Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination
Abstract
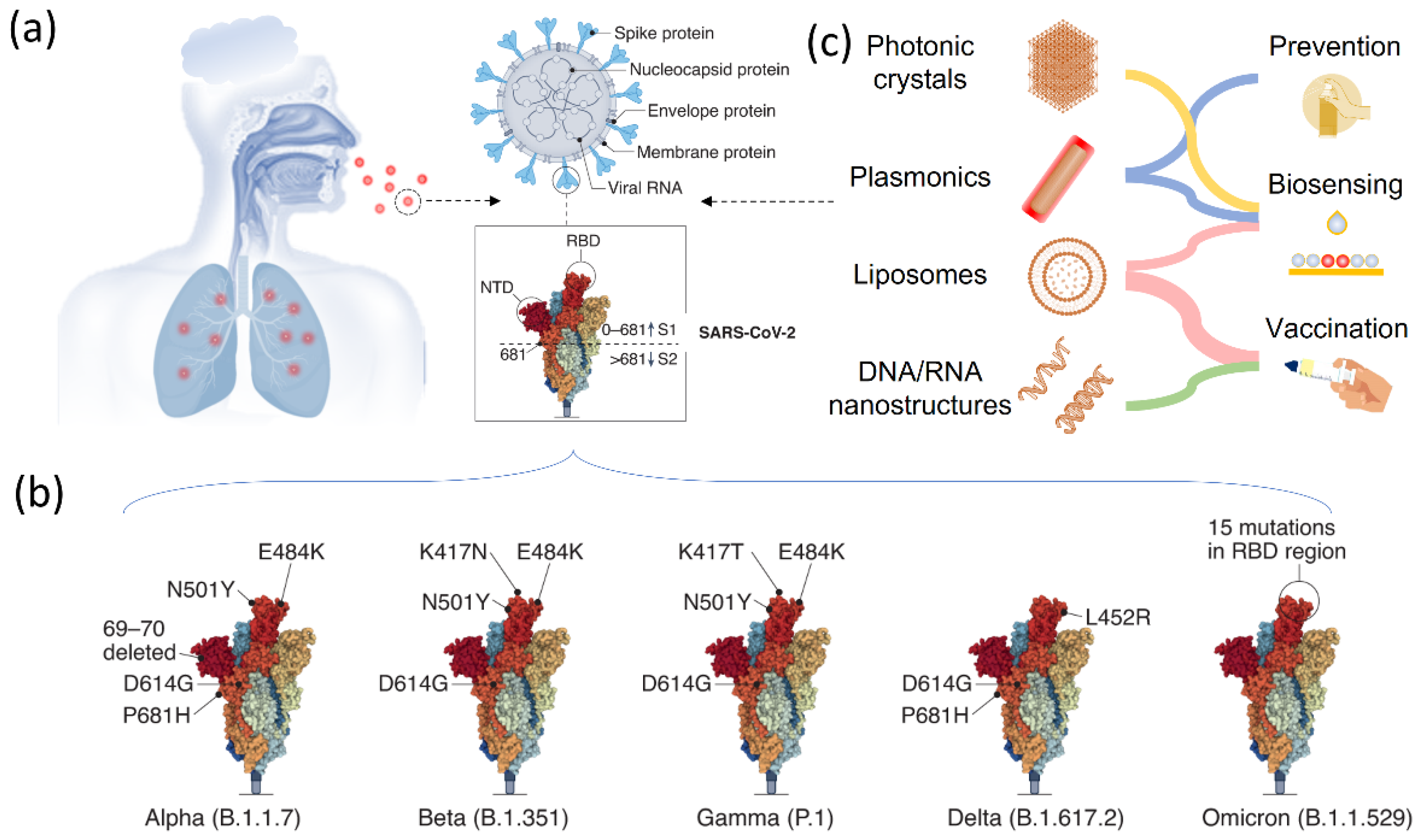
:1. Introduction
2. SARS-CoV-2 and VOCs
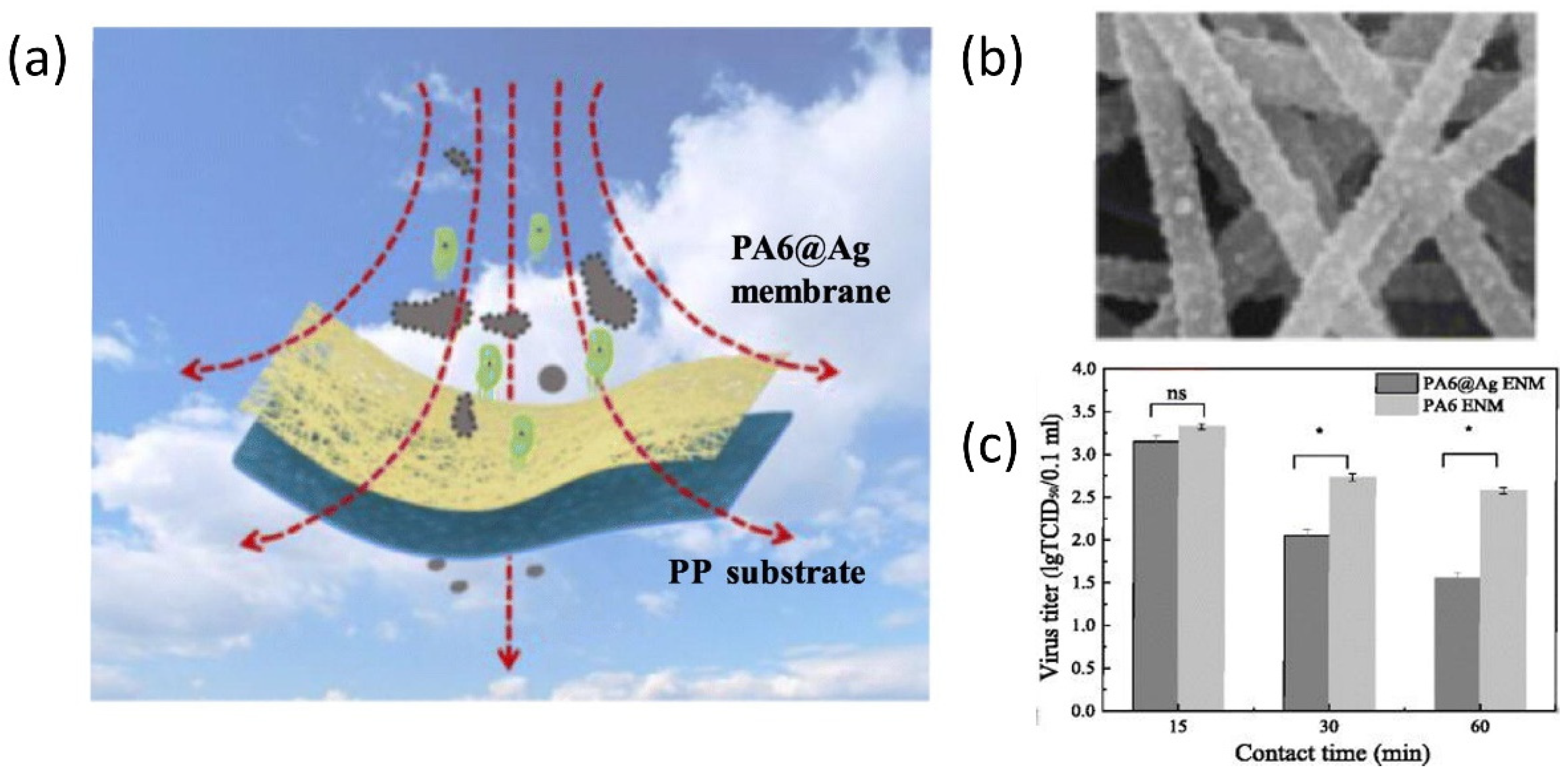
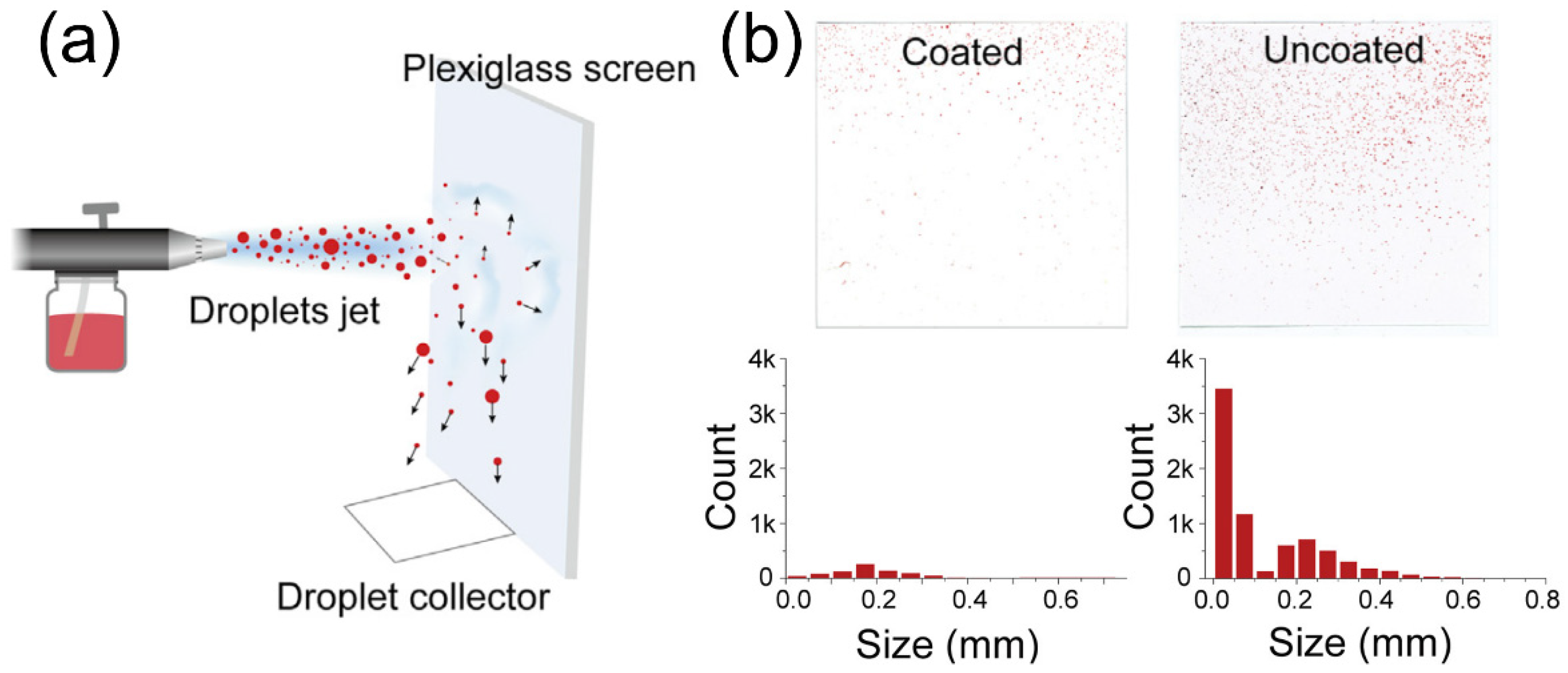
3. Nanostructured Materials for COVID-19 Prevention
4. Responsive Nanostructured Materials for Viral Disease Biosensing
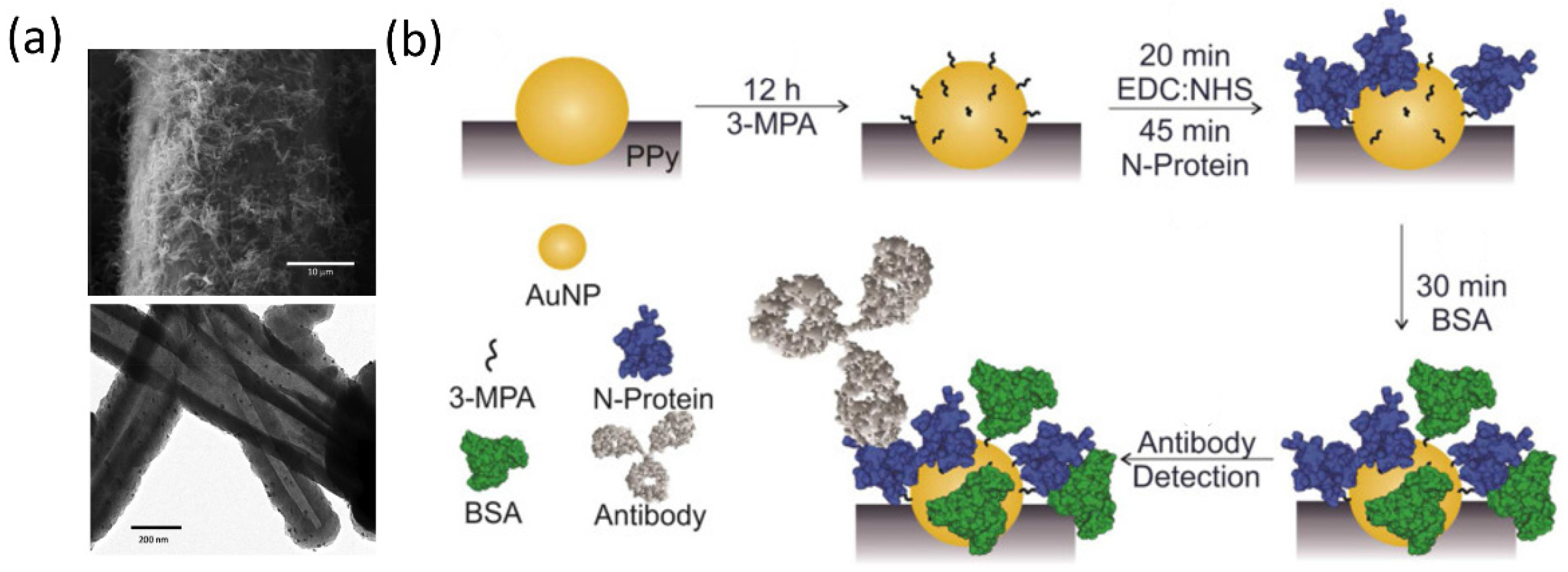
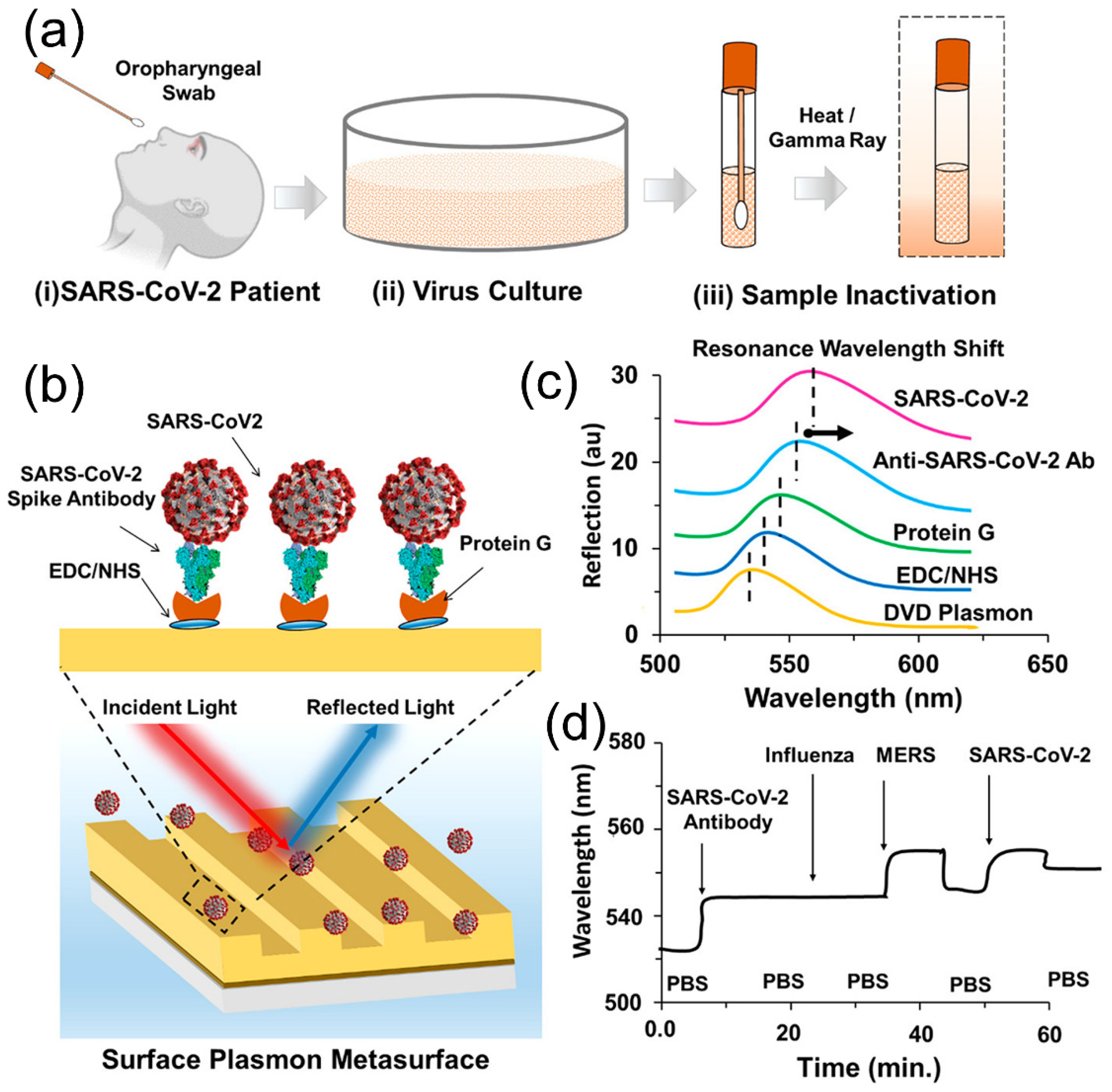
4.1. Responsive Plasmonic Nanostructures for Biosensing of Coronavirus
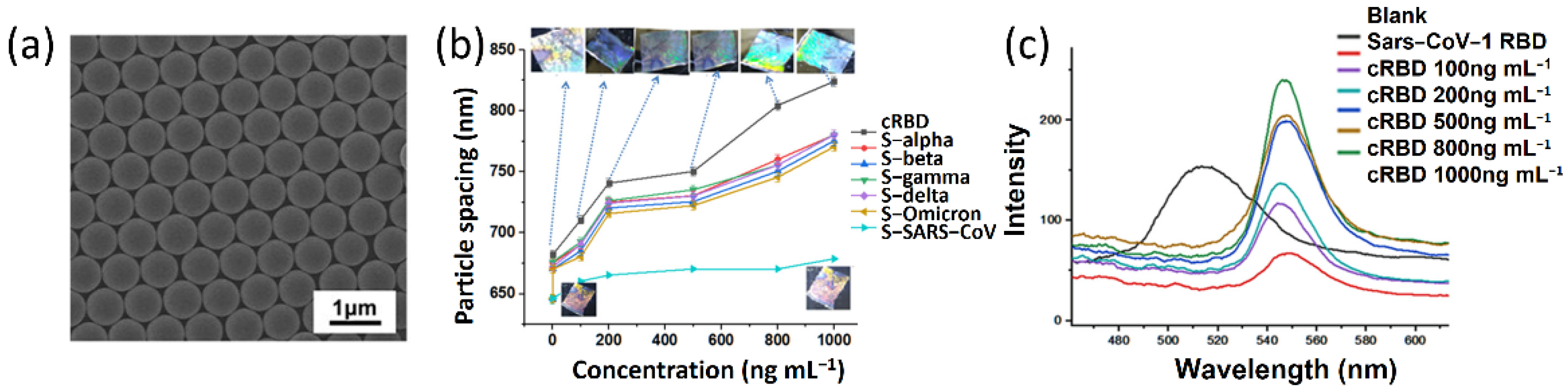
4.2. Responsive Photonic Crystals for Biosensing of Viral Disease
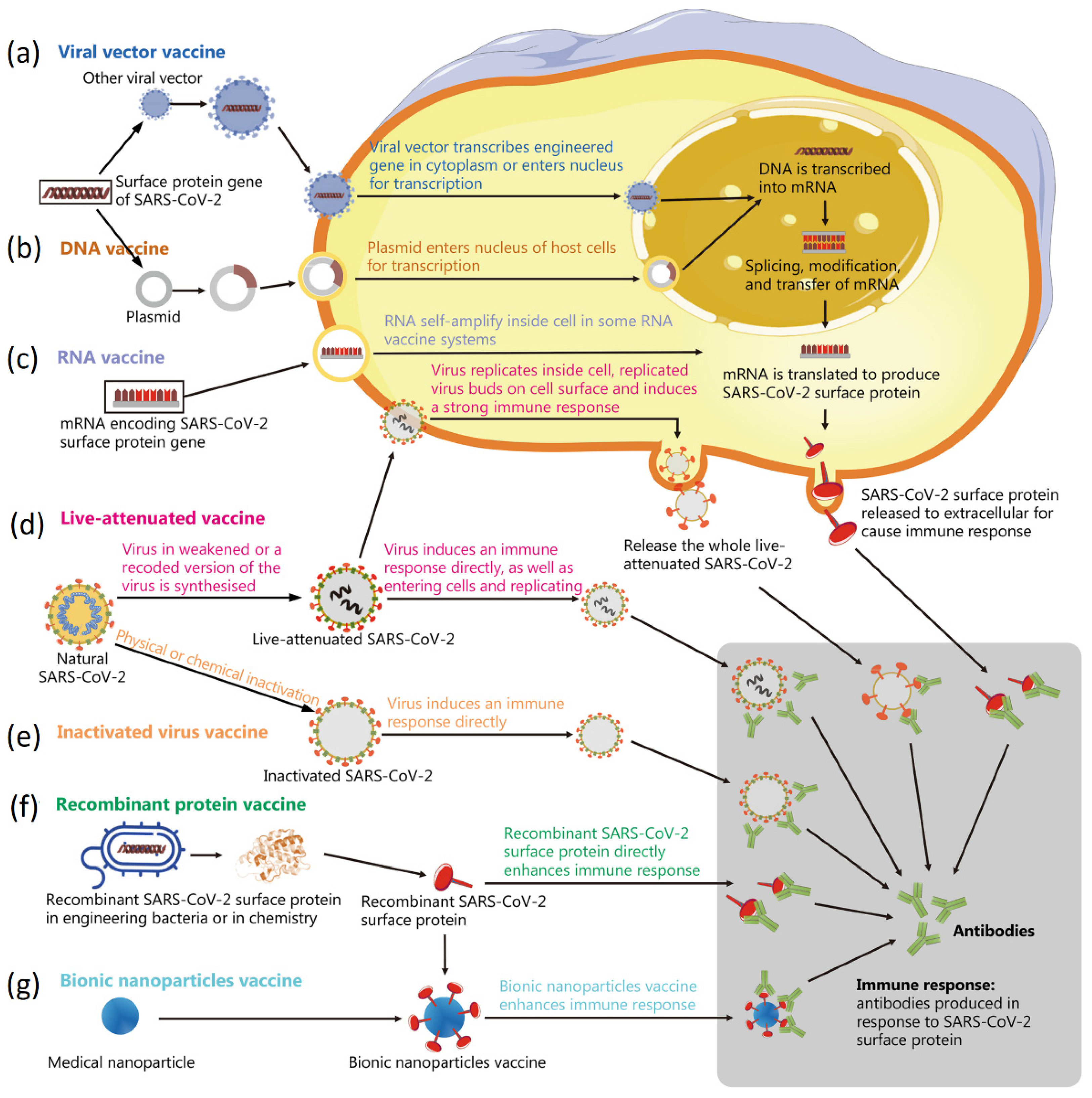
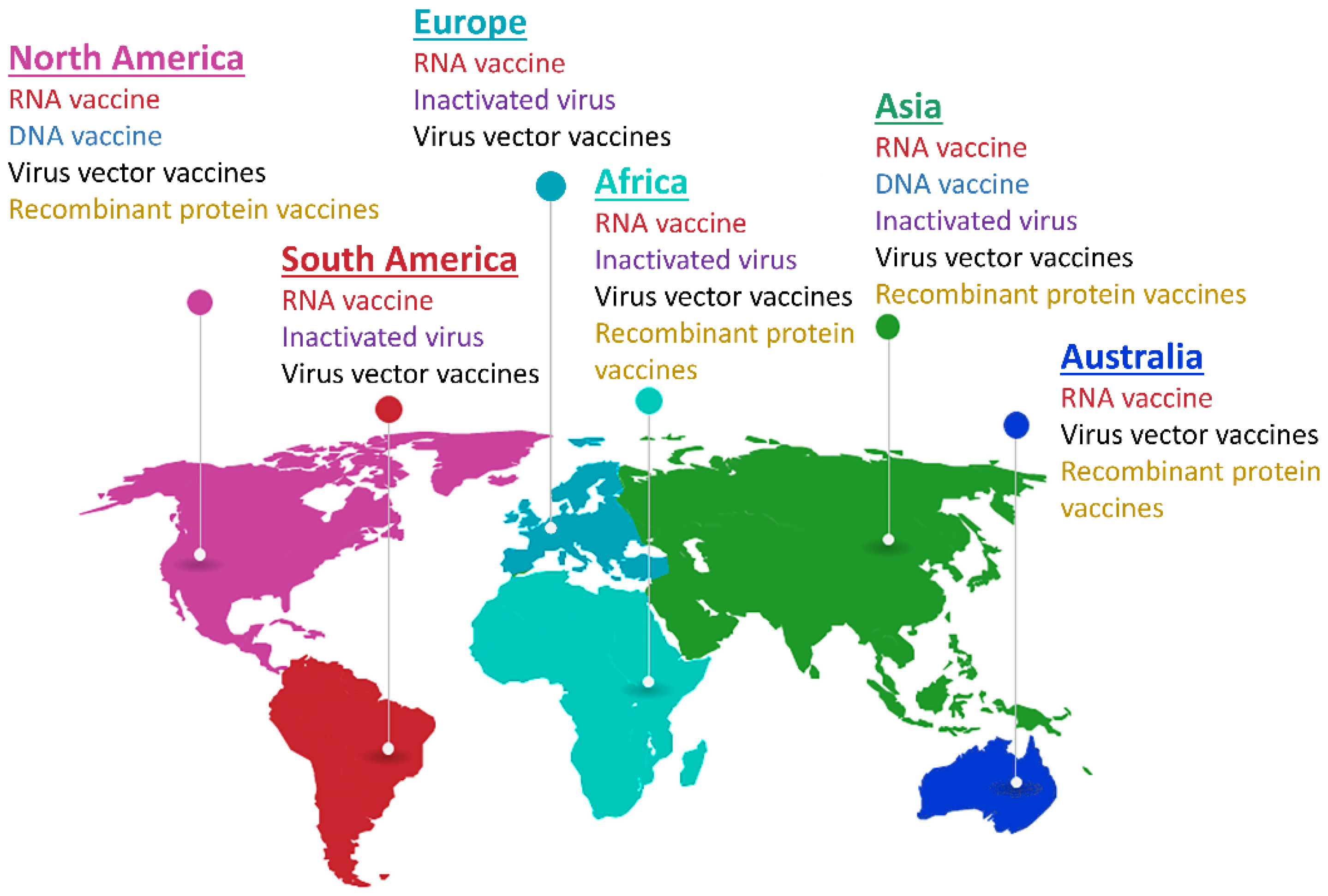
5. Nanotechnology in Viral Disease Vaccination
5.1. Delivery of mRNA Using Lipid Nanoparticles
5.2. Assembly of Viral Protein Subunits for Vaccination
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C.; Henry, B.M. Updated picture of SARS-CoV-2 variants and mutations. Diagnosis 2021, 9, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K. Rapid diagnostic testing for SARS-CoV-2. N. Engl. J. Med. 2022, 386, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global impact of the first year of COVID-19 vaccination: A mathematical modelling study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Ozili, P.K.; Arun, T. Spillover of COVID-19: Impact on the Global Economy. In Managing Inflation and Supply Chain Disruptions in the Global Economy; IGI Global: Hershey, PA, USA, 2022; pp. 41–61. [Google Scholar]
- Hasan, I.; Dhawan, P.; Rizvi, S.; Dhir, S. Data analytics and knowledge management approach for COVID-19 prediction and control. Int. J. Inf. Technol. 2022, 1–18. [Google Scholar] [CrossRef]
- Hadj Hassine, I. COVID-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef]
- Sagulkoo, P.; Plaimas, K.; Suratanee, A.; Vissoci Reiche, E.M.; Maes, M. Immunopathogenesis and immunogenetic variants in COVID-19. Curr. Pharm. Des. 2022, 28, 1780–1797. [Google Scholar]
- Ciotti, M.; Ciccozzi, M.; Pieri, M.; Bernardini, S. The COVID-19 pandemic: Viral variants and vaccine efficacy. Crit. Rev. Clin. Lab. Sci. 2022, 59, 66–75. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef]
- Rasmi, Y.; Saloua, K.S.; Nemati, M.; Choi, J.R. Recent progress in nanotechnology for COVID-19 prevention, diagnostics and treatment. Nanomaterials 2021, 11, 1788. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, F.; Yin, Y. Smart materials by nanoscale magnetic assembly. Adv. Funct. Mater. 2020, 30, 1903467. [Google Scholar] [CrossRef]
- Pishva, P.; Yüce, M. Nanomaterials to tackle the COVID-19 pandemic. Emergent Mater. 2021, 4, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Srivastava, N.; Mishra, P.; Malhotra, B.D. Prospects of nanomaterials-enabled biosensors for COVID-19 detection. Sci. Total Environ. 2021, 754, 142363. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Yin, Y. Colloidal self-assembly approaches to smart nanostructured materials. Chem. Rev. 2021, 122, 4976–5067. [Google Scholar] [CrossRef]
- Ghaemi, F.; Amiri, A.; Bajuri, M.Y.; Yuhana, N.Y.; Ferrara, M. Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19. Sustain. Cities Soc. 2021, 72, 103046. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Cheng, L.; Gong, H.; Yin, S.; Gong, Q.; Li, Y.; Liu, Z. PEG-functionalized iron oxide nanoclusters loaded with chlorin e6 for targeted, NIR light induced, photodynamic therapy. Biomaterials 2013, 34, 9160–9170. [Google Scholar] [CrossRef]
- Abbasinia, M.; Karimie, S.; Haghighat, M.; Mohammadfam, I. Application of nanomaterials in personal respiratory protection equipment: A literature review. Safety 2018, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, X.; Han, L.; Zhu, C.; Xin, H.; Yin, Y. Multicolor Photonic Pigments for Rotation-Asymmetric Mechanochromic Devices. Adv. Mater. 2022, 34, 2107398. [Google Scholar] [CrossRef]
- Fenzl, C.; Hirsch, T.; Wolfbeis, O.S. Photonic crystals for chemical sensing and biosensing. Angew. Chem. Int. Ed. 2014, 53, 3318–3335. [Google Scholar] [CrossRef]
- Joshi, N.; Shukla, S.; Narayan, R.J. Novel photonic methods for diagnosis of SARS-CoV-2 infection. Transl. Biophotonics 2022, 4, e202200001. [Google Scholar] [CrossRef] [PubMed]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2021, 55, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jeong, M.; Hur, S.; Cho, Y.; Park, J.; Jung, H.; Seo, Y.; Woo, H.; Nam, K.; Lee, K.; et al. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci. Adv. 2021, 7, eabf4398. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Dincer, C. The impact of biosensing in a pandemic outbreak: COVID-19. Biosens. Bioelectron. 2020, 163, 112274. [Google Scholar] [CrossRef]
- Tang, Z.; Kong, N.; Zhang, X.; Liu, Y.; Hu, P.; Mou, S.; Liljeström, P.; Shi, J.; Tan, W.; Kim, J.S.; et al. A materials-science perspective on tackling COVID-19. Nat. Rev. Mater. 2020, 5, 847–860. [Google Scholar] [CrossRef]
- Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J.A.; Pasquali, M.; Scott, J.A.; Vitale, F.; Unal, M.A.; Mattevi, C.; et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano 2020, 14, 6383–6406. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martin-Sampedro, R.; Del Real, G.; Aranda, P. Nanotechnology responses to COVID-19. Adv. Healthc. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef]
- Huang, X.; Kon, E.; Han, X.; Zhang, X.; Kong, N.; Mitchell, M.J.; Peer, D.; Tao, W. Nanotechnology-based strategies against SARS-CoV-2 variants. Nat. Nanotechnol. 2022, 17, 1027–1037. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef]
- Ye, G.; Liu, B.; Li, F. Cryo-EM structure of a SARS-CoV-2 omicron spike protein ectodomain. Nat. Commun. 2022, 13, 1214. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhang, X.; Li, F. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale 2022, 14, 1054–1074. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, C.; Xu, X.-F.; Xu, W.; Liu, S.-W. Structural and functional properties of SARS-CoV-2 spike protein: Potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef]
- Langel, S.N.; Johnson, S.; Martinez, C.I.; Tedjakusuma, S.N.; Peinovich, N.; Dora, E.G.; Kuehl, P.J.; Irshad, H.; Barrett, E.G.; Werts, A.D.; et al. Adenovirus type 5 SARS-CoV-2 vaccines delivered orally or intranasally reduced disease severity and transmission in a hamster model. Sci. Transl. Med. 2022, 14, eabn6868. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Finsterbusch, K.; Wu, M.; Harvey, R.; Hussain, S.; Greco, M.; Liu, Y.; Kjaer, S.; Swanton, C.; et al. SARS-CoV-2 S2–targeted vaccination elicits broadly neutralizing antibodies. Sci. Transl. Med. 2022, 14, eabn3715. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Liao, H.-Y.; Chen, X.; Wang, S.-W.; Cheng, C.-W.; Shahed-Al-Mahmud, M.; Liu, Y.-M.; Mohapatra, A.; Chen, T.-H.; Lo, J.M.; et al. Vaccination with SARS-CoV-2 spike protein lacking glycan shields elicits enhanced protective responses in animal models. Sci. Transl. Med. 2022, 14, eabm0899. [Google Scholar] [CrossRef]
- Callaway, E. The coronavirus is mutating—Does it matter? Nature 2020, 585, 174–178. [Google Scholar] [CrossRef]
- Williams, T.C.; Burgers, W.A. SARS-CoV-2 evolution and vaccines: Cause for concern? Lancet Respir. Med. 2021, 9, 333–335. [Google Scholar] [CrossRef]
- Ahmad, S.U.; Kiani, B.H.; Abrar, M.; Jan, Z.; Zafar, I.; Ali, Y.; Alanazi, A.M.; Malik, A.; Rather, M.A.; Ahmad, A. A comprehensive genomic study, mutation screening, phylogenetic and statistical analysis of SARS-CoV-2 and its variant omicron among different countries. J. Infect. Public Health 2022, 15, 878–891. [Google Scholar] [CrossRef]
- Escalera, A.; Gonzalez-Reiche, A.S.; Aslam, S.; Mena, I.; Laporte, M.; Pearl, R.L.; Fossati, A.; Rathnasinghe, R.; Alshammary, H.; van de Guchte, A.; et al. Mutations in SARS-CoV-2 variants of concern link to increased spike cleavage and virus transmission. Cell Host Microbe 2022, 30, 373–387.e7. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; de Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Abdool Karim, S.S.; de Oliveira, T. New SARS-CoV-2 variants—Clinical, public health, and vaccine implications. N. Engl. J. Med. 2021, 384, 1866–1868. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.-M.; et al. SARS-CoV-2 variants and vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef]
- Li, Z.; Yin, Y. Stimuli-responsive optical nanomaterials. Adv. Mater. 2019, 31, 1807061. [Google Scholar] [CrossRef]
- Yoshida, M.; Lahann, J. Smart nanomaterials. ACS Nano 2008, 2, 1101–1107. [Google Scholar] [CrossRef]
- Aflori, M. Smart nanomaterials for biomedical applications—A review. Nanomaterials 2021, 11, 396. [Google Scholar] [CrossRef]
- Murray, W.A.; Barnes, W.L. Plasmonic materials. Adv. Mater. 2007, 19, 3771–3782. [Google Scholar] [CrossRef]
- Zeng, J.; Gong, M.; Wang, D.; Li, M.; Xu, W.; Li, Z.; Li, S.; Zhang, D.; Yan, Z.; Yin, Y. Direct synthesis of water-dispersible magnetic/plasmonic heteronanostructures for multimodality biomedical imaging. Nano Lett. 2019, 19, 3011–3018. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; Zeng, J. Recent Advances in Responsive Optical Nanomaterials. Front. Chem. 2021, 9, 760187. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Q.; Wu, C.; Li, Y.; Cheng, C.; Yin, Y. Magnetically tunable plasmon coupling of Au nanoshells enabled by space-free confined growth. Nano Lett. 2020, 20, 8242–8249. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Yin, Y. Colloidal assembly and active tuning of coupled plasmonic nanospheres. Trends Chem. 2020, 2, 593–608. [Google Scholar] [CrossRef]
- Nel, A.E.; Miller, J.F. Nano-enabled COVID-19 vaccines: Meeting the challenges of durable antibody plus cellular immunity and immune escape. ACS Nano 2021, 15, 5793–5818. [Google Scholar] [CrossRef]
- Han, K.S.; Lee, S.; Kim, M.; Park, P.; Lee, M.H.; Nah, J. Electrically activated ultrathin PVDF-TrFE air filter for high-efficiency PM1.0 filtration. Adv. Funct. Mater. 2019, 29, 1903633. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Cao, S.; Li, S.; Chen, F.; Yuan, S.; Xu, C.; Zhou, J.; Feng, X.; Ma, X.; et al. Roll-to-roll production of metal-organic framework coatings for particulate matter removal. Adv. Mater. 2017, 29, 1606221. [Google Scholar] [CrossRef] [PubMed]
- Gu, G.Q.; Han, C.B.; Lu, C.X.; He, C.; Jiang, T.; Gao, Z.L.; Li, C.J.; Wang, Z.L. Triboelectric nanogenerator enhanced nanofiber air filters for efficient particulate matter removal. ACS Nano 2017, 11, 6211–6217. [Google Scholar] [CrossRef] [PubMed]
- Han, C.B.; Jiang, T.; Zhang, C.; Li, X.; Zhang, C.; Cao, X.; Wang, Z.L. Removal of particulate matter emissions from a vehicle using a self-powered triboelectric filter. ACS Nano 2015, 9, 12552–12561. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Ultra-light 3D nanofibre-nets binary structured nylon 6–polyacrylonitrile membranes for efficient filtration of fine particulate matter. J. Mater. Chem. A 2015, 3, 23946–23954. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Yin, X.; Yu, J.; Ding, B. Anti-deformed polyacrylonitrile/polysulfone composite membrane with binary structures for effective air filtration. ACS Appl. Mater. Interfaces 2016, 8, 8086–8095. [Google Scholar] [CrossRef]
- Choi, D.Y.; Jung, S.-H.; Song, D.K.; An, E.J.; Park, D.; Kim, T.-O.; Jung, J.H.; Lee, H.M. Al-coated conductive fibrous filter with low pressure drop for efficient electrostatic capture of ultrafine particulate pollutants. ACS Appl. Mater. Interfaces 2017, 9, 16495–16504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-H.; Zhu, Q.-H.; Zhang, L.; Yong, F.; Zhang, Z.; Wang, S.-L.; Wang, Y.; He, L.; Tao, G.-H. High-performance particulate matter including nanoscale particle removal by a self-powered air filter. Nat. Commun. 2020, 11, 1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Lan, W.; Hossen, M.A.; Qin, W.; Lee, K. Electrospun antibacterial and antiviral poly (ε-caprolactone)/zein/Ag bead-on-string membranes and its application in air filtration. Mater. Today Adv. 2021, 12, 100173. [Google Scholar] [CrossRef]
- Borojeni, I.A.; Gajewski, G.; Riahi, R.A. Application of Electrospun Nonwoven Fibers in Air Filters. Fibers 2022, 10, 15. [Google Scholar] [CrossRef]
- Ding, L.-G.; Wang, S.; Yao, B.-J.; Wu, W.-X.; Kan, J.-L.; Liu, Y.; Wu, J.; Dong, Y.-B. Covalent organic framework based multifunctional self-sanitizing face masks. J. Mater. Chem. A 2022, 10, 3346–3358. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, D.; Li, Z.; Shen, L.; Li, R.; Zhang, M.; Jiao, Y.; Xu, Y.; Lin, H. A new strategy to accelerate co-deposition of plant polyphenol and amine for fabrication of antibacterial nanofiltration membranes by in-situ grown Ag nanoparticles. Sep. Purif. Technol. 2022, 280, 119866. [Google Scholar] [CrossRef]
- Murali, G.; Lee, M.; Modigunta, J.K.R.; Kang, B.; Kim, J.; Park, E.; Kang, H.; Lee, J.; Park, Y.H.; Park, S.Y. Ultraviolet–Ozone-Activation-Driven Ag Nanoparticles Grown on Plastic Substrates for Antibacterial Applications. ACS Appl. Nano Mater. 2022, 5, 8767–8774. [Google Scholar] [CrossRef]
- Guo, C.; Cheng, F.; Liang, G.; Zhang, S.; Jia, Q.; He, L.; Duan, S.; Fu, Y.; Zhang, Z.; Du, M. Copper-based polymer-metal–organic framework embedded with Ag nanoparticles: Long-acting and intelligent antibacterial activity and accelerated wound healing. Chem. Eng. J. 2022, 435, 134915. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kong, W.; Zhou, J.; Hou, T.; Zhang, X.; Zhou, L.; Sun, M.; Liu, S.; Yang, B. Cross-Linking of Centrifugally Spun Starch/Polyvinyl Alcohol (ST/PVA) Composite Ultrafine Fibers and Antibacterial Activity Loaded with Ag Nanoparticles. ACS Omega 2022, 7, 7706–7714. [Google Scholar] [CrossRef]
- Ju, Y.; Han, T.; Yin, J.; Li, Q.; Chen, Z.; Wei, Z.; Zhang, Y.; Dong, L. Bumpy structured nanofibrous membrane as a highly efficient air filter with antibacterial and antiviral property. Sci. Total Environ. 2021, 777, 145768. [Google Scholar] [CrossRef] [PubMed]
- Saud, Z.; Richards, C.A.; Williams, G.; Stanton, R.J. Anti-viral organic coatings for high touch surfaces based on smart-release, Cu2+ containing pigments. Prog. Org. Coat. 2022, 172, 107135. [Google Scholar] [CrossRef] [PubMed]
- Raj, B.; Padhy, A.K.; Basu, S.; Mohapatra, M. Perspective of Organic-Based Antimicrobial Coating Materials: Implication Toward COVID-19. In COVID-19 Pandemic; Springer: Berlin/Heidelberg, Germany, 2022; pp. 75–89. [Google Scholar]
- Yu, Z.; Kadir, M.; Liu, Y.; Huang, J. Droplet-capturing coatings on environmental surfaces based on cosmetic ingredients. Chem 2021, 7, 2201–2211. [Google Scholar] [CrossRef]
- Yin, S.; Li, Z.; Cheng, L.; Wang, C.; Liu, Y.; Chen, Q.; Gong, H.; Guo, L.; Li, Y.; Liu, Z. Magnetic PEGylated Pt 3 Co nanoparticles as a novel MR contrast agent: In vivo MR imaging and long-term toxicity study. Nanoscale 2013, 5, 12464–12473. [Google Scholar] [CrossRef]
- Song, X.; Gong, H.; Yin, S.; Cheng, L.; Wang, C.; Li, Z.; Li, Y.; Wang, X.; Liu, G.; Liu, Z. Ultra-small iron oxide doped polypyrrole nanoparticles for in vivo multimodal imaging guided photothermal therapy. Adv. Funct. Mater. 2014, 24, 1194–1201. [Google Scholar] [CrossRef]
- Preethi, M.; Roy, L.; Lahkar, S.; Borse, V. Outlook of various diagnostics and nanodiagnostic techniques for COVID-19. Biosens. Bioelectron. 2022, 100276. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Yin, L.; Li, X.; Chen, S.; Peng, L.; Liu, G.; Ye, S.; Zhang, W.; Man, S. A smartphone-based visual biosensor for CRISPR-Cas powered SARS-CoV-2 diagnostics. Biosens. Bioelectron. 2022, 195, 113646. [Google Scholar] [CrossRef]
- Djaileb, A.; Charron, B.; Jodaylami, M.H.; Thibault, V.; Coutu, J.; Stevenson, K.; Forest, S.; Live, L.S.; Boudreau, D.; Pelletier, J.N.; et al. A rapid and quantitative serum test for SARS-CoV-2 antibodies with portable surface plasmon resonance sensing. ChemRxiv 2020. [Google Scholar] [CrossRef]
- FDA. EUA Authorized Serology Test Performance [WWW Document]. 2020. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (accessed on 21 May 2022).
- Wang, S.; Shu, J.; Lyu, A.; Huang, X.; Zeng, W.; Jin, T.; Cui, H. Label-free immunoassay for sensitive and rapid detection of the SARS-CoV-2 antigen based on functionalized magnetic nanobeads with chemiluminescence and immunoactivity. Anal. Chem. 2021, 93, 14238–14246. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Lin, C.; Long, L.; Hu, J.; He, J.; Zeng, H.; Huang, Z.; Li, Z.-Y.; Tanemura, M. Human ACE2-functionalized gold “virus-trap” nanostructures for accurate capture of SARS-CoV-2 and single-virus SERS detection. Nano-Micro Lett. 2021, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- CareStartTM COVID-19 Antigen Test Kit Using NanoActTM Launched in the U.S. | BioSpace [WWW Document]. Available online: https://www.biospace.com/article/releases/carestartcovid-19-antigen-test-kit-using-nanoact-launched-in-the-u-s-/ (accessed on 21 May 2022).
- FDA. COVID-19 Rapid Test Casette [WWW Document]. 2022. Available online: https://www.fda.gov/media/138435/download (accessed on 22 May 2022).
- Chen, Z.; Zhang, Z.; Zhai, X.; Li, Y.; Lin, L.; Zhao, H.; Bian, L.; Li, P.; Yu, L.; Wu, Y. Rapid and sensitive detection of anti-SARS-CoV-2 IgG, using lanthanide-doped nanoparticles-based lateral flow immunoassay. Anal. Chem. 2020, 92, 7226–7231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, X.; Zheng, S.; Cheng, X.; Xiao, R.; Li, Q.; Wang, W.; Liu, X.; Wang, S. Development of an ultrasensitive fluorescent immunochromatographic assay based on multilayer quantum dot nanobead for simultaneous detection of SARS-CoV-2 antigen and influenza A virus. Sens. Actuators B Chem. 2021, 345, 130372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Malekjahani, A.; Udugama, B.N.; Kadhiresan, P.; Chen, H.; Osborne, M.; Franz, M.; Kucera, M.; Plenderleith, S.; Yip, L.; et al. Surveilling and tracking COVID-19 patients using a portable quantum dot smartphone device. Nano Lett. 2021, 21, 5209–5216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, F.; Xie, W.; Zhou, T.-C.; OuYang, J.; Jin, L.; Li, H.; Zhao, C.-Y.; Zhang, L.; Wei, J. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators B Chem. 2021, 327, 128899. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Behbahan, N.G.G.; Bahrani, S.; Mousavi, S.M.; Gholami, A.; Ramakrishna, S.; Firoozsani, M.; Moghadami, M.; Lankarani, K.B.; Omidifar, N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2021, 171, 112731. [Google Scholar] [CrossRef] [PubMed]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Jahan, S.; Yuan, B.; Saleh, M.S.; Ju, E.; Gao, S.J.; Panat, R. Sensing of COVID-19 antibodies in seconds via aerosol jet nanoprinted reduced-graphene-oxide-coated 3D electrodes. Adv. Mater. 2021, 33, 2006647. [Google Scholar] [CrossRef]
- Soler, M.; Estevez, M.C.; Cardenosa-Rubio, M.; Astua, A.; Lechuga, L.M. How nanophotonic label-free biosensors can contribute to rapid and massive diagnostics of respiratory virus infections: COVID-19 case. ACS Sens. 2020, 5, 2663–2678. [Google Scholar] [CrossRef]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-based testing but not real-time polymerase chain reaction correlates with severe acute respiratory syndrome coronavirus 2 viral culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef]
- Erlich, H.A.; Gelfand, D.; Sninsky, J.J. Recent advances in the polymerase chain reaction. Science 1991, 252, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Arnheim, N.; Erlich, H.A. The polymerase chain reaction. Trends Genet. 1989, 5, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Ginocchio, C.C.; McAdam, A.J. Current best practices for respiratory virus testing. J. Clin. Microbiol. 2011, 49, S44–S48. [Google Scholar] [CrossRef] [Green Version]
- Sanders, R.J.; Annest, S.J. Thoracic outlet and pectoralis minor syndromes. Semin. Vasc. Surg. 2014, 27, 86–117. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.L.; Gilbertson, B.P.; Trifkovic, S.; Brown, L.E.; McKimm-Breschkin, J.L. Influenza virus neuraminidase structure and functions. Front. Microbiol. 2019, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Wilson, I.A.; Skehel, J.J.; Wiley, D. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef]
- Yang, Q.; Gong, X.; Song, T.; Yang, J.; Zhu, S.; Li, Y.; Cui, Y.; Li, Y.; Zhang, B.; Chang, J. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosens. Bioelectron. 2011, 30, 145–150. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.-B.; Kim, M.-G. A dual gold nanoparticle conjugate-based lateral flow assay (LFA) method for the analysis of troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef]
- Sadeghi, P.; Sohrabi, H.; Hejazi, M.; Jahanban-Esfahlan, A.; Baradaran, B.; Tohidast, M.; Majidi, M.R.; Mokhtarzadeh, A.; Tavangar, S.M.; de la Guardia, M. Lateral flow assays (LFA) as an alternative medical diagnosis method for detection of virus species: The intertwine of nanotechnology with sensing strategies. TrAC Trends Anal. Chem. 2021, 145, 116460. [Google Scholar] [CrossRef]
- Liang, P.; Guo, Q.; Zhao, T.; Wen, C.-Y.; Tian, Z.; Shang, Y.; Xing, J.; Jiang, Y.; Zeng, J. Ag Nanoparticles with Ultrathin Au Shell-Based Lateral Flow Immunoassay for Colorimetric and SERS Dual-Mode Detection of SARS-CoV-2 IgG. Anal. Chem. 2022, 94, 8466–8473. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Bai, Y.; Yin, Y. Gold nanocups with multimodal plasmon resonance for quantum-dot random lasing. Appl. Mater. Today 2022, 26, 101358. [Google Scholar] [CrossRef]
- Olson, J.; Dominguez-Medina, S.; Hoggard, A.; Wang, L.-Y.; Chang, W.-S.; Link, S. Optical characterization of single plasmonic nanoparticles. Chem. Soc. Rev. 2015, 44, 40–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic plasmonic nanostructures for colorimetric sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.; Oliveira, O.N., Jr. Plasmonic biosensing: Focus review. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical properties and applications of plasmonic-metal nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Liu, N.; Hentschel, M.; Weiss, T.; Alivisatos, A.P.; Giessen, H. Three-dimensional plasmon rulers. Science 2011, 332, 1407–1410. [Google Scholar] [CrossRef]
- Hentschel, M.; Schaferling, M.; Weiss, T.; Liu, N.; Giessen, H. Three-dimensional chiral plasmonic oligomers. Nano Lett. 2012, 12, 2542–2547. [Google Scholar] [CrossRef]
- Li, Z.; Ye, Z.; Han, L.; Fan, Q.; Wu, C.; Ding, D.; Xin, H.L.; Myung, N.V.; Yin, Y. Polarization-modulated multidirectional photothermal actuators. Adv. Mater. 2021, 33, 2006367. [Google Scholar] [CrossRef]
- Li, Z.; Jin, J.; Yang, F.; Song, N.; Yin, Y. Coupling magnetic and plasmonic anisotropy in hybrid nanorods for mechanochromic responses. Nat. Commun. 2020, 11, 2883. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, J.; Jin, J.; Yang, F.; Aleisa, R.; Yin, Y. Creation and reconstruction of thermochromic Au nanorods with surface concavity. J. Am. Chem. Soc. 2021, 143, 15791–15799. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Poon, W.; Ye, Z.; Qi, F.; Park, B.H.; Yin, Y. Magnetic Field-Modulated Plasmonic Scattering of Hybrid Nanorods for FFT-Weighted OCT Imaging in NIR-II. ACS Nano 2022, 16, 12738–12746. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, Z.; Tian, F.; Ye, Z.; Zhou, X.; Zhong, X.; Chen, Q.; Yang, M.; Liu, Z.; Yin, Y. Fast Fourier Transform-weighted Photoacoustic Imaging by In Vivo Magnetic Alignment of Hybrid Nanorods. Nano Lett. 2022, 22, 5158–5166. [Google Scholar] [CrossRef]
- Jain, P.K.; Huang, X.; El-Sayed, I.H.; El-Sayed, M.A. Noble metals on the nanoscale: Optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc. Chem. Res. 2008, 41, 1578–1586. [Google Scholar] [CrossRef]
- Pereira-Barros, M.A.; Daeid, N.N.; Adegoke, O. Rapid and selective aptamer-based fluorescence detection of salivary lysozyme using plasmonic metal-enhanced fluorescence of ZnSSe alloyed quantum dots-gold nanoparticle nanohybrid. J. Photochem. Photobiol. A Chem. 2021, 418, 113384. [Google Scholar] [CrossRef]
- Hou, S.; Chen, Y.; Lu, D.; Xiong, Q.; Lim, Y.; Duan, H. A Self-Assembled Plasmonic Substrate for Enhanced Fluorescence Resonance Energy Transfer. Adv. Mater. 2020, 32, 1906475. [Google Scholar] [CrossRef]
- Choi, J.-H.; Choi, J.-W. Metal-enhanced fluorescence by bifunctional Au nanoparticles for highly sensitive and simple detection of proteolytic enzyme. Nano Lett. 2020, 20, 7100–7107. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.-H. Highly sensitive flexible SERS-based sensing platform for detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef]
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B Chem. 2021, 329, 129196. [Google Scholar] [CrossRef]
- Dina, N.E.; Tahir, M.A.; Bajwa, S.Z.; Amin, I.; Valev, V.K.; Zhang, L. SERS-based antibiotic susceptibility testing: Towards point-of-care clinical diagnosis. Biosens. Bioelectron. 2022, 114843. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Gupta, R.K.; Prakash, J. Engineering metal oxide semiconductor nanostructures for enhanced charge transfer: Fundamentals and emerging SERS applications. J. Mater. Chem. C 2022, 10, 73–95. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, K.; Zhang, Y.; Yang, Z.; Qian, Z.; Li, N.; Li, L.; Jiang, G.; Wang, T.; Zong, S. Wearable SERS Sensor Based on Omnidirectional Plasmonic Nanovoids Array with Ultra-High Sensitivity and Stability. Small 2022, 18, 2201508. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Kurniawan, D.; Gholami, A.; Rahmanian, V.; Omidifar, N.; Chiang, W.-H. Recent Advances in Inflammatory Diagnosis with Graphene Quantum Dots Enhanced SERS Detection. Biosensors 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Saada, H.; Pagneux, Q.; Boukherroub, R. Plasmonic Approaches for the Detection of SARS-CoV-2 Viral Particles. Biosensors 2022, 12, 548. [Google Scholar] [CrossRef]
- Blumenfeld, N.R.; Bolene, M.A.E.; Jaspan, M.; Ayers, A.G.; Zarrandikoetxea, S.; Freudman, J.; Shah, N.; Tolwani, A.M.; Hu, Y.; Chern, T.L.; et al. Multiplexed reverse-transcriptase quantitative polymerase chain reaction using plasmonic nanoparticles for point-of-care COVID-19 diagnosis. Nat. Nanotechnol. 2022, 17, 984–992. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Wang, Y.; Wu, Y.; Liu, Z.; Liu, S. Sensitive detection of organophosphorus pesticides based on the localized surface plasmon resonance and fluorescence dual-signal readout. Anal. Chim. Acta 2022, 1235, 340536. [Google Scholar] [CrossRef]
- Yue, W.; Liu, C.; Zha, Z.; Liu, R.; Gao, J.; Shafi, M.; Feng, J.; Jiang, S. Composite substrate of graphene/Ag nanoparticles coupled with a multilayer film for surface-enhanced Raman scattering biosensing. Opt. Express 2022, 30, 13226–13237. [Google Scholar] [CrossRef]
- Beck, F.; Horn, C.; Baeumner, A.J. Dry-reagent microfluidic biosensor for simple detection of NT-proBNP via Ag nanoparticles. Anal. Chim. Acta 2022, 1191, 339375. [Google Scholar] [CrossRef]
- Beck, F.; Horn, C.; Baeumner, A.J. Ag nanoparticles outperform Au nanoparticles for the use as label in electrochemical point-of-care sensors. Anal. Bioanal. Chem. 2022, 414, 475–483. [Google Scholar] [CrossRef]
- Liu, L.; Liang, X.; Qiu, G.; Guo, C.; Chan, Y.K.; Wu, C.M.L. Self-Assembly Silver Nanoparticles Decorated on Gold Nanoislands for Label-Free Localized Surface Plasmon Resonance Biosensing. Adv. Mater. Interfaces 2022, 9, 2200339. [Google Scholar] [CrossRef]
- Gao, C.; Hu, Y.; Wang, M.; Chi, M.; Yin, Y. Fully alloyed Ag/Au nanospheres: Combining the plasmonic property of Ag with the stability of Au. J. Am. Chem. Soc. 2014, 136, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, Z.; Ravaine, S.; He, M.; Song, Y.; Yin, Y.; Zheng, H.; Teng, J.; Zhang, A. From colloidal particles to photonic crystals: Advances in self-assembly and their emerging applications. Chem. Soc. Rev. 2021, 50, 5898–5951. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Jan, M.R. Ultrasensitive electrical biosensing of proteins and DNA: Carbon-nanotube derived amplification of the recognition and transduction events. J. Am. Chem. Soc. 2004, 126, 3010–3011. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A review on impedimetric biosensors. Artif. Cells Nanomed. Biotechnol. 2016, 44, 248–262. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Volpe, J.; Bach-Toledo, L.; Kurpel, K.C.; Deller, A.E.; Soares, A.L.; Nardin, J.M.; Marchesi, L.F.; Simas, F.F.; Oliveira, C.C.; et al. Development of polypyrrole (nano) structures decorated with gold nanoparticles toward immunosensing for COVID-19 serological diagnosis. Mater. Today Chem. 2022, 24, 100817. [Google Scholar] [CrossRef]
- Chen, S.; Sun, Y.; Fan, F.; Chen, S.; Zhang, Y.; Zhang, Y.; Meng, X.; Lin, J.-M. Present status of microfluidic PCR chip in nucleic acid detection and future perspective. TrAC Trends Anal. Chem. 2022, 157, 116737. [Google Scholar] [CrossRef]
- Garzarelli, V.; Chiriacò, M.S.; Cereda, M.; Autuori, I.; Ferrara, F. Miniaturized Real-Time PCR systems for SARS-CoV-2 detection at the Point-of-Care. Clin. Chim. Acta 2022, 536, 104–111. [Google Scholar] [CrossRef]
- Ahmed, W.; Simpson, S.L.; Bertsch, P.M.; Bibby, K.; Bivins, A.; Blackall, L.L.; Bofill-Mas, S.; Bosch, A.; Brandão, J.; Choi, P.M.; et al. Minimizing errors in RT-PCR detection and quantification of SARS-CoV-2 RNA for wastewater surveillance. Sci. Total Environ. 2022, 805, 149877. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.-S.; Lee, D.-I.; Jeong, S.; Kang, G.-H.; Jang, Y.S.; Kim, W.; Choi, H.Y.; Kim, J.G.; Choi, S.-H. The application of a deep learning system developed to reduce the time for RT-PCR in COVID-19 detection. Sci. Rep. 2022, 12, 1234. [Google Scholar] [CrossRef]
- Nguyen, P.Q.M.; Wang, M.; Ann Maria, N.; Li, A.Y.; Tan, H.Y.; Xiong, G.M.; Tan, M.-K.M.; Bhagat, A.A.S.; Ong, C.W.; Lim, C.T. Modular micro-PCR system for the onsite rapid diagnosis of COVID-19. Microsyst. Nanoeng. 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Chen, L.; Hu, J.; Trick, A.Y.; Chen, F.-E.; Hsieh, K.; Zhao, Y.; Coleman, B.; Kruczynski, K.; Pisanic, T.R., II. Magnetofluidic immuno-PCR for point-of-care COVID-19 serological testing. Biosens. Bioelectron. 2022, 195, 113656. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, I.; Citu, C.; Bratosin, F.; Malita, D.; Romosan, I.; Gurban, C.V.; Bota, A.V.; Turaiche, M.; Bratu, M.L.; Pilut, C.N. The Impact of Multiplex PCR in Diagnosing and Managing Bacterial Infections in COVID-19 Patients Self-Medicated with Antibiotics. Antibiotics 2022, 11, 437. [Google Scholar] [CrossRef]
- Bucharskaya, A.B.; Khlebtsov, N.G.; Khlebtsov, B.N.; Maslyakova, G.N.; Navolokin, N.A.; Genin, V.D.; Genina, E.A.; Tuchin, V.V. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: Challenges and prospects. Materials 2022, 15, 1606. [Google Scholar] [CrossRef]
- Muzzi, B.; Albino, M.; Gabbani, A.; Omelyanchik, A.; Kozenkova, E.; Petrecca, M.; Innocenti, C.; Balica, E.; Lavacchi, A.; Scavone, F. Star-Shaped Magnetic-Plasmonic Au@ Fe3O4 Nano-Heterostructures for Photothermal Therapy. ACS Appl. Mater. Interfaces 2022, 14, 29087–29098. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Xiong, Y.; Cai, S.; Li, H.; Zhang, X.; Sun, Q.; Yang, R. Plasmon-Enhanced Photothermal Properties of Au@ Ti3C2Tx Nanosheets for Antibacterial Applications. Nanoscale 2022, 14, 16572–16580. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Cheng, X.; Sun, D.; Guo, Y.; Wang, N.; Ruan, J.; Hu, Y.; Zhao, M.; Zhao, T.; Feng, H. Synthesis of multi-branched Au nanocomposites with distinct plasmon resonance in NIR-II window and controlled CRISPR-Cas9 delivery for synergistic gene-photothermal therapy. Biomaterials 2022, 287, 121621. [Google Scholar] [CrossRef]
- Li, Z.; Myung, N.V.; Yin, Y. Light-powered soft steam engines for self-adaptive oscillation and biomimetic swimming. Sci. Robot. 2021, 6, eabi4523. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, Y.; Cai, Z.; Liu, R.; Xu, X.; Li, R.; Wang, J.-J.; Yang, D.A. Three-dimensional/two-dimensional photonic crystal hydrogels for biosensing. J. Mater. Chem. C 2021, 9, 5840–5857. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Zhang, X.; Wang, D.; Xu, W.; Yin, Y. Magnetic assembly of nanocubes for orientation-dependent photonic responses. Nano Lett. 2019, 19, 6673–6680. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Yang, S. Chemical aspects of three-dimensional photonic crystals. Chem. Rev. 2010, 110, 547–574. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Meng, Z.; Wang, F.; Wang, Q.; Xue, M.; Xu, Z. A 2-D photonic crystal hydrogel for selective sensing of glucose. J. Mater. Chem. A 2014, 2, 9559–9565. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizvi, A.S.; Irfan, M.; Yan, D.; Khan, R.U.; Rafique, B.; Xue, M.; Meng, Z.H.; Qu, F. Glycated albumin based photonic crystal sensors for detection of lipopolysaccharides and discrimination of Gram-negative bacteria. Anal. Chim. Acta 2020, 1117, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Smith, N.L.; Zhang, J.-T.; Asher, S.A. Two-dimensional photonic crystal chemical and biomolecular sensors. Anal. Chem. 2015, 87, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Kwak, D.H.; Punihaole, D.; Hong, Z.; Velankar, S.S.; Liu, X.; Asher, S.A. A photonic crystal protein hydrogel sensor for Candida albicans. Angew. Chem. 2015, 127, 13228–13232. [Google Scholar] [CrossRef]
- Cai, Z.; Luck, L.A.; Punihaole, D.; Madura, J.D.; Asher, S.A. Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition. Chem. Sci. 2016, 7, 4557–4562. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Sasmal, A.; Liu, X.; Asher, S.A. Responsive photonic crystal carbohydrate hydrogel sensor materials for selective and sensitive lectin protein detection. ACS Sens. 2017, 2, 1474–1481. [Google Scholar] [CrossRef]
- Cai, Z.; Xu, X.; Meng, Z.; Rafique, B.; Liu, R. Colloidal Photonic Crystal Sensors. Funct. Mater. Colloid. Self-Assem. 2022, 237–275. [Google Scholar]
- Zhang, Y.; Sun, Y.; Liu, J.; Guo, P.; Cai, Z.; Wang, J.-J. Polymer-infiltrated SiO2 inverse opal photonic crystals for colorimetrically selective detection of xylene vapors. Sens. Actuators B Chem. 2019, 291, 67–73. [Google Scholar] [CrossRef]
- Murtaza, G.; Rizvi, A.S.; Xue, M.; Qiu, L.; Meng, Z. Consensus Receptor-Binding Domain-Targeted Aptamer Selection and Designing of a Photonic Crystal-Decorated Aptasensor for SARS-CoV-2. Anal. Chem. 2022, 94, 7391–7399. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Qiu, L.; Shea, K.J.; Meng, Z.; Xue, M. Dyeing and functionalization of wearable silk fibroin/cellulose composite by nanocolloidal array. ACS Appl. Mater. Interfaces 2019, 11, 39163–39170. [Google Scholar] [CrossRef] [PubMed]
- Boxer, M.; Mazloumi, M.; Snell, P.; Rochon, P.; Sabat, R.G. Large-area photonic crystals, quasicrystals, and Moiré quasicrystals fabricated on azobenzene molecular glass films by pyramidal interference lithography. Opt. Mater. Express 2022, 12, 4362–4374. [Google Scholar] [CrossRef]
- Kawasaki, D.; Yamada, H.; Sueyoshi, K.; Hisamoto, H.; Endo, T. Imprinted Photonic Crystal-Film-Based Smartphone-Compatible Label-Free Optical Sensor for SARS-CoV-2 Testing. Biosensors 2022, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.-L.; Vaddi, Y.; Bin-Alam, M.S.; Cheng, L.; Alaee, R.; Upham, J.; Huttunen, M.J.; Dolgaleva, K.; Reshef, O.; Boyd, R.W. Fourier-Engineered Plasmonic Lattice Resonances. ACS Nano 2022, 16, 5696–5703. [Google Scholar] [CrossRef]
- Kelavuori, J.; Vanyukov, V.; Stolt, T.; Karvinen, P.; Rekola, H.; Hakala, T.K.; Huttunen, M.J. Thermal Control of Plasmonic Surface Lattice Resonances. Nano Lett. 2022, 22, 3879–3883. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B. Photonic and Plasmonic Metasensors. Laser Photonics Rev. 2022, 16, 2100328. [Google Scholar] [CrossRef]
- Scarabelli, L.; Vila-Liarte, D.; Mihi, A.; Liz-Marzán, L.M. Templated Colloidal Self-Assembly for Lattice Plasmon Engineering. Acc. Mater. Res. 2021, 2, 816–827. [Google Scholar] [CrossRef]
- Gupta, V.; Aftenieva, O.; Probst, P.T.; Sarkar, S.; Steiner, A.M.; Vogel, N.; Fery, A.; König, T.A. Advanced Colloidal Sensors Enabled by an Out-of-Plane Lattice Resonance. Adv. Photonics Res. 2022, 3, 2200152. [Google Scholar] [CrossRef]
- Vinnacombe-Willson, G.A.; Conti, Y.; Jonas, S.J.; Weiss, P.S.; Mihi, A.; Scarabelli, L. Surface Lattice Plasmon Resonances by Direct In Situ Substrate Growth of Gold Nanoparticles in Ordered Arrays. Adv. Mater. 2022, 34, 2205330. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Cao, Z.; Zhou, J.; Ong, H.C.; Jin, W.; Du, J.; Lei, D. High-Q Circular Dichroism Resonances in Plasmonic Lattices with Chiral Unit Cells. Adv. Funct. Mater. 2022, 32, 2204095. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Wang, S.-H.; Lo, S.-C.; Yong, W.-H.; Ho, Y.-L.; Delaunay, J.-J.; Tsai, W.-S.; Wei, P.-K. Sensitive Oligonucleotide Detection Using Resonant Coupling between Fano Resonance and Image Dipoles of Gold Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 14012–14024. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Samanta, T.; Guchhait, S.; Mitra, P.; Shunmugam, R.; Ghosh, N. Fano Resonance in Plasmonic Crystals Enables High-Sensitive Arsenite Detection. Plasmonics 2022, 17, 2015–2021. [Google Scholar] [CrossRef]
- Ahmed, R.; Guimarães, C.F.; Wang, J.; Soto, F.; Karim, A.H.; Zhang, Z.; Reis, R.L.; Akin, D.; Paulmurugan, R.; Demirci, U. Large-Scale functionalized metasurface-based SARS-CoV-2 detection and quantification. ACS Nano 2022, 16, 15946–15958. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Ortiz-Ospina, E.; Roser, M.; Hasell, J.; Appel, C.; Giattino, C.; Rodés-Guirao, L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021, 5, 947–953. [Google Scholar] [CrossRef]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 vaccination hesitancy in the United States: A rapid national assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef]
- Peng, X.-L.; Cheng, J.-S.-Y.; Gong, H.-L.; Yuan, M.-D.; Zhao, X.-H.; Li, Z.; Wei, D.-X. Advances in the design and development of SARS-CoV-2 vaccines. Mil. Med. Res. 2021, 8, 1–31. [Google Scholar] [CrossRef]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. [Google Scholar] [CrossRef]
- Shafaati, M.; Saidijam, M.; Soleimani, M.; Hazrati, F.; Mirzaei, R.; Amirheidari, B.; Tanzadehpanah, H.; Karampoor, S.; Kazemi, S.; Yavari, B. A brief review on DNA vaccines in the era of COVID-19. Future Virol. 2022, 17, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Bekker, L.-G. DNA vaccines join the fight against COVID-19. Lancet 2022, 399, 1281–1282. [Google Scholar] [CrossRef] [PubMed]
- Babuadze, G.G.; Fausther-Bovendo, H.; deLaVega, M.-A.; Lillie, B.; Naghibosadat, M.; Shahhosseini, N.; Joyce, M.A.; Saffran, H.A.; Lorne Tyrrell, D.; Falzarano, D.; et al. Two DNA vaccines protect against severe disease and pathology due to SARS-CoV-2 in Syrian hamsters. NPJ Vaccines 2022, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Hogan, M.J.; Pardi, N. mRNA Vaccines in the COVID-19 Pandemic and beyond. Annu. Rev. Med. 2022, 73, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Yang, H.; Zhang, M.; Liu, Q.; Alotaibi, G.; Irfan, M.; He, H.; Chang, J.; Liang, X.-J.; Weng, Y. mRNA vaccines for COVID-19 and diverse diseases. J. Control. Release 2022, 345, 314–333. [Google Scholar] [CrossRef]
- Rubin, E.J.; Longo, D.L. COVID-19 mRNA vaccines—Six of one, half a dozen of the other. Mass. Med. Soc. 2022, 386, 183–185. [Google Scholar] [CrossRef]
- Xue, J.-B.; Lai, D.-Y.; Jiang, H.-W.; Qi, H.; Guo, S.-J.; Zhu, Y.-S.; Xu, H.; Zhou, J.; Tao, S.-C. Landscape of the RBD-specific IgG, IgM, and IgA responses triggered by the inactivated virus vaccine against the Omicron variant. Cell Discov. 2022, 8, 15. [Google Scholar] [CrossRef]
- Premikha, M.; Chiew, C.J.; Wei, W.E.; Leo, Y.S.; Ong, B.; Lye, D.C.; Lee, V.J.; Tan, K.B. Comparative Effectiveness of mRNA and Inactivated Whole-Virus Vaccines Against Coronavirus Disease 2019 Infection and Severe Disease in Singapore. Clin. Infect. Dis. 2022, 75, 1442–1445. [Google Scholar] [CrossRef]
- Hotez, P.J.; Bottazzi, M.E. Whole inactivated virus and protein-based COVID-19 vaccines. Annu. Rev. Med. 2022, 73, 55–64. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Zarandi, P.K.; Zinatizadeh, M.; Yousefi, M.H.; Amani, J.; Rezaei, N. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed. Pharmacother. 2022, 146, 112527. [Google Scholar] [CrossRef]
- Karbiener, M.; Farcet, M.R.; Zollner, A.; Masuda, T.; Mori, M.; Moschen, A.R.; Kreil, T.R. Calibrated comparison of SARS-CoV-2 neutralizing antibody levels in response to protein-, mRNA-, and vector-based COVID-19 vaccines. NPJ Vaccines 2022, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Vesikari, T. Trimeric S protein COVID-19 vaccine needs to find its place. Lancet 2022, 399, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.J.; Kulkarni, J.A.; van der Meel, R.; Cullis, P.R.; Vader, P.; Schiffelers, R.M. State-of-the-art design and rapid-mixing production techniques of lipid nanoparticles for nucleic acid delivery. Small Methods 2018, 2, 1700375. [Google Scholar] [CrossRef] [Green Version]
- Salvatori, G.; Luberto, L.; Maffei, M.; Aurisicchio, L.; Roscilli, G.; Palombo, F.; Marra, E. SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. J. Transl. Med. 2020, 18, 222. [Google Scholar] [CrossRef]
- Belouzard, S.; Millet, J.K.; Licitra, B.N.; Whittaker, G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses 2012, 4, 1011–1033. [Google Scholar] [CrossRef] [Green Version]
- Bongini, P.; Trezza, A.; Bianchini, M.; Spiga, O.; Niccolai, N. A possible strategy to fight COVID-19: Interfering with spike glycoprotein trimerization. Biochem. Biophys. Res. Commun. 2020, 528, 35–38. [Google Scholar] [CrossRef]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef]
- Granwehr, B.P. In adults who had not had COVID-19, Novavax vaccine had 90% efficacy at ≥7 d after the second dose. Ann. Intern. Med. 2022, 175, JC52. [Google Scholar] [CrossRef]
- Parums, D.V. First Approval of the Protein-Based Adjuvanted Nuvaxovid (NVX-CoV2373) Novavax Vaccine for SARS-CoV-2 Could Increase Vaccine Uptake and Provide Immune Protection from Viral Variants. Med. Sci. Monit. 2022, 28, e936521–e936523. [Google Scholar] [CrossRef]
- Bangaru, S.; Ozorowski, G.; Turner, H.L.; Antanasijevic, A.; Huang, D.; Wang, X.; Torres, J.L.; Diedrich, J.K.; Tian, J.-H.; Portnoff, A.D. Structural analysis of full-length SARS-CoV-2 spike protein from an advanced vaccine candidate. Science 2020, 370, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.; Raghunandan, R.; Wu, Y.; Liu, Y.; Massare, M.; Nathan, M.; Zhou, B.; Lu, H.; Boddapati, S.; Li, J. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE 2012, 7, e50852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, V.; Fries, L.; Wu, Y.; Agrawal, S.; Cho, I.; Thomas, D.N.; Spindler, M.; Lindner, E.; Hahn, T.; Plested, J. Improved titers against influenza drift variants with a nanoparticle vaccine. N. Engl. J. Med. 2018, 378, 2346–2348. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Zhu, Z.; Lin, J.; Cheung, C.F.; Lu, V.L.; Yan, F.; Chan, C.-Y.; Li, G. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano 2020, 14, 6213–6221. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X.; et al. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 2177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, R.K.; Tiwari, R.; Sarangi, A.K.; Islam, M.R.; Chakraborty, C.; Dhama, K. Omicron (B. 1.1. 529) variant of SARS-CoV-2: Concerns, challenges, and recent updates. J. Med. Virol. 2022, 94, 2336–2342. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Zhang, Y.; Yang, E.S.; Schramm, C.A.; Shi, W.; Pegu, A.; Oloniniyi, O.K.; Henry, A.R.; Darko, S.; et al. Ultrapotent antibodies against diverse and highly transmissible SARS-CoV-2 variants. Science 2021, 373, eabh1766. [Google Scholar] [CrossRef]
- De Gasparo, R.; Pedotti, M.; Simonelli, L.; Nickl, P.; Muecksch, F.; Cassaniti, I.; Percivalle, E.; Lorenzi, J.C.; Mazzola, F.; Magrì, D.; et al. Bispecific IgG neutralizes SARS-CoV-2 variants and prevents escape in mice. Nature 2021, 593, 424–428. [Google Scholar] [CrossRef]





| Diagnostic Techniques | Advantages | Disadvantages | |
|---|---|---|---|
| Conventional diagnostic techniques | CT scan | Early screening of infection, no sampling, non-invasive | Non-specific, X-ray exposure, operational only by technicians |
| X-ray imaging | Low cost, no sampling, non-invasive | Non-specific, false negatives, operational only by technicians, X-ray exposure | |
| MRI | Non-invasive infection monitoring, 3D imaging | Costly, only available in technical labs | |
| RT-PCR | High accuracy and sensitivity, sequence-specific sensing of coronavirus | Long detection time, high cost, only operational for trained experts | |
| CRISPR | Lost cost, highly sensitive, integrated to portable devices | Necessary specific CRISPR sequences | |
| Biosensing techniques based on responsive nanostructures | LSPR sensing | Colorimetric changes, easy operation, low cost, available for point-of-care detection, fast detection | Large-scale production of noble metal nanoparticles, complicated fabrication |
| SERS sensing | Highly sensitive, specific to virus, quantitative detection | Raman spectroscope needed | |
| Fluorescent biosensing | Highly sensitive and accuracy, low detection limit | Possible fluorescence quenching | |
| Electrochemical biosensing | Highly sensitive, label-free | Energy consumption, possible incorrect positives, low reproducibility | |
| Piezoelectric biosensing | Highly sensitive and specific, label-free and fast detection | Complicated sample preparation and pretreatment | |
| Type | Nanostructures | Surface Chemistry | Target | Sensitivity | Specificity | References |
|---|---|---|---|---|---|---|
| Colorimetricbiosensors | Au nanoislands | Thiol chemistry | DNA sequences of SARS-CoV-2 | 1.32 × 105 copies/μL | – | [79]. |
| Au nanoparticles | Thiol chemistry | N-gene | 1 copy µL−1 | – | [80]. | |
| Au thin films | Carbodiimide chemistry | Anti-SARS-CoV-2 antibodies | 1 μg/mL | – | [81]. | |
| Au nanoparticles | – | S and nucleocapsid protein | 96.7% | 100% | [82]. | |
| Magnetic beads/Au nanoparticles | Au−N and Au−S bonds and hydrophobic interactions | N protein | 69 fg mL−1 | – | [83]. | |
| Au nanoneedles array | Thiol chemistry | Virus via S protein | 80 copies mL−1 | – | [84]. | |
| Cellulose nanobeads | – | Nucleocapsidprotein | 88.4% | 100% | [85]. | |
| Au nanoparticles | – | S protein | 100% | 97.5% | [86]. | |
| Fluorescentbiosensors | Lanthanide-Doped Nanoparticles | Carbodiimide chemistry | Anti-SARS-CoV-2 IgG | – | – | [87]. |
| SiO2@QDs | Carbodiimide chemistry | SARS-CoV-2antigen | 5 pg/mL | – | [88]. | |
| CdSe/ZnS quantum dots | Carbodiimide chemistry | Antibodies | 90% | 100% | [89]. | |
| Electrochemical biosensors | Au@Fe3O4/carbon electrodes | Thiol chemistry | Viral RNA | 3 aM | – | [90]. |
| GO-Au NS | Carbodiimide chemistry | Glycoproteins | 0.0048 μAμg.mL−1.cm−2 | – | [91]. | |
| Graphene-ssDNA-AuNP/Au Electrode | Thiol chemistry | Viral RNA | 231 (copies/μL)−1 | ~100% | [92]. | |
| rGO/3D printed 3D electrode | Carbodiimide chemistry | Antibodies to spike S1 protein | 1 × 10−12 M | – | [93]. |
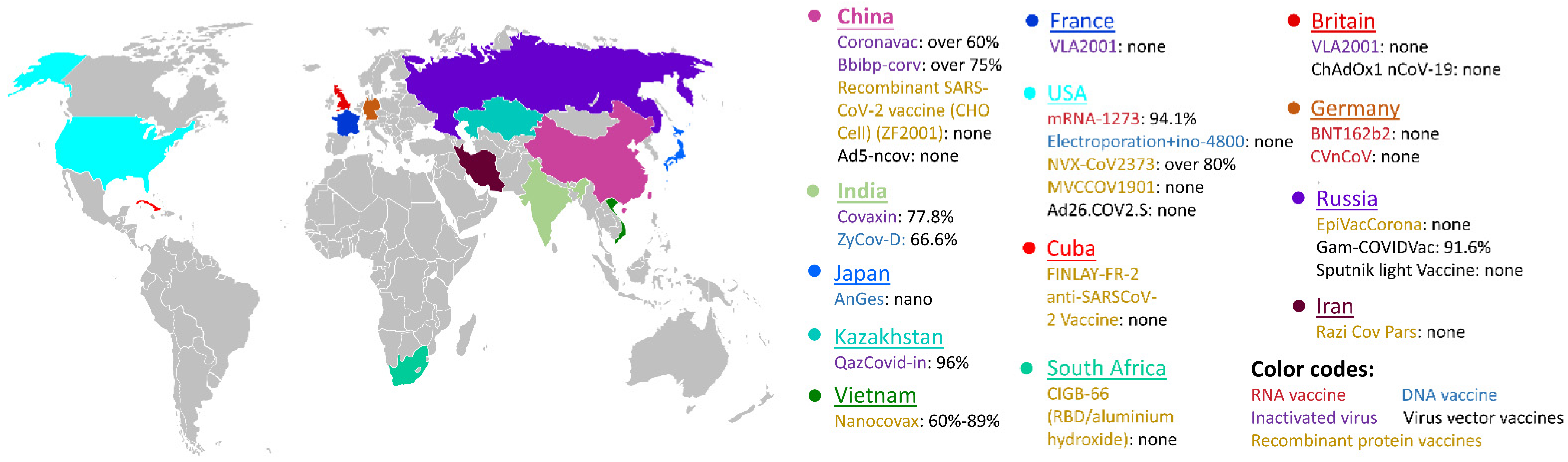
| Type | Company or Vaccine Name | Phase 3 | Efficacy (%) | Dose | Storage (°C) |
|---|---|---|---|---|---|
| mRNA vaccine | Sinovac | NCT04582344 | 50 | 2 (14−day interval) | 2–8 |
| Pfizer/BioNTech | NCT04368728 | 95 | 2 (21 days apart) | −70 | |
| Moderna | NCT04470427 | 94 | 2 (28 days apart) | −20 | |
| CureVac | NCT04652102 | 47 | 2 (28 days apart) | 2–8 | |
| DNA vaccine | AnGes | NCT04655625 | none | 2 (14− and 28−day interval) | RT |
| Inactivated virus | Sinopharm | NCT04510207 | 79 | 2 (21−day interval) | 2–8 |
| Virus vector vaccines | AstraZeneca | NCT04324606 | 62–90 | 2 (28−day interval) | 2–8 |
| Gameleya | NCT04530396 | 91.6 | 2 (21−day interval) | −18 | |
| Johnson & Johnson | NCT04505722 | 66–85.4 | 1 | 2–8 | |
| Recombinant protein vaccines | Novavax | NCT04636697 | 60–89 | 2 (21−day interval) | 2–8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, Z. Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination. Biosensors 2022, 12, 1129. https://doi.org/10.3390/bios12121129
Wang L, Li Z. Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination. Biosensors. 2022; 12(12):1129. https://doi.org/10.3390/bios12121129
Chicago/Turabian StyleWang, Lifeng, and Zhiwei Li. 2022. "Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination" Biosensors 12, no. 12: 1129. https://doi.org/10.3390/bios12121129
APA StyleWang, L., & Li, Z. (2022). Smart Nanostructured Materials for SARS-CoV-2 and Variants Prevention, Biosensing and Vaccination. Biosensors, 12(12), 1129. https://doi.org/10.3390/bios12121129




