1972, 29, 421–425. [Google Scholar] [CrossRef]
- Babson, A.L.; Phillips, G.E. A rapid colorimetric assay for serum lactic dehydrogenase. Clin. Chim. Acta. 1965, 12, 210–215. [Google Scholar] [CrossRef]
- Burd, J.F.; Usategui-Gomez, M. A colorimetric assay for serum lactate dehydrogenase. Clin. Chim. Acta 1973, 46, 223–227. [Google Scholar] [CrossRef]
- Debnam, P.M.; Shearer, G. Colorimetric assay for substrates of NADP+−dependent dehydrogenases based on reduction of a tetrazolium dye to its soluble formazan. Anal. Biochem. 1997, 250, 253–255. [Google Scholar] [CrossRef]
- Allain, C.C.; Henson, C.P.; Nadel, M.K.; Knoblesdorff, A.J. Rapid single-step kinetic colorimetric assay for lactate dehydrogenase in serum. Clin. Chem. 1973, 19, 223–227. [Google Scholar] [CrossRef]
- Papaneophytou, C.; Zervou, M.E.; Theofanous, A. Optimization of a Colorimetric Assay to Determine Lactate Dehydrogenase B Activity Using Design of Experiments. SLAS Discov. Adv. Sci. Drug Discov. 2021, 26, 383–399. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: The NADPH network. Redox Rep. 2018, 23, 47–56. [Google Scholar] [CrossRef]
- Muthuchamy, M.; Muneeswaran, T.; Rajivgandhi, G.; Franck, Q.; Muthusamy, A.; Ji-Ming, S. Biologically synthesized copper and zinc oxide nanoparticles for important biomolecules detection and antimicrobial applications. Mater. Toda. Commun. 2020, 22, 100766. [Google Scholar] [CrossRef]
- Stanko, A.P.; Soki, J.; Brkic, D.V.; Plecko, V. Lactate dehydrogenase activity in Bacteroides fragilis group strains with induced resistance to metronidazole. J. Glob. Antimicrob. Resist. 2016, 5, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.N.; de Campos Moura, M.N.; Lima, J.; Reis, B.F. Automatic flow procedure for the determination of glycerol in wine using enzymatic reaction and spectrophotometry. Microchem. J. 2004, 77, 107–112. [Google Scholar] [CrossRef]
- Moran, J.H.; Schnellmann, R.G. A rapid beta-NADH-linked fluorescence assay for lactate dehydrogenase in cellular death. J. Pharmacol. Toxicol. Methods 1996, 36, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zeng, G.M.; Shen, G.L.; Zhang, Y.; Li, Y.P.; Fan, C.Z.; Liu, C.; Niu, C.G. Highly sensitive sensor for detection of NADH based on catalytic growth of Au nanoparticles on glassy carbon electrode. Anal. Bioanal. Chem. 2009, 393, 1677–1684. [Google Scholar] [CrossRef]
- Frattini, D.; Hyun, K.; Kwon, Y. Direct electrochemistry of lactate dehydrogenase in aqueous solution system containing L(+)−lactic acid, beta-nicotinamide adenine dinucleotide, and its reduced form. J. Ind. Eng. Chem. 2019, 80, 508–515. [Google Scholar] [CrossRef]
- Di, J.W.; Cheng, J.J.; Xu, Q.A.; Zheng, H.I.; Zhuang, J.Y.; Sun, Y.B.; Wang, K.Y.; Mo, X.Y.; Bi, S.P. Direct electrochemistry of lactate dehydrogenase immobilized on silica sol-gel modified gold electrode and its application. Biosens. Bioelectron. 2007, 23, 682–687. [Google Scholar] [CrossRef]
- O’Mahony, A.M.; Hardacre, C.; Compton, R.G. Selecting Room-Temperature Ionic Liquids to Optimize Voltammetric Responses: The Oxidation of NADH. J. Electrochem. Soc. 2010, 157, F49. [Google Scholar] [CrossRef]
- Filip, J.; Sefcovicova, J.; Tomcik, P.; Gemeiner, P.; Tkac, J. A hyaluronic acid dispersed carbon nanotube electrode used for a mediatorless NADH sensing and biosensing. Talanta 2011, 84, 355–361. [Google Scholar] [CrossRef]
- Arvinte, A.; Valentini, F.; Radoi, A.; Arduini, F.; Tamburri, E.; Rotariu, L.; Palleschi, G.; Bala, C. The NADH electrochemical detection performed at carbon nanofibers modified glassy carbon electrode. Electroanalysis 2007, 19, 1455–1459. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Karagozler, A.E.; Russell, G.C.; Zimmer, H.; Mark, H.B., Jr. Electrochemistry and detection of some organic and biological molecules at conducting poly(3−methylthiophene) electrodes. Biosens. Bioelectron. 1991, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Valentini, F.; Amine, A.; Orlanducci, S.; Terranova, M.L.; Palleschi, G. Carbon nanotube purification: Preparation and characterization of carbon nanotube paste electrodes. Anal. Chem. 2003, 75, 5413–5421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Tanner, J.A.; Li, H.W.; Wu, Y.Q. A novel fluorescence probe of Plasmodium vivax lactate dehydrogenase based on adenosine monophosphate protected bimetallic nanoclusters. Talanta 2020, 213, 120850. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Ren, X.L.; Meng, X.W.; Li, H.B.; Tang, F.Q. Optical analysis of lactate dehydrogenase and glucose by CdTe quantum dots and their dual simultaneous detection. Biosens. Bioelectron. 2011, 26, 3488–3493. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Chakma, B.; Patra, S.; Goswami, P. Hairpin stabilized fluorescent silver nanoclusters for quantitative detection of NAD(+) and monitoring NAD(+)/NADH based enzymatic reactions. Anal. Chim. Acta 2017, 956, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.L.; Yang, L.Q.; Tang, F.Q.; Yan, C.M.; Ren, J. Enzyme biosensor based on NAD-sensitive quantum dots. Biosens. Bioelectron. 2010, 26, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Koyappayil, A.; Kim, H.T.; Lee, M.H. An efficient and rapid synthesis route to highly fluorescent copper microspheres for the selective and sensitive excitation wavelength-dependent dual-mode sensing of NADH. Sens. Actuators B Chem. 2021, 327, 128887. [Google Scholar] [CrossRef]
- Deore, L.A.; Freund, M.S. Reactivity of poly(anilineboronic acid) with NAD(+) and NADH. Chem. Mater. 2005, 17, 2918–2923. [Google Scholar] [CrossRef]
- Gebicki, J.; Marcinek, A.; Zielonka, J. Transient species in the stepwise interconversion of NADH and NAD(+). Acc. Chem. Res. 2004, 37, 379–386. [Google Scholar] [CrossRef]
- Titov, D.V.; Cracan, V.; Goodman, R.P.; Peng, J.; Grabarek, Z.; Mootha, V.K. Complementation of mitochondrial electron transport chain by manipulation of the NAD(+)/NADH ratio. Science 2016, 352, 231–235. [Google Scholar] [CrossRef]
- Maynard, A.G.; Kanarek, N. NADH Ties One-Carbon Metabolism to Cellular Respiration. Cell Metab. 2020, 31, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Bilan, D.S.; Belousov, V.V. Genetically encoded probes for NAD(+)/NADH monitoring. Free Radical Biol. Med. 2016, 100, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.G.; Raju, R.P. Regulation of NAD(+) metabolism in aging and disease. Metabolism 2022, 126, 154923. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Landick, R.; Raman, S. A Regulatory NADH/NAD plus Redox Biosensor for Bacteria. Acs. Synth. Biol. 2019, 8, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Amjad, S.; Nisar, S.; Bhat, A.A.; Shah, A.R.; Frenneaux, M.P.; Fakhro, K.; Haris, M.; Reddy, R.; Patay, Z.; Baur, J.; et al. Role of NAD(+) in regulating cellular and metabolic signaling pathways. Mol. Metab. 2021, 49, 101195. [Google Scholar] [CrossRef]
- Siu, V.S.; Lu, M.H.; Hsieh, K.Y.; Raines, K.; Asaad, Y.A.; Patel, K.; Afzali-Ardakani, A.; Wen, B.; Budd, R. Toward a Quantitative Colorimeter for Point-of-Care Nitrite Detection. Acs. Omega 2022, 7, 11126–11134. [Google Scholar] [CrossRef]
- Lee, S.; Manjunatha, D.H.; Jeon, W.; Ban, C. Cationic Surfactant-Based Colorimetric Detection of Plasmodium Lactate Dehydrogenase, a Biomarker for Malaria, Using the Specific DNA Aptamer. PLoS ONE 2014, 9, e100847. [Google Scholar] [CrossRef]
- Kannan, B.; Jahanshahi-Anbuhi, S.; Pelton, R.H.; Li, Y.F.; Filipe, C.D.M.; Brennan, J.D. Printed Paper Sensors for Serum Lactate Dehydrogenase using Pullulan-Based Inks to Immobilize Reagents. Anal. Chem. 2015, 87, 9288–9293. [Google Scholar] [CrossRef]
- Arias-Alpízar, K.; Sánchez-Cano, A.; Prat-Trunas, J.; de la Serna Serna, E.; Alonso, O.; Sulleiro, E.; Sánchez-Montalvá, A.; Diéguez, A.; Baldrich, E. Malaria quantitative POC testing using magnetic particles, a paper microfluidic device and a hand-held fluorescence reader. Biosens. Bioelectron. 2022, 215, 114513. [Google Scholar] [CrossRef]
- Halvorsen, C.P.; Olson, L.; Araujo, A.C.; Karlsson, M.; Nguyen, T.T.; Khu, D.T.K.; Le, H.T.T.; Nguyen, H.T.B.; Winbladh, B.; Russom, A. A rapid smartphone-based lactate dehydrogenase test for neonatal diagnostics at the point of care. Sci. Rep. 2019, 9, 9301. [Google Scholar] [CrossRef]
- Passos, M.L.C.; Saraiva, M. Detection in UV-visible spectrophotometry: Detectors, detection systems, and detection strategies. Measurement 2019, 135, 896–904. [Google Scholar] [CrossRef]
- Hikosaka, K.; Kim, J.; Kajita, M.; Kanayama, A.; Miyamoto, Y. Platinum nanoparticles have an activity similar to mitochondrial NADH: Ubiquinone oxidoreductase. Colloids Surf. B Biointerfaces 2008, 66, 195–200. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.R.; Souza, R.O.; Dias, L.B.; Ribas, T.B.; de Oliveira, L.C.F.; Sumida, D.H.; Dornelles, R.C.M.; Nakamune, A.; Chaves-Neto, A.H. The effects of storage time and temperature on the stability of salivary phosphatases, transaminases and dehydrogenase. Arch. Oral Biol. 2018, 85, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kaya, H.O.; Cetin, A.E.; Azimzadeh, M.; Topkaya, S.N. Pathogen detection with electrochemical biosensors: Advantages, challenges and future perspectives. J. Electroanal. Chem. 2021, 882, 114989. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Dzyadevych, S.V.; Soldatkin, A.P. Electrochemical biosensors based on multienzyme systems: Main groups, advantages and limitations—A review. Anal. Chim. Acta 2020, 1111, 114–131. [Google Scholar] [CrossRef]
- Hong, M.Y.; Chang, J.Y.; Yoon, H.C.; Kim, H.S. Development of a screen-printed amperometric biosensor for the determination of L−lactate dehydrogenase level. Biosens. Bioelectron. 2002, 17, 13–18. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, X.X.; Yang, J.; He, Y.C.; Li, Y.C. Application of Multiplex Microfluidic Electrochemical Sensors in Monitoring Hematological Tumor Biomarkers. Anal. Chem. 2020, 92, 11981–11986. [Google Scholar] [CrossRef]
- Lee, K.; Song, G.; Kwon, J.; Kim, J.; Yang, H. Electrochemical Detection of Glucose and Lactate Dehydrogenase Using a Zwitterionic Phenazine Compound as an Electron Mediator for NADH Oxidation. Electroanalysis 2022, 34, 1499–1506. [Google Scholar] [CrossRef]
- Ruiz-Vega, G.; Arias-Alpízar, K.; de la Serna, E.; Borgheti-Cardoso, L.N.; Sulleiro, E.; Molina, I.; Fernàndez-Busquets, X.; Sánchez-Montalvá, A.; del Campo, F.J.; Baldrich, E. Electrochemical POC device for fast malaria quantitative diagnosis in whole blood by using magnetic beads, Poly-HRP and microfluidic paper electrodes. Biosens. Bioelectron. 2020, 150, 111925. [Google Scholar] [CrossRef]
- Yi, Y.H.; Li, Y.; Li, W.Z.; Cheng, M.J.; Wu, M.S.; Miao, J.F.; Kang, W.; Xu, Y.Y. Electrochemical Immunosensor for Lactate Dehydrogenase Detection Through Analyte-driven Catalytic Reaction on Multi-walled Carbon Nanotubes and Gold Nanoparticle Modified Carbon Electrode. Electroanal 2022, 34, 1187–1192. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhou, W.H.; Zhao, R.Z.; Yang, Z.D.; Li, W.; Chao, D.L.; Qiao, S.Z.; Zhao, D.Y. Sulfur-Based Aqueous Batteries: Electrochemistry and Strategies. J. Am. Chem. Soc. 2021, 143, 15475–15489. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Electrochemistry of Graphene: New Horizons for Sensing and Energy Storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef]
- Jaugstetter, M.; Blanc, N.; Kratz, M.; Tschulik, K. Electrochemistry under confinement. Chem. Soc. Rev. 2022, 51, 2491–2543. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, S.A.; Huynh, T.; Chang, T.C.; Anderson, C.E.; McDermott, J.J.; Oncina, C.I.; Weigl, B.H.; Nichols, K.P. Wash-Free, Digital Immunoassay in Polydisperse Droplets. Anal. Chem. 2020, 92, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Hemben, A.; Ashley, J.; Tothill, I.E. An immunosensor for parasite lactate dehydrogenase detection as a malaria biomarker—Comparison with commercial test kit. Talanta 2018, 187, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Chen, G.C.; Zhang, Y.J.; Wu, F.; Wang, Q.B. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J. Am. Chem. Soc. 2020, 142, 14789–14804. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef]
- Gui, R.J.; Jin, H.; Bu, X.N.; Fu, Y.X.; Wang, Z.H.; Liu, Q.Y. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coord. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Fan, S.N.; Li, X.Q.; Ma, F.H.; Yang, M.H.; Su, J.; Chen, X. Sulfur quantum dot based fluorescence assay for lactate dehydrogenase activity detection. J. Photoch. Photobio. A. 2022, 430, 113989. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.W.; Wu, Y.Q. A highly selective and sensitive fluorescent probe for lactate dehydrogenase based on ultrabright adenosine monophosphate capped gold nanoclusters. RSC Adv. 2017, 7, 13438–13443. [Google Scholar] [CrossRef]
- He, X.L.; Tan, L.F.; Wu, X.L.; Yan, C.M.; Chen, D.; Meng, X.W.; Tang, F.Q. Electrospun quantum dots/polymer composite porous fibers for turn-on fluorescent detection of lactate dehydrogenase. J. Mater. Chem. 2012, 22, 18471–18478. [Google Scholar] [CrossRef]
- Kenry; Geldert, A.; Zhang, X.; Zhang, H.; Lim, C.T. Highly Sensitive and Selective Aptamer-Based Fluorescence Detection of a Malarial Biomarker Using Single-Layer MoS2 Nanosheets. Acs. Sens. 2016, 1, 1315–1321. [Google Scholar] [CrossRef]
- Jenie, S.N.A.; Prieto-Simon, B.; Voelcker, N.H. Development of L-lactate dehydrogenase biosensor based on porous silicon resonant microcavities as fluorescence enhancers. Biosens. Bioelectron. 2015, 74, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Minopoli, A.; Della Ventura, B.; Campanile, R.; Tanner, J.A.; Offenhausser, A.; Mayer, D.; Velotta, R. Randomly positioned gold nanoparticles as fluorescence enhancers in apta-immunosensor for malaria test. Microchim. Acta 2021, 188, 88. [Google Scholar] [CrossRef]
- Singh, N.K.; Jain, P.; Das, S.; Goswami, P. Dye Coupled Aptamer-Captured Enzyme Catalyzed Reaction for Detection of Pan Malaria and P. falciparum Species in Laboratory Settings and Instrument-Free Paper-Based Platform. Anal. Chem. 2019, 91, 4213–4221. [Google Scholar] [CrossRef]
- Sanchez-Cano, A.; Ruiz-Vega, G.; Vicente-Gomez, S.; de la Serna, E.; Sulleiro, E.; Molina, I.; Sanchez-Montalva, A.; Baldrich, E. Development of a Fast Chemiluminescent Magneto-Immunoassay for Sensitive Plasmodium falciparum Detection in Whole Blood. Anal. Chem. 2021, 93, 12793–12800. [Google Scholar] [CrossRef]
- Figueroa-Miranda, G.; Liang, Y.; Suranglikar, M.; Stadler, M.; Samane, N.; Tintelott, M.; Lo, Y.; Tanner, J.A.; Vu, X.T.; Knoch, J.; et al. Delineating charge and capacitance transduction in system-integrated graphene-based BioFETs used as aptasensors for malaria detection. Biosens. Bioelectron. 2022, 208, 114219. [Google Scholar] [CrossRef]
- Cao, X.E.; Kim, J.; Mehta, S.; Erickson, D. Two-Color Duplex Platform for Point-of-Care Differential Detection of Malaria and Typhoid Fever. Anal. Chem. 2021, 93, 12175–12180. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Ricks, K.M.; Bitting, A.L.; Mudenda, L.; Wright, D.W. Simultaneous capture and sequential detection of two malarial biomarkers on magnetic microparticles. Talanta 2016, 161, 443–449. [Google Scholar] [CrossRef]
- Markwalter, C.F.; Davis, K.M.; Wright, D.W. Immunomagnetic capture and colorimetric detection of malarial biomarker Plasmodium falciparum lactate dehydrogenase. Anal. Biochem. 2016, 493, 30–34. [Google Scholar] [CrossRef]
- Sancho-Fornes, G.; Avella-Oliver, M.; Carrascosa, J.; Fernandez, E.; Brun, E.M.; Maquieira, Á. Disk-based one-dimensional photonic crystal slabs for label-free immunosensing. Biosens. Bioelectron. 2019, 126, 315–323. [Google Scholar] [CrossRef] [PubMed]


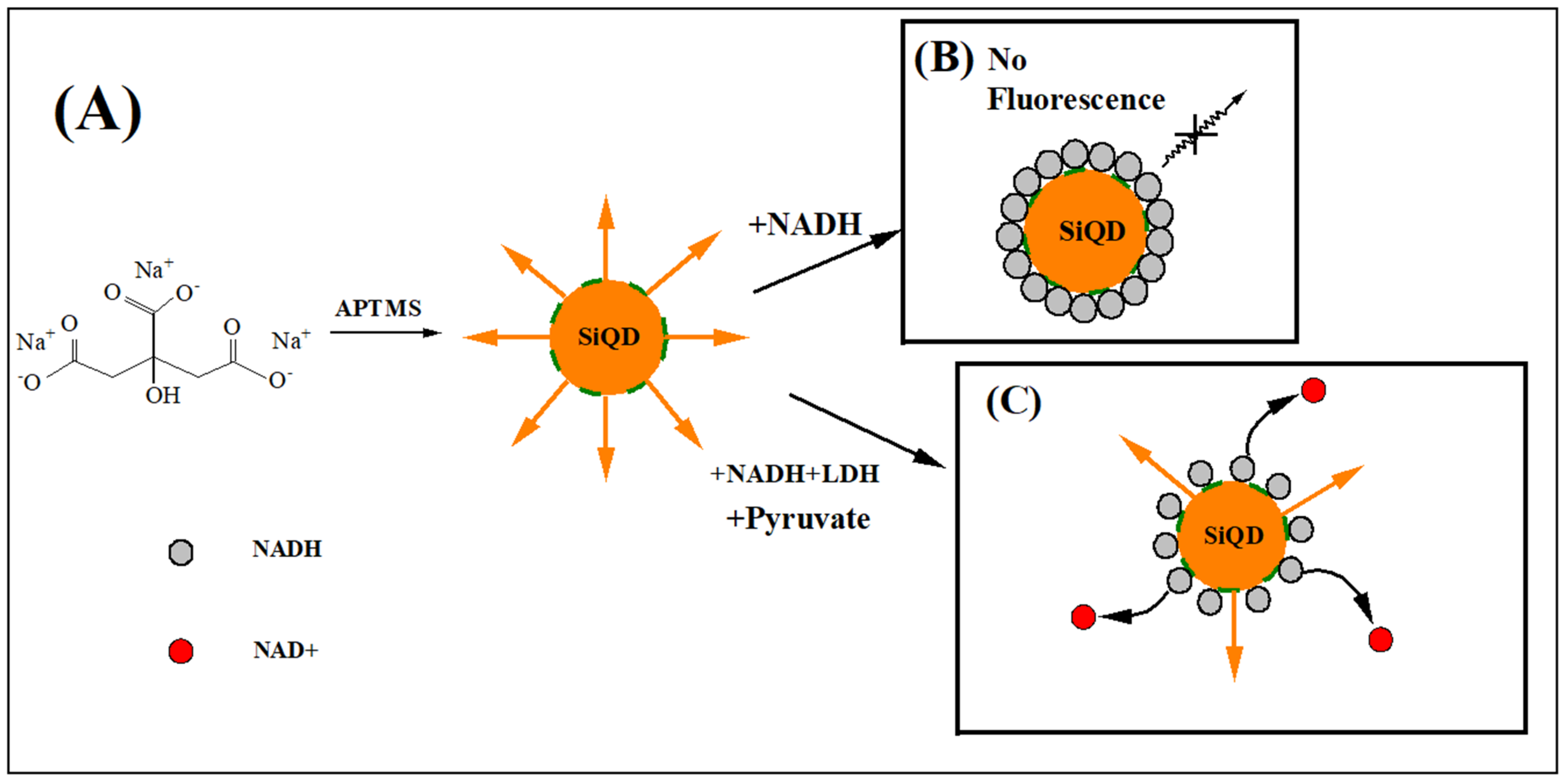
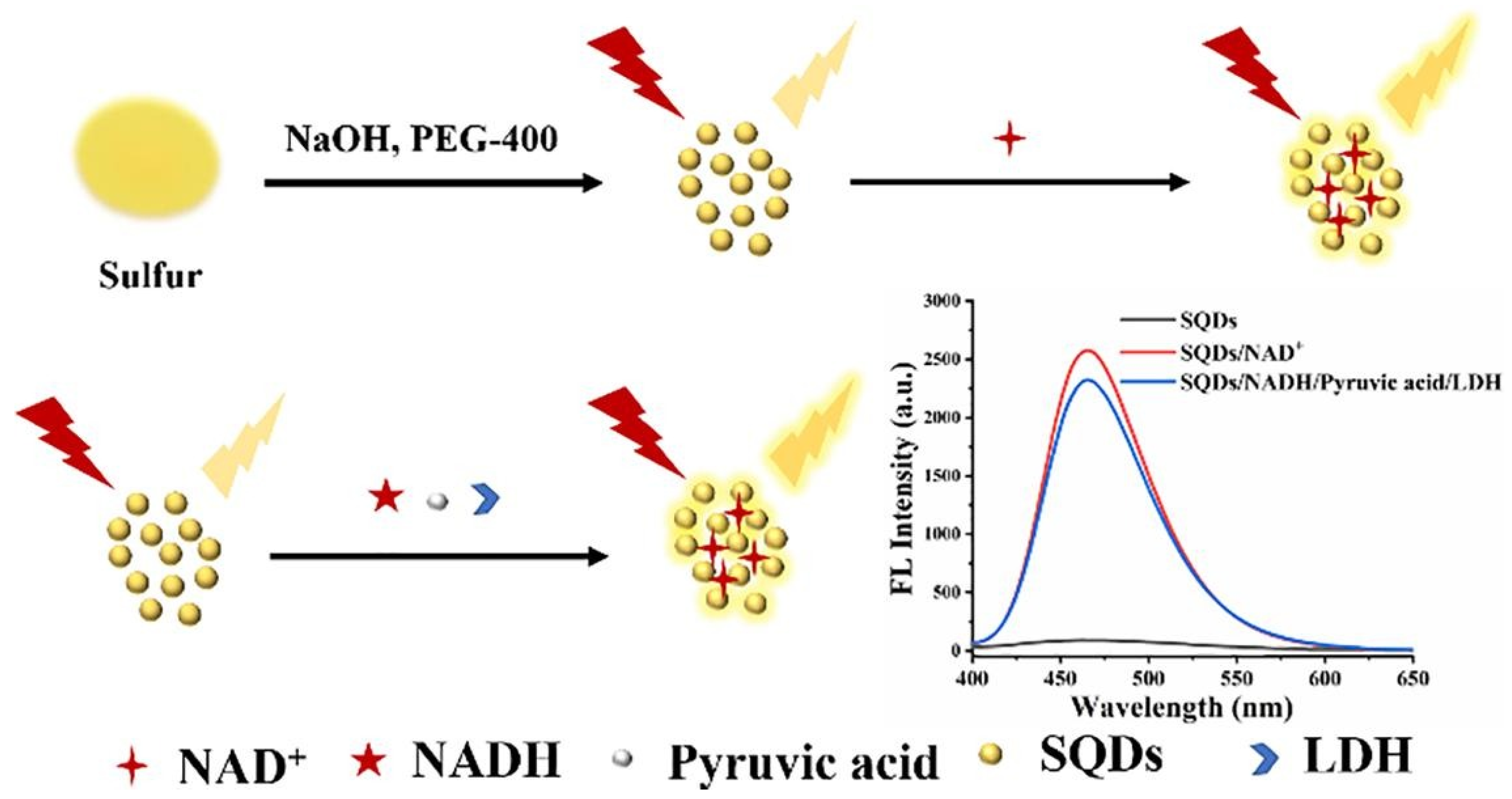
| Analytical Method | Materials | Detection Limit (U L−1) | Linear Range (U L−1) | Sensor Response Time (min) | Correlation Coefficient | Reference | Real Sample Test |
|---|---|---|---|---|---|---|---|
| Fluorescence | CdTe QDs | / | 250–6000 | 15 | 0.996 | [84] | Serum |
| Fluorescence | CdTe/CdS QDs | 75 | 150–1500 | 20 | 0.996 | [86] | / |
| Fluorescence | AuNCs@AMP | 0.8 | 8.0–400 | 30 | 0.996 | [120] | Serum |
| Fluorescence | Au−AgNCs@AMP | 0.93 | 92.5–925 | 10 | 0.997 | [83] | / |
| Fluorescence | SiQDs | 970 | 0.77–385 × 103 | 20 | 0.997 | [12] | Serum |
| Fluorescence | SQDs | 262.41 | 0.5–40 × 103 | 60 | 0.991 | [119] | Serum |
| Fluorescence | pSi | 80 | 0.16–6.5 × 103 | 10 | 0.984 | [123] | / |
| Fluorescence | AuNPs | 9.25 × 10−2 | 7.5 × 10−2–7.5 × 104 | 120 | / | [124] | Blood |
| Fluorescence | CdSe QDs/PCL | / | 200–2400 | 10 | 0.998 | [121] | / |
| Fluorescence | Paper−based Sensor | 0.225 | 0.2–3.13 | ˂20 | 0.997 | [99] | Blood |
| Fluorescence | Aptamer-Coated Magnetic Beads | 1.51 × 10−2 | 9.25 × 10−3–9.25 × 102 | 60 | 0.990 | [125] | / |
| Fluorescence | MoS2 nanosheets | 5.09 | 0–578.13 | 10 | 0.990 | [122] | / |
| Fluorescence | Magnetic Beads | 2.75 × 10−2 | 0.025–6.25 | 15 | / | [126] | Blood |
| Electrochemistry | Glassy Carbon Electrode | 0.21 | 0.55–275 | 60 | 0.991 | [110] | / |
| Electrochemistry | Screen-printed Electrode | 50 | 50–500 | 10 | 0.998 | [106] | / |
| Electrochemistry | N−Mo2C/SPE | 25 | 60–700 | / | 0.991 | [107] | Plasma |
| Electrochemistry | MP−dsSPCE | 50 | 3.13–25 | ˂20 | 0.990 | [109] | Blood |
| Electrochemistry | rGO−2DBioFET | 7.22 × 10−5 | 7.22 × 10−4–9.25 × 103 | / | 0.990 | [127] | Serum |
| Electrochemistry | mPPS | 0.5 | / | / | / | [108] | Serum |
| Colorimetry | Pullulan-Based Inks | 13 | 0–225 | 5 | / | [98] | Serum |
| Colorimetry | LFA | 2.5 | 1.25–125 | 15 | 0.960 | [128] | Serum |
| Colorimetry | mAb−functionalized Magnetic Beads | 2.41 × 10−2 | 6.48 × 10−2–46.25 | 15 | / | [129] | Blood |
| Colorimetry | Magnetic Beads | 0.24 | / | 30 | / | [130] | / |
| Colorimetry | Paper-based Sensor | 0.39 | / | ˂20 | / | [99] | Blood |
| Colorimetry | Aptamer-Coated Magnetic Beads | 0.57 | 9.25 × 10−3–9.25 × 102 | 60 | 0.990 | [125] | / |
| Colorimetry | Magnetic Beads | 0.03 | 0.1–6.25 | 20 | / | [126] | Blood |
| Chemiluminescence | Magnetic Beads | 5 × 10−3 | 0.01–6.25 | 1 | / | [126] | Blood |
| Immunoassay | TiO | 12.8 | 1–100 | 30 | 0.996 | [131] | / |
| Immunoassay | Microfluidic Microplate | 6.25 × 10−3 | / | ˂90 | / | [43] | Serum |
| Immunoassay | SPGE | 0.45 | 0–0.17 | 120 | 0.991 | [115] | Serum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Qi, M.; Yang, M. Current Status and Future Perspectives of Lactate Dehydrogenase Detection and Medical Implications: A Review. Biosensors 2022, 12, 1145. https://doi.org/10.3390/bios12121145
Zhou Y, Qi M, Yang M. Current Status and Future Perspectives of Lactate Dehydrogenase Detection and Medical Implications: A Review. Biosensors. 2022; 12(12):1145. https://doi.org/10.3390/bios12121145
Chicago/Turabian StyleZhou, Yangzhe, Min Qi, and Minghui Yang. 2022. "Current Status and Future Perspectives of Lactate Dehydrogenase Detection and Medical Implications: A Review" Biosensors 12, no. 12: 1145. https://doi.org/10.3390/bios12121145
APA StyleZhou, Y., Qi, M., & Yang, M. (2022). Current Status and Future Perspectives of Lactate Dehydrogenase Detection and Medical Implications: A Review. Biosensors, 12(12), 1145. https://doi.org/10.3390/bios12121145





