What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications
Abstract
1. Introduction
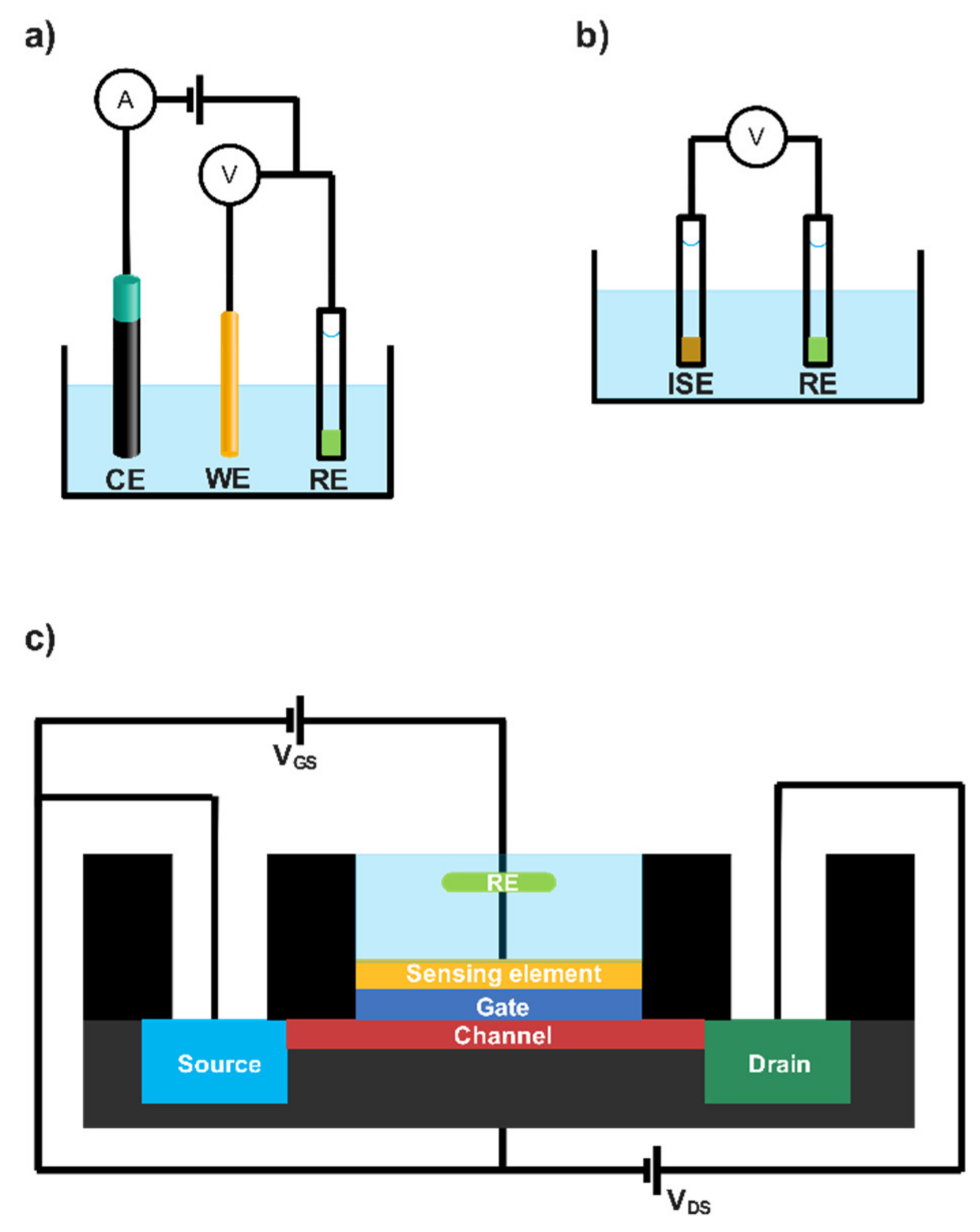
2. Electrochemical (Bio)Sensor Transduction Approaches
2.1. Amperometric Lactate Biosensors
2.2. Potentiometric and Conductometric Lactate Biosensors
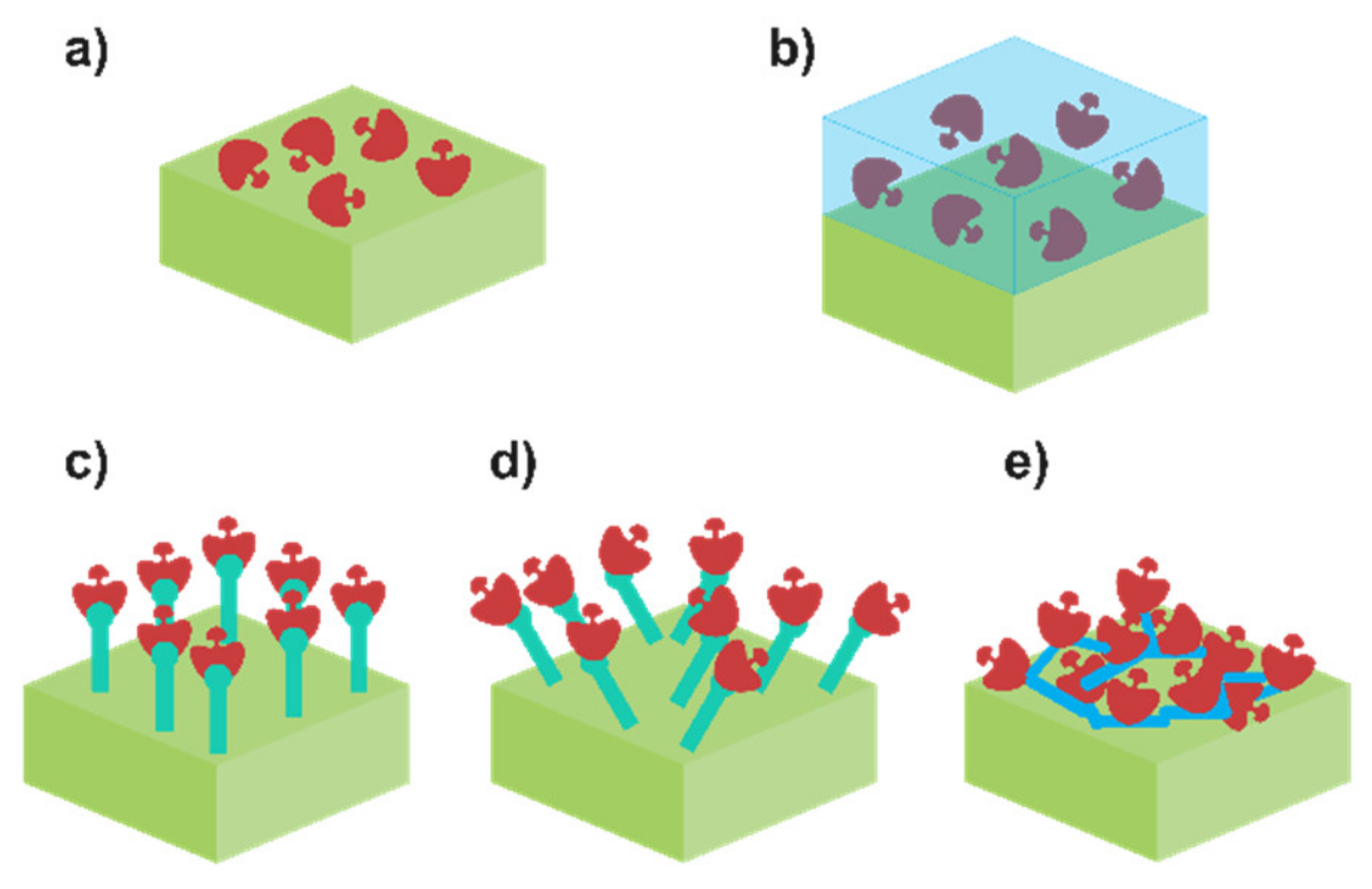
2.3. Enzyme Immobilisation Influence and Viable Approaches
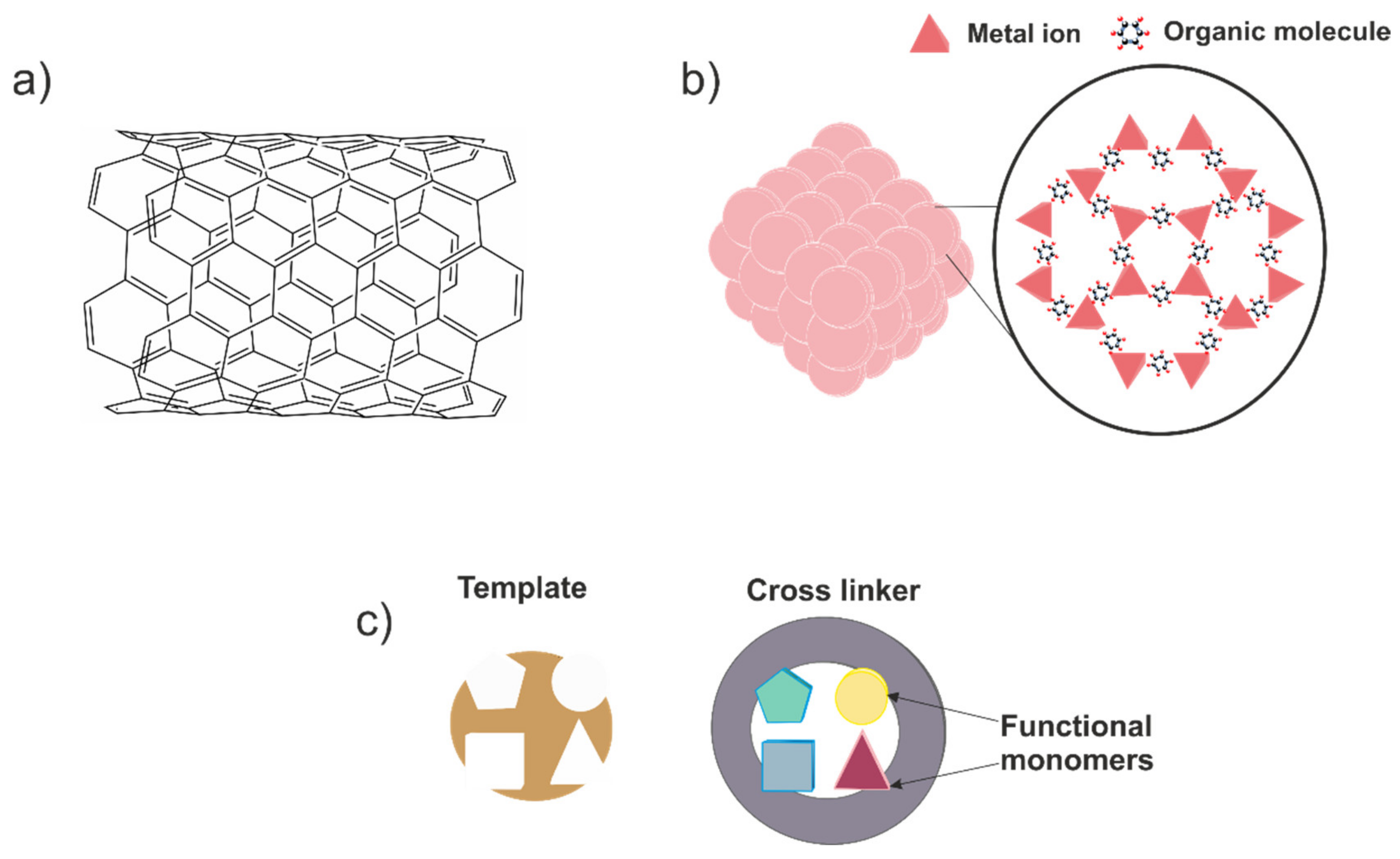
2.4. Non-Enzymatic Sensors
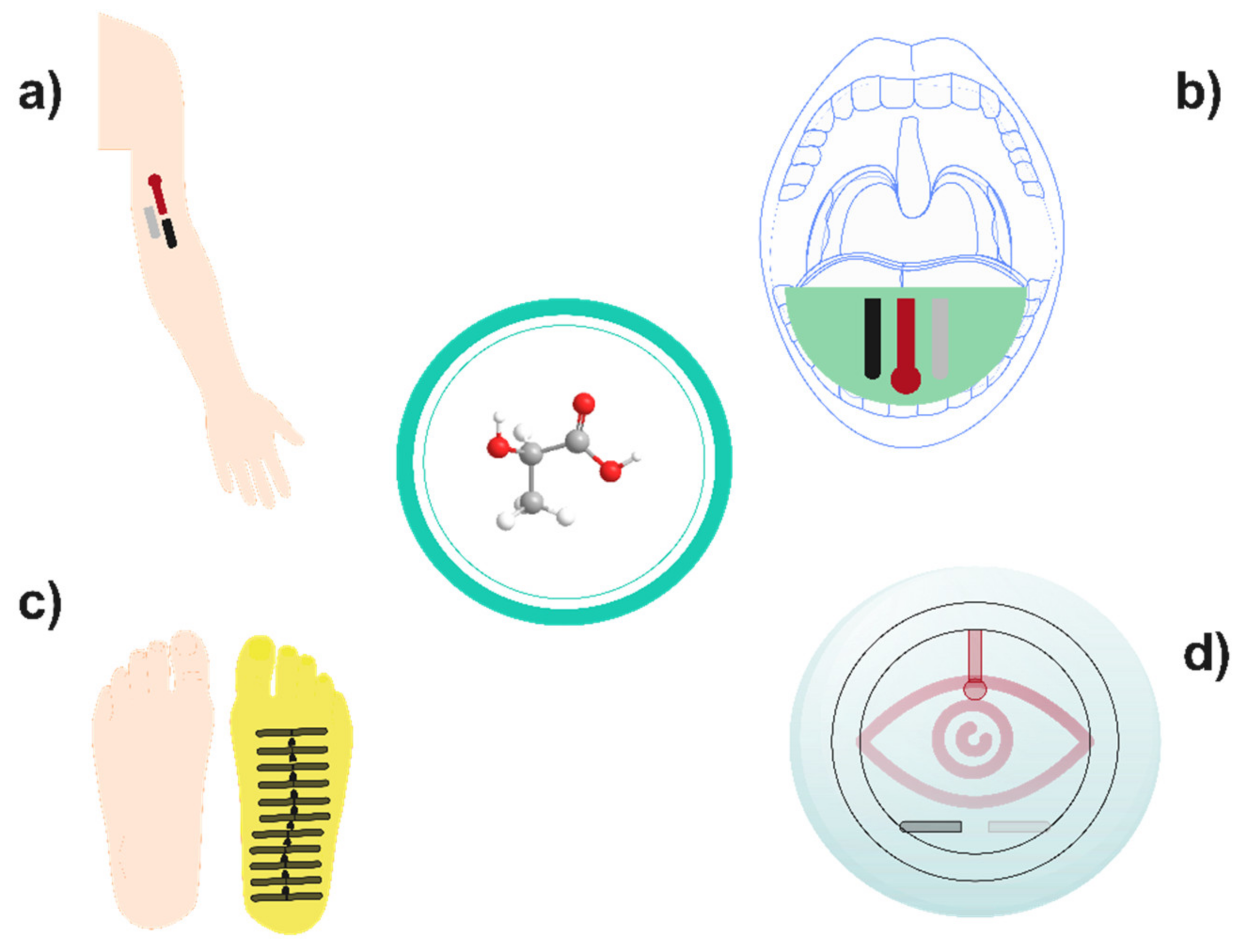
3. Implementation of Lactate Biosensors on Biomedicine: Real-Time Health Care
| Biosensor | Electrode | Immobilisation Process | LRR (μM) | Sensi. (µA/mM) | LOD (µM) | Tr (s) | Lifetime (Days) | Samp. | App. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Lactate Oxidase Biosensors | ||||||||||
| MWCNT/TTF/LOx/Chit | Carbon ink | Crosslinking | 1000–20,000 | 0.644 | - | - | 152 | Sweat | Physical exercise intensity monitoring | [80] |
| PB/LOx/Chit/AuNWs | AuNWs | - | 0–30,000 | 0.69 | 137 | 10 | 6 | Sweat | Physical exercise intensity monitoring | [104] |
| LOx/BSA/PEGDE/β-cysteamine/AuNNs/Au | Au | Crosslinking | 1000–25,000 | 0.65 | 54 | - | 28 | Sweat | - | [105] |
| LOx/PtNPs/GO/Au/SFNFs | Au | Entrapment | 400–6000 | - | - | - | - | Sweat | Physical exercise intensity monitoring | [82] |
| ETH 500-PVC- DOS/LOx/PB/SPE | Carbon ink | - | 1000–25,000 | 0.0094 | 110 | 50 | - | Sweat | Physical exercise intensity monitoring | [75] |
| LOx/PANHS/GO/Pd/Polyamide | Pd | Crosslinking | 1000–100,000 | - | 1000 | - | - | Sweat | - | [89] |
| Nafion-LOx/PPy/MWCNT/PA6 | Pt | - | 0.001–1000 | - | - | 0.8 | - | Sweat | Physical exercise intensity monitoring | [90] |
| PDDA/LOx/ZnO/MWCNT/PG | PG | Adsorption | 200–2000 | 7.3 | 6 | 6 | 120 | Serum | - | [106] |
| LOx/sol-gel/MWCNTs/GCE | GCE | Sol–gel | 200–2000 | 6.031 | 0.3 | 5 | 28 | Serum | - | [107] |
| PB-PPD-LOx-mouthguard | Prussian blue graphite ink | Electropolymeric entrapment | 100–1000 | 0.00055 | 50 | - | - | Saliva | Health and physical exercise monitoring | [84] |
| Pt/o-PD/PEG/BSA/Chit-LOX-Pt-Ceria-AO | Pt | Adsorption | 0.0001–15,500 | - | 0.0001 | 6 | 21 | Rat tissues | Monitoring in vitro and in vivo tissues during hypoxia conditions | [108] |
| LOx/cMWCNT/CuNPs/PANI/PGE | PGE | Covalent | 1–2500 | - | 0.25 | 5 | 140 | Plasma | Lactate acidosis diagnosis | [96] |
| Nafion/LOx-GO-Ch/PB/SPE | Graphite | - | 1000–50,000 | 0.072 | 0.02 | - | - | Buffer solution | - | [109] |
| Au/MoO3/LOx/Nafion | Au | - | 500–8000 | 0.87 | 150 | 10 | 16 | - | - | [110] |
| HRP-PEGDGE-Os/Chit-LOx/polyphenol | Graphite paste | Crosslinking | 100–1000 | 0.763 | 13 | - | 91 | Saliva | - | [111] |
| MWCNT/FcMe/Chit/HRP/BSA/LOx/SPBGE | SPCE | Entrapment | 30.4–243.9 | 3.42 | 22.6 | - | 150 | Embryonic cell culture | Growth evaluation of embryo | [19] |
| Lactate Dehydrogenase Biosensors | ||||||||||
| LDH/RGO-AuNPs/SPCE | SPCE | Entrapment | 10–5000 | 77 | 0.13 | 8 | 25 | Serum | Cancer biomarker detection | [112] |
| LDHNPs/Au | Au | Covalent binding | 0.01–55,000 | 10.83 | 0.01 | 2.5 | 210 | Serum | Cardiogenic shock diagnosis | [94] |
| LDH/GrONPs/PGE | PGE | Covalent binding | 5000–50,000 | - | 0.1 | 5 | 60 | Serum | Lactate acidosis diagnosis | [97] |
| AuNP-cysteamine-LDH/Nafion/MWE | W | - | 500–7000 | 2.45 | 411 | - | 18 | Serum | Lactate acidosis diagnosis | [91] |
| LDH-NAD+/Fe3O4NPs/MWCNTs/GCE | GCE | Covalent binding | 50–500 | 7.67 | 5 | - | 14 | Serum | - | [92] |
| LDH/MWCNTs/Chit/Au | Au | Covalent binding | 0–120 | - | 15 | 8 | 10 | Blood | Lactate acidosis diagnosis | [98] |
| LDH/MWCT-MB | CPE | Crosslinking | 100–10,000 | 0.42 | 7.5 | - | - | Blood | Physical exercise intensity monitoring | [113] |
| LDH/MG/SWNT/GCE | GCE | Crosslinking | 200–10,000 | 0.0256 | 160 | - | 8 | Rat cardiomyocyte cell culture | Monitoring of cardiomyocytes during hypoxia | [114] |
| LDH-NAD+/pTTABA/DPC | DPC | Covalent binding | 0.5–4000 | 0.02 | 0.112 | - | 60 | Extracellular matrix of cancer cells | Cancer diagnosis and antitumour activity evaluation | [95] |
| LDH-GPT/SPCE | SPCE | - | 100–1000 | 0.033 | 5 | 300 | - | Cell cultures, sweat | Growth evaluation of cells, physical exercise intensity monitoring | [115] |
| NADH/LDH/Nano-CeO2/GCE | GCE | Electrostatic interactions | 200–2000 | 571.19 | 50 | 4 | - | Buffer solution | - | [93] |
| Other Enzyme and Non-enzyme Sensors | ||||||||||
| FC b2/nAu-Au | Au | - | 300–2000 | 5.33 | - | - | 91 | Sweat, saliva | - | [116] |
| MIPs-AgNWs | Carbon | - | 1–100,000 | 0.0045 | 0.22 | - | 212 | Sweat | Physical exercise intensity monitoring | [60] |
| Cu2(NDC)2/PDHP | PDHP | - | 50–22,250 | 114 | 25 | 5 | - | Sweat | - | [117] |
| NH2-GP-Cu3(btc)2 | GP | - | 0.05–22.6 | - | 5 | - | - | Sweat | - | [57] |
| SPCE-NiCo (layered double hydroxide) | SPCE | - | 2–26 | 4.70 | 400 | - | 28 | Sweat | Physical exercise intensity monitoring | [58] |
| Commercial Biosensors (Lactate Oxidase-Based) | ||||||||||
| StatStrip® Lactate | - | - | 300–20,000 | - | - | 13 | 91 | Blood (0.6 µL) | Medical monitoring | [118] |
| StatStrip Xpress® Lactate | - | - | 300–20,000 | - | - | 13 | 91 | Blood (0.6 µL) | Medical monitoring | [118] |
| Lactate Plus Version 2 | - | - | 300–25,000 | - | - | 13 | - | Blood (0.6 µL) | Physical performance monitoring | [119] |
| LactatEDGE | - | - | 700–22,000 | - | - | 45 | 91 | Blood (0.3 µL) | Physical performance monitoring | [120] |
| Lactate Pro 2 (LT-1730) | - | - | 500–25,000 | - | - | 15 | - | Blood (0.3 µL) | Physical performance monitoring | [121] |
| Lactate Scout 4 | - | - | 500–25,000 | - | - | 10 | Blood (0.2 µL) | Physical performance monitoring | [122] | |
| Biosen C-Line (glucose and lactate) | - | - | 500–40,000 | - | - | 20–45 | 50 | Blood, plasma or serum (20 µL) | Medical monitoring | [123] |
4. Rise of Lactate Biosensors on the Food Industries: Proficient Quality Control
| Biosensor | Electrode | Immobilisation Process | LRR (μM) | Sens. (µA/mM) | LOD (µM) | Tr (s) | Lifetime (Days) | Samp. | App. | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| LOx/3,4DHS–AuNP/SPCE | SPCE | - | 2.6–800 | 5.1 | 2.6 | - | 30 | White wine, yoghurt, beer | Quality evaluation | [135] |
| laponite/Chit/LOx/GCE | GCE | - | 10–700 | 11.41 | 3.8 | 4 | 30 | White wine, beer, fermented milk | - | [134] |
| LOx-PVC-NH2-Quinhydrone- Graphite | Graphite | - | 50–10,000 | - | 20 | 10 | - | Buttermilk, pickle Juice | - | [136] |
| LOx-Pt&Pd-Nafion-carbon | Carbon | - | 50–800 | - | 0.1 | 5 | 28 | Wine | Quality evaluation | [124] |
| LOx–Cu-MOF/Chit/Pt/SPCE | SPCE | Crosslinking | 0.75–1000 | 14.65 | 0.75 | - | 50 | Red wine, white wine | Control of malolactic fermentation | [133] |
| PtNPs/GCNF–PEI–GA–LOx–Gly–SPCE | SPCE | Covalent | 10–2000 | 0.025 | 6.9 | - | 547 | Wine, ciders | Analysis of lactic acid | [135] |
| LODP/HRP-AuIDE | AuIDE | - | 0.05–210 | - | 0.05 | - | 35 | Yogurt | - | [133] |
| GOx-LOx-BSA-GA-Au | Au | Covalent | 5–1000 | 0.75 | - | 10 | 85 | Red wine, white wine | Quality evaluation | [134] |
| N-eicosane-MWCN-LOx-HRP-GPE | GPE | - | 5–244 | 3.47 | 0.96 | 65 | 456 | Beverages, wines, sauces | - | [136] |
| DLDH/DP-TTF-Au | Au | Entrapment | 11–42 | 4.095 | 0.34 | - | 9 | Beer | Simultaneous determination of lactate enantiomers | [125] |
| GA-LDH/AuNPs-ERGO-PAH/SPE | SPE | Crosslinking | 500–3000 | - | 1 | - | 49 | Yoghurt, wine | Quality evaluation | [41] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2021, 10, 1229–1251. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Barsan, M.M.; Korpan, Y.; Brett, C.M.A. L-lactate selective impedimetric bienzymatic biosensor based on lactate dehydrogenase and pyruvate oxidase. Electrochim. Acta 2017, 231, 209–215. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Loaiza, O.A.; Añorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabañero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Tsafrakidou, P.; Sameli, N.; Bosnea, L.; Chorianopoulos, N.; Samelis, J. Assessment of the spoilage microbiota in minced free-range chicken meat during storage at 4 C in retail modified atmosphere packages. Food Microbiol. 2021, 99, 103822. [Google Scholar] [CrossRef] [PubMed]
- Rassaei, L.; Olthuis, W.; Tsujimura, S.; Sudhölter, E.J.R.; Van Den Berg, A. Lactate biosensors: Current status and outlook. Anal. Bioanal. Chem. 2014, 406, 123–137. [Google Scholar] [CrossRef]
- Amin, S.; Tahira, A.; Solangi, A.; Mazzaro, R.; Ibupoto, Z.H.; Vomiero, A. A sensitive enzyme-free lactic acid sensor based on NiO nanoparticles for practical applications. Anal. Methods 2019, 11, 3578–3583. [Google Scholar] [CrossRef]
- Fuernau, G. Lactate and other biomarkers as treatment target in cardiogenic shock. Curr. Opin. Crit. Care 2019, 25, 403–409. [Google Scholar] [CrossRef]
- Seheult, J.; Fitzpatrick, G.; Boran, G. Lactic acidosis: An update. Clin. Chem. Lab. Med. 2017, 55, 322–333. [Google Scholar] [CrossRef]
- Levitt, D.G.; Levitt, J.E.; Levitt, M.D. Quantitative Assessment of Blood Lactate in Shock: Measure of Hypoxia or Beneficial Energy Source. BioMed Res. Int. 2020, 2020, 2608318. [Google Scholar] [CrossRef]
- Ebner, M.; Pagel, C.F.; Sentler, C.; Harjola, V.; Lerchbaumer, M.H.; Stangl, K.; Pieske, B.; Hasenfu, G.; Konstantinides, S.V.; Lankeit, M. Venous lactate improves the prediction of in-hospital adverse outcomes in normotensive pulmonary embolism. Eur. J. Intern. Med. 2021, 86, 25–31. [Google Scholar] [CrossRef]
- Sauer, C.M.; Gómez, J.; Botella, M.R.; Ziehr, D.R.; Oldham, W.M.; Gavidia, G.; Rodríguez, A.; Elbers, P.; Girbes, A.; Bodi, M.; et al. Understanding critically ill sepsis patients with normal serum lactate levels: Results from U.S. and European ICU cohorts. Sci. Rep. 2021, 11, 20076. [Google Scholar] [CrossRef] [PubMed]
- Nasu, T.; Ueda, K.; Kawashima, S.; Okishio, Y.; Kunitatsu, K. Prediction of early acute kidney injury after trauma using prehospital systolic blood pressure and lactate levels: A prospective validation study. Injury 2022, 53, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takayama, W.; Murata, K.; Otomo, Y. The impact of lactate clearance on outcomes according to infection sites in patients with sepsis: A retrospective observational study. Sci. Rep. 2021, 11, 22394. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Lahoz, A. Tumor Microenvironment-Derived Metabolites: A Guide to Find New Metabolic Therapeutic Targets and Biomarkers. Cancers 2021, 13, 3230. [Google Scholar] [CrossRef]
- Powell, C.L.; Davidson, A.R.; Brown, A.M. Universal Glia to Neurone Lactate Transfer in the Nervous System: Physiological Functions and Pathological Consequences. Biosensors 2020, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Naylor, E.; Aillon, D.V.; Barrett, B.S.; Wilson, G.S.; Johnson, D.A.; Johnson, D.A.; Harmon, H.P.; Gabbert, S.; Petillo, P.A. Lactate as a biomarker for sleep. Sleep 2012, 35, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H.; Cummins, G.; Kremer, J.; Bernassau, A.; Brown, A.; Bridle, H.L.; Schulze, H.; Bachmann, T.T.; et al. Sensors for fetal hypoxia and metabolic acidosis: A review. Sensors 2018, 186, 2648. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef]
- Barker, S.B.; Summerson, W.H. the Colorimetric Determination of Lactic Acid in Biological Material. J. Biol. Chem. 1941, 138, 535–554. [Google Scholar] [CrossRef]
- Alhusban, A.A.; Albustanji, S.; Hamadneh, L.A.; Shallan, A.I. High performance liquid chromatography–tandem mass spectrometry method for correlating the metabolic changes of lactate, pyruvate and L-Glutamine with induced tamoxifen resistant MCF-7 cell line potential molecular changes. Molecules 2021, 26, 4824. [Google Scholar] [CrossRef] [PubMed]
- Briones, M.; Busó-Rogero, C.; Catalán-Gómez, S.; García-Mendiola, T.; Pariente, F.; Redondo-Cubero, A.; Lorenzo, M.E. ZnO nanowire-based fluorometric enzymatic assays for lactate and cholesterol. Microchim. Acta 2020, 187, 180. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Guardigli, M.; Calabria, D.; Calabretta, M.; Cevenini, L.; Michelini, E. A 3D-printed device for a smartphone-based chemiluminescence biosensor for lactate in oral fluid and sweat. Analyst 2014, 139, 6494–6501. [Google Scholar] [CrossRef] [PubMed]
- Goh, J.H.; Mason, A.; Al-Shamma’a, A.I.; Field, M.; Shackcloth, M.; Browning, P. Non invasive microwave sensor for the detection of lactic acid in cerebrospinal fluid (CSF). J. Phys. Conf. Ser. 2011, 307, 012017. [Google Scholar] [CrossRef]
- Sartain, F.K.; Yang, X.; Lowe, C.R. Holographic lactate sensor. Anal. Chem. 2006, 78, 5664–5670. [Google Scholar] [CrossRef]
- Hvinden, I.C.; Berg, H.E.; Sachse, D.; Skaga, E.; Skottvoll, F.S.; Lundanes, E.; Sandberg, C.J.; Vik-mo, E.O.; Rise, F.; Wilson, S.R. Nuclear Magnetic Resonance Spectroscopy to Identify Metabolite Biomarkers of Nonresponsiveness to Targeted Therapy in Glioblastoma Tumor Stem Cells. J. Proteome Res. 2020, 18, 2012–2020. [Google Scholar] [CrossRef]
- Gajovic, N.; Binyamin, G.; Warsinke, A.; Scheller, F.W.; Heller, A. Operation of a miniature redox hydrogel-based pyruvate sensor in undiluted deoxygenated calf serum. Anal. Chem. 2000, 72, 2963–2968. [Google Scholar] [CrossRef]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Pinheiro, K.M.P.; Duarte, L.M.; Duarte-Junior, G.F.; Coltro, W.K.T. Chip-based separation of organic and inorganic anions and multivariate analysis of wines according to grape varieties. Talanta 2021, 231, 122381. [Google Scholar] [CrossRef]
- Teipel, J.C.; Hausler, T.; Sommerfeld, K.; Scharinger, A.; Walch, S.G.; Lachenmeier, D.W.; Kuballa, T. Application of 1H nuclear magnetic resonance spectroscopy as spirit drinks screener for quality and authenticity control. Foods 2020, 9, 1355. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors 2020, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Milagres, M.P.; Brandão, S.C.C.; Magalhães, M.A.; Minim, V.P.R.; Minim, L.A. Development and validation of the high performance liquid chromatography-ion exclusion method for detection of lactic acid in milk. Food Chem. 2012, 135, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Hird, S.J.; Lau, B.P.Y.; Schuhmacher, R.; Krska, R. Liquid chromatography-mass spectrometry for the determination of chemical contaminants in food. TrAC Trends Anal. Chem. 2014, 59, 59–72. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the biosensors for lactate and pyruvate detection for medical applications: A review. TrAC Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Nonenzymatic sensor for lactate detection in human sweat. Anal. Chem. 2017, 89, 11198–11202. [Google Scholar] [CrossRef]
- Md Shakhih, M.F.; Rosslan, A.S.; Noor, A.M.; Ramanathan, S.; Lazim, A.M.; Wahab, A.A. Enzymatic and non-enzymatic electrochemical sensor for lactate detection in human biofluids. J. Electrochem. Soc. 2021, 168, 067502. [Google Scholar] [CrossRef]
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate detection sensors for food, clinical and biological applications: A review. Environ. Chem. Lett. 2021, 19, 1135–1152. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Jang, M.; Park, H.S.; Lee, J.Y. One-Pot electrochemical fabrication of high performance amperometric enzymatic biosensors using polypyrrole and polydopamine. J. Ind. Eng. Chem. 2021, 97, 316–325. [Google Scholar] [CrossRef]
- Booth, M.A.; Gowers, S.A.N.; Hersey, M.; Samper, I.C.; Park, S.; Anikeeva, P.; Hashemi, P.; Stevens, M.M.; Boutelle, M.G. Fiber-Based Electrochemical Biosensors for Monitoring pH and Transient Neurometabolic Lactate. Anal. Chem. 2021, 93, 6646–6655. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully printed wearable microfluidic devices for high-throughput sweat sampling and multiplexed electrochemical analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef]
- Istrate, O.M.; Rotariu, L.; Bala, C. Amperometric L-Lactate biosensor based upon a gold nanoparticles/reduced graphene oxide/polyallylamine hydrochloride modified screen-printed graphite electrode. Chemosensors 2021, 9, 74. [Google Scholar] [CrossRef]
- Hiraka, K.; Kojima, K.; Tsugawa, W.; Asano, R.; Ikebukuro, K.; Sode, K. Rational engineering of Aerococcus viridans L-lactate oxidase for the mediator modification to achieve quasi-direct electron transfer type lactate sensor. Biosens. Bioelectron. 2020, 151, 111974. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Topolnikova, Y.V.; Dzyadevych, S.V.; Soldatkin, A.P. Novel multiplexed biosensor system for the determination of lactate and pyruvate in blood serum. Electroanalysis 2019, 31, 1625–1631. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Sugiura, M.; Kamo, N.; Shinbo, T. Potentiometric enzyme electrode for lactate. Anal. Chem. 1979, 51, 100–104. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Shah, S.M.U.A.; Khun, K.; Willander, M. Electrochemical L-lactic acid sensor based on immobilized ZnO nanorods with lactate oxidase. Sensors 2012, 12, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Lupu, A.; Valsesia, A.; Bretagnol, F.; Colpo, P.; Rossi, F. Development of a potentiometric biosensor based on nanostructured surface for lactate determination. Sens. Actuators B Chem. 2007, 127, 606–612. [Google Scholar] [CrossRef]
- Schuck, A.; Kim, H.E.; Moreira, J.K.; Lora, P.S.; Kim, Y.S. A graphene-based enzymatic biosensor using a common-gate field-effect transistor for l-lactic acid detection in blood plasma samples. Sensors 2021, 21, 1852. [Google Scholar] [CrossRef] [PubMed]
- Minamiki, T.; Tokito, S.; Minami, T. Fabrication of a flexible biosensor based on an organic field-effect transistor for lactate detection. Anal. Sci. 2019, 35, 103–106. [Google Scholar] [CrossRef]
- Kim, S.; Yang, W.S.; Kim, H.J.; Lee, H.N.; Park, T.J.; Seo, S.J.; Park, Y.M. Highly sensitive non-enzymatic lactate biosensor driven by porous nanostructured nickel oxide. Ceram. Int. 2019, 45, 23370–23376. [Google Scholar] [CrossRef]
- Chang, A.S.; Memon, N.N.; Amin, S.; Chang, F.; Aftab, U.; Abro, M.I.; Dad Chandio, A.; Shah, A.A.; Ibupoto, M.H.; Ansari, M.A.; et al. Facile non-enzymatic lactic acid sensor based on cobalt oxide nanostructures. Electroanalysis 2019, 31, 1296–1303. [Google Scholar] [CrossRef]
- Hussain, M.M.; Hussain, M.M.; Hussain, M.M.; Asiri, A.M.; Asiri, A.M.; Rahman, M.M.; Rahman, M.M.; Hussain, M.M. A non-enzymatic electrochemical approach for l-lactic acid sensor development based on CuO·MWCNT nanocomposites modified with a Nafion matrix. New J. Chem. 2020, 44, 9775–9787. [Google Scholar] [CrossRef]
- Choi, Y.M.; Lim, H.; Lee, H.N.; Park, Y.M.; Park, J.S.; Kim, H.J. Selective nonenzymatic amperometric detection of lactic acid in human sweat utilizing a multi-aalled carbon nanotube (MWCNT)-polypyrrole core-shell nanowire. Biosensors 2020, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Sedenho, G.C.; Lee, P.T.; Toh, H.S.; Salter, C.; Johnston, C.; Stradiotto, N.R.; Compton, R.G. Nanoelectrocatalytic oxidation of lactic acid using nickel nanoparticles. J. Phys. Chem. C 2015, 119, 6896–6905. [Google Scholar] [CrossRef]
- 5Wu, Y.T.; Tsao, P.K.; Chen, K.J.; Lin, Y.C.; Aulia, S.; Chang, L.Y.; Ho, K.C.; Chang, C.Y.; Mizuguchi, H.; Yeh, M.H. Designing bimetallic Ni-based layered double hydroxides for enzyme-free electrochemical lactate biosensors. Sens. Actuators B Chem. 2021, 346, 130505. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Maduraiveeran, G. Ultra-fine nickel sulfide nanoclusters @ nickel sulfide microsphere as enzyme-free electrode materials for sensitive detection of lactic acid. J. Electroanal. Chem. 2020, 874, 114465. [Google Scholar] [CrossRef]
- Wang, Z.; Gui, M.; Asif, M.; Yu, Y.; Dong, S.; Wang, H.; Wang, W.; Wang, F.; Xiao, F.; Liu, H. A facile modular approach to the 2D oriented assembly MOF electrode for non-enzymatic sweat biosensors. Nanoscale 2018, 10, 6629–6638. [Google Scholar] [CrossRef]
- Wang, Y.X.; Tsao, P.K.; Rinawati, M.; Chen, K.J.; Chen, K.Y.; Chang, C.Y.; Yeh, M.H. Designing ZIF-67 derived NiCo layered double hydroxides with 3D hierarchical structure for Enzyme-free electrochemical lactate monitoring in human sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar] [CrossRef]
- Pereira, T.C.; Stradiotto, N.R. Electrochemical sensing of lactate by using an electrode modified with molecularly imprinted polymers, reduced graphene oxide and gold nanoparticles. Microchim. Acta 2019, 186, 764. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, D.; Xu, C.; Ge, Y.; Liu, X.; Wei, Q.; Huang, L.; Ren, X.; Wang, C.; Wang, Y. Wearable electrochemical biosensor based on molecularly imprinted Ag nanowires for noninvasive monitoring lactate in human sweat. Sens. Actuators B Chem. 2020, 320, 128325. [Google Scholar] [CrossRef]
- Heo, S.G.; Yang, W.S.; Kim, S.; Park, Y.M.; Park, K.T.; Oh, S.J.; Seo, S.J. Synthesis, characterization and non-enzymatic lactate sensing performance investigation of mesoporous copper oxide (CuO) using inverse micelle method. Appl. Surf. Sci. 2021, 555, 149638. [Google Scholar] [CrossRef]
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55. [Google Scholar] [CrossRef]
- Burmeister, J.J.; Palmer, M.; Gerhardt, G.A. L-lactate measures in brain tissue with ceramic-based multisite microelectrodes. Biosens. Bioelectron. 2005, 20, 1772–1779. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, S.; Omidi, Y.; Rafii-Tabar, H. Amperometric lactate nanobiosensor based on reduced graphene oxide, carbon nanotube and gold nanoparticle nanocomposite. Microchim. Acta 2019, 186, 680. [Google Scholar] [CrossRef]
- Halliwell, C.M.; Simon, E.; Toh, C.S.; Bartlett, P.N.; Cass, A.E.G. Immobilisation of lactate dehydrogenase on poly(aniline)-poly(acrylate) and poly(aniline)-poly-(vinyl sulphonate) films for use in a lactate biosensor. Anal. Chim. Acta 2002, 453, 191–200. [Google Scholar] [CrossRef]
- Pereira, A.C.; Kisner, A.; Tarley, C.R.T.; Kubota, L.T. Development of a carbon paste electrode for lactate detection based on Meldola’s Blue adsorbed on silica gel modified with niobium oxide and lactate oxidase. Electroanalysis 2011, 23, 1470–1477. [Google Scholar] [CrossRef]
- Payne, M.E.; Zamarayeva, A.; Pister, V.I.; Yamamoto, N.A.D. Printed, flexible lactate sensors: Design considerations before performing on-body measurements. Sci. Rep. 2019, 9, 13720. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, K.; Yu, P.; Xiang, L.; Li, X.; Mao, L. A facile electrochemical method for simultaneous and on-line measurements of glucose and lactate in brain microdialysate with prussian blue as the electrocatalyst for reduction of hydrogen peroxide. Anal. Chem. 2007, 79, 9577–9583. [Google Scholar] [CrossRef]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @Prussian Blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio) sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Bakker, E.; Pretsch, E. Modern potentiometry. Angew. Chem. Int. Ed. 2007, 46, 5660–5668. [Google Scholar] [CrossRef] [PubMed]
- Shkotova, L.V.; Goriushkina, T.B.; Tran-Minh, C.; Chovelon, J.M.; Soldatkin, A.P.; Dzyadevych, S.V. Amperometric biosensor for lactate analysis in wine and must during fermentation. Mater. Sci. Eng. C 2008, 28, 943–948. [Google Scholar] [CrossRef]
- Parra, A.; Casero, E.; Vázquez, L.; Pariente, F.; Lorenzo, E. Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal. Chim. Acta 2006, 555, 308–315. [Google Scholar] [CrossRef]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Chen, C.; Cuartero, M.; Crespo, G.A.; Xuan, X.; Clara, P. Lactate biosensing for reliable on-body sweat analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef]
- Adil, K.; Belmabkhout, Y.; Pillai, R.S.; Cadiau, A.; Bhatt, P.M.; Assen, A.H.; Maurin, G.; Eddaoudi, M. Gas/vapour separation using ultra-microporous metal-organic frameworks: Insights into the structure/separation relationship. Chem. Soc. Rev. 2017, 46, 3402–3430. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137. [Google Scholar] [CrossRef]
- Lamaoui, A.; Palacios-Santander, J.M.; Amine, A.; Cubillana-Aguilera, L. Molecularly imprinted polymers based on polydopamine: Assessment of non-specific adsorption. Microchem. J. 2021, 164, 106043. [Google Scholar] [CrossRef]
- Bleiberg, B.; Steinberg, J.J.; Katz, S.D.; Wexler, J.; LeJemtel, T. Determination of plasma lactic acid concentration and specific activity using high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1991, 568, 301–308. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramírez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real-time noninvasive lactate monitoring in human perspiration. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef]
- Yeo, W.H.; Kim, Y.S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional epidermal electronics printed directly onto the skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Julian, R.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, P.W.; Tsao, Y.H.; Lin, Z.H. Utilization of self-powered electrochemical systems: Metallic nanoparticle synthesis and lactate detection. Nano Energy 2017, 42, 241–248. [Google Scholar] [CrossRef]
- Jeerapan, I.; Sempionatto, J.R.; Pavinatto, A.; You, J.M.; Wang, J. Stretchable biofuel cells as wearable textile-based self-powered sensors. J. Mater. Chem. A 2016, 4, 18342–18353. [Google Scholar] [CrossRef]
- Thomas, N.; Lähdesmäki, I.; Parviz, B.A. A contact lens with an integrated lactate sensor. Sens. Actuators B Chem. 2012, 162, 128–134. [Google Scholar] [CrossRef]
- Green, J.M.; Pritchett, R.C.; Crews, T.R.; McLester, J.R.; Tucker, D.C. Sweat lactate response between males with high and low aerobic fitness. Eur. J. Appl. Physiol. 2004, 91, 1–6. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, W.; Lin, Z.; Meng, Z.; Shi, C.; Xu, Z.; Yang, L.; Liu, X.Y. Coupling of silk fibroin nanofibrils enzymatic membrane with ultra-thin PtNPs/graphene film to acquire long and stable on-skin sweat glucose and lactate sensing. Small Methods 2021, 5, 2000926. [Google Scholar] [CrossRef]
- Santos, R.V.T.; Almeida, A.L.R.; Caperuto, E.C.; Martins, E.; Costa Rosa, L.F.B.P. Effects of a 30-km race upon salivary lactate correlation with blood lactate. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 145, 114–117. [Google Scholar] [CrossRef]
- Sakharov, D.A.; Shkurnikov, M.U.; Vagin, M.Y.; Yashina, E.I.; Karyakin, A.A.; Tonevitsky, A.G. Relationship between lactate concentrations in active muscle sweat and whole blood. Bull. Exp. Biol. Med. 2010, 150, 83–85. [Google Scholar] [CrossRef]
- Lin, K.C.; Muthukumar, S.; Prasad, S. Flex-GO (Flexible graphene oxide) sensor for electrochemical monitoring lactate in low-volume passive perspired human sweat. Talanta 2020, 214, 120810. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Qing, X.; Wang, Y.; Zhong, W.; Wang, W.; Chen, Y.; Liu, Q.; Li, M.; Wang, D. Fiber organic electrochemical transistors based on multi-walled carbon nanotube and polypyrrole composites for noninvasive lactate sensing. Anal. Bioanal. Chem. 2020, 412, 7515–7524. [Google Scholar] [CrossRef] [PubMed]
- Shankara Narayanan, J.; Slaughter, G. Lactic acid biosensor based on lactate dehydrogenase immobilized on Au nanoparticle modified microwire electrode. IEEE Sens. J. 2020, 20, 4034–4040. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Hallaj, R. Low potential detection of NADH based on Fe3O4 nanoparticles/multiwalled carbon nanotubes composite: Fabrication of integrated dehydrogenase-based lactate biosensor. Biosens. Bioelectron. 2012, 33, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J. Colloid Interface Sci. 2013, 410, 158–164. [Google Scholar] [CrossRef]
- Narwal, V.; Sharma, M.; Rani, S.; Pundir, C.S. An ultrasensitive amperometric determination of lactate by lactate dehydrogenase nanoparticles immobilized onto Au electrode. Int. J. Biol. Macromol. 2018, 115, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.K.; Gurudatt, N.G.; Akthar, M.H.; Seo, K.D.; Park, D.S.; Shim, Y.B. Nano-biosensor for the in vitro lactate detection using bi-functionalized conducting polymer/N, S-doped carbon; the effect of αCHC inhibitor on lactate level in cancer cell lines. Biosens. Bioelectron. 2020, 155, 112094. [Google Scholar] [CrossRef] [PubMed]
- Dagar, K.; Pundir, C.S. An improved amperometric L-lactate biosensor based on covalent immobilization of microbial lactate oxidase onto carboxylated multiwalled carbon nanotubes/copper nanoparticles/polyaniline modified pencil graphite electrode. Enzyme Microb. Technol. 2017, 96, 177–186. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Pundir, C.S. An amperometric lactate biosensor based on lactate dehydrogenase immobilized onto graphene oxide nanoparticles-modified pencil graphite electrode. Eng. Life Sci. 2016, 16, 786–794. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, R.; Singh, S.; Dhull, V. Fabrication of biosensor for determination of L-lactate using elite nanomaterials based LDH-CMWCNT-MB/Chitosan/SWCNT-Au electrode. Anal. Bioanal. Electrochem. 2018, 10, 1031–1052. [Google Scholar]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef]
- Yu, M.; Li, Y.T.; Hu, Y.; Tang, L.; Yang, F.; Lv, W.L.; Zhang, Z.Y.; Zhang, G.J. Gold nanostructure-programmed flexible electrochemical biosensor for detection of glucose and lactate in sweat. J. Electroanal. Chem. 2021, 882, 115029. [Google Scholar] [CrossRef]
- Wang, Y.T.; Bao, Y.J.; Lou, L.; Li, J.J.; Du, W.J.; Zhu, Z.Q.; Peng, H.; Zhu, J.Z. A novel L-lactate sensor based on enzyme electrode modified with ZnO nanoparticles and multiwall carbon nanotubes. Proc. IEEE Sens. 2010, 2010, 33–37. [Google Scholar] [CrossRef]
- Huang, J.; Song, Z.; Li, J.; Yang, Y.; Shi, H.; Wu, B.; Anzai, J.; Osa, T.; Chen, Q. A highly-sensitive l-lactate biosensor based on sol-gel film combined with multi-walled carbon nanotubes (MWCNTs) modified electrode. Mater. Sci. Eng. C 2007, 27, 29–34. [Google Scholar] [CrossRef]
- Sardesai, N.P.; Ganesana, M.; Karimi, A.; Leiter, J.C.; Andreescu, S. Platinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxia. Anal. Chem. 2015, 87, 2996–3003. [Google Scholar] [CrossRef] [PubMed]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Yang, H.W.; Cherevko, S.; Chung, C.H.; Kang, D.J. α-MoO 3 nanowire-based amperometric biosensor for L-lactate detection. J. Solid State Electrochem. 2012, 16, 2197–2201. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fu, C. Os-complex-based amperometric bienzyme biosensor for continuous determination of lactate in saliva. Anal. Methods 2015, 7, 6158–6164. [Google Scholar] [CrossRef]
- Azzouzi, S.; Rotariu, L.; Benito, A.M.; Maser, W.K.; Ben Ali, M.; Bala, C. A novel amperometric biosensor based on gold nanoparticles anchored on reduced graphene oxide for sensitive detection of l-lactate tumor biomarker. Biosens. Bioelectron. 2015, 69, 280–286. [Google Scholar] [CrossRef]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.V.; Kubota, L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Chen, Z.; Lin, Y.; Yu, P.; Mao, L. Continuous electrochemical monitoring of extracellular lactate production from neonatal rat cardiomyocytes following myocardial hypoxia. Anal. Chem. 2012, 84, 5285–5291. [Google Scholar] [CrossRef]
- Rosati, G.; Gherardi, G.; Grigoletto, D.; Marcolin, G.; Cancellara, P.; Mammucari, C.; Scaramuzza, M.; De Toni, A.; Reggiani, C.; Rizzuto, R.; et al. Lactate dehydrogenase and glutamate pyruvate transaminase biosensing strategies for lactate detection on screen-printed sensors. Catalysis efficiency and interference analysis in complex matrices: From cell cultures to sport medicine. Sens. Bio-Sens. Res. 2018, 21, 54–64. [Google Scholar] [CrossRef]
- Smutok, O.; Karkovska, M.; Serkiz, R.; Vus, B.; Čenas, N.; Gonchar, M. A novel mediatorless biosensor based on flavocytochrome b2 immobilized onto gold nanoclusters for non-invasive L-lactate analysis of human liquids. Sens. Actuators B Chem. 2017, 250, 469–475. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Yu, Y.; Asif, M.; Xu, N.; Xiao, F.; Liu, H. Coffee ring–inspired approach toward oriented self-assembly of biomimetic Murray MOFs as sweat biosensor. Small 2018, 14, 1802670. [Google Scholar] [CrossRef] [PubMed]
- StatStrip®® Lactate and StatStrip Xpress®® Lactate Systems. Available online: https://novabiomedical.com/statstrip-lactate/index.php (accessed on 13 January 2022).
- Lactate Plus: Blood Lactate Measuring Meter-Version 2. Available online: https://novabiomedical.com/lactateplusmeterstore/lactate-plus-meter-4/lactate-plus-meter-10.html (accessed on 13 August 2022).
- Portable Lactate Acid Analyzer LactatEDGE. Available online: https://lactatedge.com (accessed on 13 August 2022).
- Simplified Blood Lactate Test Meter Lactate Pro2 LT-1730. Available online: https://www.arkray.co.jp/english/products/others/blood_lactate/lt-1730.html (accessed on 13 August 2022).
- Lactate Scout 4 Lactate Analyzer for Athletes. Available online: https://www.ekfdiagnostics.com/lactate-scout.html (accessed on 13 August 2022).
- Biosen C-Line Glucose and Lactate Analyzer. Available online: https://www.ekfdiagnostics.com/biosen-analyzer.html (accessed on 13 August 2022).
- Shkotova, L.; Bohush, A.; Voloshina, I.; Smutok, O.; Dzyadevych, S. Amperometric biosensor modified with platinum and palladium nanoparticles for detection of lactate concentrations in wine. SN Appl. Sci. 2019, 1, 306. [Google Scholar] [CrossRef]
- Vargas, E.; Ruiz, M.A.; Campuzano, S.; De Rivera, G.G.; López-Colino, F.; Reviejo, A.J.; Pingarrón, J.M. Implementation of a new integrated d-lactic acid biosensor in a semiautomatic FIA system for the simultaneous determination of lactic acid enantiomers. Application to the analysis of beer samples. Talanta 2016, 152, 147–154. [Google Scholar] [CrossRef]
- Alvarez-Crespo, S.L.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Amperometric glutamate biosensor based on poly(o-phenylenediamine) film electrogenerated onto modified carbon paste electrodes. Biosens. Bioelectron. 1997, 12, 739–747. [Google Scholar] [CrossRef]
- Skladal, P.; Mascini, M.; Salvadori, C.; Zannoni, G. Detection of bacterial contamination in sterile UHT milk using an l-lactate biosensor. Enzyme Microb. Technol. 1993, 15, 508–512. [Google Scholar] [CrossRef]
- Przybyt, M.; Iciek, J.; Papiewska, A.; Biernasiak, J. Application of biosensors in early detection of contamination with lactic acid bacteria during apple juice and concentrate production. J. Food Eng. 2010, 99, 485–490. [Google Scholar] [CrossRef]
- Sionek, B.; Przybylski, W.; Tambor, K. Biosensors in evaluation of quality of meat and meat products—A review. Ann. Anim. Sci. 2020, 20, 1151–1168. [Google Scholar] [CrossRef]
- Przybylski, W.; Sionek, B.; Jaworska, D.; Santé-Lhoutellier, V. The application of biosensors for drip loss analysis and glycolytic potential evaluation. Meat Sci. 2016, 117, 7–11. [Google Scholar] [CrossRef]
- Shu, H.C.; Hkanson, H.; Mattiasson, B. d-Lactic acid in pork as a freshness indicator monitored by immobilized d-lactate dehydrogenase using sequential injection analysis. Anal. Chim. Acta 1993, 283, 727–737. [Google Scholar] [CrossRef]
- Smit, N.J.; Howatson, G.; Greenfield, R. Blood lactate levels as a biomarker for angling-induced stress in tigerfish Hydrocynus vittatus from the Okavango Delta, Botswana. Afr. J. Aquat. Sci. 2009, 34, 255–259. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual range lactate oxidase-based screen printed amperometric biosensor for analysis of lactate in diversified samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Shkotova, L.V.; Piechniakova, N.Y.; Kukla, O.L.; Dzyadevych, S.V. Thin-film amperometric multibiosensor for simultaneous determination of lactate and glucose in wine. Food Chem. 2016, 197, 972–978. [Google Scholar] [CrossRef]
- Bravo, I.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Reagent-less and robust biosensor for direct determination of lactate in food samples. Sensors 2017, 17, 144. [Google Scholar] [CrossRef]
- Uygun, H.D.E.; Tinkilic, N.; Attar, A.; Isildak, I. Development of potentiometric lactate biosensor based on composite pH sensor. J. New Mater. Electrochem. Syst. 2016, 19, 151–156. [Google Scholar] [CrossRef]
- Nguyen-Boisse, T.T.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. Highly sensitive conductometric biosensors for total lactate, D- and L-lactate determination in dairy products. Sens. Actuators B Chem. 2013, 179, 232–239. [Google Scholar] [CrossRef]
- Zanini, V.P.; López De Mishima, B.; Solís, V. An amperometric biosensor based on lactate oxidase immobilized in laponite-chitosan hydrogel on a glassy carbon electrode. Application to the analysis of l-lactate in food samples. Sens. Actuators B Chem. 2011, 155, 75–80. [Google Scholar] [CrossRef]
- Loaiza, O.A.; Lamas-Ardisana, P.J.; Añorga, L.; Jubete, E.; Ruiz, V.; Borghei, M.; Cabañero, G.; Grande, H.J. Graphitized carbon nanofiber-Pt nanoparticle hybrids as sensitive tool for preparation of screen printing biosensors. Detection of lactate in wines and ciders. Bioelectrochemistry 2015, 101, 58–65. [Google Scholar] [CrossRef]
- Monošík, R.; Streďanský, M.; Greif, G.; Šturdík, E. A rapid method for determination of l-lactic acid in real samples by amperometric biosensor utilizing nanocomposite. Food Control 2012, 23, 238–244. [Google Scholar] [CrossRef]



| Enzymatic Sensors | ||||||
|---|---|---|---|---|---|---|
| Sensor Architecture | Trans. | LOD (µM) | Sensitivity | LRR (mM) | Sample Applied | Ref |
| PtE-PDA/PPy/LOx | Amp. | - | 37.53 µA mM−1 cm−2 | 0–0.5 | PBS | [38] |
| PtµE-poly-m-phenylene diamine/poly(ethylene glycol) diglycidyl ether)-LOx | Amp. | 19 ± 7 | 2.63 ± 0.66 nA mM−1 | 0–1.0 | Cerebrospinal fluid in mouse brain | [39] |
| CE-fSWCNTs/Chit-PBNPs/Chit LOx | Amp. | 200 | - | 1–25 | Human sweat | [40] |
| SPE-ERGO-PAH-AuNPs/LDH-GA | Amp. | 1 | 1.08 µA mM−1·cm−2 | 0–3 | Wine | [41] |
| SPCE-PEGDGE/AvLOx | Amp. | 25 | 13 µA mM−1 cm−2 | 0–1 | PBS | [42] |
| PtE-poly(phenylenedi- amine)/LOx/glycerol/PVA-SbQ | Amp. | 5 | 204 nA mM−1 | 0.005–1 | Blood serum | [43] |
| Au-PB-Chit/CNTs-LOx-Chit/CNts | Amp. | - | 220 nA mM−1 | 0–30 | Sweat | [44] |
| PVC/FC-LDH | Pot. | - | 52 mV decade −1 | - | Tris buffer | [45] |
| AuE-LOx/GA/ZnO nanorod | Pot. | 0.1 | 41.33 ± 1.58 mV decade−1 | 0.0001–1 | PBS | [46] |
| Si3N4-PAA/NHS-EDC/LOx | Pot. | 0.0002 | 49.7 mV decade−1 | 0–0.00005 | PBS | [47] |
| Ti-Au/Nafion/Chit/LDH/GA | GFET | - | - | 0–7.5 | Human plasma | [48] |
| Au-Os redox polymer/LOx | OFETs | - | - | 0–10 | PBS | [49] |
| Non-enzymatic sensors | ||||||
| GCE-Nafion/NiO | Amp. | 27 | 62.35 μA mM−1 cm−2 | 0.01–7.75 | NaOH | [50] |
| GCE-Nafion/Co3O4 | Volt. | 6 | - | 0.5–3.0 | NaOH | [51] |
| Amp. | 10 | |||||
| GCE-CuO/MWCNTs/Nafion | Volt. | 0.088 | 633.0 pA mM−1 cm−2 | 0.0001–10 | Serum samples | [52] |
| Ti-PTFE/PPy-MWCNTs | Amp. | 51 | 2.9 µAmM−1 cm−2 | 1–15 | Sweat | [53] |
| BDD-NiNPs | Amp. | 0.72 ± 0.09 | (24.70 ± 0.36) μA L C−1 mol−1 | 6–120 | NaOH | [54] |
| SPCE-NiCo layered double hydroxide | Amp. | 533 | 30.59 ± 0.34 μA mM−1 cm−2 | 5–25 | NaOH | [55] |
| NiF-NiS-NC@NiS-MS | Amp. | 0.5 | 0.39 μA μM−1 | 0.0005–0.085 | Urine | [56] |
| NH2-GP-Cu3(btc)2 | Amp. | 5 | 0.029 mA mM−1 cm−2 | 0.05–22.6 | Sweat | [57] |
| SPCE-NiCo (layered double hydroxide) | Amp. | 400 | 83.98 μA mM−1 cm−2 | 2–26 | Sweat | [58] |
| GCE-rGO-AuNPs-MIP | Amp. | 9 × 10−5 | 1.9 × 105 μA L mol−1 | 1 × 10−7–1 10−6 | Sugarcane vinasse | [59] |
| CE-AgNWs-MIP | Amp. | 0.22 | 4.5 × 10−6 A M−1 | 0.001–100 | Sweat | [60] |
| GCE-Nafion/CuO | Amp. | - | 80.33 μA mM−1 | 0.01–27.76 | - | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors 2022, 12, 919. https://doi.org/10.3390/bios12110919
García-Guzmán JJ, Sierra-Padilla A, Palacios-Santander JM, Fernández-Alba JJ, Macías CG, Cubillana-Aguilera L. What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors. 2022; 12(11):919. https://doi.org/10.3390/bios12110919
Chicago/Turabian StyleGarcía-Guzmán, Juan José, Alfonso Sierra-Padilla, José María Palacios-Santander, Juan Jesús Fernández-Alba, Carmen González Macías, and Laura Cubillana-Aguilera. 2022. "What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications" Biosensors 12, no. 11: 919. https://doi.org/10.3390/bios12110919
APA StyleGarcía-Guzmán, J. J., Sierra-Padilla, A., Palacios-Santander, J. M., Fernández-Alba, J. J., Macías, C. G., & Cubillana-Aguilera, L. (2022). What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors, 12(11), 919. https://doi.org/10.3390/bios12110919










