Feasibility Analysis and Implementation of Head-Mounted Electrical Impedance Respiratory Monitoring
Abstract
1. Introduction
2. Feasibility Analysis
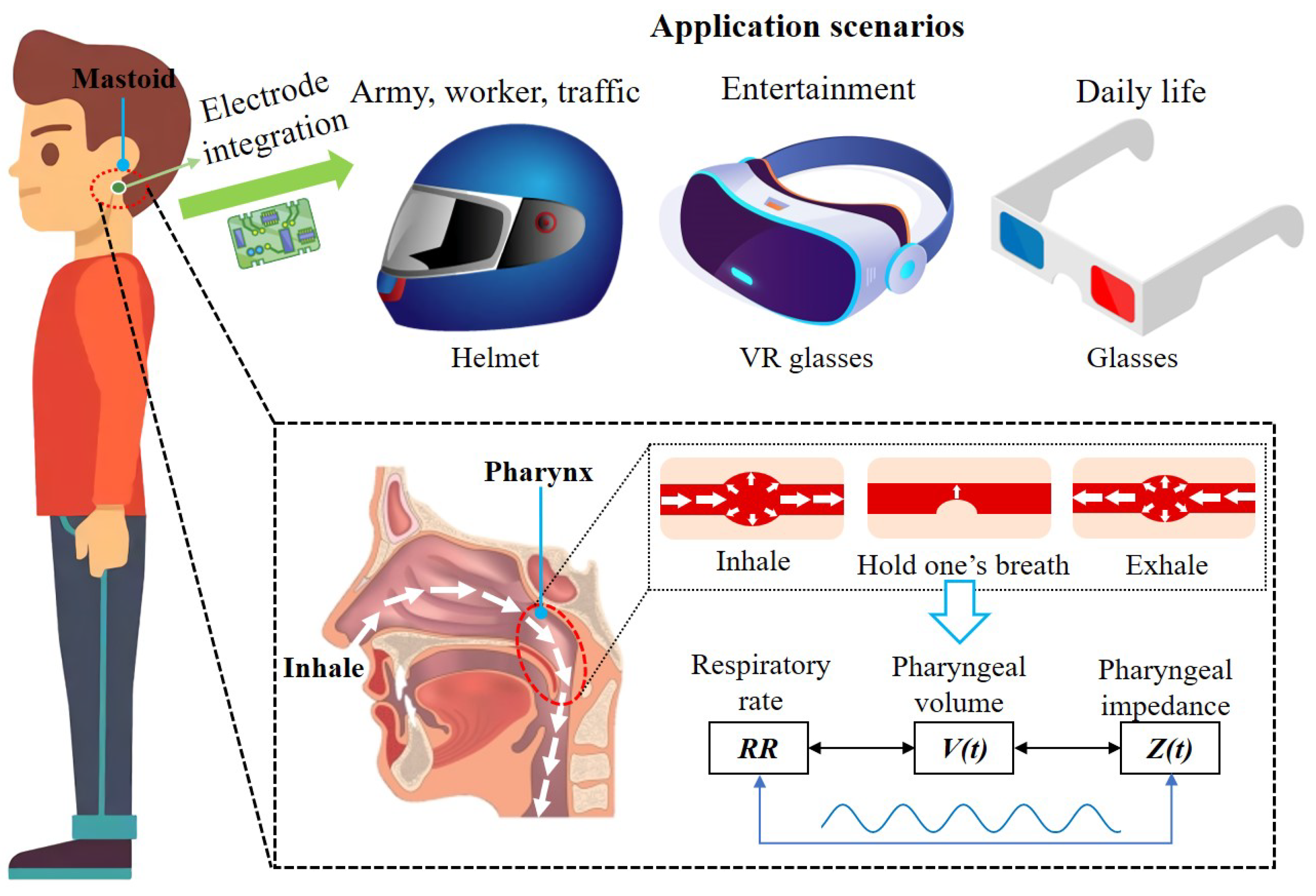
2.1. Anatomical Structure Analysis
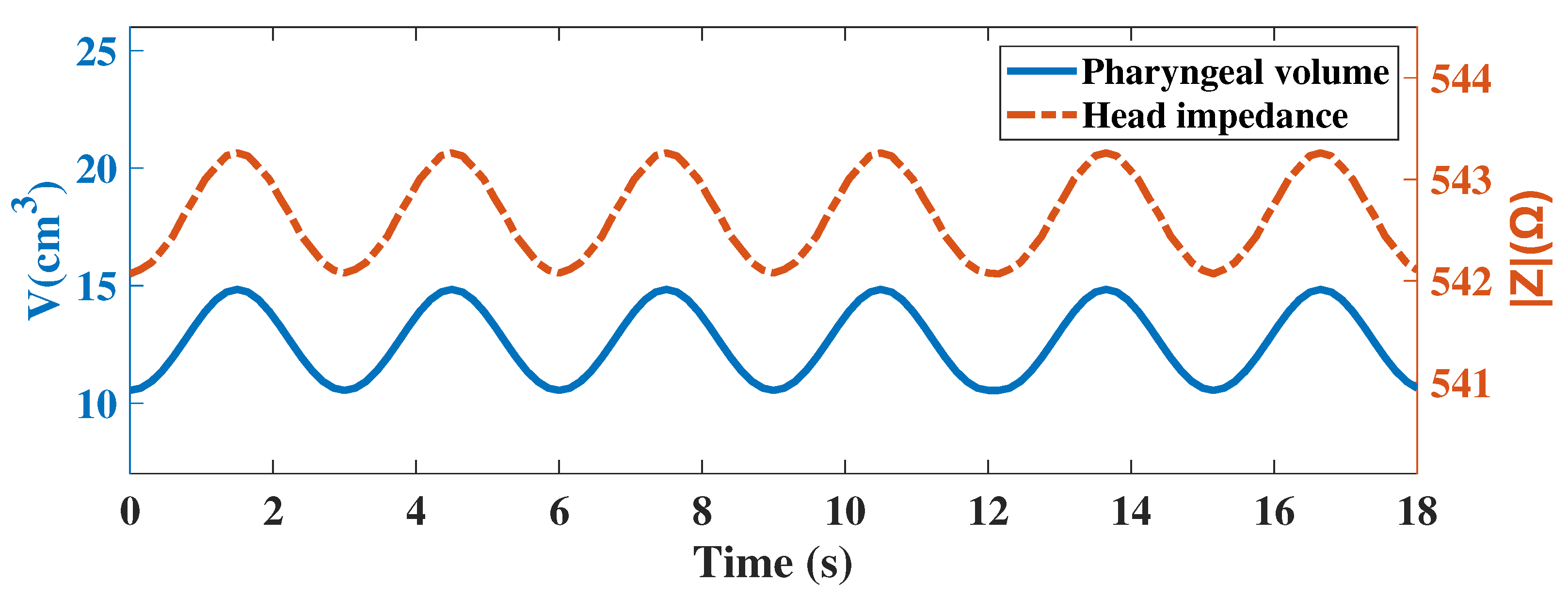
2.2. Simulation Analysis
3. Systems Design
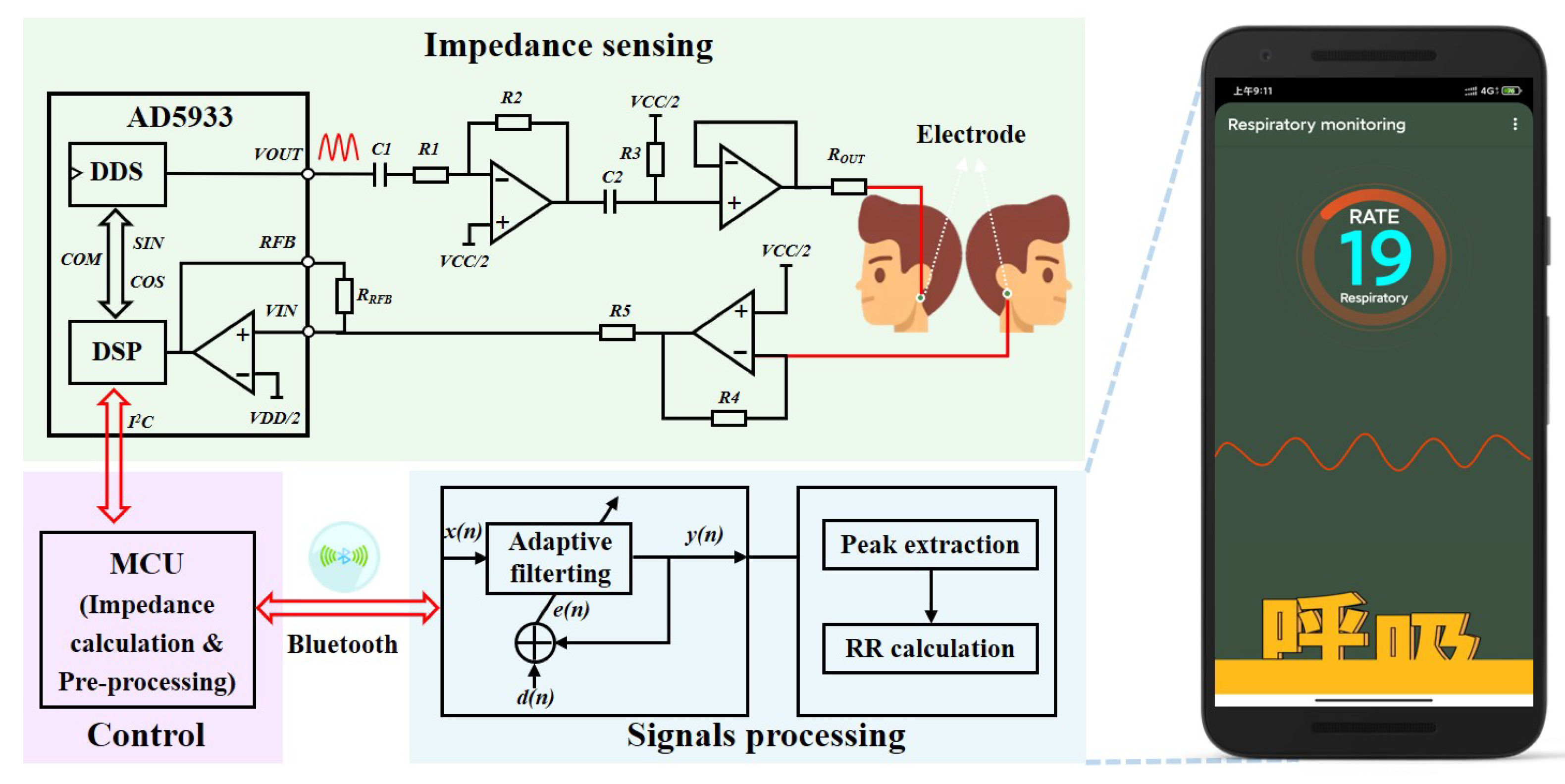
3.1. Electrical Impedance Sensing
3.2. Signal Processing
3.2.1. Adaptive Filtering for Noise Reduction
3.2.2. Calculation of Respiration Rate
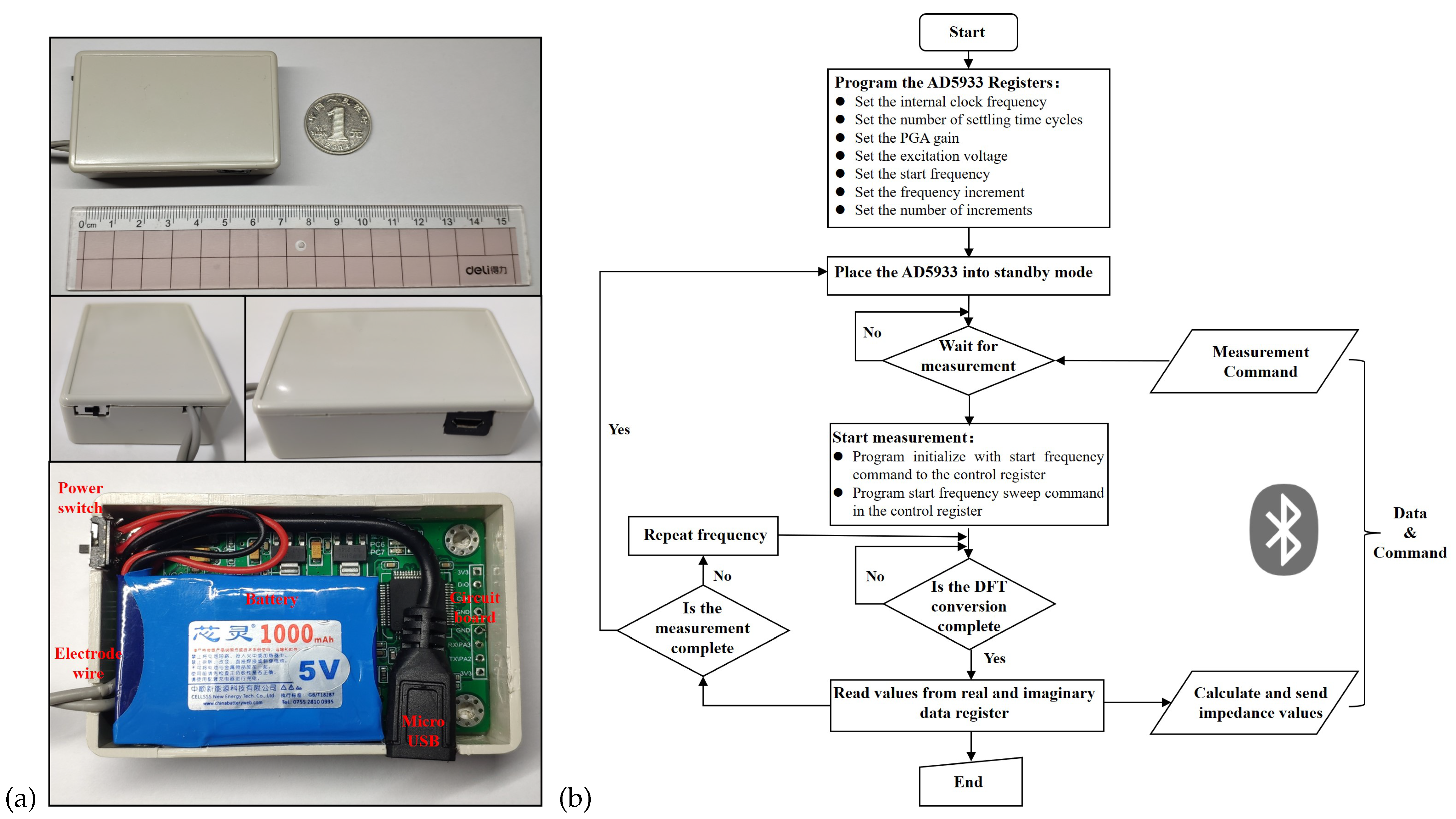
3.2.3. Electronic Devices and Software Processes
4. Experiments and Results
4.1. Experimental Protocol
4.2. Experimental Results
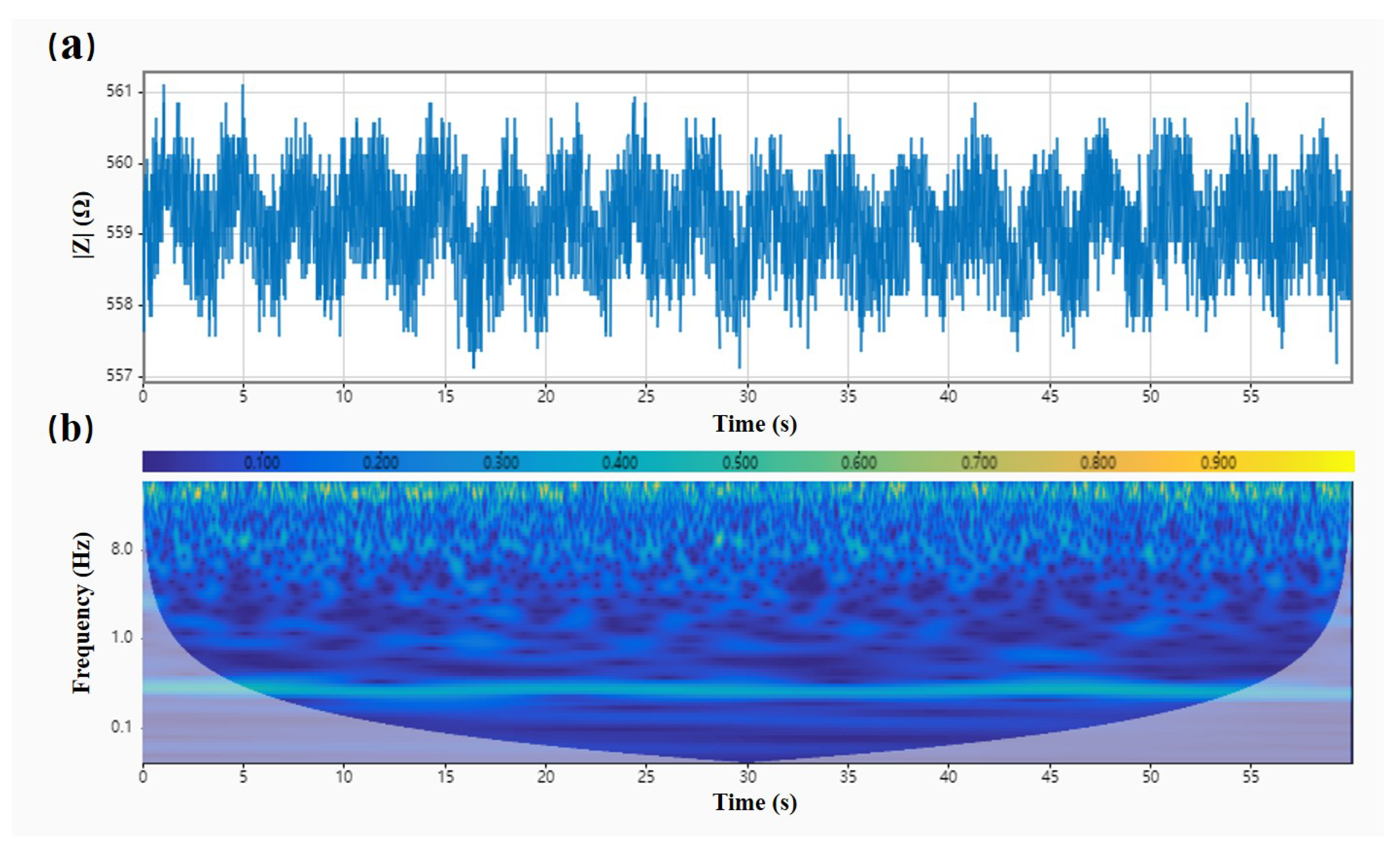
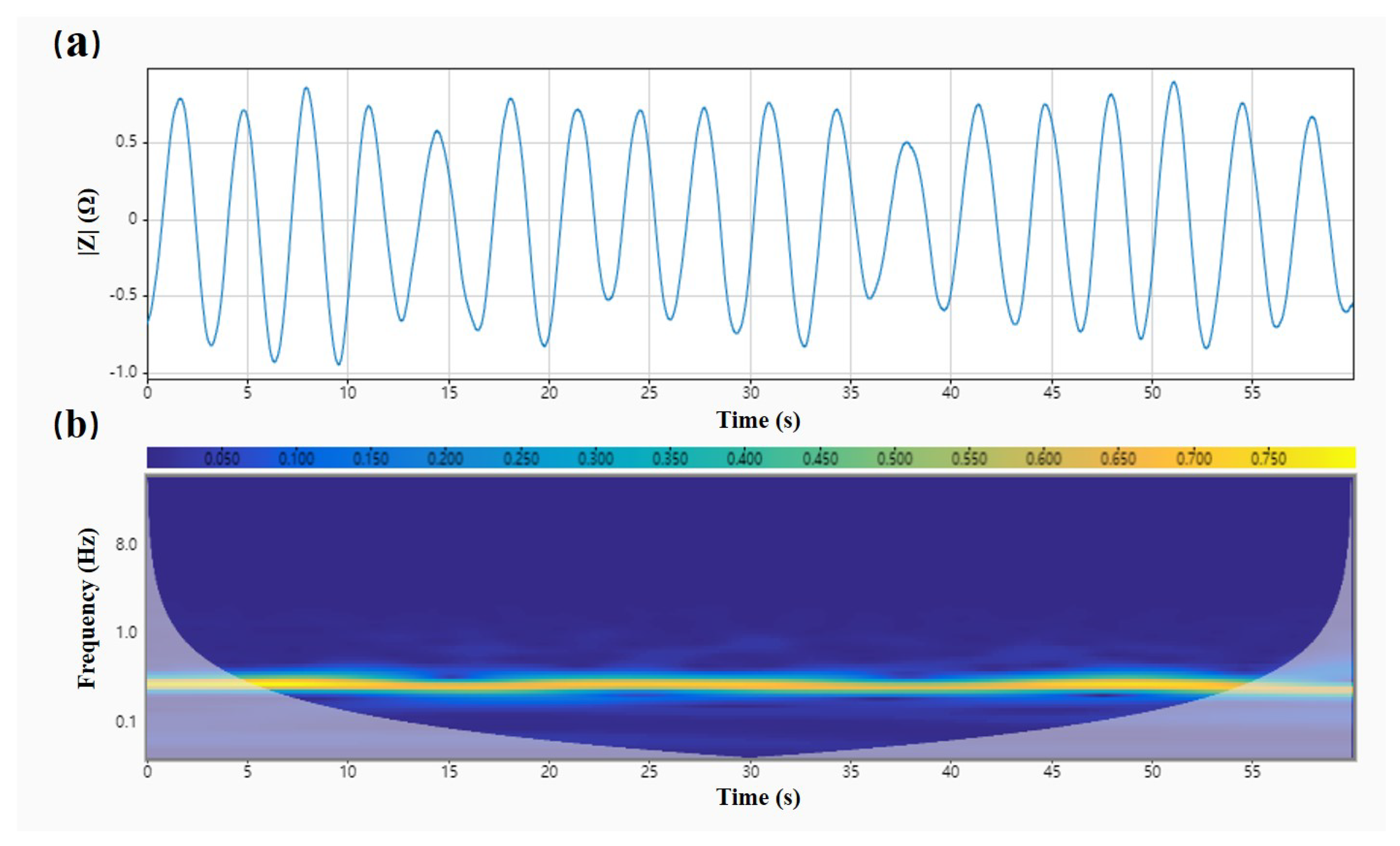
4.2.1. Noise Reduction Processing
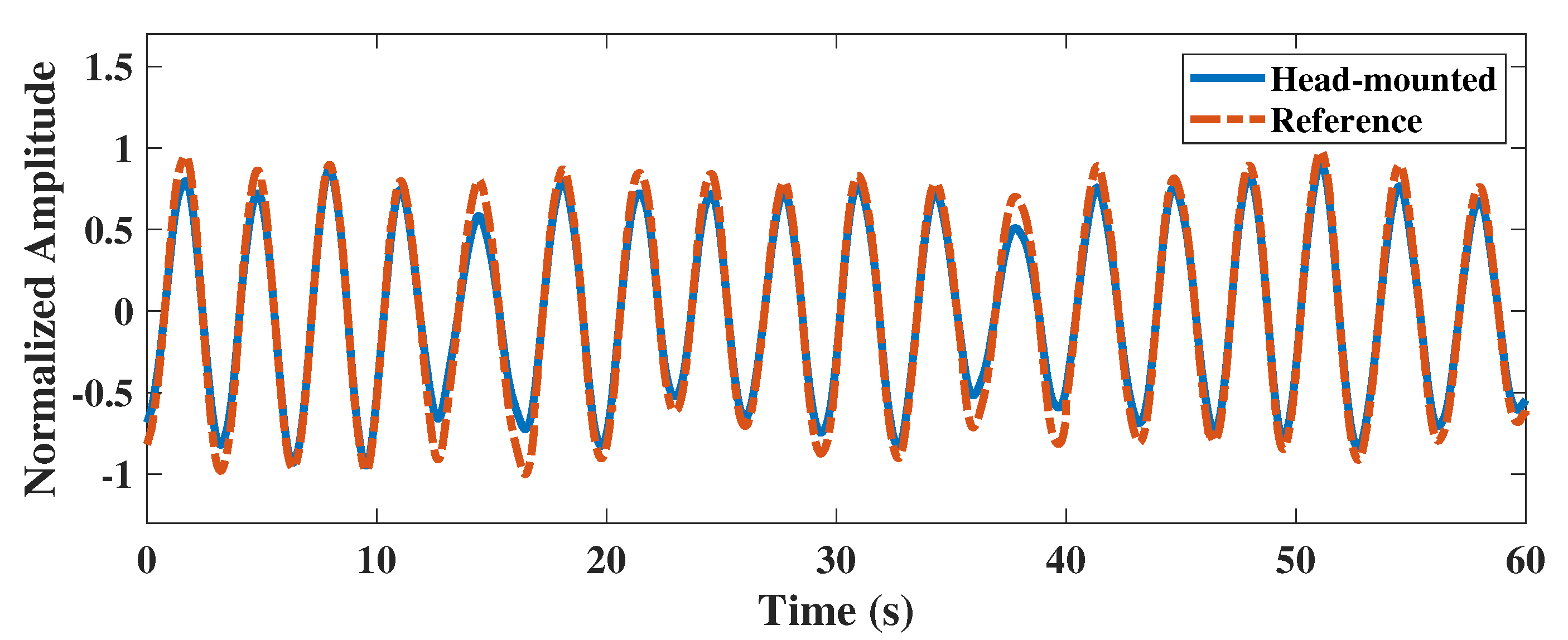
4.2.2. Performance Evaluation
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-based methods for measuring respiratory rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Nicolo, A.; Sacchetti, M.; Schena, E. Contactless methods for measuring respiratory rate: A review. IEEE Sens. J. 2020, 21, 12821–12839. [Google Scholar] [CrossRef]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing systems for respiration monitoring: A technical systematic review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhu, R.; Que, R.Y. A wireless portable system with microsensors for monitoring respiratory diseases. IEEE Trans. Biomed. Eng. 2012, 59, 3110–3116. [Google Scholar] [PubMed]
- Kontaxis, S.; Lázaro, J.; Corino, V.D.; Sandberg, F.; Bailón, R.; Laguna, P.; Sörnmo, L. ECG-derived respiratory rate in atrial fibrillation. IEEE Trans. Biomed. Eng. 2019, 67, 905–914. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Elvin, C.S.M.; Janice, L.H.Y.; Ng, S.H.; Teo, J.T.; Wu, R. Textile fiber optic microbend sensor used for heartbeat and respiration monitoring. IEEE Sens. J. 2014, 15, 757–761. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Mao, S. Respiration monitoring with RFID in driving environments. IEEE J. Sel. Areas Commun. 2020, 39, 500–512. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Liu, D.; Wang, Y.; Zhang, Y.; Bai, O.; Sun, J. Human emotion classification based on multiple physiological signals by wearable system. Technol. Health Care 2018, 26, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Folschweiller, S.; Sauer, J.F. Respiration-driven brain oscillations in emotional cognition. Front. Neural Circuits 2021, 15, 761812. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.A.; Ahmed, B.; Al Rihawi, R.; Gutierrez-Osuna, R. Gaming away stress: Using biofeedback games to learn paced breathing. IEEE Trans. Affect. Comput. 2018, 11, 519–531. [Google Scholar] [CrossRef]
- Chourpiliadis, C.; Bhardwaj, A. Physiology, Respiratory Rate; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rodríguez-Molinero, A.; Narvaiza, L.; Ruiz, J.; Gálvez-Barrón, C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J. Am. Geriatr. Soc. 2013, 61, 2238–2240. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, M.; Tavassolian, N. Accurate Doppler radar-based cardiopulmonary sensing using chest-wall acceleration. IEEE J. Electromagn. Microwaves Med. Biol. 2018, 3, 41–47. [Google Scholar] [CrossRef]
- Hurtado, D.E.; Abusleme, A.; Chávez, J.A. Non-invasive continuous respiratory monitoring using temperature-based sensors. J. Clin. Monit. Comput. 2020, 34, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Zhao, H.; Lin, X.; Liu, S.; Liu, Y.; Liu, X.; Fei, T.; Zhang, T. Ultrafast response polyelectrolyte humidity sensor for respiration monitoring. Acs Appl. Mater. Interfaces 2019, 11, 6483–6490. [Google Scholar] [CrossRef] [PubMed]
- Subbe, C.P.; Kinsella, S. Continuous monitoring of respiratory rate in emergency admissions: Evaluation of the RespiraSense™ sensor in acute care compared to the industry standard and gold standard. Sensors 2018, 18, 2700. [Google Scholar] [CrossRef]
- Rahman, T.; Page, R.; Page, C.; Bonnefoy, J.R.; Cox, T.; Shaffer, T.H. pneuRIPTM: A novel respiratory inductance plethysmography monitor. J. Med. Devices 2017, 11, 0110101–0110106. [Google Scholar] [CrossRef] [PubMed]
- Simić, M.; Stavrakis, A.K.; Sinha, A.; Premčevski, V.; Markoski, B.; Stojanović, G.M. Portable Respiration Monitoring System with an Embroidered Capacitive Facemask Sensor. Biosensors 2022, 12, 339. [Google Scholar] [CrossRef]
- Fan, W.; He, Q.; Meng, K.; Tan, X.; Zhou, Z.; Zhang, G.; Yang, J.; Wang, Z.L. Machine-knitted washable sensor array textile for precise epidermal physiological signal monitoring. Sci. Adv. 2020, 6, eaay2840. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Zheng, Y.; Gu, T.; Zhou, X.; Dorizzi, B. C-FMCW based contactless respiration detection using acoustic signal. Proc. ACM Interactive Mobile Wearable Ubiquitous Technol. 2018, 1, 1–20. [Google Scholar] [CrossRef]
- Pramudita, A.A.; Suratman, F.Y. Low-Power Radar System for Noncontact Human Respiration Sensor. IEEE Trans. Instrum. Meas. 2021, 70, 1–15. [Google Scholar] [CrossRef]
- Manullang, M.C.T.; Lin, Y.H.; Lai, S.J.; Chou, N.K. Implementation of Thermal Camera for Non-Contact Physiological Measurement: A Systematic Review. Sensors 2021, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Reyes, B.A.; Chon, K.H. Estimation of respiratory rates using the built-in microphone of a smartphone or headset. IEEE J. Biomed. Health Inf. 2015, 20, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, D.; Wang, L.; Zheng, Y.; Gu, T.; Dorizzi, B.; Zhou, X. Contactless respiration monitoring using ultrasound signal with off-the-shelf audio devices. IEEE Internet Things J. 2018, 6, 2959–2973. [Google Scholar] [CrossRef]
- Sel, K.; Ibrahim, B.; Jafari, R. ImpediBands: Body coupled bio-impedance patches for physiological sensing proof of concept. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Wu, F.; Han, W.; Yuce, M.R. A Wearable Bioimpedance Chest Patch for Real-Time Ambulatory Respiratory Monitoring. IEEE Trans. Biomed. Eng. 2022, 69, 2970–2981. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, E.; Gambari, G.; Boero, F.; Fedeli, A.; Pastorino, M.; Randazzo, A. A breath monitoring approach based on electrical impedance measurements. IEEE J. Electromagn. Microwaves Med. Biol. 2020, 5, 179–186. [Google Scholar] [CrossRef]
- Yan, H.; Yang, X.; Li, X.; Gao, Y.; Vasić, Ž.L.; Cifrek, M. Finite element modeling and experimental analysis of bladder volume body surface monitoring method. In Proceedings of the 2022 IEEE MTT-S International Microwave Biomedical Conference (IMBioC), Suzhou, China, 16–18 May 2022; pp. 87–89. [Google Scholar]
- Schrunder, A.F.; Rodriguez, S.; Rusu, A. A Finite Element Analysis and Circuit Modelling Methodology for Studying Electrical Impedance Myography of Human Limbs. IEEE Trans. Biomed. Eng. 2021, 69, 244–255. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Sanchez, B. A framework for modeling bioimpedance measurements of nonhomogeneous tissues: A theoretical and simulation study. Physiol. Meas. 2021, 42, 055007. [Google Scholar] [CrossRef]
- Schwartz, S.; Geisbush, T.R.; Mijailovic, A.; Pasternak, A.; Darras, B.T.; Rutkove, S.B. Optimizing electrical impedance myography measurements by using a multifrequency ratio: A study in Duchenne muscular dystrophy. Clin. Neurophysiol. 2015, 126, 202–208. [Google Scholar] [CrossRef]
- Zhong, J.; Li, Z.; Takakuwa, M.; Inoue, D.; Hashizume, D.; Jiang, Z.; Shi, Y.; Ou, L.; Nayeem, M.O.G.; Umezu, S.; et al. Smart face mask based on an ultrathin pressure sensor for wireless monitoring of breath conditions. Adv. Mater. 2022, 34, 2107758. [Google Scholar] [CrossRef]
- Cotur, Y.; Olenik, S.; Asfour, T.; Bruyns-Haylett, M.; Kasimatis, M.; Tanriverdi, U.; Gonzalez-Macia, L.; Lee, H.S.; Kozlov, A.S.; Güder, F. Bioinspired Stretchable Transducer for Wearable Continuous Monitoring of Respiratory Patterns in Humans and Animals (Adv. Mater. 33/2022). Adv. Mater. 2022, 34, 2270240. [Google Scholar] [CrossRef]
- Chen, A.; Halton, A.J.; Rhoades, R.D.; Booth, J.C.; Shi, X.; Bu, X.; Wu, N.; Chae, J. Wireless wearable ultrasound sensor on a paper substrate to characterize respiratory behavior. ACS Sens. 2019, 4, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Mongan, W.M.; Rasheed, I.; Liu, Y.; Anday, E.; Dion, G.; Fontecchio, A.; Kurzweg, T.; Dandekar, K.R. Ensemble learning approach via kalman filtering for a passive wearable respiratory monitor. IEEE J. Biomed. Health Inf. 2018, 23, 1022–1031. [Google Scholar] [CrossRef] [PubMed]


| Tissue | Tissue Thickness | Conductivity (S/m) | Relative Permittivity |
|---|---|---|---|
| Skin | thickness = 2 mm | 4.33 × 10−2 | 1.63 × 102 |
| Skull | thickness = 10 mm | 2.06 × 10−2 | 2.64 × 102 |
| Cervical vertebra | - | 2.06 × 10−2 | 2.64 × 102 |
| Brain | - | 1.28 × 10−1 | 5.46 × 103 |
| Muscle | - | 3.52 × 10−1 | 1.01 × 104 |
| Pharynx (gas) | volume = 10.54∼ 14.84 cm | 0.00 × 100 | 1.00 × 100 |
| Trachea (gas) | radius = 8 mm | 0.00 × 100 | 1.00 × 100 |
| Electrode | radius = 10 mm | 5.00 × 105 | 1.00 × 100 |
| Category | Values |
|---|---|
| Effective sample size | 16 |
| Mean (head-mounted) | 18.781 |
| Mean (reference) | 18.594 |
| Mean of the difference | 0.188 |
| Standard deviation | 0.443 |
| 95% CI (Mean of the difference) | −0.048∼0.423 |
| 95% CI (Difference) | −0.680∼1.055 |
| t | 1.695 |
| p | 0.111 |
| Coefficient of repeatability | 0.917 |
| Reference, Year | Type of Sensor | Wearing Style | Data Transmission and Processing | Reported Characteristics |
|---|---|---|---|---|
| [32], 2022 | Flexible pressure sensor | Face mask | Wifi, PC, Matlab | Ultrathin self-powered, sensors affect the air permeability of the face mask. |
| [18], 2022 | Textile capacitive sensor | Face mask | UART, mobile app | Disposable and consumable, lightweight handheld device. |
| [33], 2022 | Pressure sensor | Waist belt | Wifi, SD card, PC | ASiT does not require calibration and is sufficiently sensitive, low air permeability. |
| [19], 2020 | All-textile sensor array | Chest, abdomen, or wrist | Wirelessly, mobile app | Washable, high stability and comfort, textiles easily stained. |
| [34], 2019 | Ultrasonic sensor | Abdomen-apposed rib cage | Bluetooth, mobile app | Wireless wearable measurement, relatively complex circuit, signal susceptible to interference. |
| [35], 2019 | Radio frequency identification | Shoulder | Radiofrequency, PC | Passive radiofrequency identification, low power consumption, signal susceptible to interference. |
| [26], 2022 | Bioimpedance | Chest patch | Bluetooth, LoRa, on device in real time | Real-world scenarios evaluation, chest movement interferes with the measurement signal. |
| This work | Bioimpedance | Head-mounted (mastoid) | Bluetooth, on device, and mobile app | Pharyngeal respiratory monitoring, comfortable to wear, easy to integrated and reusable. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Yang, X.; Liu, Y.; He, W.; Liao, Y.; Yang, J.; Gao, Y. Feasibility Analysis and Implementation of Head-Mounted Electrical Impedance Respiratory Monitoring. Biosensors 2022, 12, 934. https://doi.org/10.3390/bios12110934
Yan H, Yang X, Liu Y, He W, Liao Y, Yang J, Gao Y. Feasibility Analysis and Implementation of Head-Mounted Electrical Impedance Respiratory Monitoring. Biosensors. 2022; 12(11):934. https://doi.org/10.3390/bios12110934
Chicago/Turabian StyleYan, Hongli, Xudong Yang, Yanyan Liu, Wanting He, Yipeng Liao, Jiejie Yang, and Yueming Gao. 2022. "Feasibility Analysis and Implementation of Head-Mounted Electrical Impedance Respiratory Monitoring" Biosensors 12, no. 11: 934. https://doi.org/10.3390/bios12110934
APA StyleYan, H., Yang, X., Liu, Y., He, W., Liao, Y., Yang, J., & Gao, Y. (2022). Feasibility Analysis and Implementation of Head-Mounted Electrical Impedance Respiratory Monitoring. Biosensors, 12(11), 934. https://doi.org/10.3390/bios12110934





