Review: Advanced Atomic Force Microscopy Modes for Biomedical Research
Abstract
:1. Introduction
2. AFM Basic Principles
2.1. Cantilever Probe
2.2. Nano-Positioners
2.3. Controller Electronics
2.4. AFM Imaging Modes
3. AFM Biomedical Imaging Considerations
4. High-Speed AFM
4.1. AFM Instrument Modification
4.2. HSAFM Biomedical Applications
5. Mechanobiology with AFM
6. AFM Molecular Species Characterization
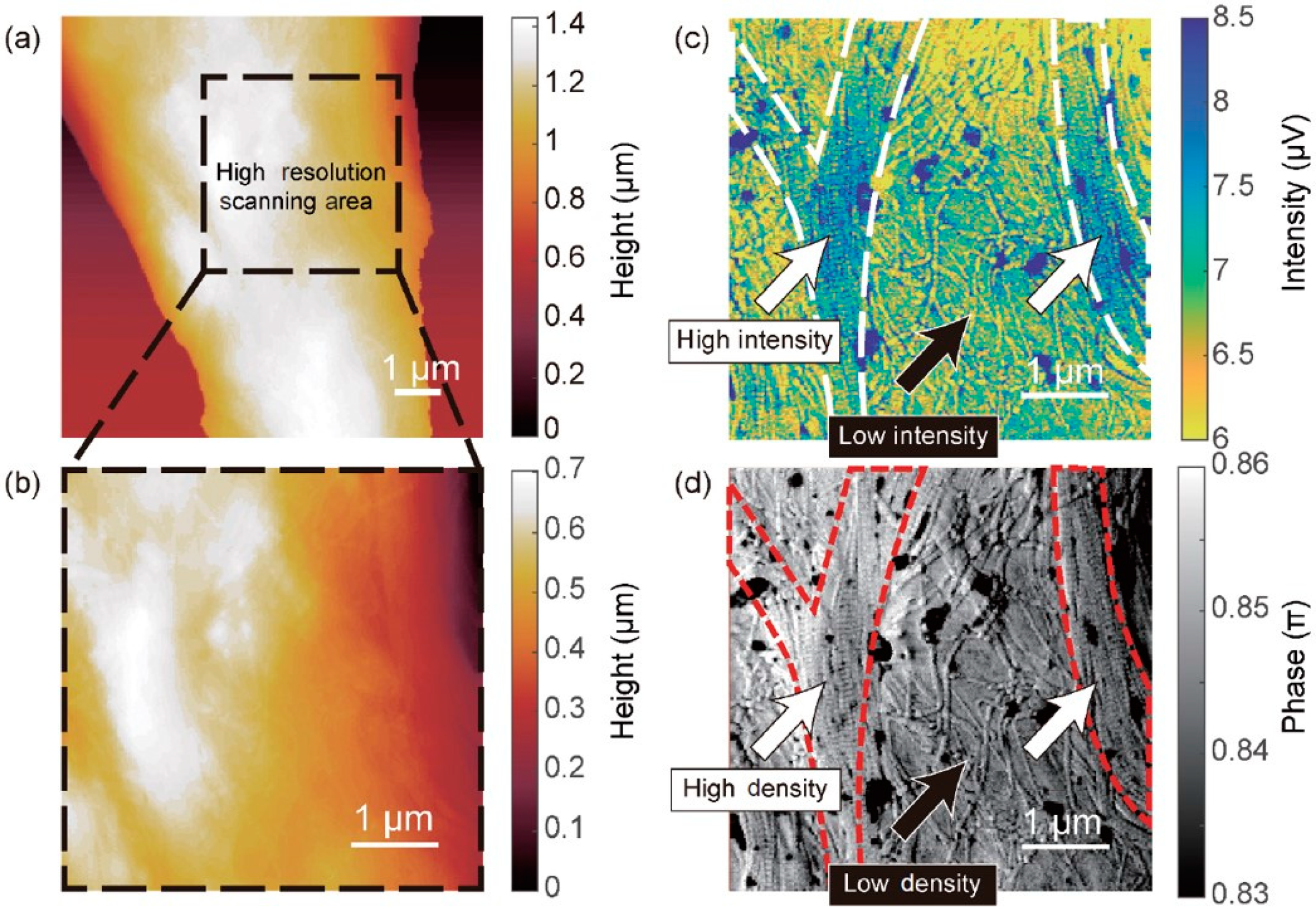
6.1. Tip-Enhanced Scanning Near-Field Optical Microscopy
6.2. AFM Molecular Spectroscopy Biomedical Applications
7. Probe-Based Nano-Manipulation and Nanofabrication
7.1. AFM Sample Surface Modification
7.2. AFM Biomedical Sample Manipulation
8. Outlook and Future Perspectives
8.1. Additional Modes and Applications
8.2. AFM Instrumentation
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| SPM | Scanning probe microscopy |
| STM | Scanning tunneling microscopy |
| TERS | Tip-enhanced Raman spectroscopy |
| SVM | Scanning voltage microscopy |
| SThM | Scanning thermal microscopy |
| PiFM | Photo-induced Force Microscopy |
| PC/CAFM | Photoconductive/Conductive Atomic Force Microscopy |
| SSRM | Scanning spreading resistance microscopy |
| AM | Amplitud modulation |
| PM | Phase modulation |
| FM | Frequency modulation |
| KPFM | Kelvin probe force microscopy |
| PFT | Peak force tapping |
| PFM | Piezoresponse force microscopy |
| PFQNM | Peak force quantitative nanomechanics |
| SCM | Scanning capacitance microscopy |
| SMM | Scanning microwave microscopy |
| EFM | Electrostatic force microscopy |
| MFM | Magnetic Force Microscopy |
| CFM | Chemical force microscopy |
| c-PTIR | Contact-mode photo-thermal induced resonance |
| s-SNOM | Scattering-type scanning near-field optical microscopy |
| nano-FTIR | Nanoscale Fourier Transformation Infrared Spectroscopy |
| FluidFM | Fluid force microscopy |
| SPL | Scanning probe lithography |
| SICM | Scanning Ion Conductance microscopy |
| SECM | Scanning electrochemical microscopy |
| LFM | Lateral force microscopy |
| FFM | Friction force microscopy |
| HSAFM | High-speed atomic force microscopy |
| SMFS | Single molecule force spectroscopy |
| SCFS | Single-cell force spectroscopy |
References
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic force microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mou, J.; Czajkowsky, D.M.; Zhang, Y.; Shao, Z. High-resolution atomic-force microscopy of DNA: The pitch of the double helix. FEBS Lett. 1995, 371, 279–282. [Google Scholar] [PubMed] [Green Version]
- Miyata, K.; Tracey, J.; Miyazawa, K.; Haapasilta, V.; Spijker, P.; Kawagoe, Y.; Foster, A.S.; Tsukamoto, K.; Fukuma, T. Dissolution processes at step edges of calcite in water investigated by high-speed frequency modulation atomic force microscopy and simulation. Nano Lett. 2017, 17, 4083–4089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, F.; Quigley, J.; Zhang, X.; Yang, C.; Wang, Y.; Youcef-Toumi, K. A modular low-cost atomic force microscope for precision mechatronics education. Mechatronics 2021, 76, 102550. [Google Scholar] [CrossRef]
- Rangelow, I.W.; Ivanov, T.; Ahmad, A.; Kaestner, M.; Lenk, C.; Bozchalooi, I.S.; Xia, F.; Youcef-Toumi, K.; Holz, M.; Reum, A. Review Article: Active scanning probes: A versatile toolkit for fast imaging and emerging nanofabrication. J. Vac. Sci. Technol. B 2017, 35, 06G101. [Google Scholar] [CrossRef] [Green Version]
- Janus, P.; Grabiec, P.; Sierakowski, A.; Gotszalk, T.; Rudek, M.; Kopiec, D.; Majstrzyk, W.; Boetsch, G.; Koehler, B. Design, Technology, and Application of Integrated Piezoresistive Scanning Thermal Microscopy (SThM) Microcantilever; Scanning Microscopies 2014; Postek, M.T., Newbury, D.E., Platek, S.F., Maugel, T.K., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2014; Volume 9236, pp. 154–164. [Google Scholar] [CrossRef]
- Song, Y.; Bhushan, B. Atomic force microscopy dynamic modes: Modeling and applications. J. Phys. Condens. Matter 2008, 20, 225012. [Google Scholar] [CrossRef]
- Giessibl, F.J.; Morita, S. Non-contact AFM. J. Phys. Condens. Matter 2012, 24, 080301. [Google Scholar] [CrossRef]
- Shi, J.; Hu, Y.; Hu, S.; Ma, J.; Su, C. Method and Apparatus of Using Peak Force Tapping Mode to Measure Physical Properties of a Sample. U.S. Patent 8650660, 11 February 2014. [Google Scholar]
- Monclus, M.; Young, T.; Di Maio, D. AFM indentation method used for elastic modulus characterization of interfaces and thin layers. J. Mater. Sci. 2010, 45, 3190–3197. [Google Scholar] [CrossRef]
- Eppell, S.J.; Friedenberg, D.; Payton, O.; Picco, L.; Zypman, F.R. Euler–Bernoulli theory accurately predicts atomic force microscope cantilever shape during non-equilibrium snap-to-contact motion. Nanotechnology 2020, 31, 185702. [Google Scholar] [CrossRef]
- Sokolov, I.; Dokukin, M.E. Imaging of soft and biological samples using AFM ringing mode. In Nanoscale Imaging; Springer: Berlin/Heidelberg, Germany, 2018; pp. 469–482. [Google Scholar]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J. Local infrared microspectroscopy with subwavelength spatial resolution with an atomic force microscope tip used as a photothermal sensor. Opt. Lett. 2005, 30, 2388–2390. [Google Scholar] [CrossRef]
- Sifat, A.A.; Jahng, J.; Potma, E.O. Photo-induced force microscopy (PiFM)–principles and implementations. Chem. Soc. Rev. 2022, 51, 4208–4222. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Jin, M.; Belkin, M.A. Tip-enhanced infrared nanospectroscopy via molecular expansion force detection. Nat. Photonics 2014, 8, 307–312. [Google Scholar] [CrossRef]
- Nowak, D.; Morrison, W.; Wickramasinghe, H.K.; Jahng, J.; Potma, E.; Wan, L.; Ruiz, R.; Albrecht, T.R.; Schmidt, K.; Frommer, J.; et al. Nanoscale chemical imaging by photoinduced force microscopy. Sci. Adv. 2016, 2, e1501571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa-Zeiser, A.; Weilandt, E.; Hild, S.; Marti, O. The simultaneous measurement of elastic, electrostatic and adhesive properties by scanning force microscopy: Pulsed-force mode operation. Meas. Sci. Technol. 1997, 8, 1333. [Google Scholar] [CrossRef]
- Xia, F.; Bozchalooi, I.S.; Youcef-Toumi, K. Induced vibration contact detection for minimizing cantilever tip-sample interaction forces in jumping mode atomic force microscopy. In Proceedings of the 2017 American Control Conference (ACC), Seattle, WA, USA, 24–26 May 2017; pp. 4141–4146. [Google Scholar]
- Pürckhauer, K.; Weymouth, A.J.; Pfeffer, K.; Kullmann, L.; Mulvihill, E.; Krahn, M.P.; Müller, D.J.; Giessibl, F.J. Imaging in biologically-relevant environments with AFM using stiff qPlus sensors. Sci. Rep. 2018, 8, 9330. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Yang, C.; Wang, Y.; Youcef-Toumi, K.; Reuter, C.; Ivanov, T.; Holz, M.; Rangelow, I.W. Lights Out! Nano-Scale Topography Imaging of Sample Surface in Opaque Liquid Environments with Coated Active Cantilever Probes. Nanomaterials 2019, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
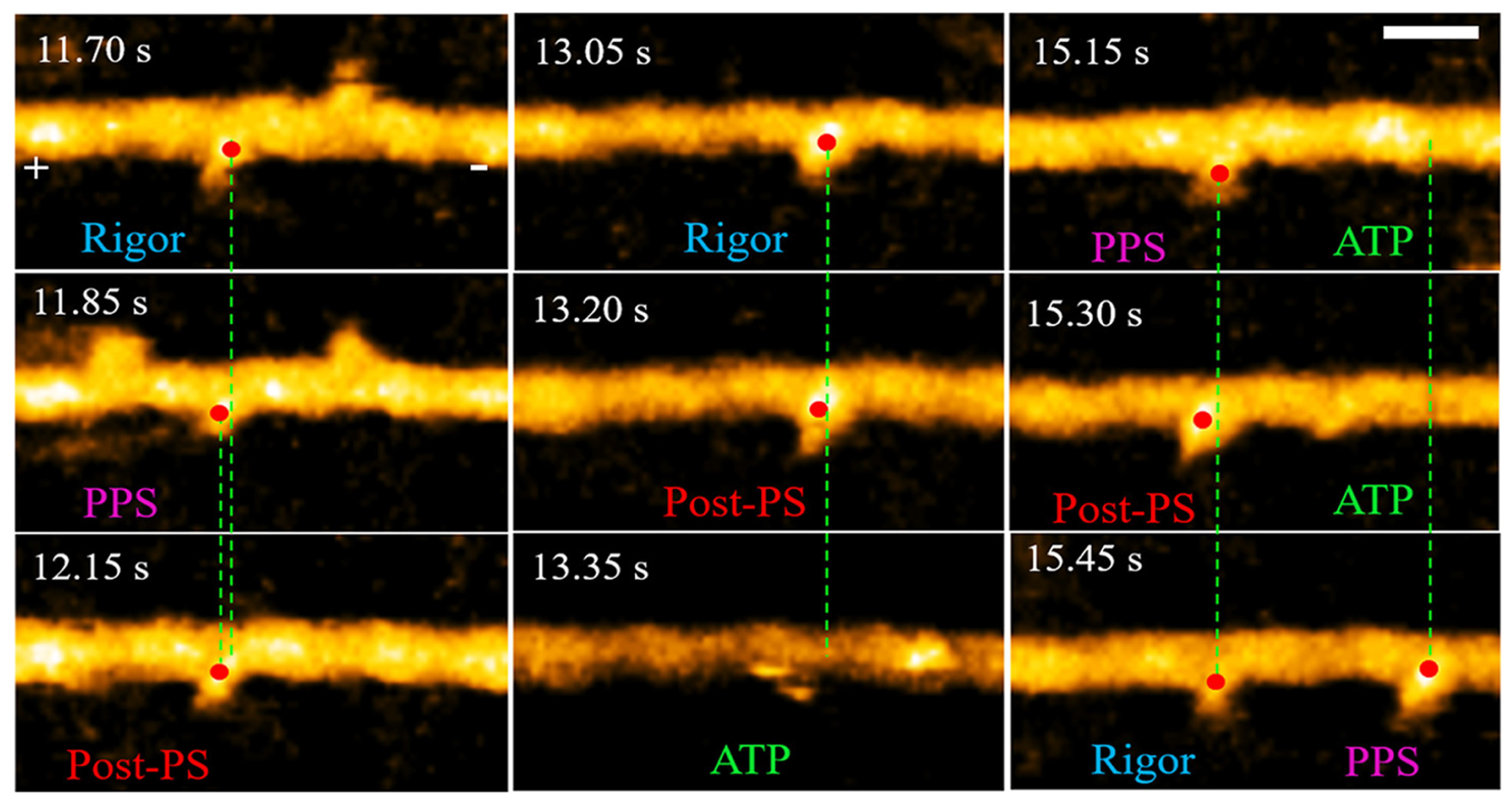
- Matusovsky, O.S.; Kodera, N.; MacEachen, C.; Ando, T.; Cheng, Y.S.; Rassier, D.E. Millisecond Conformational Dynamics of Skeletal Myosin II Power Stroke Studied by High-Speed Atomic Force Microscopy. ACS Nano 2021, 15, 2229–2239. [Google Scholar] [CrossRef]
- Viani, M.B.; Schäffer, T.E.; Paloczi, G.T.; Pietrasanta, L.I.; Smith, B.L.; Thompson, J.B.; Richter, M.; Rief, M.; Gaub, H.E.; Plaxco, K.W.; et al. Fast imaging and fast force spectroscopy of single biopolymers with a new atomic force microscope designed for small cantilevers. Rev. Sci. Instrum. 1999, 70, 4300–4303. [Google Scholar] [CrossRef]
- Ando, T.; Kodera, N.; Takai, E.; Maruyama, D.; Saito, K.; Toda, A. A high-speed atomic force microscope for studying biological macromolecules. Proc. Natl. Acad. Sci. USA 2001, 98, 12468–12472. [Google Scholar] [CrossRef]
- Adams, J.D.; Nievergelt, A.; Erickson, B.W.; Yang, C.; Dukic, M.; Fantner, G.E. High-speed imaging upgrade for a standard sample scanning atomic force microscope using small cantilevers. Rev. Sci. Instrum. 2014, 85, 093702. [Google Scholar] [CrossRef]
- Braunsmann, C.; Seifert, J.; Rheinlaender, J.; Schäffer, T.E. High-speed force mapping on living cells with a small cantilever atomic force microscope. Rev. Sci. Instrum. 2014, 85, 073703. [Google Scholar] [CrossRef] [PubMed]
- Pedrak, R.; Ivanov, T.; Ivanova, K.; Gotszalk, T.; Abedinov, N.; Rangelow, I.W.; Edinger, K.; Tomerov, E.; Schenkel, T.; Hudek, P. Micromachined atomic force microscopy sensor with integrated piezoresistive sensor and thermal bimorph actuator for high-speed tapping-mode atomic force microscopy phase-imaging in higher eigenmodes. J. Vac. Sci. Technol. Microelectron. Nanometer Struct. Process. Meas. Phenom. 2003, 21, 3102–3107. [Google Scholar] [CrossRef]
- Yong, Y.K.; Moheimani, S.O.R.; Kenton, B.J.; Leang, K.K. Invited Review Article: High-speed flexure-guided nanopositioning: Mechanical design and control issues. Rev. Sci. Instrum. 2012, 83, 121101. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yan, J.; Dukic, M.; Hosseini, N.; Zhao, J.; Fantner, G.E. Design of a high-bandwidth tripod scanner for high speed atomic force microscopy. Scanning 2016, 38, 889–900. [Google Scholar] [CrossRef] [Green Version]
- Schitter, G.; Astrom, K.J.; DeMartini, B.E.; Thurner, P.J.; Turner, K.L.; Hansma, P.K. Design and Modeling of a High-Speed AFM-Scanner. IEEE Trans. Control Syst. Technol. 2007, 15, 906–915. [Google Scholar] [CrossRef]
- Herfst, R.; Dekker, B.; Witvoet, G.; Crowcombe, W.; de Lange, D.; Sadeghian, H. A miniaturized, high frequency mechanical scanner for high speed atomic force microscope using suspension on dynamically determined points. Rev. Sci. Instrum. 2015, 86, 113703. [Google Scholar] [CrossRef] [Green Version]
- Xia, F.; Truncale, S.; Wang, Y.; Youcef-Toumi, K. Design and Control of a Multi-actuated High-bandwidth and Large-range Scanner for Atomic Force Microscopy. In Proceedings of the 2018 Annual American Control Conference (ACC), Milwaukee, WI, USA, 27–29 June 2018; pp. 4330–4335. [Google Scholar] [CrossRef]
- Yang, C.; Xia, F.; Wang, Y.; Truncale, S.; Youcef-Toumi, K. Design and Control of a Multi-Actuated Nanopositioning Stage with Stacked Structure. In Proceedings of the 2019 American Control Conference (ACC), Philadelphia, PA, USA, 10–12 July 2019; pp. 3782–3788. [Google Scholar]
- Yang, C.; Li, C.; Xia, F.; Zhu, Y.; Zhao, J.; Youcef-Toumi, K. Charge controller with decoupled and self-compensating configurations for linear operation of piezoelectric actuators in a wide bandwidth. IEEE Trans. Ind. Electron. 2018, 66, 5392–5402. [Google Scholar] [CrossRef]
- Yang, C.; Verbeek, N.; Xia, F.; Wang, Y.; Youcef-Toumi, K. Modeling and Control of Piezoelectric Hysteresis: A Polynomial-Based Fractional Order Disturbance Compensation Approach. IEEE Trans. Ind. Electron. 2020, 68, 3348–3358. [Google Scholar] [CrossRef]
- Yang, C.; Verbeek, N.; Xia, F.; Wang, Y.; Youcef-Toumi, K. Statically Stable Charge Sensing Method for Precise Displacement Estimation of Piezoelectric Stack-Based Nanopositioning. IEEE Trans. Ind. Electron. 2020, 68, 8550–8560. [Google Scholar] [CrossRef]
- Srinivasan, K.; Shaw, F. Analysis and Design of Repetitive Control Systems using the Regeneration Spectrum. In Proceedings of the 1990 American Control. Sheraton Harbor Island Hotel, San Diego, CA, USA, 23–25 May 1990; pp. 1150–1155. [Google Scholar] [CrossRef]
- Xia, F.; Yang, C.; Wang, Y.; Youcef-Toumi, K. Bandwidth Based Repetitive Controller Design for a Modular Multi-actuated AFM Scanner. In Proceedings of the 2019 American Control, Philadelphia, PA, USA, 10–12 July 2019; pp. 3776–3781. [Google Scholar] [CrossRef]
- Xia, F.; Yang, C.; Wang, Y.; Youcef-Toumi, K. Model and Controller Design for High-speed Atomic Force Microscope Imaging and Autotuning. In Proceedings of the ASPE Spring Topical Meeting on Design and Control of Precision Mechatronic Systems, Online, 6–8 May 2020; American Society for Precision Engineering: Raleigh, NC, USA, 2020. [Google Scholar]
- Bozchalooi, I.S.; Houck, A.C.; AlGhamdi, J.; Youcef-Toumi, K. Design and control of multi-actuated atomic force microscope for large-range and high-speed imaging. Ultramicroscopy 2016, 160, 213–224. [Google Scholar] [CrossRef]
- Soltani Bozchalooi, I.; Youcef-Toumi, K. Multi-actuation and PI control: A simple recipe for high-speed and large-range atomic force microscopy. Ultramicroscopy 2014, 146, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Holz, M.; Reuter, C.; Reum, A.; Ahmad, A.; Hofmann, M.; Ivanov, T.; Rangelow, I.; Stauffenberg, J.; Manske, E.; Du, C.; et al. High throughput AFM inspection system with parallel active cantilevers. Photomask Technol. 2019, 11148, 111481E. [Google Scholar]
- Axmann, M.; Sezgin, E.; Karner, A.; Novacek, J.; Brodesser, M.D.; Röhrl, C.; Preiner, J.; Stangl, H.; Plochberger, B. Receptor-Independent Transfer of Low Density Lipoprotein Cargo to Biomembranes. Nano Lett. 2019, 19, 2562–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haruyama, T.; Sugano, Y.; Kodera, N.; Uchihashi, T.; Ando, T.; Tanaka, Y.; Konno, H.; Tsukazaki, T. Single-Unit Imaging of Membrane Protein-Embedded Nanodiscs from Two Oriented Sides by High-Speed Atomic Force Microscopy. Structure 2019, 27, 152–160.e3. [Google Scholar] [CrossRef] [Green Version]
- Heath, G.R.; Scheuring, S. Advances in high-speed atomic force microscopy (HS-AFM) reveal dynamics of transmembrane channels and transporters. Curr. Opin. Struct. Biol. 2019, 57, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.C.; Chipot, C.; Scheuring, S. Annexin-V stabilizes membrane defects by inducing lipid phase transition. Nat. Commun. 2020, 11, 230. [Google Scholar] [CrossRef] [Green Version]
- Nievergelt, A.P.; Kammer, C.; Brillard, C.; Kurisinkal, E.; Bastings, M.M.C.; Karimi, A.; Fantner, G.E. Large-Range HS-AFM Imaging of DNA Self-Assembly through In Situ Data-Driven Control. Small Methods 2019, 3, 1900031. [Google Scholar] [CrossRef]
- Xu, X.; Nakano, T.; Tsuda, M.; Kanamoto, R.; Hirayama, R.; Uzawa, A.; Ide, H. Direct observation of damage clustering in irradiated DNA with atomic force microscopy. Nucleic Acids Res. 2020, 48, e18. [Google Scholar] [CrossRef]
- Spokoini-Stern, R.; Stamov, D.; Jessel, H.; Aharoni, L.; Haschke, H.; Giron, J.; Unger, R.; Segal, E.; Abu-Horowitz, A.; Bachelet, I. Visualizing the structure and motion of the long noncoding RNA HOTAIR. RNA 2020, 26, 629–636. [Google Scholar] [CrossRef]
- Lin, Y.C.; Guo, Y.R.; Miyagi, A.; Levring, J.; MacKinnon, R.; Scheuring, S. Force-induced conformational changes in PIEZO1. Nature 2019, 573, 230–234. [Google Scholar] [CrossRef]
- Lim, K.; Kodera, N.; Wang, H.; Mohamed, M.S.; Hazawa, M.; Kobayashi, A.; Yoshida, T.; Hanayama, R.; Yano, S.; Ando, T.; et al. High-Speed AFM Reveals Molecular Dynamics of Human Influenza A Hemagglutinin and Its Interaction with Exosomes. Nano Lett. 2020, 20, 6320–6328. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.S.; Mohamed, M.S.; Wang, H.; Hartono; Hazawa, M.; Kobayashi, A.; Voon, D.C.C.; Kodera, N.; Ando, T.; Wong, R.W. Direct visualization of avian influenza H5N1 hemagglutinin precursor and its conformational change by high-speed atomic force microscopy. Biochim. Biophys. Acta-(Bba)-Gen. Subj. 2020, 1864, 129313. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Foster, S.J.; Fantner, G.E.; Hobbs, J.K.; Dufrêne, Y.F. Scratching the surface: Bacterial cell envelopes at the nanoscale. MBio 2020, 11, e03020-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, M.S.; Hazawa, M.; Kobayashi, A.; Guillaud, L.; Watanabe-Nakayama, T.; Nakayama, M.; Wang, H.; Kodera, N.; Oshima, M.; Ando, T.; et al. Spatiotemporally tracking of nano-biofilaments inside the nuclear pore complex core. Biomaterials 2020, 256, 120198. [Google Scholar] [CrossRef]
- Ando, T.; Uchihashi, T.; Scheuring, S. Filming Biomolecular Processes by High-Speed Atomic Force Microscopy. Chem. Rev. 2014, 114, 3120–3188. [Google Scholar] [CrossRef]
- Umakoshi, T.; Fukuda, S.; Iino, R.; Uchihashi, T.; Ando, T. High-speed near-field fluorescence microscopy combined with high-speed atomic force microscopy for biological studies. Biochim. Biophys. Acta-(BBA)-Gen. Subj. 2020, 1864, 129325. [Google Scholar] [CrossRef]
- Ando, T. High-speed atomic force microscopy and its future prospects. Biophys. Rev. 2018, 10, 285–292. [Google Scholar] [CrossRef]
- Sinha, R.; Verdonschot, N.; Koopman, B.; Rouwkema, J. Tuning cell and tissue development by combining multiple mechanical signals. Tissue Eng. Part B Rev. 2017, 23, 494–504. [Google Scholar] [CrossRef]
- Nikolaev, N.I.; Müller, T.; Williams, D.J.; Liu, Y. Changes in the stiffness of human mesenchymal stem cells with the progress of cell death as measured by atomic force microscopy. J. Biomech. 2014, 47, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Mierke, C.T. The fundamental role of mechanical properties in the progression of cancer disease and inflammation. Rep. Prog. Phys. 2014, 77, 076602. [Google Scholar] [CrossRef]
- Kristi, N.; Gafur, A.; Kong, L.; Ma, X.; Ye, Z.; Wang, G. Atomic Force Microscopy in Mechanoimmunology Analysis: A New Perspective for Cancer Immunotherapy. Biotechnol. J. 2020, 15, 1900559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J. Friction Determination by Atomic Force Microscopy in Field of Biochemical Science. Micromachines 2018, 9, 313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Z.; Wang, J.; Chen, G.; Deng, L. Imaging and determining friction forces of specific interactions between human IgG and rat anti-human IgG. J. Biol. Phys. 2011, 37, 417–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnham, N.A.; Dominguez, D.D.; Mowery, R.L.; Colton, R.J. Probing the surface forces of monolayer films with an atomic-force microscope. Phys. Rev. Lett. 1990, 64, 1931. [Google Scholar] [CrossRef]
- Maugis, D. Adhesion of spheres: The JKR-DMT transition using a Dugdale model. J. Colloid Interface Sci. 1992, 150, 243–269. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Wang, W.H.; Hardy, S.D.; Geahlen, R.L.; Raman, A. Measuring nanoscale viscoelastic parameters of cells directly from AFM force-displacement curves. Sci. Rep. 2017, 7, 1541. [Google Scholar] [CrossRef] [Green Version]
- Minelli, E.; Ciasca, G.; Sassun, T.E.; Antonelli, M.; Papi, M.; Palmieri, V.; Maulucci, G.; Santoro, A.; Giangaspero, F.; Delfini, R.; et al. Neural Network Approach for the Analysis of AFM Force-Distance Curves for Brain Cancer Diagnosis. Biophys. J. 2018, 114, 353a. [Google Scholar] [CrossRef]
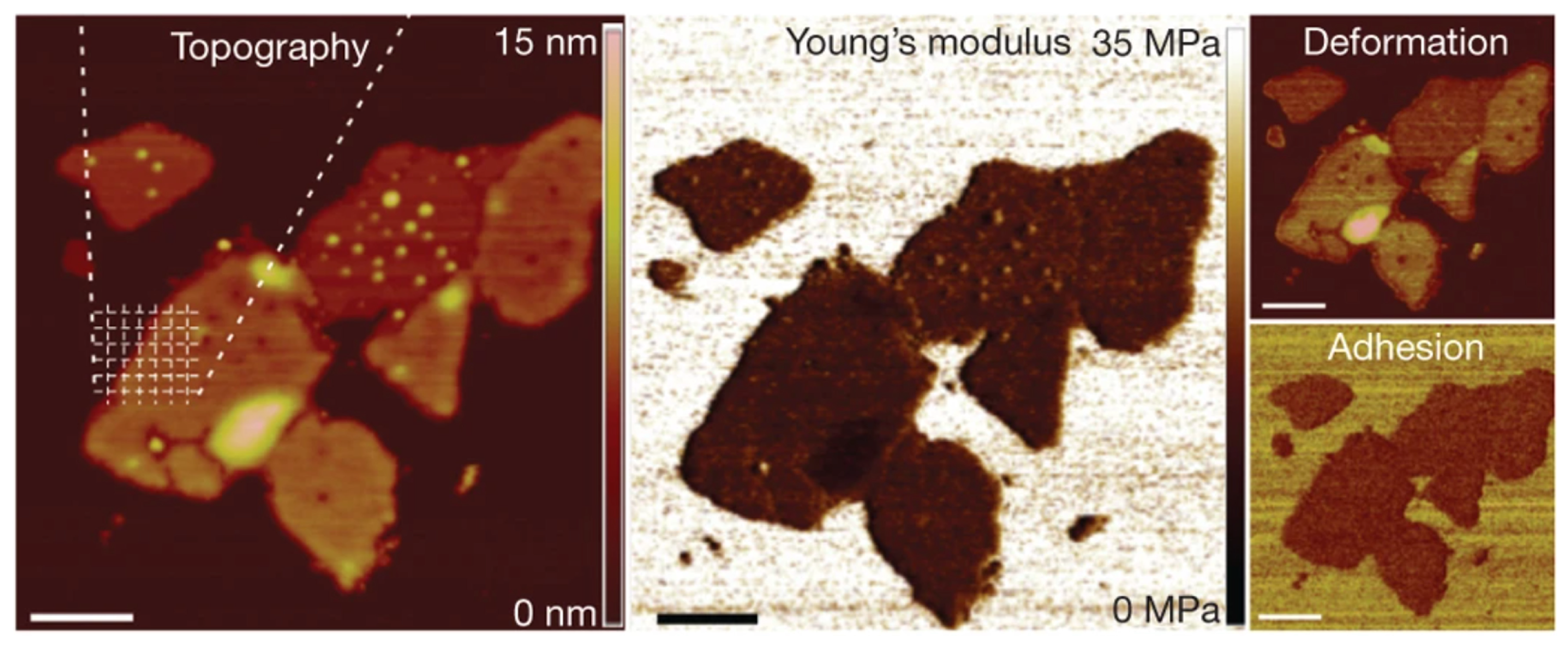
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. AFM PeakForce QNM mode: Evidencing nanometer-scale mechanical properties of chitin-silica hybrid nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef]
- Hu, J.; Chen, S.; Huang, D.; Zhang, Y.; Lü, S.; Long, M. Global mapping of live cell mechanical features using PeakForce QNM AFM. Biophys. Rep. 2020, 6, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Schillers, H.; Medalsy, I.; Hu, S.; Slade, A.L.; Shaw, J.E. PeakForce Tapping resolves individual microvilli on living cells. J. Mol. Recognit. 2016, 29, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Efremov, Y.M.; Shpichka, A.; Kotova, S.; Timashev, P. Viscoelastic mapping of cells based on fast force volume and PeakForce Tapping. Soft Matter 2019, 15, 5455–5463. [Google Scholar] [CrossRef]
- Nia, H.T.; Bozchalooi, I.S.; Li, Y.; Han, L.; Hung, H.H.; Frank, E.; Youcef-Toumi, K.; Ortiz, C.; Grodzinsky, A. High-bandwidth AFM-based rheology reveals that cartilage is most sensitive to high loading rates at early stages of impairment. Biophys. J. 2013, 104, 1529–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufrêne, Y.F.; Martínez-Martín, D.; Medalsy, I.; Alsteens, D.; Müller, D.J. Multiparametric imaging of biological systems by force-distance curve–based AFM. Nat. Methods 2013, 10, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Lai, C.Y.; Olukan, T.; Chiesa, M. Multifrequency AFM: From origins to convergence. Nanoscale 2017, 9, 5038–5043. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, M.G.; Moore, S.I.; Zawierta, M.; Fleming, A.J.; Putrino, G.; Yong, Y.K. Multimodal atomic force microscopy with optimized higher eigenmode sensitivity using on-chip piezoelectric actuation and sensing. Nanotechnology 2019, 30, 085503. [Google Scholar] [CrossRef] [PubMed]
- Schuh, A.; Bozchalooi, I.S.; Rangelow, I.W.; Youcef-Toumi, K. Multi-eigenmode control for high material contrast in bimodal and higher harmonic atomic force microscopy. Nanotechnology 2015, 26, 235706. [Google Scholar] [CrossRef]
- Schuh, A.; Bozchalooi, I.S.; Rangelow, I.W.; Youcef-Toumi, K. Estimator based multi-eigenmode control of cantilevers in multifrequency Atomic Force Microscopy. In Proceedings of the 2015 American Control, Chicago, IL, USA, 1–3 July 2015; pp. 1905–1910. [Google Scholar]
- Seifert, J.; Kirchhelle, C.; Moore, I.; Contera, S. Mapping cellular nanoscale viscoelasticity and relaxation times relevant to growth of living Arabidopsis thaliana plants using multifrequency AFM. Acta Biomater. 2021, 121, 371–382. [Google Scholar] [CrossRef]
- Al-Rekabi, Z.; Contera, S. Multifrequency AFM reveals lipid membrane mechanical properties and the effect of cholesterol in modulating viscoelasticity. Proc. Natl. Acad. Sci. USA 2018, 115, 2658–2663. [Google Scholar] [CrossRef] [Green Version]
- Benaglia, S.; Gisbert, V.G.; Perrino, A.P.; Amo, C.A.; Garcia, R. Fast and high-resolution mapping of elastic properties of biomolecules and polymers with bimodal AFM. Nat. Protoc. 2018, 13, 2890–2907. [Google Scholar] [CrossRef]
- Dokukin, M.E.; Sokolov, I. Nanoscale compositional mapping of cells, tissues, and polymers with ringing mode of atomic force microscopy. Sci. Rep. 2017, 7, 11828. [Google Scholar] [CrossRef]
- Siamantouras, E.; Hills, C.E.; Liu, K.K.; Squires, P.E. Examining Cell-Cell Interactions in the Kidney Using AFM Single-Cell Force Spectroscopy. Methods Mol. Biol. 2020, 2067, 189–201. [Google Scholar] [PubMed]
- Baier, D.; Müller, T.; Mohr, T.; Windberger, U. Red Blood Cell Stiffness and Adhesion Are Species-Specific Properties Strongly Affected by Temperature and Medium Changes in Single Cell Force Spectroscopy. Molecules 2021, 26, 2771. [Google Scholar] [CrossRef]
- Zemła, J.; Danilkiewicz, J.; Orzechowska, B.; Pabijan, J.; Seweryn, S.; Lekka, M. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol. 2018, 73, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.J.; Engel, A. Atomic force microscopy and spectroscopy of native membrane proteins. Nat. Protoc. 2007, 2, 2191–2197. [Google Scholar] [CrossRef]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Á.G.; Bonyár, A.; Székács, I.; Horvath, R. Analysis of single-cell force-spectroscopy data of Vero cells recorded by FluidFM BOT. In Proceedings of the 2020 IEEE 26th International Symposium for Design and Technology in Electronic Packaging (SIITME), Pitești, Romania, 21–24 October 2020; pp. 21–25. [Google Scholar] [CrossRef]
- Quack, M. Fundamental Symmetries and Symmetry Violations from High Resolution Spectroscopy. In Handbook of High-Resolution Spectroscopy; American Cancer Society: Atlanta, GA, USA, 2011. [Google Scholar] [CrossRef]
- Benavente, L.; Coetsier, C.; Venault, A.; Chang, Y.; Causserand, C.; Bacchin, P.; Aimar, P. FTIR mapping as a simple and powerful approach to study membrane coating and fouling. J. Membr. Sci. 2016, 520, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Ihli, J.; Marchant, W.J.; Zeng, M.; Chen, L.; Wehbe, K.; Cinque, G.; Cespedes, O.; Kapur, N.; Meldrum, F.C. Synchrotron FTIR mapping of mineralization in a microfluidic device. Lab Chip 2017, 17, 1616–1624. [Google Scholar] [CrossRef]
- Huth, F.; Govyadinov, A.; Amarie, S.; Nuansing, W.; Keilmann, F.; Hillenbrand, R. Nano-FTIR Absorption Spectroscopy of Molecular Fingerprints at 20 nm Spatial Resolution. Nano Lett. 2012, 12, 3973–3978. [Google Scholar] [CrossRef]
- Qiao, Z.; Xue, M.; Zhao, Y.; Huang, Y.; Zhang, M.; Chang, C.; Chen, J. Infrared nanoimaging of nanoscale sliding dislocation of collagen fibrils. Nano Res. 2021. [Google Scholar] [CrossRef]
- Yang, Z.; Tang, D.; Hu, J.; Tang, M.; Zhang, M.; Cui, H.L.; Wang, L.; Chang, C.; Fan, C.; Li, J.; et al. Near-Field Nanoscopic Terahertz Imaging of Single Proteins. Small 2021, 17, 2005814. [Google Scholar] [CrossRef]
- Amenabar, I.; Poly, S.; Nuansing, W.; Hubrich, E.; Govyadinov, A.; Huth, F.; Krutokhvostov, R.; Zhang, L.; Knez, M.; Heberle, J.; et al. Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy. Nat. Commun. 2013, 4, 2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zancajo, V.M.R.; Lindtner, T.; Eisele, M.; Huber, A.J.; Elbaum, R.; Kneipp, J. FTIR Nanospectroscopy Shows Molecular Structures of Plant Biominerals and Cell Walls. Anal. Chem. 2020, 92, 13694–13701. [Google Scholar] [CrossRef] [PubMed]
- Cernescu, A.; Szuwarzyński, M.; Kwolek, U.; Wydro, P.; Kepczynski, M.; Zapotoczny, S.; Nowakowska, M.; Quaroni, L. Label-Free Infrared Spectroscopy and Imaging of Single Phospholipid Bilayers with Nanoscale Resolution. Anal. Chem. 2018, 90, 10179–10186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamage, S.; Howard, M.; Makita, H.; Cross, B.; Hastings, G.; Luo, M.; Abate, Y. Probing structural changes in single enveloped virus particles using nano-infrared spectroscopic imaging. PLoS ONE 2018, 13, e0199112. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.; Flores-Borges, D.N.; Bittencourt, P.R.; Mayer, J.L.; Kiyota, E.; Araújo, P.; Jansen, S.; Freitas, R.O.; Oliveira, R.S.; Mazzafera, P. Infrared Nanospectroscopy Reveals the Chemical Nature of Pit Membranes in Water-Conducting Cells of the Plant Xylem. Plant Physiol. 2018, 177, 1629–1638. [Google Scholar] [CrossRef]
- Amenabar, I.; Poly, S.; Goikoetxea, M.; Nuansing, W.; Lasch, P.; Hillenbrand, R. Hyperspectral infrared nanoimaging of organic samples based on Fourier transform infrared nanospectroscopy. Nature Commun. 2017, 8, 14402. [Google Scholar] [CrossRef] [Green Version]
- Amrania, H.; Drummond, L.; Coombes, R.C.; Shousha, S.; Woodley-Barker, L.; Weir, K.; Hart, W.; Carter, I.; Phillips, C.C. New IR imaging modalities for cancer detection and for intra-cell chemical mapping with a sub-diffraction mid-IR s-SNOM. Faraday Discuss. 2016, 187, 539–553. [Google Scholar] [CrossRef] [Green Version]
- Lucidi, M.; Tranca, D.E.; Nichele, L.; Ünay, D.; Stanciu, G.A.; Visca, P.; Holban, A.M.; Hristu, R.; Cincotti, G.; Stanciu, S.G. SSNOMBACTER: A collection of scattering-type scanning near-field optical microscopy and atomic force microscopy images of bacterial cells. GigaScience 2020, 9, giaa129. [Google Scholar] [CrossRef]
- Ji, B.; Kenaan, A.; Gao, S.; Cheng, J.; Cui, D.; Yang, H.; Wang, J.; Song, J. Label-free detection of biotoxins via a photo-induced force infrared spectrum at the single-molecular level. Analyst 2019, 144, 6108–6117. [Google Scholar] [CrossRef]
- Ajaezi, G.C.; Eisele, M.; Contu, F.; Lal, S.; Rangel-Pozzo, A.; Mai, S.; Gough, K.M. Near-field infrared nanospectroscopy and super-resolution fluorescence microscopy enable complementary nanoscale analyses of lymphocyte nuclei. Analyst 2018, 143, 5926–5934. [Google Scholar] [CrossRef]
- Birarda, G.; Delneri, A.; Lagatolla, C.; Parisse, P.; Cescutti, P.; Vaccari, L.; Rizzo, R. Multi-technique microscopy investigation on bacterial biofilm matrices: A study on Klebsiella pneumoniae clinical strains. Anal. Bioanal. Chem. 2019, 411, 7315–7325. [Google Scholar] [CrossRef] [PubMed]
- Custance, O.; Perez, R.; Morita, S. Atomic force microscopy as a tool for atom manipulation. Nat. Nanotechnol. 2009, 4, 803. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Abe, M.; Hirayama, S.; Oyabu, N.; Custance, Ó.; Morita, S. Atom inlays performed at room temperature using atomic force microscopy. Nat. Mater. 2005, 4, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Rangelow, I. Scanning proximity probes for nanoscience and nanofabrication. Microelectron. Eng. 2006, 83, 1449–1455. [Google Scholar] [CrossRef]
- Durrani, Z.; Jones, M.; Abualnaja, F.; Wang, C.; Kaestner, M.; Lenk, S.; Lenk, C.; Rangelow, I.W.; Andreev, A. Room-temperature single dopant atom quantum dot transistors in silicon, formed by field-emission scanning probe lithography. J. Appl. Phys. 2018, 124, 144502. [Google Scholar] [CrossRef]
- Fan, P.; Gao, J.; Mao, H.; Geng, Y.; Yan, Y.; Wang, Y.; Goel, S.; Luo, X. Scanning Probe Lithography: State-of-the-Art and Future Perspectives. Micromachines 2022, 13, 228. [Google Scholar] [CrossRef]
- Borodin, B.R.; Benimetskiy, F.A.; Davydov, V.Y.; Eliseyev, I.A.; Lepeshov, S.I.; Bogdanov, A.A.; Alekseev, P.A. Mechanical scanning probe lithography of nanophotonic devices based on multilayer TMDCs. J. Phys. Conf. Ser. 2021, 2015, 012020. [Google Scholar] [CrossRef]
- Howell, S.T.; Grushina, A.; Holzner, F.; Brugger, J. Thermal scanning probe lithography—A review. Microsyst. Nanoeng. 2020, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Albisetti, E.; Carroll, K.; Lu, X.; Curtis, J.; Petti, D.; Bertacco, R.; Riedo, E. Thermochemical scanning probe lithography of protein gradients at the nanoscale. Nanotechnology 2016, 27, 315302. [Google Scholar] [CrossRef] [Green Version]
- Martinez, J.; Martínez, R.V.; Garcia, R. Silicon Nanowire Transistors with a Channel Width of 4 nm Fabricated by Atomic Force Microscope Nanolithography. Nano Lett. 2008, 8, 3636–3639. [Google Scholar] [CrossRef]
- Maynor, B.W.; Filocamo, S.F.; Grinstaff, M.W.; Liu, J. Direct-Writing of Polymer Nanostructures: Poly(thiophene) Nanowires on Semiconducting and Insulating Surfaces. J. Am. Chem. Soc. 2002, 124, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Hirt, L.; Ihle, S.; Pan, Z.; Dorwling-Carter, L.; Reiser, A.; Wheeler, J.M.; Spolenak, R.; Vörös, J.; Zambelli, T. Template-Free 3D Microprinting of Metals Using a Force-Controlled Nanopipette for Layer-by-Layer Electrodeposition. Adv. Mater. 2016, 28, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.; Shi, F.; Edinger, K.; Güthner, P.; Rangelow, I. Nanofabrication with scanning nanonozzle ‘Nanojet’. Microelectron. Eng. 2001, 57–58, 1035–1042. [Google Scholar] [CrossRef]
- Morello, A.; Tosi, G.; Mohiyaddin, F.; Schmitt, V.; Mourik, V.; Botzem, T.; Laucht, A.; Pla, J.; Tenberg, S.; Savytskyy, R.; et al. Scalable quantum computing with ion-implanted dopant atoms in silicon. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018; pp. 6–12. [Google Scholar]
- Kawai, S.; Foster, A.S.; Canova, F.F.; Onodera, H.; Kitamura, S.i.; Meyer, E. Atom manipulation on an insulating surface at room temperature. Nat. Commun. 2014, 5, 4403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillaume-Gentil, O.; Potthoff, E.; Ossola, D.; Dörig, P.; Zambelli, T.; Vorholt, J.A. Force-Controlled Fluidic Injection into Single Cell Nuclei. Small 2013, 9, 1904–1907. [Google Scholar] [CrossRef]
- Ribeiro-Palau, R.; Zhang, C.; Watanabe, K.; Taniguchi, T.; Hone, J.; Dean, C.R. Twistable electronics with dynamically rotatable heterostructures. Science 2018, 361, 690–693. [Google Scholar] [CrossRef] [Green Version]
- Ilg, M.; Weis, C.D.; Schwartz, J.; Persaud, A.; Ji, Q.; Chi Lo, C.; Bokor, J.; Hegyi, A.; Guliyev, E.; Rangelow, I.W.; et al. Improved single ion implantation with scanning probe alignment. J. Vac. Sci. Technol. Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2012, 30, 06FD04. [Google Scholar] [CrossRef]
- Meister, A.; Gabi, M.; Behr, P.; Studer, P.; Vörös, J.; Niedermann, P.; Bitterli, J.; Polesel-Maris, J.; Liley, M.; Heinzelmann, H.; et al. FluidFM: Combining Atomic Force Microscopy and Nanofluidics in a Universal Liquid Delivery System for Single Cell Applications and Beyond. Nano Lett. 2009, 9, 2501–2507. [Google Scholar] [CrossRef]
- Li, M.; Liu, L.; Zambelli, T. FluidFM for single-cell biophysics. Nano Res. 2021, 15, 773–786. [Google Scholar] [CrossRef]
- Li, W.; Sancho, A.; Chung, W.L.; Vinik, Y.; Groll, J.; Zick, Y.; Medalia, O.; Bershadsky, A.D.; Geiger, B. Differential cellular responses to adhesive interactions with galectin-8- and fibronectin-coated substrates. J. Cell Sci. 2021, 134, jcs252221. [Google Scholar] [CrossRef]
- Sztilkovics, M.; Gerecsei, T.; Peter, B.; Saftics, A.; Kurunczi, S.; Szekacs, I.; Szabo, B.; Horvath, R. Single-cell adhesion force kinetics of cell populations from combined label-free optical biosensor and robotic fluidic force microscopy. Sci. Rep. 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathelié-Guinlet, M.; Viela, F.; Dehullu, J.; Filimonava, S.; Rauceo, J.M.; Lipke, P.N.; Dufrêne, Y.F. Single-cell fluidic force microscopy reveals stress-dependent molecular interactions in yeast mating. Commun. Biol. 2021, 4, 33. [Google Scholar] [CrossRef]
- Wysotzki, P.; Sancho, A.; Gimsa, J.; Groll, J. A comparative analysis of detachment forces and energies in initial and mature cell-material interaction. Colloids Surfaces B Biointerfaces 2020, 190, 110894. [Google Scholar] [CrossRef] [PubMed]
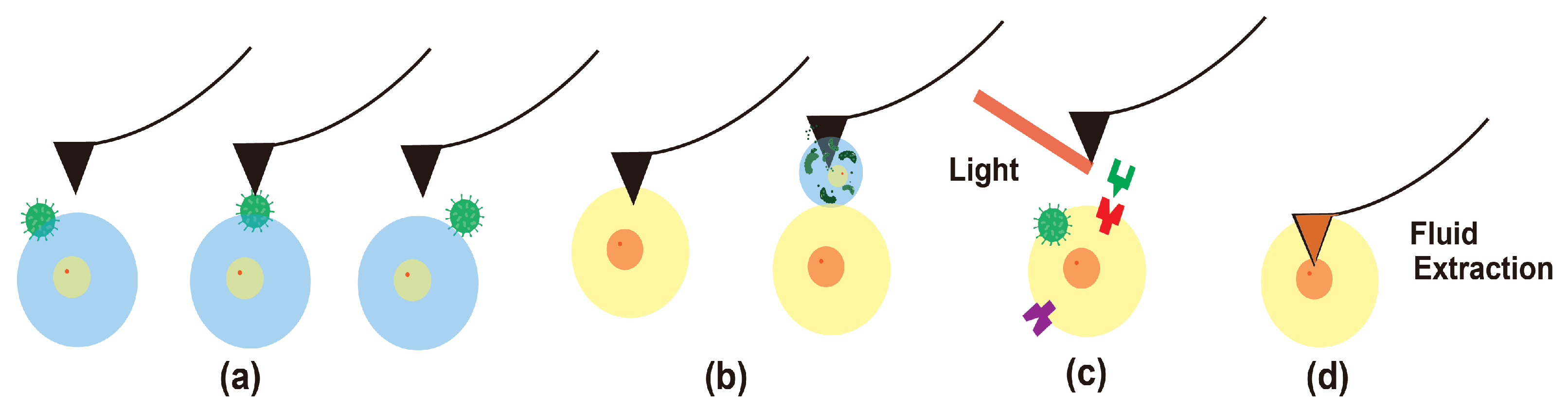
- Higgins, S.G.; Stevens, M.M. Extracting the contents of living cells. Science 2017, 356, 379–380. [Google Scholar] [CrossRef]
- Guillaume-Gentil, O.; Grindberg, R.V.; Kooger, R.; Dorwling-Carter, L.; Martinez, V.; Ossola, D.; Pilhofer, M.; Zambelli, T.; Vorholt, J.A. Tunable single-cell extraction for molecular analyses. Cell 2016, 166, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Guillaume-Gentil, O.; Rey, T.; Kiefer, P.; Ibáñez, A.J.; Steinhoff, R.; Brönnimann, R.; Dorwling-Carter, L.; Zambelli, T.; Zenobi, R.; Vorholt, J.A. Single-Cell Mass Spectrometry of Metabolites Extracted from Live Cells by Fluidic Force Microscopy. Anal. Chem. 2017, 89, 5017–5023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Harris, B.; Zheng, Y.; Celik, U.; Na, L.; Faller, R.; Chen, X.; Haudenschild, D.R.; Liu, G.y. New Means to Control Molecular Assembly. J. Phys. Chem. C 2020, 124, 6405–6412. [Google Scholar] [CrossRef]
- Müller-Renno, C.; Remmel, D.; Braun, M.; Boonrod, K.; Krczal, G.; Ziegler, C. Producing Plant Virus Patterns with Defined 2D Structure. Phys. Status Solidi 2021, 218, 2100259. [Google Scholar] [CrossRef]
- Ventrici de Souza, J.; Liu, Y.; Wang, S.; Dörig, P.; Kuhl, T.L.; Frommer, J.; Liu, G.y. Three-Dimensional Nanoprinting via Direct Delivery. J. Phys. Chem. B 2018, 122, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Zambelli, T.; Aebersold, M.J.; Behr, P.; Han, H.; Hirt, L.; Martinez, V.; Guillaume-Gentil, O.; Vörös, J. FluidFM: Development of the Instrument as well as Its Applications for 2D and 3D Lithography. In Open-Space Microfluidics; Chapter 14; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 295–323. [Google Scholar] [CrossRef]
- Dufrêne, Y.F.; Ando, T.; Garcia, R.; Alsteens, D.; Martinez-Martin, D.; Engel, A.; Gerber, C.; Müller, D.J. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol. 2017, 12, 295–307. [Google Scholar] [CrossRef]
- Cheong, L.Z.; Zhao, W.; Song, S.; Shen, C. Lab on a tip: Applications of functional atomic force microscopy for the study of electrical properties in biology. Acta Biomater. 2019, 99, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Kontomaris, S.V.; Grant, C.; Alexandratou, E. Atomic force microscopy on biological materials related to pathological conditions. Scanning 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Maver, U.; Velnar, T.; Gaberšček, M.; Planinšek, O.; Finšgar, M. Recent progressive use of atomic force microscopy in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 96–111. [Google Scholar] [CrossRef] [Green Version]
- Last, J.A.; Russell, P.; Nealey, P.F.; Murphy, C.J. The applications of atomic force microscopy to vision science. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6083–6094. [Google Scholar] [CrossRef]
- Chang, Z.; Paoletti, P.; Hansen, M.L.; Beck, H.C.; Chen, P.Y.; Rasmussen, L.M.; Akhtar, R. AFM characterization of the internal mammary artery as a novel target for arterial stiffening. Scanning 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Rhee, Y.; Park, J.H.; Lee, G.J.; Kim, K.S.; Park, J.H.; Park, Y.G.; Park, H.K. Effects of fluoride treatment on phosphoric acid-etching in primary teeth: An AFM observation. Micron 2010, 41, 498–506. [Google Scholar] [CrossRef]
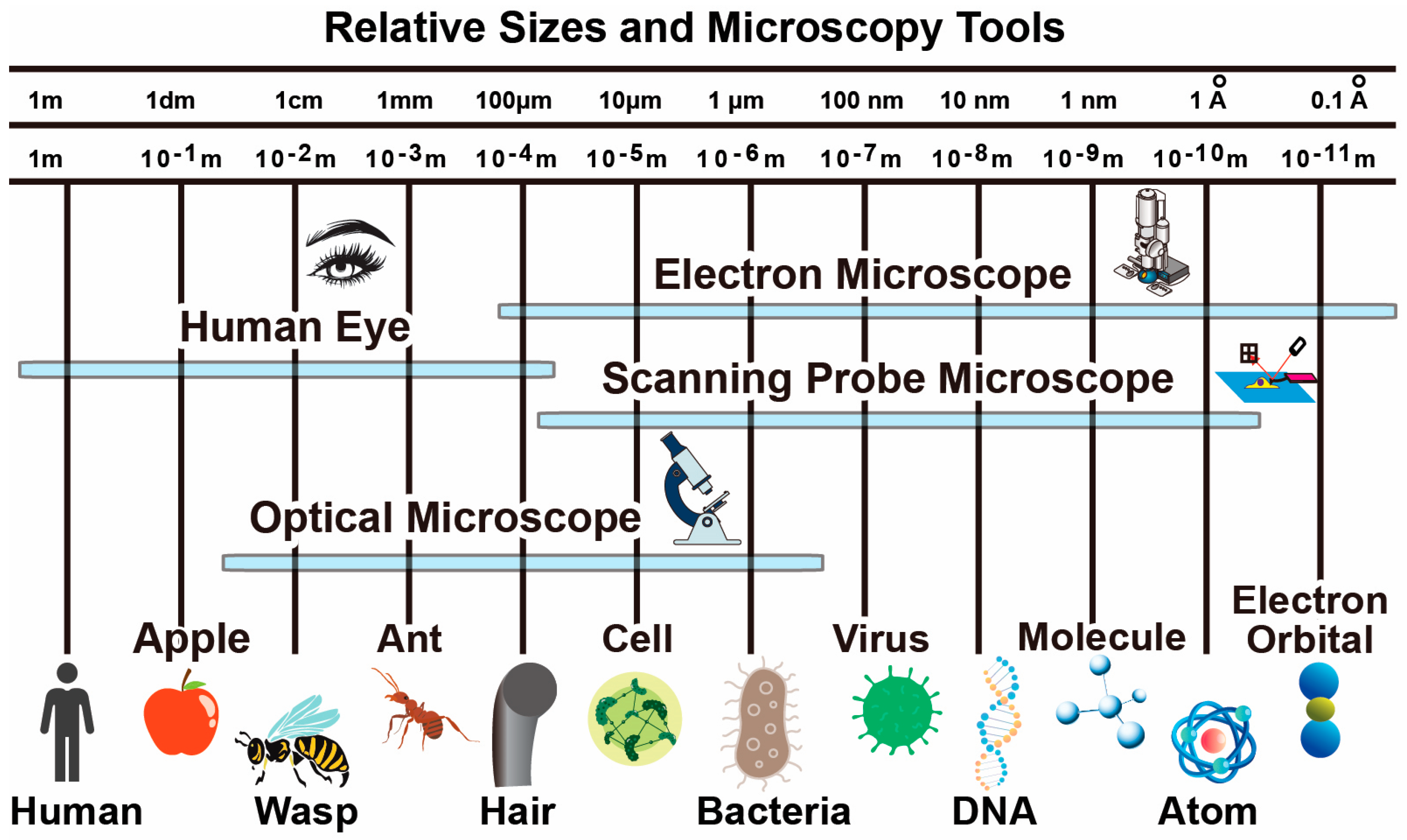


| Microscopy | Optical | SEM | TEM | AFM | STM |
|---|---|---|---|---|---|
| Resolution | 200 nm | 10 nm | nm | 2 nm | nm |
| Typical Image Size | 1000 m | 1000 m | 100 m | 100 m | m |
| Typical Frame Rate | 100 FPS | 20 FPS | 20 FPS | 0.1 FPS | 0.1 FPS |
| Main Modality | 2D image | 3D image | 2D projection | 3D Topography | 3D density of states |
| Environment | vacuum, air, liquid | vacuum | vacuum | vacuum, air, liquid | vacuum, air |
| Category | Mode Name | Modalities | Modification | Main Benefits | Main Limitations |
|---|---|---|---|---|---|
| Contact | Constant height | topography | no z control | simple control & high-speed | changing force, lateral friction |
| Constant force | topography | standard z PID | easy high-speed | lateral friction | |
| Lateral force | friction | twist detection | roughness | sample scratching | |
| PFM | piezoelectric | electrical bias | Domain identification | lateral friction | |
| SMM | impedance | network analyzer | versatile samples | lateral friction | |
| SVM | voltage | conductive path | voltage measurement | conductive probe wear | |
| PC/CAFM | current | conductive path | current measurement | conductive probe wear | |
| SSRM | resistance | conductive path | resistance measurement | sample property spreading | |
| SCM | capacitance | conductive path | capacitance measurement | ambient water meniscus trap | |
| SThM | temperature | thermal filament | temperature mapping | filament-tip offset | |
| TERS | Raman | optical parts | chemical species mapping | laser focusing overhead | |
| Dynamic | AM tapping | topography | lock-in amplifier | amplitude control | slower imaging |
| PM tapping | topography | lock-in amplifier | phase control | slower imaging | |
| FM Tapping | topography | phase-locked loop | Quality factor robustness | frequency instability | |
| Non-contact | topography | lock-in amplifier | minimal sample damage | ambient water meniscus trap | |
| Multifrequency | nanomechanics | lock-in amplifier | stiffness, damping, etc. | available cantilever resonance | |
| KPFM | surface potential | conductive path | chemical potential mapping | slow operation | |
| EFM | electrostatic | conductive path | electrostatic force | ambient water meniscus trap | |
| MFM | magnetic | magnetized tip | magnetic force | stray magnetic field effect | |
| s-SNOM | spectroscopy | optical parts | chemical species spectroscopy | light path access | |
| Jumping | Force volume | nanomechanics | algorithm | full indentation curve | slower imaging |
| Peak force | topography | algorithm | easy experiment setup | proprietary technology | |
| PFQNM | nanomechanics | algorithm | easy quantitative mechanics | proprietary technology | |
| Ringing | nanomechanics | algorithm | stiffness, adhesion, etc. | proprietary technology | |
| Hybrid | C-resonance | nanomechanics | algorithm | stiffness, damping, etc. | sample scratching |
| AFM-IR | photothermal | light source | spectroscopy contrast | absorption rate | |
| CFM | chemical forces | probe tip functionalization | chemical species interaction | tip/sample preparation |
| Category | Principles | Modification | Application Examples | Reference |
|---|---|---|---|---|
| lithography | mechanical | larger interaction force | pattern on nanophotonic devices | [109] |
| thermal | heatable probe tip | low-force data storage patterning | [110] | |
| thermo-chemical | heatable probe, reactions | protein gradient patterning | [111] | |
| oxidation | heated probe, reactions | nano-wire transistors | [112] | |
| dip-pen | positioning control sequence | electrostatic/chemical patterning | [113] | |
| field-emission | controlled electrical bias | quantum dot transistors | [107] | |
| Deposition | fluidFM printing | hollow cantilever & tip aperture | 3D metal printing, self-assembly | [114] |
| nanojet | particle system & tip aperture | local particle placement | [115] | |
| ion implantation | ion source & tip aperture | local-doping on silicon | [116] | |
| manipulation | pick & place | functionalized tip | fix atomic defects | [117] |
| inject & sample | pressure system & hollow tip | collect cell substances | [118] | |
| move & twist | flexible motion control system | stacking 2D material flakes | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, F.; Youcef-Toumi, K. Review: Advanced Atomic Force Microscopy Modes for Biomedical Research. Biosensors 2022, 12, 1116. https://doi.org/10.3390/bios12121116
Xia F, Youcef-Toumi K. Review: Advanced Atomic Force Microscopy Modes for Biomedical Research. Biosensors. 2022; 12(12):1116. https://doi.org/10.3390/bios12121116
Chicago/Turabian StyleXia, Fangzhou, and Kamal Youcef-Toumi. 2022. "Review: Advanced Atomic Force Microscopy Modes for Biomedical Research" Biosensors 12, no. 12: 1116. https://doi.org/10.3390/bios12121116
APA StyleXia, F., & Youcef-Toumi, K. (2022). Review: Advanced Atomic Force Microscopy Modes for Biomedical Research. Biosensors, 12(12), 1116. https://doi.org/10.3390/bios12121116






