A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials and Reagents
2.2. Instruments
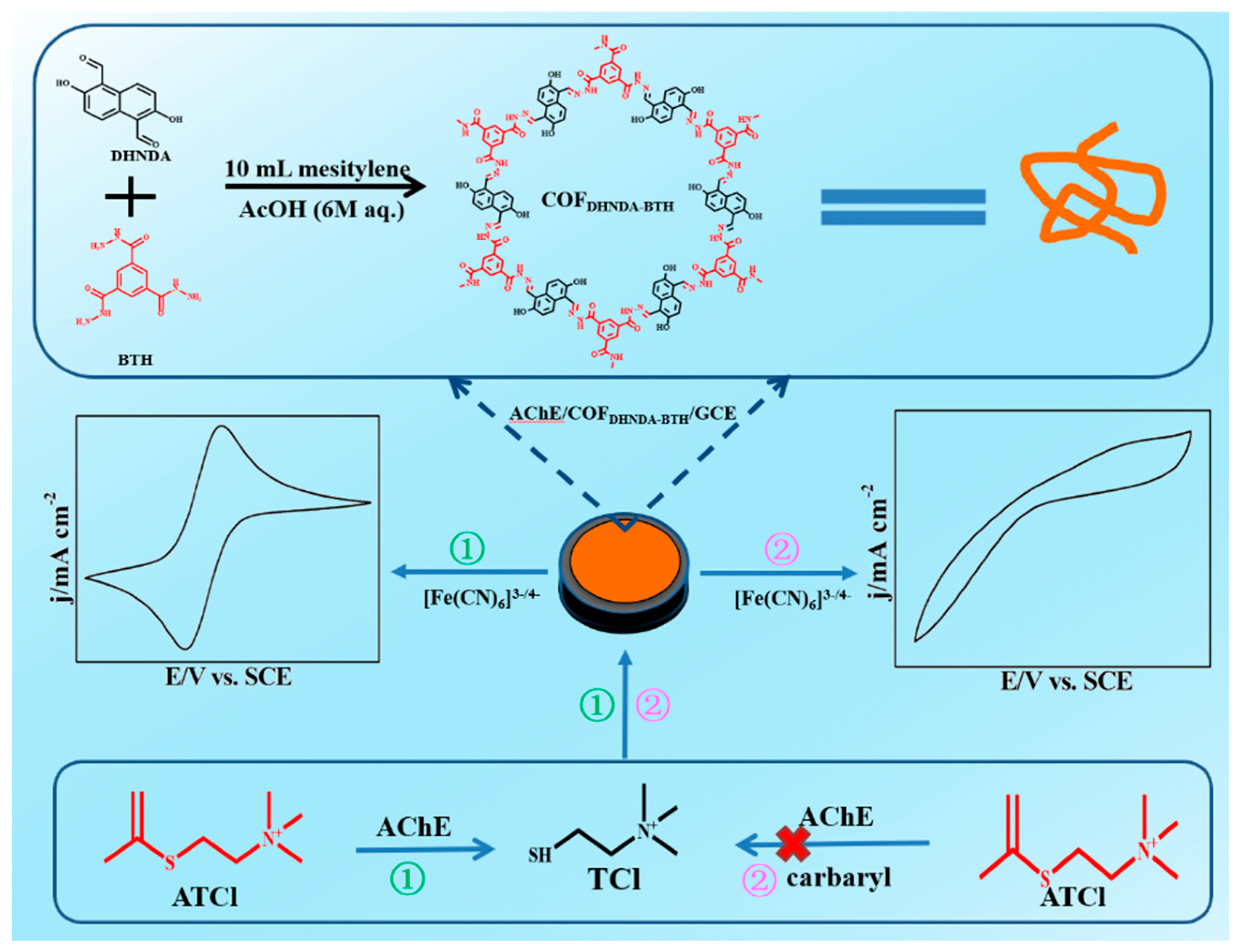
2.3. Preparation of COFDHNDA-BTH
2.4. Preparation of AChE/COFDHNDA-BTH/Glassy Carbon Electrode (GCE)
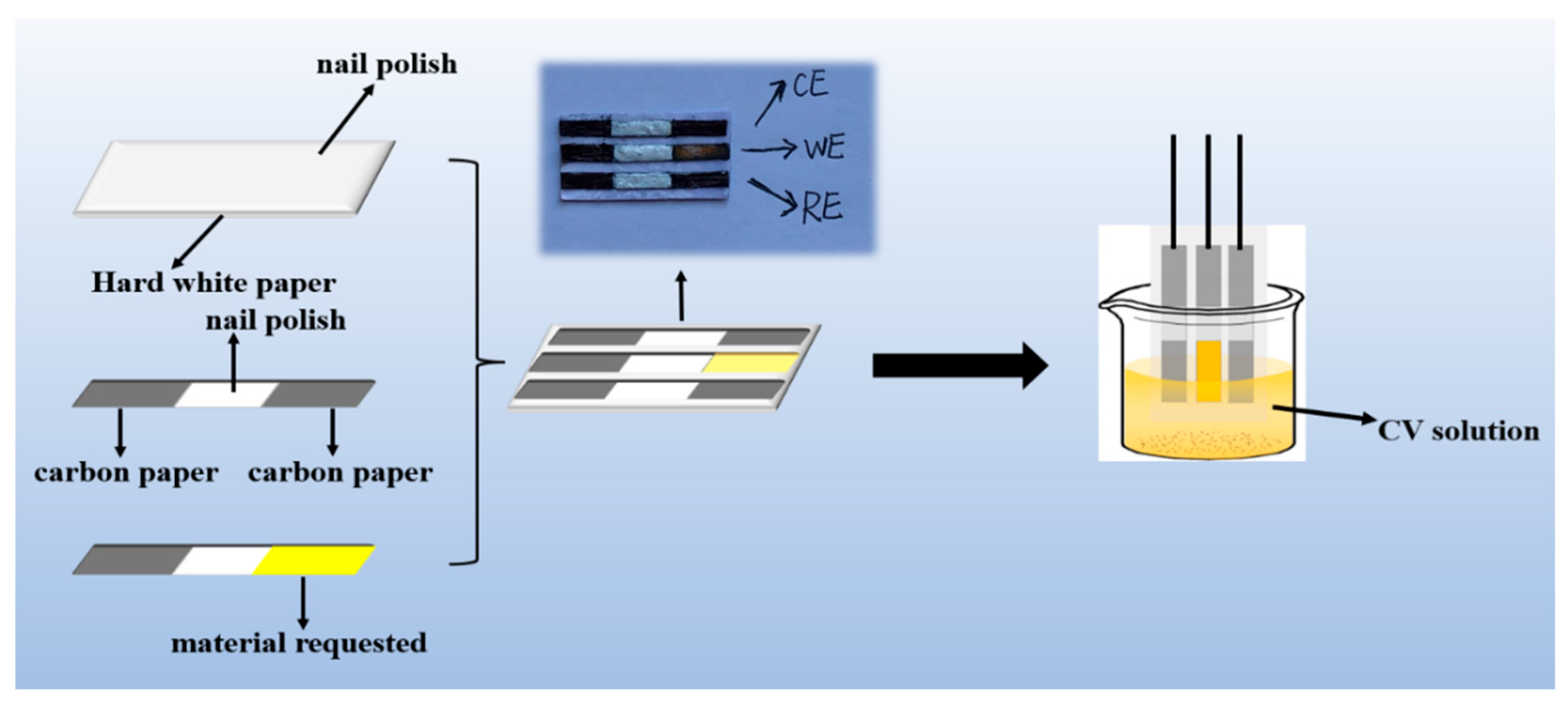
2.5. Preparation of Paper-Based Electrode
2.6. Preparation of AChE/COFDHNDA-BTH/Portable Paper-Based Electrode
3. Results and Discussion
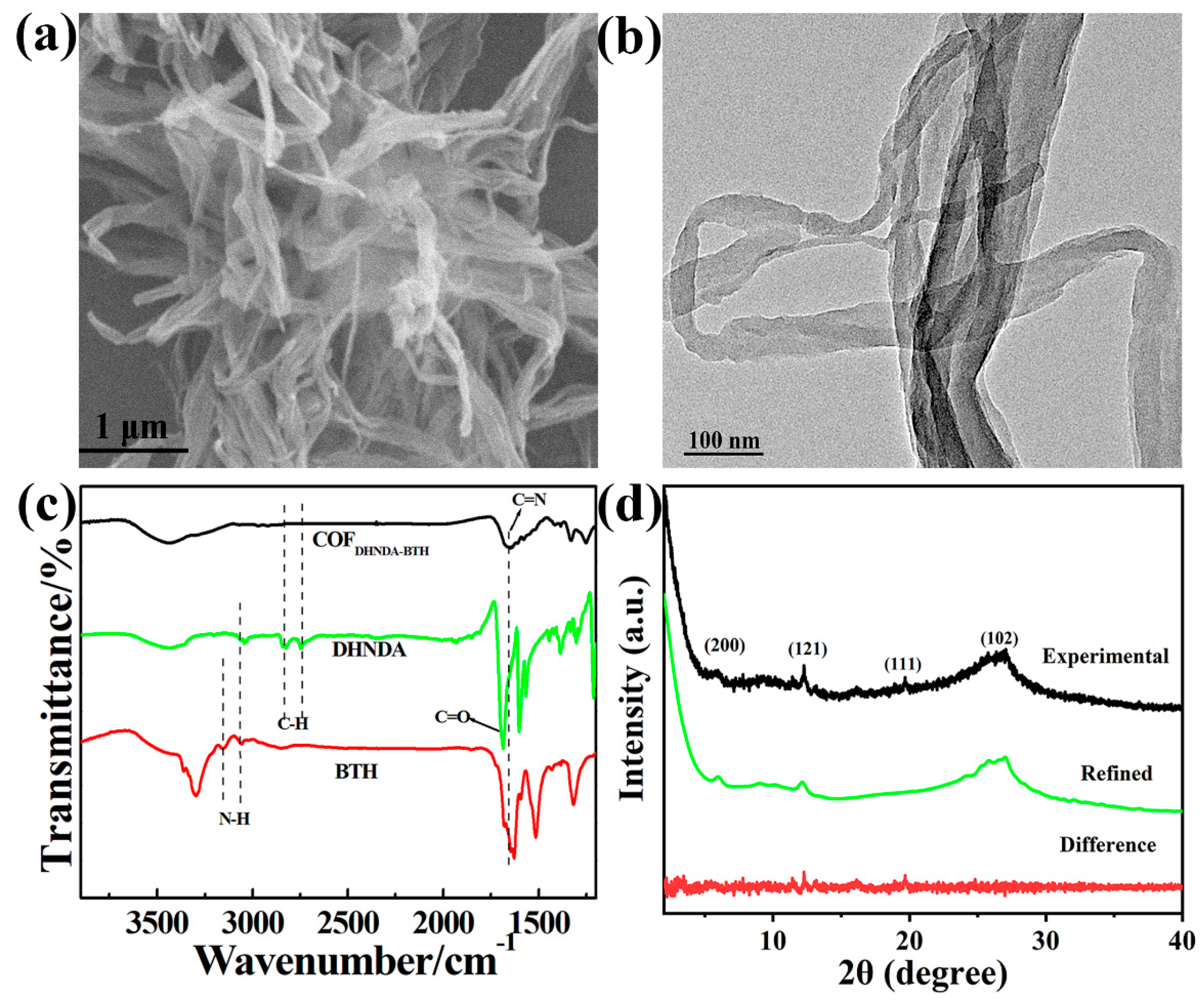
3.1. Characterization of COFDHNDA-BTH
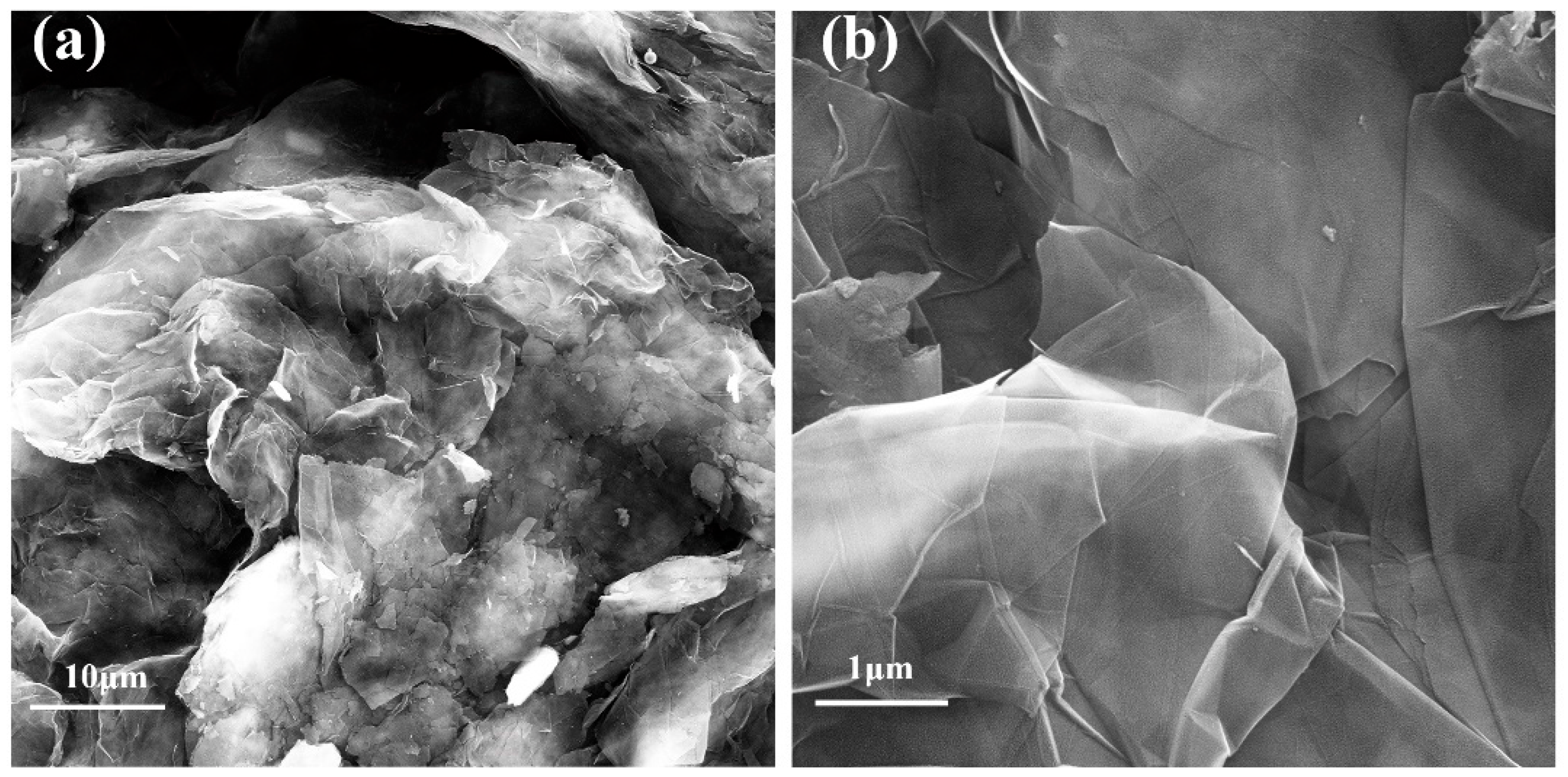
3.2. Characterization of Commercial Carbon Paper
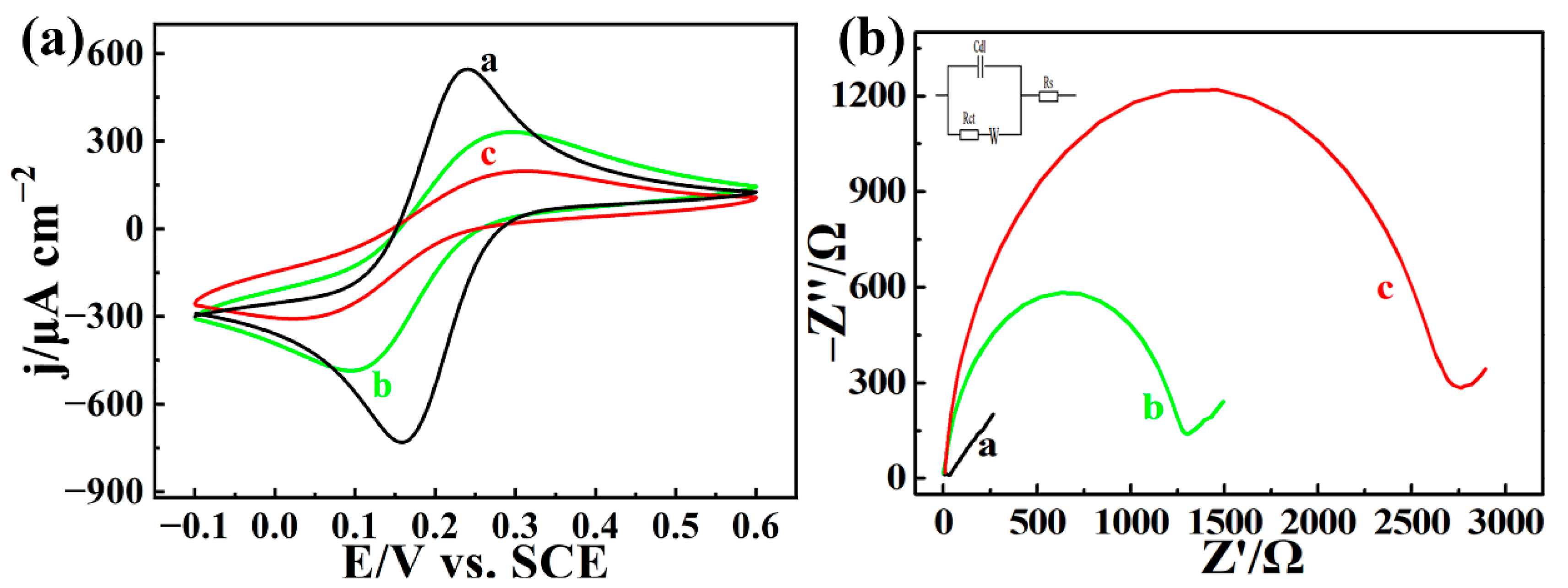
3.3. Electrochemical Behaviors of AChE/COFDHNDA-BTH/GCE
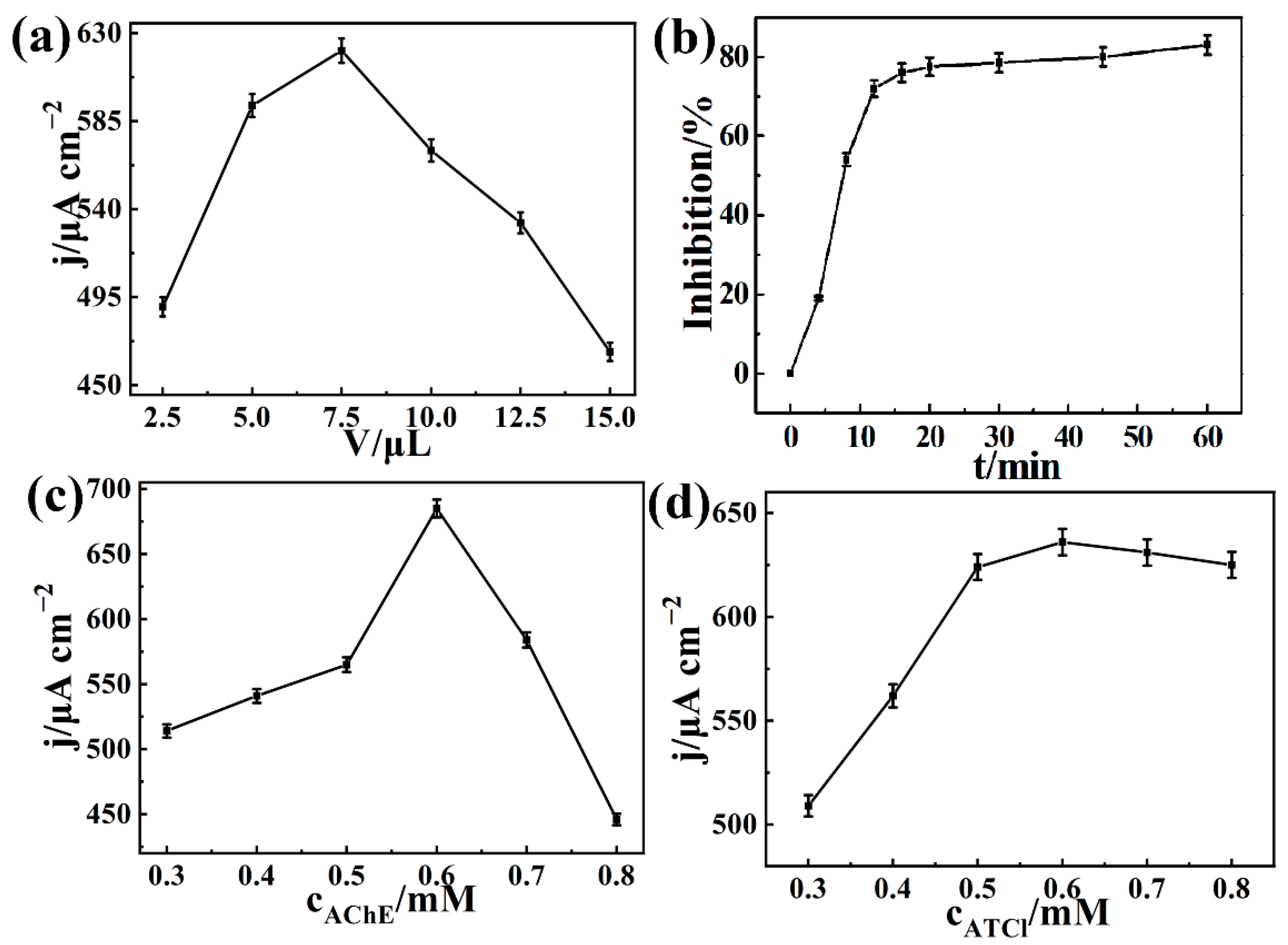
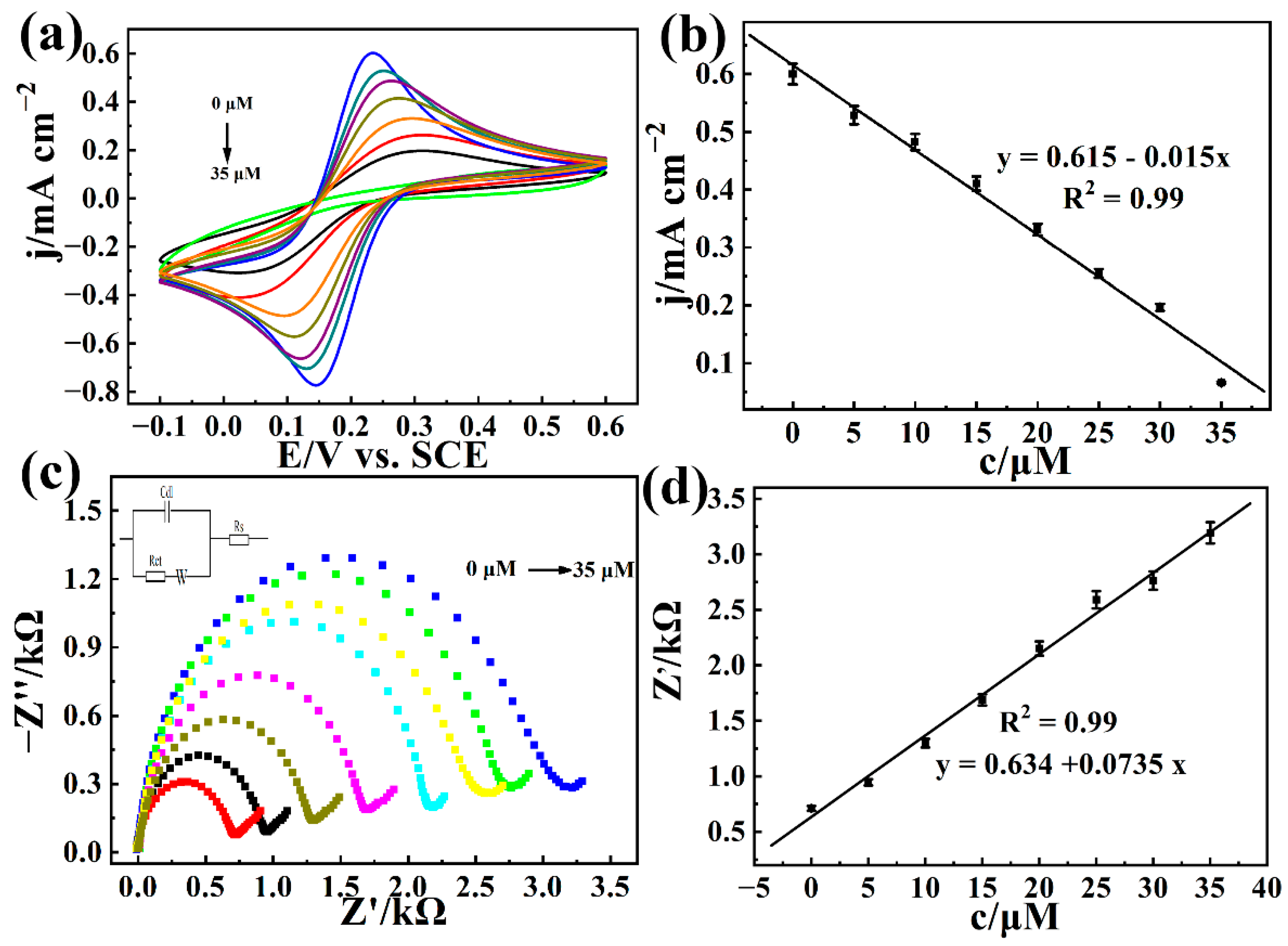
3.4. Electrochemical Detection of Carbaryl Based on AChE/COFDHNDA-BTH/GCE
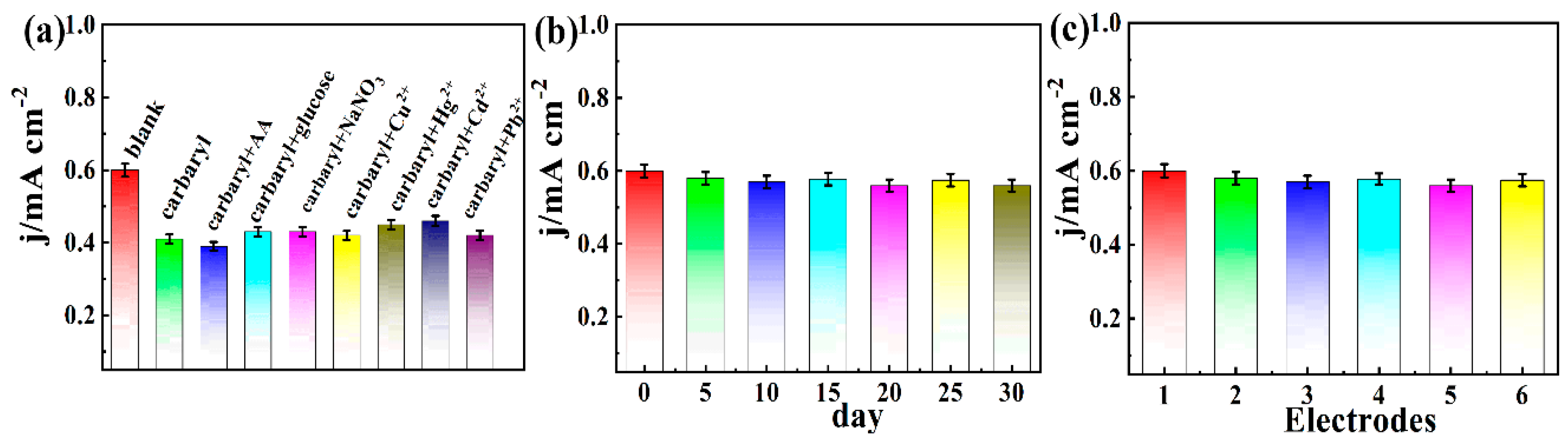
3.5. Selectivity, Stability, and Repeatability of Electrochemical Carbaryl Biosensors
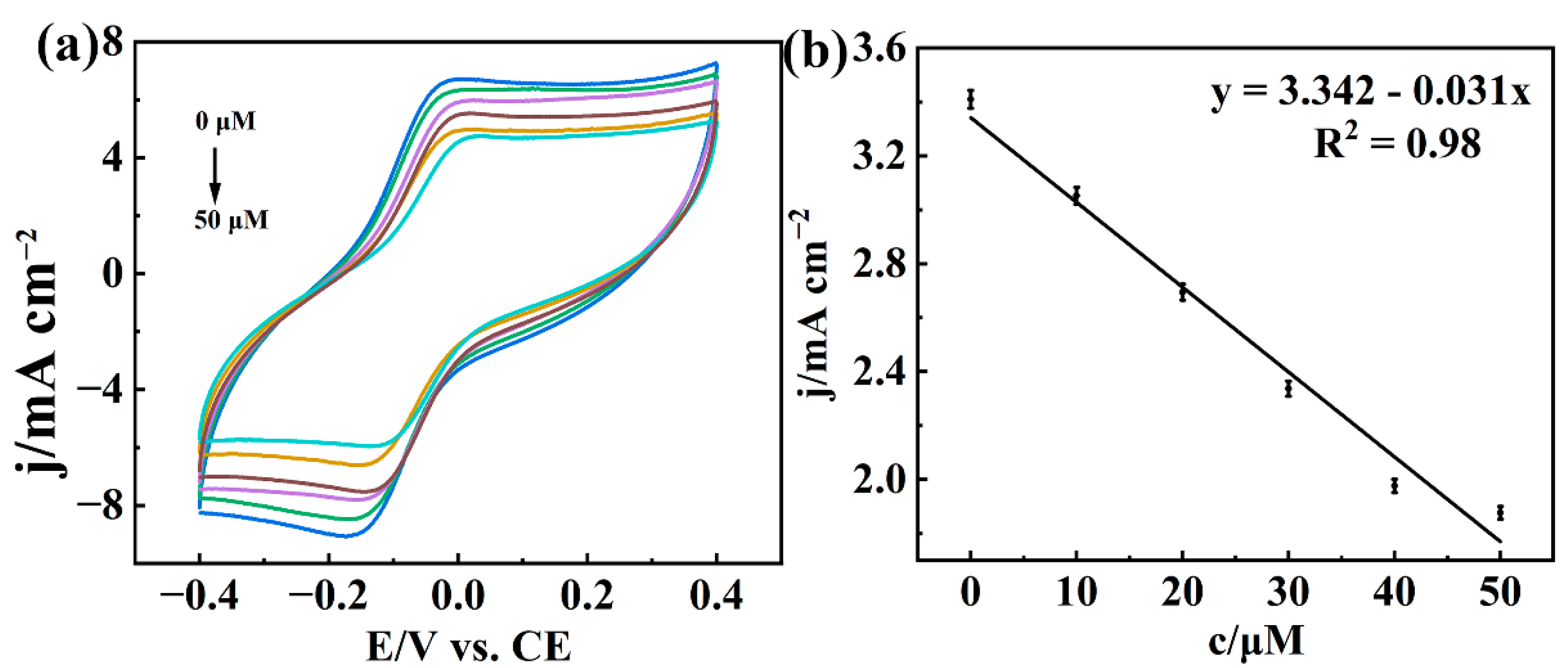
3.6. Actual Sample Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartolucci, C.; Antonacci, A.; Arduini, F.; Moscone, D.; Fraceto, L.; Campos, E.; Attaallah, R.; Amine, A.; Zanardi, C.; Cubillana-Aguilera, L.; et al. Green nanomaterials fostering agrifood sustainability. TrAC Trends Anal. Chem. 2020, 125, 115840. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.; Gomes, N.; Shimizu, F.; Machado, S.; Oliveira, O. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. Chem. Eng. J. 2021, 408, 127279. [Google Scholar] [CrossRef]
- Paschoalin, R.; Gomes, N.; Almeida, G.; Bilatto, S.; Farinas, C.; Machado, S.; Mattoso, L.; Oliveira, O.; Raymundo-Pereira, P. Wearable sensors made with solution-blow spinning poly (lactic acid) for non-enzymatic pesticide detection in agriculture and food safety. Biosens. Bioelectron. 2022, 199, 113875. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Sonwal, S.; Lee, M.; Rani, G.; Lee, E.; Jeon, T.; Kang, S.; Oh, M.; Huh, Y. Colorimetric based on-site sensing strategies for the rapid detection of pesticides in agricultural foods: New horizons, perspectives, and challenges. Coord. Chem. Rev. 2021, 446, 214061. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, Y.; Ke, Z.; Zhang, L.; Zhang, M.; Zhou, Y.; Wang, H.; Wu, C.; Qiu, J.; Hong, Q. Substrate preference of carbamate hydrolase CehA reveals its environmental behavior. J. Hazard. Mater. 2021, 403, 123677. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Zhao, X.; Yan, X.; Han, X.; Zhu, Z.; Wang, C.; Jia, X.; Liu, F.; Sun, P.; Liu, X.; et al. Background-free sensing platform for on-site detection of carbamate pesticide through upconversion nanoparticles-based hydrogel suit. Biosens. Bioelectron. 2021, 194, 113598. [Google Scholar] [CrossRef]
- Madrigal, J.; Jones, R.; Gunier, R.; Whitehead, T.; Reynolds, P.; Metayer, C.; Ward, M. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ. Res. 2021, 201, 111501. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, H.; Xiao, Z.; Fu, H.; Shen, Y.; Luo, L.; Wang, H.; Lei, H.; Hongsibsong, S.; Xu, Z. Rational hapten design to produce high-quality antibodies against carbamate pesticides and development of immunochromatographic assays for simultaneous pesticide screening. J. Hazard. Mater. 2021, 412, 126823. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Liu, F.; Laborda, P. Chromatographic methods for detection and quantification of carbendazim in food. J. Agric. Food Chem. 2020, 68, 11880–11894. [Google Scholar] [CrossRef]
- Zha, Y.; Li, Y.; Hu, P.; Lu, S.; Ren, H.; Liu, Z.; Yang, H.; Zhou, Y. Duplex-specific nuclease-triggered fluorescence immunoassay based on dual-functionalized AuNP for acetochlor, metolachlor, and propisochlor. Anal. Chem. 2021, 93, 13886–13892. [Google Scholar] [CrossRef]
- Zhao, Y.; Ruan, X.; Song, Y.; Smith, J.; Vasylieva, N.; Hammock, B.; Lin, Y.; Du, D. Smartphone-based dual-channel immunochromatographic test strip with polymer quantum dot labels for simultaneous detection of cypermethrin and 3-phenoxybenzoic acid. Anal. Chem. 2021, 93, 13658–13666. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Yang, M.; Rasooly, A. Dual signal amplification electrochemical biosensor for monitoring the activity and inhibition of the Alzheimer’s related protease β-secretase. Anal. Chem. 2016, 21, 10559–10565. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Qu, F.; Lu, L. A photoelectrochemical aptasensor based on pn heterojunction CdS-Cu2O nanorod arrays with enhanced photocurrent for the detection of prostate-specific antigen. Anal. Bioanal. Chem. 2020, 412, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Gai, P.; Gu, C.; Hou, T.; Li, F. Integration of biofuel cell-based self-powered biosensing and homogeneous electrochemical strategy for ultrasensitive and easy-to-use bioassays of microRNA. ACS Appl. Mater. Interfaces 2018, 10, 9325–9331. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Li, F. Nucleic acid-functionalized metal-organic framework for ultrasensitive immobilization-free photoelectrochemical biosensing. Biosens. Bioelectron. 2021, 173, 112832. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, X.; Wu, K.; Lv, S.; Zhu, C. A ratiometric rganic fluorescent nanogel thermometer for highly sensitive temperature sensing. Biosensors 2022, 12, 702. [Google Scholar] [CrossRef]
- Shaw, D.S.; Honeychurch, K.C. Nanosensor applications in plant science. Biosensors 2022, 12, 675. [Google Scholar] [CrossRef]
- Song, Y.; Chen, J.; Sun, M.; Gong, C.; Shen, Y.; Wang, L. A simple electrochemical biosensor based on AuNPs/MPS/Au electrode sensing layer for monitoring carbamate pesticides in real samples. J. Hazard. Mater. 2016, 304, 103–109. [Google Scholar] [CrossRef]
- Liang, H.; Wang, L.; Yang, Y.; Song, Y.; Wang, L. A novel biosensor based on multienzyme microcapsules constructed from covalent-organic framework. Biosens. Bioelectron. 2021, 193, 113553. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- D’Aurelio, R.; Chianella, I.; Goode, J.A.; Tothill, I.E. Molecularly imprinted nanoparticles based sensor for cocaine detection. Biosensors 2020, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Gigli, V.; Tortolini, C.; Capecchi, E.; Angeloni, A.; Lenzi, A.; Antiochia, R. Novel amperometric biosensor based on tyrosinase/chitosan nanoparticles for sensitive and interference-free detection of total catecholamine. Biosensors 2022, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wu, N.; Wang, L.; Song, Y.; Du, Y.; Ma, G. A novel acetylcholinesterase biosensor based on electroactive organic framework for ratiometric detection of carbaryl. Biosensors 2022, 12, 625. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Lie, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B 2020, 323, 128701. [Google Scholar] [CrossRef]
- Loguercio, L.; Thesing, A.; Demingos, P.; de Albuquerque, C.; Rodrigues, R.; Brolo, A.; Santos, J. Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sens. Actuators B 2021, 339, 129875. [Google Scholar] [CrossRef]
- Ivanov, A.; Davletshina, R.; Sharafieva, I.; Evtugyn, G. Electrochemical biosensor based on polyelectrolyte complex. Talanta 2019, 194, 723–730. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Qiu, S.; Zhang, Q.; Tang, W.; Liu, H.; Guo, Y.; Ma, Y.; Guo, X.; Liu, Y. A flexible acetylcholinesterase-modified graphene for chiral pesticide sensor. J. Am. Chem. Soc. 2019, 141, 14643–14649. [Google Scholar] [CrossRef]
- Montali, L.; Calabretta, M.; Lopreside, A.; D’Elia, M.; Guardigli, M.; Michelini, E. Multienzyme chemiluminescent foldable biosensor for on-site detection of acetylcholinesterase inhibitors. Biosens. Bioelectron. 2020, 162, 112232. [Google Scholar] [CrossRef]
- Ramírez-Santana, M.; Zúñiga-Venegas, L.; Corral, S.; Roeleveld, N.; Groenewoud, H.; Velden, K.; Scheepers, P.; Pancetti, F. Association between cholinesterase’s inhibition and cognitive impairment: A basis for prevention policies of environmental pollution by organophosphate and carbamate pesticides. J. Chile Environ. Res. 2020, 186, 109539. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pang, S.; Zhang, W.; Lin, Z.; Bhatt, P.; Chen, S. Insights into the microbial degradation and biochemical mechanisms of carbamates. J. Chemosphere 2021, 279, 130500. [Google Scholar] [CrossRef]
- Pundir, C.; Malik, A.; Preety. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar]
- Song, Y.; Zhang, M.; Wang, L.; Wan, L.; Xiao, X.; Ye, S.; Wang, J. A novel biosensor based on acetylecholinesterase/prussian blue–chitosan modified electrode for detection of carbaryl pesticides. Electrochim. Acta 2011, 56, 7267–7271. [Google Scholar] [CrossRef]
- Pinyou, P.; Blay, V.; Muresan, L.; Noguer, T. Enzyme-modified electrodes for biosensors and biofuel cells. Mater. Horiz. 2019, 6, 1336–1358. [Google Scholar] [CrossRef]
- He, Y.; Hu, F.; Zhao, J.; Yang, G.; Zhang, Y.; Chen, S.; Yuan, R. Bifunctional moderator-powered ratiometric electrochemiluminescence enzymatic biosensors for detecting organophosphorus pesticides based on dual-signal combined nanoprobes. Anal. Chem. 2021, 93, 8783–8790. [Google Scholar] [CrossRef] [PubMed]
- Kadambar, V.; Bellare, M.; Bollella, P.; Katz, E.; Melman, A. Electrochemical control of the catalytic activity of immobilized enzymes. Chem. Commun. 2020, 56, 13800–13803. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.R. Immobilization of lipases–A review. Part I: Enzyme immobilization. ChemBioEng Rev. 2019, 6, 157–166. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered covalent organic frameworks, COFs and PAFs: From preparation to application. Coord. Chem. Rev. 2016, 311, 85–124. [Google Scholar] [CrossRef]
- Xu, F.; Xu, H.; Chen, X.; Wu, D.; Wu, Y.; Liu, H.; Gu, G.; Fu, R.; Jiang, D. Radical covalent organic frameworks: A general strategy to immobilize open-accessible polyradicals for high-performance capacitive energy storage. Angew. Chem. Int. Ed. 2015, 54, 6814–6818. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Yang, L.; Li, Y.; Kuang, L.; Song, Y.; Wang, L. Covalent organic frameworks for fluorescent sensing: Recent developments and future challenges. Coord. Chem. Rev. 2021, 440, 213957. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, C.M.; Hatamie, A.; Simchi, A.; Willander, M.; Malhotra, B.D. Nanomaterial-modified conducting paper: Fabrication, properties, and emerging biomedical applications. Global. Chall. 2019, 12, 1900041. [Google Scholar] [CrossRef]
- Gu, F.; Chen, K.; Du, Y.; Song, Y.; Wang, L. CeO2-NiO/N, O-rich porous carbon derived from covalent-organic framework for enhanced Li-storage. Chem. Eng. J. 2022, 442, 136298. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Thomas, A. Covalent organic frameworks (COFs) for electrochemical applications. Chem. Soc. Rev. 2021, 50, 6871–6913. [Google Scholar] [CrossRef]
- Wu, N.; Wang, L.; Xie, Y.; Du, Y.; Song, Y.; Wang, L. Double signal ratiometric electrochemical riboflavin sensor based on macroporous carbon/electroactive thionine-contained covalent organic framework. J. Colloid Interface Sci. 2022, 608, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, Y.; Liang, H.; Wu, N.; Peng, X.; Wang, L.; Song, Y. A novel N, S-rich COF and its derived hollow N, S-doped carbon@ Pd nanorods for electrochemical detection of Hg2+ and paracetamol. J. Hazard. Mater. 2021, 409, 124528. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, M.; Kuang, L.; Li, Y.; Chen, L.; Lin, C.; Song, Y. Benzotrithiophene-based covalent organic frameworks for real-time visual onsite assays of enrofloxacin. Biosens. Bioelectron. 2022, 214, 114527. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Huang, R.; Gu, F.; Du, Y.; Song, Y. A novel hollow Co3O4@ N-doped carbon nanobubble film composite for high-performance anode of lithium-ion batteries. Compos. Part B 2021, 224, 109247. [Google Scholar] [CrossRef]
- Wang, L.; Liang, H.; Xu, M.; Wang, L.; Xie, Y.; Song, Y. Ratiometric electrochemical biosensing based on double-enzymes loaded on two-dimensional dual-pore COFETTA-TPAL. Sens. Actuators B 2019, 298, 126859. [Google Scholar] [CrossRef]
- Liang, H.; Luo, Y.; Li, Y.; Song, Y.; Wang, L. An immunosensor using electroactive COF as signal probe for electrochemical detection of carcinoembryonic antigen. Anal. Chem. 2022, 94, 5352–5358. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Xie, Y.; Song, Y.; Wang, L. Ratiometric electrochemical sensing and biosensing based on multiple redox-active state COFDHTA-TTA. Sens. Actuators B 2019, 281, 1009–1015. [Google Scholar] [CrossRef]
- Santos, C.S.; Mossanha, R.; Pessôa, C.A. Biosensor for carbaryl based on gold modified with PAMAM-G4 dendrimer. J. Appl. Electrochem. 2015, 45, 325–334. [Google Scholar] [CrossRef]
- Caetano, J.; Machado, S.A.S. Determination of carbaryl in tomato “in natura” using an amperometric biosensor based on the inhibition of acetylcholinesterase activity. Sens. Actuators B 2008, 129, 40–46. [Google Scholar] [CrossRef]
- Pedrosa, V.A.; Caetano, J.; Machado, S.A.; Bertotti, M. Determination of parathion and carbaryl pesticides in water and food samples using a self-assembled monolayer/acetylcholinesterase electrochemical biosensor. Sensors 2008, 8, 4600–4610. [Google Scholar] [CrossRef]
- da Silva, M.K.; Vanzela, H.C.; Defavari, L.M.; Cesarino, I. Determination of carbamate pesticide in food using a biosensor based on reduced graphene oxide and acetylcholinesterase enzyme. Sens. Actuators B 2018, 277, 555–561. [Google Scholar] [CrossRef]
- Cesarino, I.; Moraes, F.C.; Lanza, M.R.; Machado, S.A. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline–carbon nanotubes. Food. Chem. 2012, 135, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gong, Z.; Cao, Y.; Wang, X. Acetylcholinesterase biosensor based on poly (diallyldimethylammonium chloride)-multi-walled carbon nanotubes-graphene hybrid film. Nano-Micro Lett. 2013, 5, 47–56. [Google Scholar] [CrossRef]
- Moraes, F.C.; Mascaro, L.H.; Machado, S.A.; Brett, C.M. Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode. Talanta 2009, 79, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Fei, A.; Huan, J.; Mao, H.; Wang, K. Effective amperometric biosensor for carbaryl detection based on covalent immobilization acetylcholinesterase on multiwall carbon nanotubes/graphene oxide nanoribbons nanostructure. J. Electroanal. Chem. 2015, 740, 8–13. [Google Scholar] [CrossRef]
- Ha, T.B.; Le, H.T.; Cao, H.H.; Binh, N.T.; Nguyen, H.L.; Dang, L.H.; Do, Q.P.; Nguyen, D.T.; Lam, T.D.; Nguyen, V.A. Electro-immobilization of acetylcholinesterase using polydopamine for carbaryl microsensor. J. Electron. Mater. 2018, 47, 1686–1693. [Google Scholar] [CrossRef]
| Electrode | LOD(μM) | Linear Range(μM) | Reference |
|---|---|---|---|
| Au/PAMAM/GLUT/AChE | 0.032 | 1.0–9.0 | [50] |
| Carbe/CoPcf/AChE | 2 | 5.0–75.0 | [51] |
| Au/MPA/AChE | 0.035 | 2.0–30.0 | [52] |
| GC/rGO/AChE | 0.0019 | 0.2–10 | [53] |
| GC/PANI/MWCNT/AChE | 1.4 | 9.9–49.6 | [54] |
| AChE/PDDA-MWCNTs-GR/GCE | 0.001 | 0.255–14.9 | [55] |
| GC/MWCNT/CoPc | 0.0055 | 0.33–6.61 | [56] |
| AChE–MWCNTs/GONRs/GCE | 0.0017 | 0.005–5.0 | [57] |
| AChE/PDA-Gr/PPNWs/IdlE | 0.04 | 0.25–7.45 | [58] |
| AChE/COFDHNDA-BTH/GCE | 0.23 0.13 | 0.69–35 0.39–35 | This work |
| Sample | Added (μM) | Founded (μM) | Average Value (μM) | Recovery (%) | RSD (%, n = 3) |
|---|---|---|---|---|---|
| 1 | 0 | – | – | – | |
| 2 | 5 | 4.51, 5.10, 4.56 | 4.72 | 94.4 | 6.93 |
| 3 | 10 | 10.6, 10.4, 10.8 | 10.6 | 106 | 1.89 |
| 4 | 20 | 20.4, 19.8, 20.6 | 20.3 | 101.3 | 2.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Wu, N.; Wang, L.; Chen, L. A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors 2022, 12, 899. https://doi.org/10.3390/bios12100899
Xiao Y, Wu N, Wang L, Chen L. A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors. 2022; 12(10):899. https://doi.org/10.3390/bios12100899
Chicago/Turabian StyleXiao, Yawen, Na Wu, Li Wang, and Lili Chen. 2022. "A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection" Biosensors 12, no. 10: 899. https://doi.org/10.3390/bios12100899
APA StyleXiao, Y., Wu, N., Wang, L., & Chen, L. (2022). A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors, 12(10), 899. https://doi.org/10.3390/bios12100899




