A Flexible Optoelectronic Device for Continuous Cerebral Blood Flow Monitoring
Abstract
1. Introduction
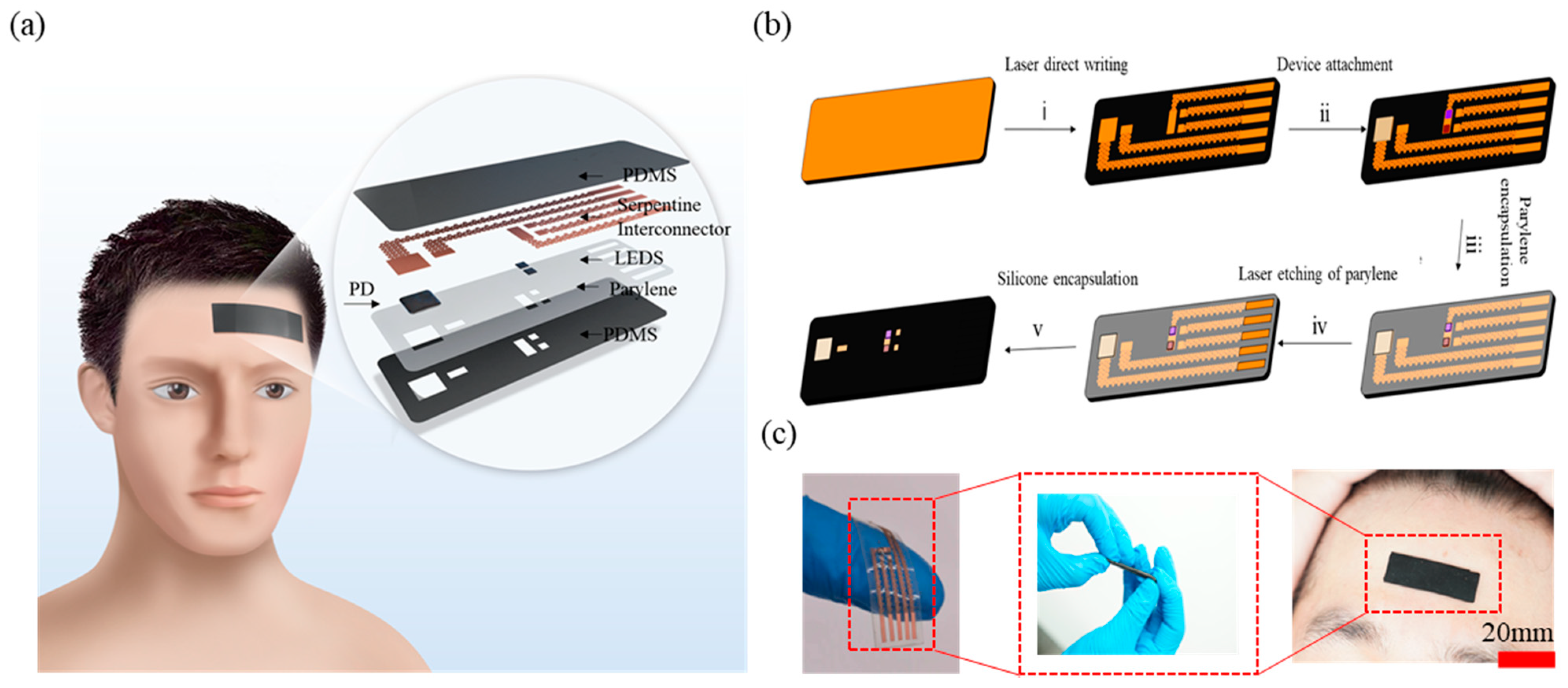
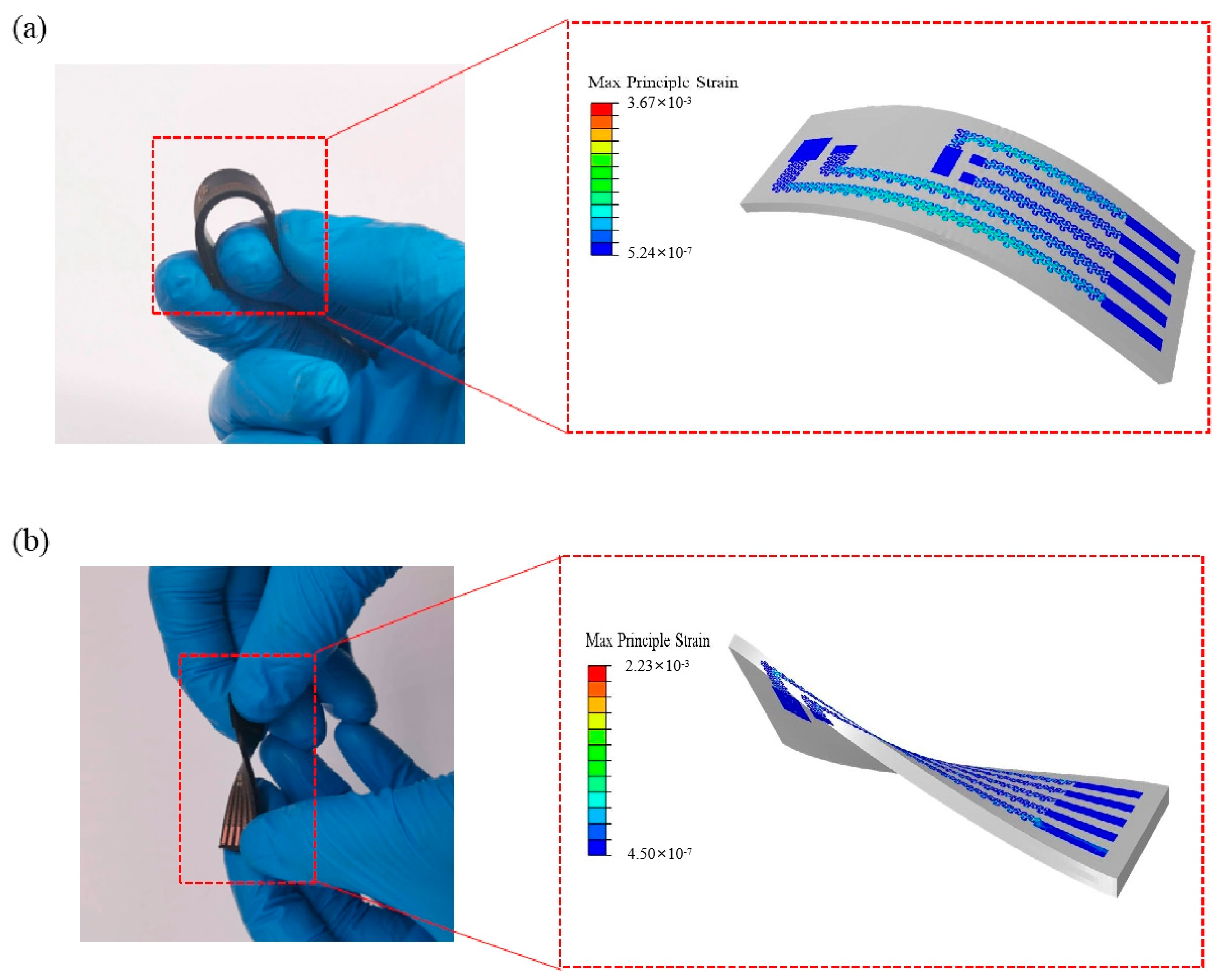
2. Materials and Methods
3. Results and Discussion
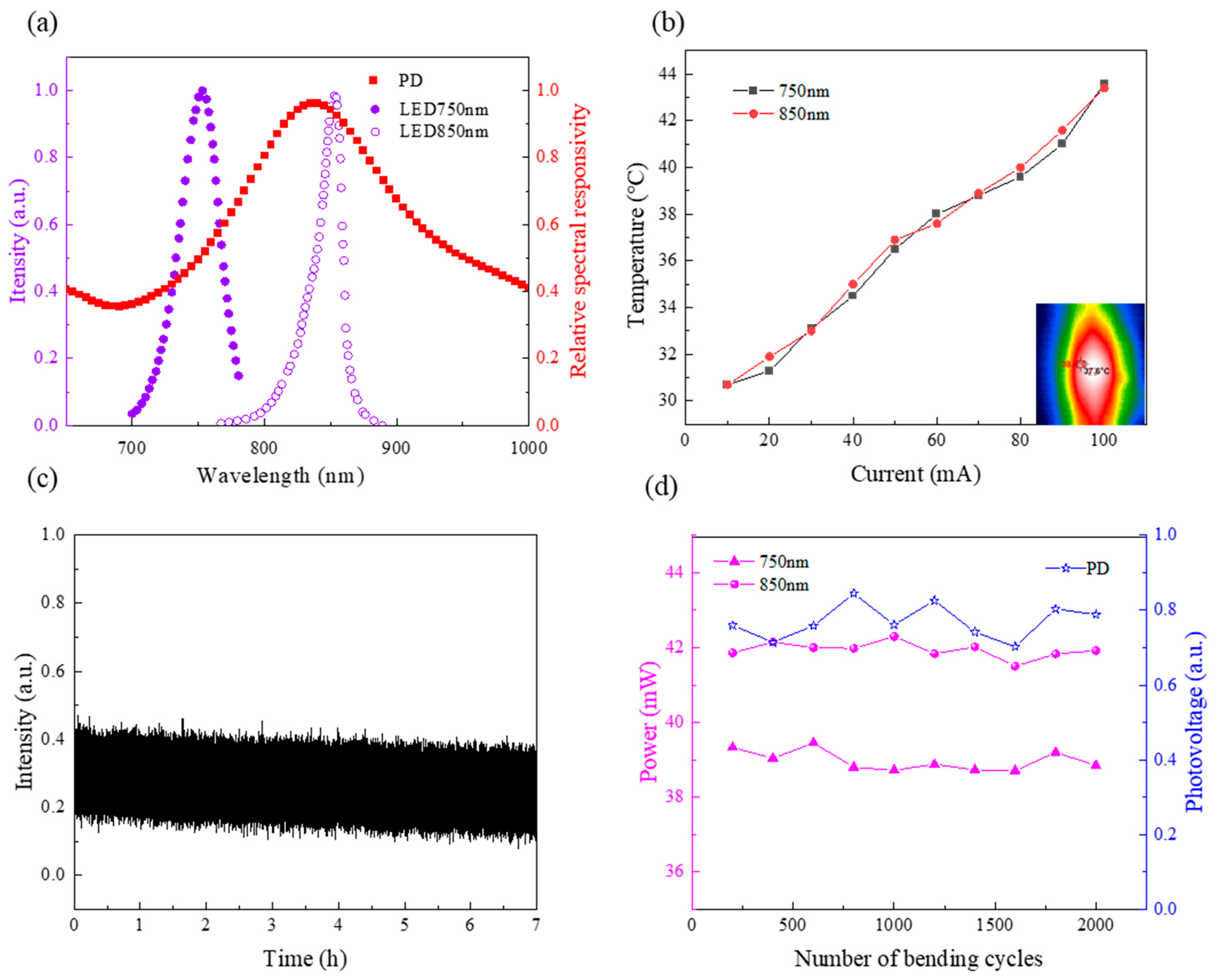
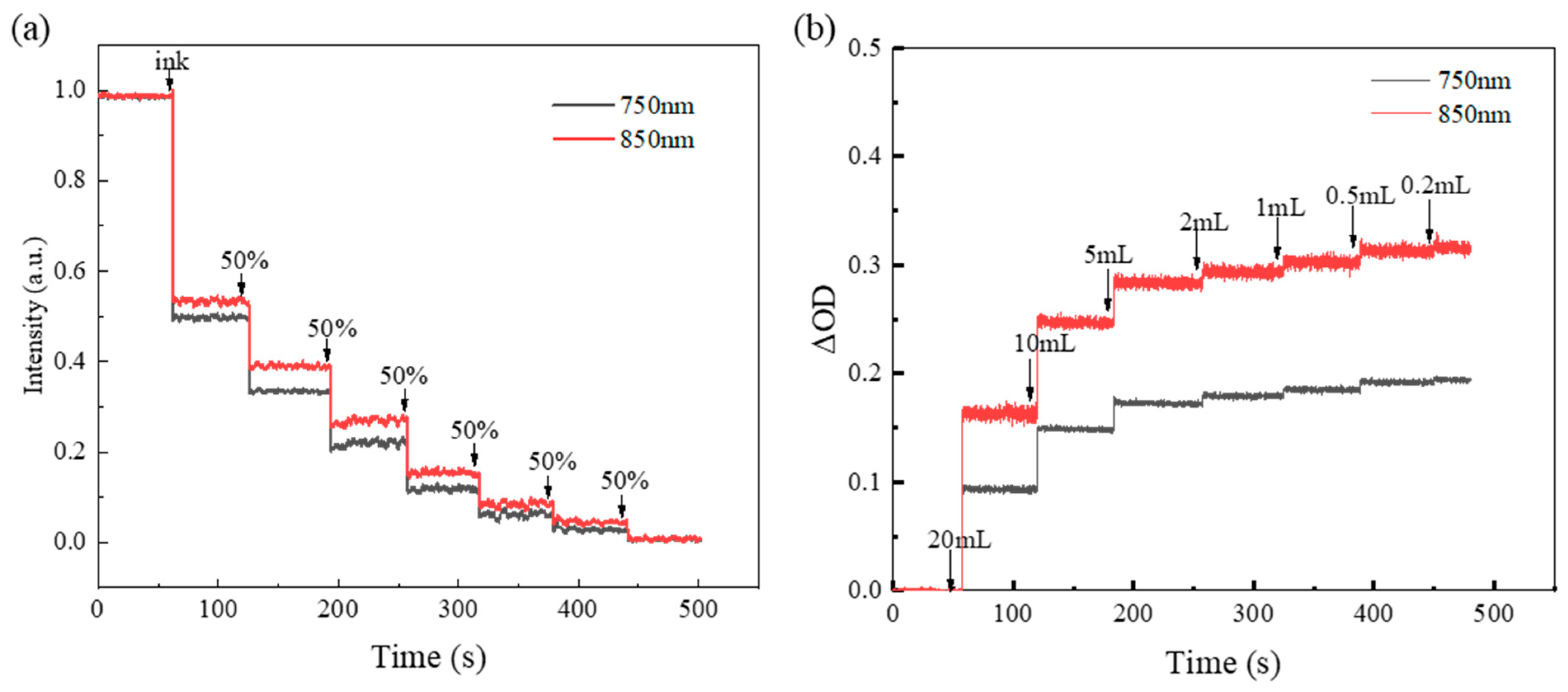
3.1. In Vitro Experiment
3.2. Forearm Block Experiment
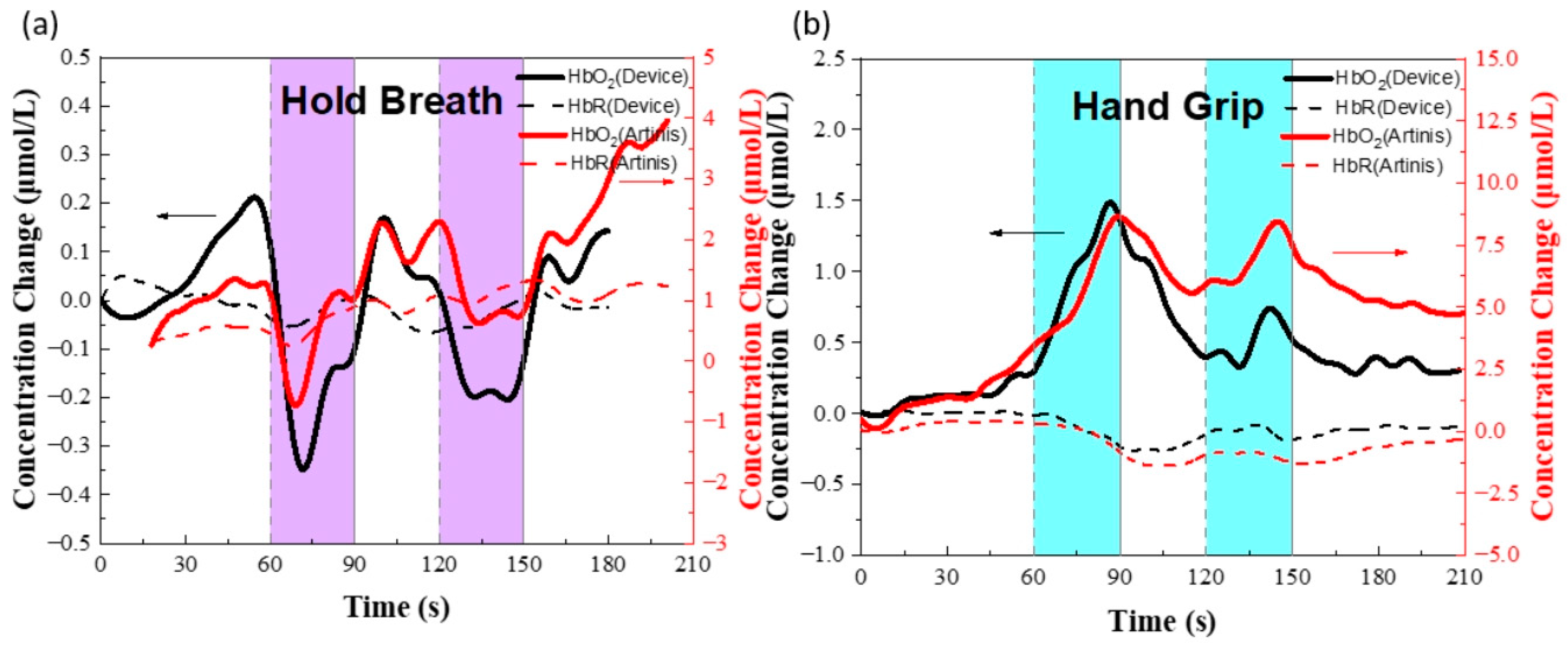
3.3. Prefrontal Cortex fNIRS Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The Present and Future Use of Functional Near-infrared Spectroscopy (FNIRS) for Cognitive Neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.J.; Lal, T.N.; Schröder, M.; Hinterberger, T.; Widman, G.; Elger, C.E.; Schölkopf, B.; Birbaumer, N. Classifying Event-Related Desynchronization in EEG, ECoG and MEG Signals. In Pattern Recognition; Franke, K., Müller, K.-R., Nickolay, B., Schäfer, R., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2006; Volume 4174, pp. 404–413. [Google Scholar]
- Yeom, G.S.; Song, I.; Warkad, S.D.; Shinde, P.B.; Kim, T.; Park, S.; Nimse, S.B. Development of a Novel Benzimidazole-Based Probe and Portable Fluorimeter for the Detection of Cysteine in Human Urine. Biosensors 2021, 11, 420. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, T.; Zwang, T.J.; Hong, G.; Zhao, Y.; Viveros, R.D.; Fu, T.-M.; Gao, T.; Lieber, C.M. Bioinspired Neuron-like Electronics. Nat. Mater. 2019, 18, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.W.; Wang, M.H.; Ge, C.F.; Xie, Z.Q.; Guo, Z.J.; Hong, W.; Gu, X.W.; Wang, L.C.; Yi, Z.; Jiang, C.; et al. Flexible bioelectrodes with enhanced wrinkle microstructures for reliable electrochemical modification and neuromodulation in vivo. Biosens. Bioelectron. 2019, 135, 181–191. [Google Scholar] [CrossRef]
- Martínez-Montes, E.; Valdés-Sosa, P.A.; Miwakeichi, F.; Goldman, R.I.; Cohen, M.S. Concurrent EEG/FMRI Analysis by Multiway Partial Least Squares. NeuroImage 2004, 22, 1023–1034. [Google Scholar] [CrossRef]
- Choi, S.-I.; Choi, G.-Y.; Lee, H.-T.; Hwang, H.-J.; Shin, J. Classification of Mental Arithmetic and Resting-State Based on Ear-EEG. In Proceedings of the 2018 6th International Conference on Brain-Computer Interface (BCI), Gangwon, Korea, 15–17 January 2018; pp. 1–4. [Google Scholar]
- Ferrari, M.; Quaresima, V. A Brief Review on the History of Human Functional Near-Infrared Spectroscopy (FNIRS) Development and Fields of Application. NeuroImage 2012, 63, 921–935. [Google Scholar] [CrossRef]
- Thornbury, J.R.; Fryback, D.G.; Turski, P.A.; Javid, M.J.; McDonald, J.V.; Beinlich, B.R.; Gentry, L.R.; Sackett, J.F.; Dasbach, E.J.; Martin, P.A. Disk-Caused Nerve Compression in Patients with Acute Low-Back Pain: Diagnosis with MR, CT Myelography, and Plain CT. Radiology 1993, 186, 731–738. [Google Scholar] [CrossRef]
- Jöbsis, F.F. Noninvasive, Infrared Monitoring of Cerebral and Myocardial Oxygen Sufficiency and Circulatory Parameters. Science 1977, 198, 1264–1267. [Google Scholar] [CrossRef]
- Scholkmann, F.; Kleiser, S.; Metz, A.J.; Zimmermann, R.; Mata Pavia, J.; Wolf, U.; Wolf, M. A Review on Continuous Wave Functional Near-Infrared Spectroscopy and Imaging Instrumentation and Methodology. NeuroImage 2014, 85, 6–27. [Google Scholar] [CrossRef]
- Ahmad Tarar, A.; Mohammad, U.; Srivastava, K.S. Wearable Skin Sensors and Their Challenges: A Review of Transdermal, Optical, and Mechanical Sensors. Biosensors 2020, 10, 56. [Google Scholar] [CrossRef]
- Paulmurugan, K.; Vijayaragavan, V.; Ghosh, S.; Padmanabhan, P.; Gulyás, B. Brain–Computer Interfacing Using Functional Near-Infrared Spectroscopy (FNIRS). Biosensors 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Soraghan, C.; Matthews, F.; Markham, C.; Pearlmutter, B.A.; O’Neill, R.; Ward, T.E. A 12-Channel, Real-Time Near-Infrared Spectroscopy Instrument for Brain-Computer Interface Applications. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–24 August 2008; pp. 5648–5651. [Google Scholar]
- Coyle, S.M.; Ward, T.E.; Markham, C.M. Brain-Computer Interface Using a Simplified Functional near-Infrared Spectroscopy System. J. Neural Eng. 2007, 4, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, G.; Leeb, R.; Wriessnegger, S.C.; Pfurtscheller, G. Development, Set-up and First Results for a One-Channel near-Infrared Spectroscopy System/Entwicklung, Aufbau Und Vorläufige Ergebnisse Eines Einkanal- Nahinfrarot-Spektroskopie-Systems. Biomed. Tech. Eng. 2008, 53, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Everdell, N.L.; Airantzis, D.; Kolvya, C.; Suzuki, T.; Elwell, C.E. A Portable Wireless Near-Infrared Spatially Resolved Spectroscopy System for Use on Brain and Muscle. Med. Eng. Phys. 2013, 35, 1692–1697. [Google Scholar] [CrossRef]
- Yaqub, M.A.; Woo, S.-W.; Hong, K.-S. Compact, Portable, High-Density Functional Near-Infrared Spectroscopy System for Brain Imaging. IEEE Access 2020, 2020, 128224–128238. [Google Scholar] [CrossRef]
- McKendrick, R.; Mehta, R.; Ayaz, H.; Scheldrup, M.; Parasuraman, R. Prefrontal Hemodynamics of Physical Activity and Environmental Complexity During Cognitive Work. Hum. Factors 2017, 59, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Quaresima, V.; Ferrari, M. Functional Near-Infrared Spectroscopy (FNIRS) for Assessing Cerebral Cortex Function During Human Behavior in Natural/Social Situations: A Concise Review. Organ. Res. Methods 2019, 22, 46–68. [Google Scholar] [CrossRef]
- Newmanbrain FNIR BrainSpy 28 Portable Neuroimage Device—Newmanbrain. Available online: https://www.newmanbrain.com/fnir-brainspy-28/ (accessed on 20 June 2022).
- Rwei, A.Y.; Lu, W.; Wu, C.; Human, K.; Suen, E.; Franklin, D.; Fabiani, M.; Gratton, G.; Xie, Z.; Deng, Y.; et al. A Wireless, Skin-Interfaced Biosensor for Cerebral Hemodynamic Monitoring in Pediatric Care. Proc. Natl. Acad. Sci. USA 2020, 117, 31674–31684. [Google Scholar] [CrossRef]
- Strangman, G.E.; Li, Z.; Zhang, Q. Depth Sensitivity and Source-Detector Separations for Near Infrared Spectroscopy Based on the Colin27 Brain Template. PLoS ONE 2013, 8, e66319. [Google Scholar] [CrossRef]
- Zhao, K.; Li, N.; Pan, B.; Li, T. Performance Assessment of the NIRS-Based Medical System of Evaluating Therapeutic Effect. Microelectron. Reliab. 2018, 87, 188–193. [Google Scholar] [CrossRef]
- Lindkvist, M.; Granåsen, G.; Grönlund, C. Coherent Derivation of Equations for Differential Spectroscopy and Spatially Resolved Spectroscopy: An Undergraduate Tutorial. Spectrosc. Lett. 2013, 46, 243–249. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiu, L.; Sun, Y.; Huang, C.; Li, T. Optimal Hemoglobin Extinction Coefficient Data Set for Near-Infrared Spectroscopy. Biomed. Opt. Express 2017, 8, 5151–5159. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lin, Y.; Shang, Y.; He, L.; Huang, C.; Szabunio, M.; Yu, G. Simultaneous Measurement of Deep Tissue Blood Flow and Oxygenation Using Noncontact Diffuse Correlation Spectroscopy Flow-Oximeter. Sci. Rep. 2013, 3, 1358. [Google Scholar] [CrossRef]
- Diehl, R.R.; Linden, D.; Bünger, B.; Schäfer, M.; Berlit, P. Valsalva-Induced Syncope during Apnea Diving. Clin. Auton. Res. 2000, 10, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Besio, W.; Mankodiya, K. WearLight: Towards a Wearable, Configurable Functional NIR Spectroscopy System for Noninvasive Neuroimaging. IEEE Trans. Biomed. Circuits Syst. 2018, 2018, 91–102. [Google Scholar] [CrossRef]
- Kim, C.-K.; Lee, S.; Koh, D.; Kim, B.-M. Development of Wireless NIRS System with Dynamic Removal of Motion Artifacts. Biomed. Eng. Lett. 2011, 1, 254–259. [Google Scholar] [CrossRef]
- Ysehak Abay, T.; Shafqat, K.; Kyriacou, P.A. Perfusion Changes at the Forehead Measured by Photoplethysmography during a Head-Down Tilt Protocol. Biosensors 2019, 9, 71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Xu, Z.; Wang, M.; Zou, H.; Chen, Y.; Ai, J. A Flexible Optoelectronic Device for Continuous Cerebral Blood Flow Monitoring. Biosensors 2022, 12, 944. https://doi.org/10.3390/bios12110944
Ji H, Xu Z, Wang M, Zou H, Chen Y, Ai J. A Flexible Optoelectronic Device for Continuous Cerebral Blood Flow Monitoring. Biosensors. 2022; 12(11):944. https://doi.org/10.3390/bios12110944
Chicago/Turabian StyleJi, Huawei, Ze Xu, Mingyu Wang, Hong Zou, Ying Chen, and Jun Ai. 2022. "A Flexible Optoelectronic Device for Continuous Cerebral Blood Flow Monitoring" Biosensors 12, no. 11: 944. https://doi.org/10.3390/bios12110944
APA StyleJi, H., Xu, Z., Wang, M., Zou, H., Chen, Y., & Ai, J. (2022). A Flexible Optoelectronic Device for Continuous Cerebral Blood Flow Monitoring. Biosensors, 12(11), 944. https://doi.org/10.3390/bios12110944




