Rapid Detection of Attomolar SARS-CoV-2 Nucleic Acids in All-Dielectric Metasurface Biosensors
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Sequence
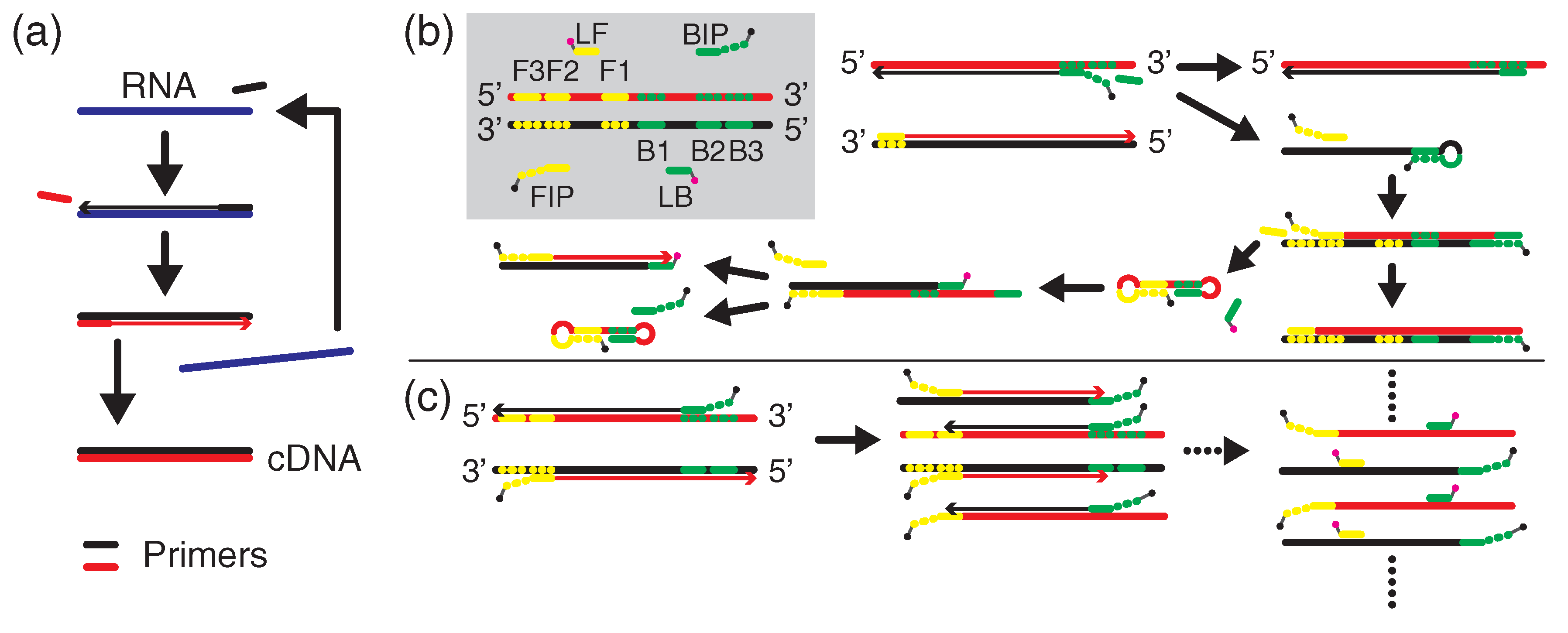
2.2. Nucleic Acid Amplification Procedures
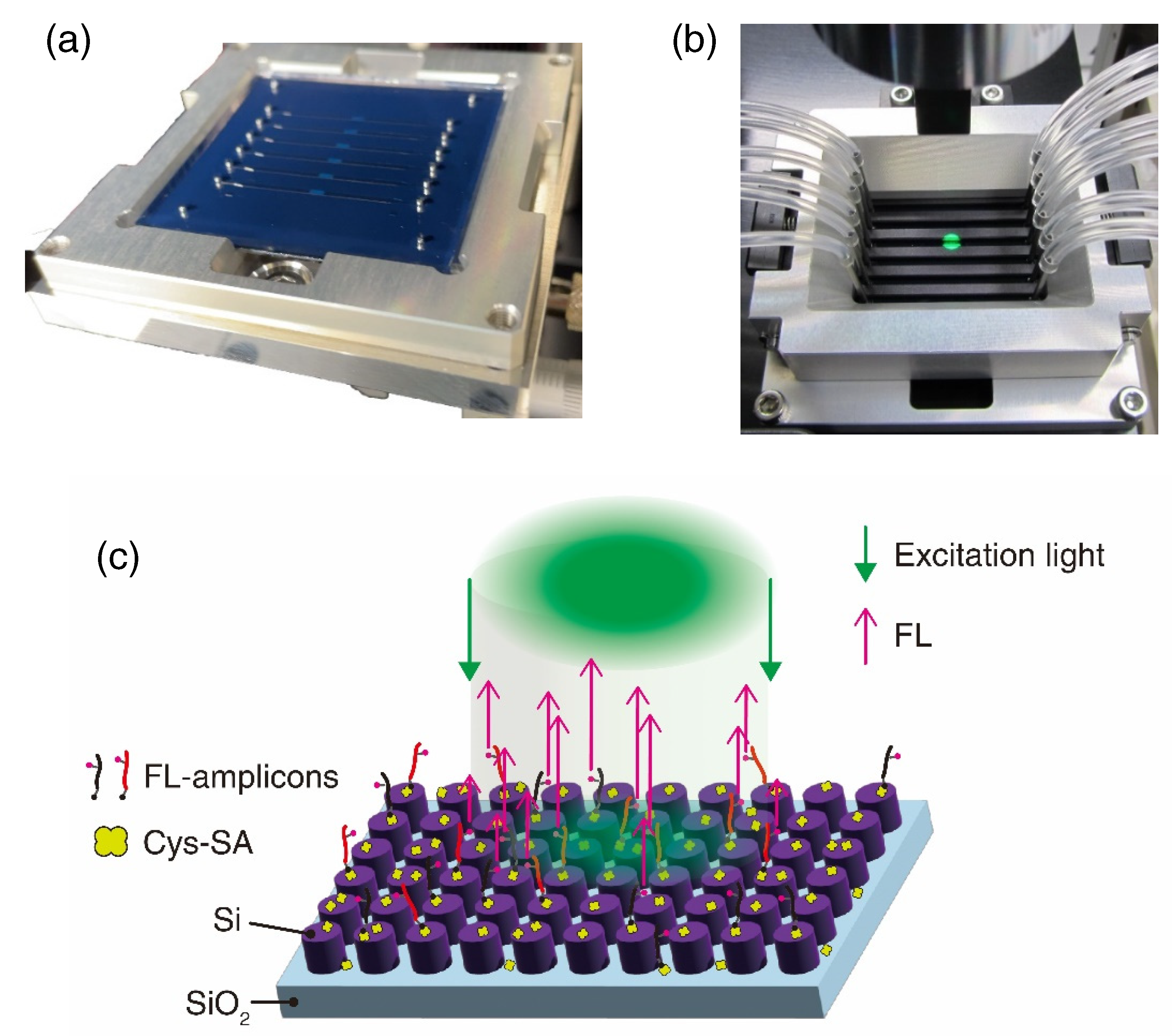
2.3. Preparation of All-Dielectric Metasurface Biosensors
2.4. Automated MF Procedures
2.5. FL Measurement
3. Results
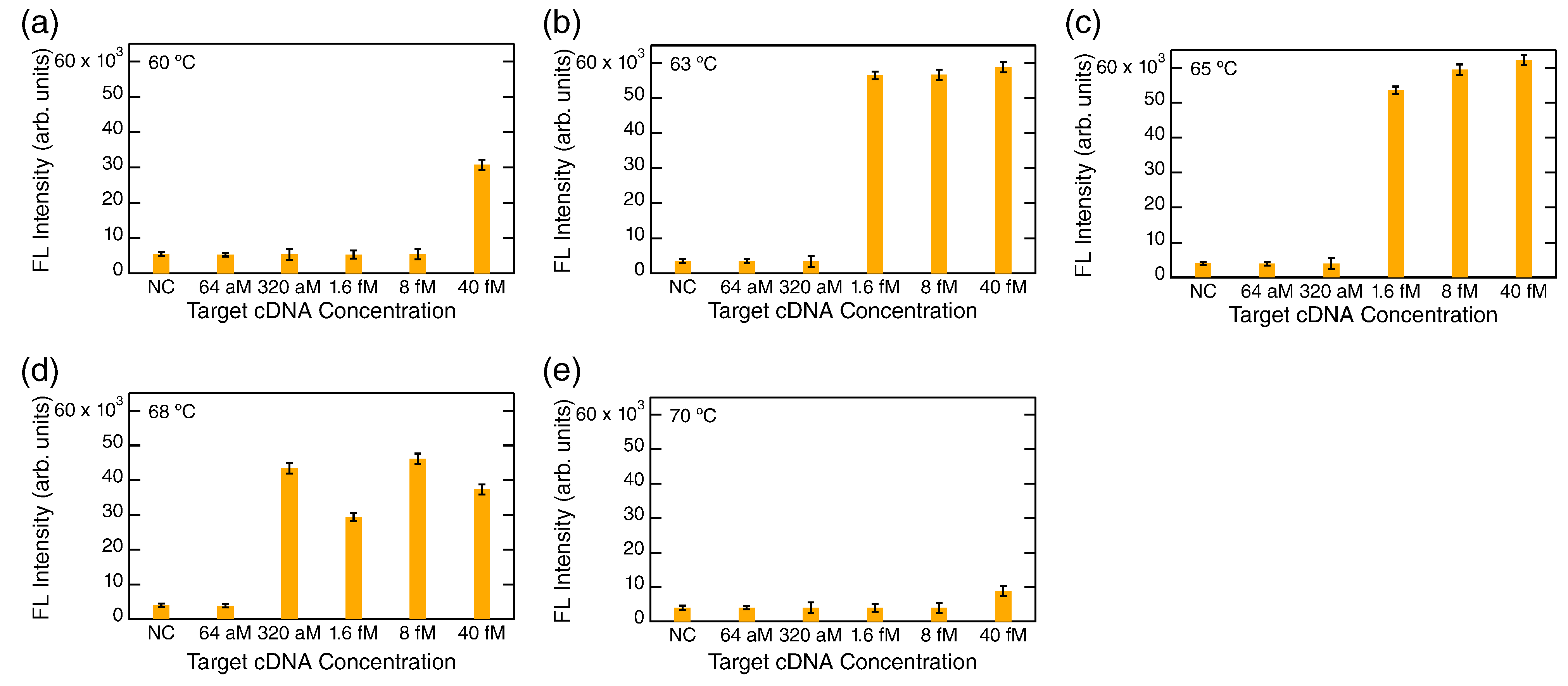
3.1. FL Detection via LAMP
3.2. FL-Enhanced Detection via PCR
4. Discussion
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| G | Guanine |
| C | Cytosine |
| T | Thymine |
| A | Adenine |
| LED | Light-emitting device |
| CCD | Charge-coupled device |
References
- Saiki, R.K.; Scharf, S.; Faloona, F.; Mullis, K.B.; Horn, G.T.; Erlich, H.A.; Arnheim, N. Enzymatic Amplification of β-Globin Genomic Sequences and Restriction Site Analysis for Diagnosis of Sickle Cell Anemia. Science 1985, 230, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, C.T.; Ririe, K.M.; Andrew, R.V.; David, D.A.; Gundry, R.A.; Balis, U.J. The LightCycler: A microvolume multisample fluorimeter with rapid temperature control. BioTechniques 1997, 22, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Sykes, P.J.; Neoh, S.H.; Brisco, M.J.; Hughes, E.; Condon, J.; Morley, A.A. Quantitation of targets for PCR by use of limiting dilution. BioTechniques 1992, 13, 444–449. [Google Scholar] [PubMed]
- Kalinina, O.; Lebedeva, I.; Brown, J.; Silver, J. Nanoliter scale PCR with TaqMan detection. Nucleic Acids Res. 1997, 25, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef]
- LOD of SARS-CoV-2 by RT-PCR. Available online: https://www.niid.go.jp/niid/ja/covid-19/9482-covid14-15.html (accessed on 6 October 2022). (In Japanese).
- Bruce, E.A.; Huang, M.L.; Perchetti, G.A.; Tighe, S.; Laaguiby, P.; Hoffman, J.J.; Gerrard, D.L.; Nalla, A.K.; Wei, Y.; Greninger, A.L.; et al. Direct RT-qPCR detection of SARS-CoV-2 RNA from patient nasopharyngeal swabs without an RNA extraction step. PLoS Biol. 2020, 18, e3000896. [Google Scholar] [CrossRef]
- Application Note “Facilitating Detection of SARS-CoV-2 Directly from Patient Samples: Precursor Studies with RT-qPCR and Colorimetric RT-LAMP Reagents” (New England BioLabs Inc.). Available online: https://www.nebj.jp/jp/info/202004_SARS_CoV2_detection_AN.pdf (accessed on 6 October 2022).
- Yin, H.; Wu, Z.; Shi, N.; Qi, Y.; Jian, X.; Zhou, L.; Tong, Y.; Cheng, Z.; Zhao, J.; Mao, H. Ultrafast multiplexed detection of SARS-CoV-2 RNA using a rapid droplet digital PCR system. Biosens. Bioelectron. 2021, 188, 113282. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Zhou, X. A CRISPR-based and post-amplification coupled SARS-CoV-2 detection with a portable evanescent wave biosensor. Biosens. Bioelectron. 2021, 190, 113418. [Google Scholar] [CrossRef]
- Chu, Y.; Qiu, J.; Wang, Y.; Wang, M.; Zhang, Y.; Han, L. Rapid and High-Throughput SARS-CoV-2 RNA Detection without RNA Extraction and Amplification by Using a Microfluidic Biochip. Chem. Eur. J. 2022, 28, e202104054. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Mori, Y.; Hirano, T.; Notomi, T. Sequence specific visual detection of LAMP reactions by addition of cationic polymers. BMC Biotechnol. 2006, 6, 3. [Google Scholar] [CrossRef]
- Varlamov, D.A.; Blagodatskikh, K.A.; Smirnova, E.V.; Kramarov, V.M.; Ignatov, K.B. Combinations of PCR and Isothermal Amplification Techniques Are Suitable for Fast and Sensitive Detection of SARS-CoV-2 Viral RNA. Front. Bioeng. Biotechnol. 2020, 8, 604793. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, T.; Tong, Y. Rapid and highly sensitive one-tube colorimetric RT-LAMP assay for visual detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 187, 113330. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M. All-Dielectric Metasurfaces with High-Fluorescence-Enhancing Capability. Appl. Sci. 2018, 8, 1328. [Google Scholar] [CrossRef]
- Ignatov, K.B.; Barsova, E.V.; Fradkov, A.F.; Blagodatskikh, K.A.; Kramarova, T.V.; Kramarov, V.M. A strong strand displacement activity of thermostable DNA polymerase markedly improves the results of DNA amplification. BioTechniques 2014, 57, 81–87. [Google Scholar] [CrossRef]
- Iwanaga, M. All-Dielectric Metasurface Fluorescence Biosensors for High-Sensitivity Antibody/Antigen Detection. ACS Nano 2020, 14, 17458–17467. [Google Scholar] [CrossRef]
- Iwanaga, M. High-Sensitivity High-Throughput Detection of Nucleic-Acid Targets on Metasurface Fluorescence Biosensors. Biosensors 2021, 11, 33. [Google Scholar] [CrossRef]
- SARS-CoV-2, BA.5.1 Sequence at National Library of Medicine, USA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OP600128 (accessed on 8 October 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- SARS-CoV-2, BA.1 Sequence at National Library of Medicine, USA. Available online: https://www.ncbi.nlm.nih.gov/nuccore/NC_045512 (accessed on 8 October 2022).
- Primer Explorer. Available online: http://primerexplorer.jp/ (accessed on 2 October 2022). (In Japanese).
- Wetmur, J.G. DNA Probes: Applications of the Principles of Nucleic Acid Hybridization. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 227–259. [Google Scholar] [CrossRef]
- Fluorescent HEX Information. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Brochure/fluorescent_dna_probes.pdf (accessed on 10 October 2022).
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Iwanaga, M. Highly sensitive wide-range target fluorescence biosensors of high-emittance metasurfaces. Biosens. Bioelectron. 2021, 190, 113423. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Nao, N.; Matsuyama, S.; Takeda, M.; Kageyama, T. An Ultra-Rapid Real-Time RT-PCR Method Using the PCR1100 to Detect Severe Acute Respiratory Syndrome Coronavirus-2. Jpn. J. Infect. Dis. 2021, 74, 29–34. [Google Scholar] [CrossRef]
- Zhang, Y.; Odiwuor, N.; Xiong, J.; Sun, L.; Nyaruaba, R.O.; Wei, H.; Tanner, N.A. Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. medRxiv 2020. [Google Scholar] [CrossRef]
- Sherrill-Mix, S.; Hwang, Y.; Roche, A.M.; Glascock, A.; Weiss, S.R.; Li, Y.; Haddad, L.; Deraska, P.; Monahan, C.; Kromer, A.; et al. Detection of SARS-CoV-2 RNA using RT-LAMP and molecular beacons. Genome Biol. 2021, 22, 169. [Google Scholar] [CrossRef]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. [Google Scholar] [CrossRef]
- Hardinge, P.; Murray, J.A.H. Full Dynamic Range Quantification using Loop-mediated Amplification (LAMP) by Combining Analysis of Amplification Timing and Variance between Replicates at Low Copy Number. Sci. Rep. 2020, 10, 916. [Google Scholar] [CrossRef]

| Role | Sequence, Shown from 5‣- to 3‣-End |
|---|---|
| Target | AAGCCTTACCGCAGAGACAGAAGAAACAGCAAACTGTGACTCTTC |
| TTCCTGCTGCAGATTTGGATGATTTCTCCAAACAATTGCAACAATCC | |
| ATGAGCCGTGCTGACTCAACTCAGGCCTAAACTCATGCAGACCACA | |
| CAAGGCAGATGGGCTATATAAACGTTTTCGCTTTTCCGTTTACGATAT | |
| ATAGTCTACTCTTGTGCAGAATGAATTCTCGTAACTACATAGCACAA | |
| GTAGATGTAGTTAACTTTAATTTCACATAGCAATCTTTAATCAGTGTG | |
| TAACATTAGGGAGGACTTGAAAGAGCCACCACATTTTCACCTACAG | |
| TGAACAATGCTAGGGAGAGCTGCCTATATGGAA | |
| F3 | CCTTACCGCAGAGACAGA |
| B3 | TCGTAAACGGAAAAGCGA |
| FIP | TGCAATTGTTTGGAGAAATCATCCAGAAACAGCAAACTGTGAC |
| BIP | ACAATCCATGAGCCGTGCTGAAACGTTTATATAGCCCATCTG |
| LF | AATCTGCAGCAGGAAGAAG |
| LB | CTCAGGCCTAAACTCATGC |
| Target | Method | LOD | Amplified Condition |
|---|---|---|---|
| RNA | RT-qPCR [7] | 9.49–16.6 aM | 40 cycles |
| RNA | RT-qPCR [31] | 1.7–5.3 aM | 20 min, 50 cycles |
| RNA | RT-PCR + LAMP [14] | 2.77 aM | 35–40 min |
| cDNA | LAMP + MSF | 320 aM | 60 min |
| cDNA | PCR + MSF | 5.86 aM | 35 cycles |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanaga, M. Rapid Detection of Attomolar SARS-CoV-2 Nucleic Acids in All-Dielectric Metasurface Biosensors. Biosensors 2022, 12, 987. https://doi.org/10.3390/bios12110987
Iwanaga M. Rapid Detection of Attomolar SARS-CoV-2 Nucleic Acids in All-Dielectric Metasurface Biosensors. Biosensors. 2022; 12(11):987. https://doi.org/10.3390/bios12110987
Chicago/Turabian StyleIwanaga, Masanobu. 2022. "Rapid Detection of Attomolar SARS-CoV-2 Nucleic Acids in All-Dielectric Metasurface Biosensors" Biosensors 12, no. 11: 987. https://doi.org/10.3390/bios12110987
APA StyleIwanaga, M. (2022). Rapid Detection of Attomolar SARS-CoV-2 Nucleic Acids in All-Dielectric Metasurface Biosensors. Biosensors, 12(11), 987. https://doi.org/10.3390/bios12110987





