Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs
Abstract
1. Introduction
2. Methodology
2.1. Functionalization of CNTs
2.2. Materials Characterization
2.3. Detection of MMP-14
2.4. Statistics
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MMPs | Matrix Metalloproteinases |
| PEX | Hemopexin |
| CNTs | Carbon nanotubes |
| SEM | Scanning Electron Microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| CV | Cyclic Voltammetry |
| EIS | Electrochemical Impedance Spectroscopy |
| PBS | Phosphate buffered saline |
| ELISA | Enzyme-linked immunosorbent assay |
| GCE | Glassy Carbon Electrode |
| SCE | Saturated Calomel Electrode |
| LOD | Limit of Detection |
| SD | Standard error intercept |
| S | Slope |
| SD | Standard deviation |
| Ipa | Peak current density |
| ΔEp | Peak width |
| RCT | Charge transfer resistance |
| ISC | Thiolated PEX-14 binding peptides |
References
- Mondal, S.; Adhikari, N.; Banerjee, S.; Amin, S.A.; Jha, T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020, 194, 112260. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef]
- Isaacson, K.J.; Jensen, M.M.; Subrahmanyam, N.B.; Ghandehari, H. Matrix-metalloproteinases as targets for controlled delivery in cancer: An analysis of upregulation and expression. J. Control. Release 2017, 259, 62–75. [Google Scholar] [CrossRef]
- Jabłońska-Trypuć, A.; Matejczyk, M.; Rosochacki, S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzyme Inhib. Med. Chem. 2016, 31, 177–183. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Bode, W.; Fernandez-Catalan, C.; Tschesche, H.; Grams, F.; Nagase, H.; Maskos, K. Structural properties of matrix metalloproteinases. Cell. Mol. Life Sci. CMLS 1999, 55, 639–652. [Google Scholar] [CrossRef]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Löffek, S.; Schilling, O.; Franzke, C.-W. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2011, 38, 191–208. [Google Scholar] [CrossRef]
- Ren, F.; Tang, R.; Zhang, X.; Madushi, W.M.; Luo, D.; Dang, Y.; Li, Z.; Wei, K.; Chen, G. Overexpression of MMP family members functions as prognostic biomarker for breast cancer patients: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0135544. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Pytliak, M.; Vargova, V.; Mechirova, V. Matrix metalloproteinases and their role in oncogenesis: A review. Oncol. Res. Treat. 2012, 35, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, M.F.; Neubert, H. An immunoaffinity liquid chromatography–tandem mass spectrometry assay for the quantitation of matrix metalloproteinase 9 in mouse serum. Anal. Biochem. 2010, 399, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Freije, R.; Klein, T.; Ooms, B.; Kauffman, H.F.; Bischoff, R. An integrated high-performance liquid chromatography–mass spectrometry system for the activity-dependent analysis of matrix metalloproteases. J. Chromatogr. A 2008, 1189, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Golizeh, M.; Abusarah, J.; Benderdour, M.; Sleno, L. Covalent binding of 4-hydroxynonenal to matrix metalloproteinase 13 studied by liquid chromatography–mass spectrometry. Chem. Res. Toxicol. 2014, 27, 1556–1565. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, P.; Li, C.; Wang, Y.; Liu, Z. Upconversion fluorescence resonance energy transfer based biosensor for ultrasensitive detection of matrix metalloproteinase-2 in blood. Anal. Chem. 2012, 84, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.; Xiao, F.; Xia, Z.; Rao, J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. 2007, 119, 4424–4427. [Google Scholar] [CrossRef]
- Xi, G.; Wang, X.; Chen, T. A reduced graphene oxide-based fluorescence resonance energy transfer sensor for highly sensitive detection of matrix metalloproteinase 2. Int. J. Nanomed. 2016, 11, 1537. [Google Scholar] [CrossRef] [PubMed]
- Tokarzewicz, A.; Romanowicz, L.; Sveklo, I.; Gorodkiewicz, E. The development of a matrix metalloproteinase-1 biosensor based on the surface plasmon resonance imaging technique. Anal. Methods 2016, 8, 6428–6435. [Google Scholar] [CrossRef]
- Shoji, A.; Kabeya, M.; Sugawara, M. Real-time monitoring of matrix metalloproteinase-9 collagenolytic activity with a surface plasmon resonance biosensor. Anal. Biochem. 2011, 419, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; He, Y.; Veidal, S.S.; Nguyen, Q.H.T.; Larsen, D.V.; Koizumi, M.; Segovia-Silvestre, T.; Zhang, C.; Zheng, Q.; Sun, S. A novel marker for assessment of liver matrix remodeling: An enzyme-linked immunosorbent assay (ELISA) detecting a MMP generated type I collagen neo-epitope (C1M). Biomarkers 2011, 16, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Barascuk, N.; Veidal, S.S.; Larsen, L.; Larsen, D.V.; Larsen, M.R.; Wang, J.; Zheng, Q.; Xing, R.; Cao, Y.; Rasmussen, L.M. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 2010, 43, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Yan, J.; Sun, L.; Chen, Y. Electrochemical impedance spectroscopy for quantization of matrix Metalloproteinase-14 based on peptides inhibiting its homodimerization and heterodimerization. Talanta 2019, 205, 120142. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chen, Y.; Chen, F.; Ma, F. Peptide-based electrochemical biosensor for matrix metalloproteinase-14 and protein-overexpressing cancer cells based on analyte-induced cleavage of peptide. Microchem. J. 2020, 157, 105103. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Vanova, V.; Mitrevska, K.; Milosavljevic, V.; Hynek, D.; Richtera, L.; Adam, V. Peptide-based electrochemical biosensors utilized for protein detection. Biosens. Bioelectron. 2021, 180, 113087. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Electrochemical nanobiosensors equipped with peptides: A review. Microchim. Acta 2022, 189, 1–30. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Topkaya, S.N.; Azimzadeh, M.; Ozsoz, M. Electrochemical biosensors for cancer biomarkers detection: Recent advances and challenges. Electroanalysis 2016, 28, 1402–1419. [Google Scholar] [CrossRef]
- Luo, X.; Davis, J.J. Electrical biosensors and the label free detection of protein disease biomarkers. Chem. Soc. Rev. 2013, 42, 5944–5962. [Google Scholar] [CrossRef]
- Joshi, P.N.; Mervinetsky, E.; Solomon, O.; Chen, Y.-J.; Yitzchaik, S.; Friedler, A. Electrochemical biosensors based on peptide-kinase interactions at the kinase docking site. Biosens. Bioelectron. 2022, 207, 114177. [Google Scholar] [CrossRef]
- Itoh, Y. MT1-MMP: A key regulator of cell migration in tissue. IUBMB Life 2006, 58, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Laudański, P.; Swiatecka, J.; Kozłowski, L.; Leśniewska, M.; Wojtukiewicz, M.; Wołczyński, S. Increased serum level of membrane type 1-matrix metalloproteinase (MT1-MMP/MMP-14) in patients with breast cancer. Folia Histochem. Cytobiol. 2010, 48, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Yonezawa, K.; Ohuchi, E.; Fujimoto, N.; Iwata, K.; Shimada, T.; Shiomi, T.; Okada, Y.; Seiki, M. Two-step sandwich enzyme immunoassay using monoclonal antibodies for detection of soluble and membrane-associated human membrane type 1-matrix metalloproteinase. J. Immunoass. Immunochem. 2002, 23, 49–68. [Google Scholar] [CrossRef]
- Atkinson, J.M.; Pennington, C.J.; Martin, S.W.; Anikin, V.A.; Mearns, A.J.; Loadman, P.M.; Edwards, D.R.; Gill, J.H. Membrane type matrix metalloproteinases (MMPs) show differential expression in non-small cell lung cancer (NSCLC) compared to normal lung: Correlation of MMP-14 mRNA expression and proteolytic activity. Eur. J. Cancer 2007, 43, 1764–1771. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogawa, S.; Kubo, H.; Murayama, Y.; Kubota, T.; Yubakami, M.; Matsumoto, T.; Ohashi, T.; Okamoto, K.; Kuriki, Y.; Hanaoka, K. Matrix metalloprotease–14 is a target enzyme for detecting peritoneal metastasis in gastric cancer. Photodiagnosis Photodyn. Ther. 2021, 35, 102420. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, J.; Młynarczyk, G.; Darewicz, B.; Bruczko-Goralewska, M.; Romanowicz, L. Dominative role of MMP-14 over MMP-15 in human urinary bladder carcinoma on the basis of its enhanced specific activity. Medicine 2020, 99, e19224. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, R.; Aljumaily, R.; Tressel, S.L.; Pradhan, R.V.; Covic, L.; Kuliopulos, A.; Zarwan, C.; Kim, Y.B.; Sharifi, S.; Agarwal, A. Selective blockade of matrix metalloprotease-14 with a monoclonal antibody abrogates invasion, angiogenesis, and tumor growth in ovarian cancer. Cancer Res. 2013, 73, 2457–2467. [Google Scholar] [CrossRef]
- Ren, Y.; Hyakusoku, H.; Sagers, J.E.; Landegger, L.D.; Welling, D.B.; Stankovic, K.M. MMP-14 (MT1-MMP) Is a Biomarker of Surgical Outcome and a Potential Mediator of Hearing Loss in Patients with Vestibular Schwannomas. Front. Cell. Neurosci. 2020, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Pacios, M.; Martín-Fernández, I.; Villa, R.; Godignon, P.; Del Valle, M.; Bartrolí, J.; Esplandiu, M.J. Carbon nanotubes as suitable electrochemical platforms for metalloprotein sensors and genosensors. In Carbon Nanotubes-Growth and Applications; IntechOpen: London, UK, 2011. [Google Scholar]
- Remacle, A.G.; Golubkov, V.S.; Shiryaev, S.A.; Dahl, R.; Stebbins, J.L.; Chernov, A.V.; Cheltsov, A.V.; Pellecchia, M.; Strongin, A.Y. Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res. 2012, 72, 2339–2349. [Google Scholar] [CrossRef]
- Piccard, H.; Van den Steen, P.E.; Opdenakker, G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J. Leukoc. Biol. 2007, 81, 870–892. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and function of human matrix metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef]
- Ma, F.; Zhu, Y.; Chen, Y.; Liu, J.; Zeng, X. Labeled and non-label electrochemical peptide inhibitor-based biosensing platform for determination of hemopexin domain of matrix metalloproteinase-14. Talanta 2019, 194, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Mathew, M.R.; Sam, S.; Keerthi, K.; Kumar, K.G. Recent advances and challenges in electrochemical biosensors for emerging and re-emerging infectious diseases. J. Electroanal. Chem. 2020, 878, 114596. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Nanomaterials in electrochemical biosensors for pesticide detection: Advances and challenges in food analysis. Microchim. Acta 2016, 183, 2063–2083. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical sensors for clinic analysis. Sensors 2008, 8, 2043–2081. [Google Scholar] [CrossRef]
- Piletsky, S.A.; Turner, A.P. Electrochemical sensors based on molecularly imprinted polymers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2002, 14, 317–323. [Google Scholar] [CrossRef]
- MT1-MMP Inhibitor, NSC405020-Calbiochem. Available online: https://www.emdmillipore.com/CA/en/product/MT1-MMP-Inhibitor-NSC405020-Calbiochem,EMD_BIO-444295 (accessed on 7 March 2022).
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Tang, Q.-Q.; Yang, D.; Hua, W.-M.; Yue, Y.-H.; Wang, B.-D.; Zhang, X.-H.; Hu, J.-H. Preparation of poly (p-styrenesulfonic acid) grafted multi-walled carbon nanotubes and their application as a solid-acid catalyst. Mater. Chem. Phys. 2011, 126, 310–313. [Google Scholar] [CrossRef]
- Zhu, Z. An overview of carbon nanotubes and graphene for biosensing applications. Nano-Micro Lett. 2017, 9, 25. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.-H.; Huang, X.-J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef]
- Osorio, A.G.; Silveira, I.C.L.; Bueno, V.L.; Bergmann, C.P. H2SO4/HNO3/HCl—Functionalization and its effect on dispersion of carbon nanotubes in aqueous media. Appl. Surf. Sci. 2008, 255, 2485–2489. [Google Scholar] [CrossRef]
- Men, X.H.; Zhang, Z.Z.; Song, H.J.; Wang, K.; Jiang, W. Functionalization of carbon nanotubes to improve the tribological properties of poly (furfuryl alcohol) composite coatings. Compos. Sci. Technol. 2008, 68, 1042–1049. [Google Scholar] [CrossRef]
- Khazaei, A.; Rad, M.N.S.; Borazjani, M.K. Organic functionalization of single-walled carbon nanotubes (SWCNTs) with some chemotherapeutic agents as a potential method for drug delivery. Int. J. Nanomed. 2010, 5, 639. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Stradolini, F.; Abate, G.; Uberti, D.; Serpelloni, M.; Carrara, S.; Sardini, E. Electrochemical detection of different p53 conformations by using nanostructured surfaces. Sci. Rep. 2019, 9, 17347. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Tung, T.T.; Losic, D. Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors 2017, 17, 1919. [Google Scholar] [CrossRef]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta BBA-Biomembr. 2006, 1758, 404–412. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Gao, C.; Jin, Y.Z.; Kong, H.; Whitby, R.L.; Acquah, S.F.; Chen, G.Y.; Qian, H.; Hartschuh, A.; Silva, S.R.P.; Henley, S. Polyurea-functionalized multiwalled carbon nanotubes: Synthesis, morphology, and Raman spectroscopy. J. Phys. Chem. B 2005, 109, 11925–11932. [Google Scholar] [CrossRef] [PubMed]
- Baibarac, M.; Baltog, I.; Godon, C.; Lefrant, S.; Chauvet, O. Covalent functionalization of single-walled carbon nanotubes by aniline electrochemical polymerization. Carbon 2004, 42, 3143–3152. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Al-Tawbini, B.; Bukhari, A.A.; Abuilaiwi, F.A.; Fettouhi, M.B. Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water. Bioinorg. Chem. Appl. 2010, 2010, 603978. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, J. Infrared Tables (short summary of common absorption frequencies). Course Notes 2004, 2620, 19. [Google Scholar]
- Guo, Y.; Li, Y.; Wang, J.; Zhu, T.; Ye, M. Effects of activated carbon properties on chlorobenzene adsorption and adsorption product analysis. Chem. Eng. J. 2014, 236, 506–512. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Guan, Y.; Ying, P.; Li, C. FT-IR spectroscopic study of the oxidation of chlorobenzene over Mn-based catalyst. Langmuir 2002, 18, 6229–6232. [Google Scholar] [CrossRef]
- Pei, Z.; Li, L.; Sun, L.; Zhang, S.; Shan, X.; Yang, S.; Wen, B. Adsorption characteristics of 1, 2, 4-trichlorobenzene, 2, 4, 6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon 2013, 51, 156–163. [Google Scholar] [CrossRef]
- Albert, S.; Keppler, K.; Lerch, P.; Quack, M.; Wokaun, A. Synchrotron-based highest resolution FTIR spectroscopy of chlorobenzene. J. Mol. Spectrosc. 2015, 315, 92–101. [Google Scholar] [CrossRef]
- Liao, L.-F.; Lien, C.-F.; Shieh, D.-L.; Chen, F.-C.; Lin, J.-L. FTIR study of adsorption and photochemistry of amide on powdered TiO 2: Comparison of benzamide with acetamide. Phys. Chem. Chem. Phys. 2002, 4, 4584–4589. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z.; Han, H. A novel impedance enhancer for amperometric biosensor based ultrasensitive detection of matrix metalloproteinase-2. Bioelectrochemistry 2019, 130, 107324. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ma, J.; Kong, D.; Zhang, Z.; Khan, A.; Yi, C.; Hu, K.; Yi, Y.; Li, J. One step electrochemical detection for matrix metalloproteinase 2 based on anodic stripping of silver nanoparticles mediated by host-guest interactions. Sens. Actuators B Chem. 2021, 330, 129379. [Google Scholar] [CrossRef]
- Munge, B.S.; Fisher, J.; Millord, L.N.; Krause, C.E.; Dowd, R.S.; Rusling, J.F. Sensitive electrochemical immunosensor for matrix metalloproteinase-3 based on single-wall carbon nanotubes. Analyst 2010, 135, 1345–1350. [Google Scholar] [CrossRef]
- Palomar, Q.; Xu, X.; Selegård, R.; Aili, D.; Zhang, Z. Peptide decorated gold nanoparticle/carbon nanotube electrochemical sensor for ultrasensitive detection of matrix metalloproteinase-7. Sens. Actuators B Chem. 2020, 325, 128789. [Google Scholar] [CrossRef]
- Yaiwong, P.; Semakul, N.; Bamrungsap, S.; Jakmunee, J.; Ounnunkad, K. Electrochemical detection of matrix metalloproteinase-7 using an immunoassay on a methylene blue/2D MoS2/graphene oxide electrode. Bioelectrochemistry 2021, 142, 107944. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, L.-Y.; Tseng, F.-Y.; Tan, Y.-C.; Li, J.; Feng, L.; Song, L.; Lai, C.-F.; Li, X.; He, J.-H. Label-free electrochemical immunosensor based on gold nanoparticle/polyethyleneimine/reduced graphene oxide nanocomposites for the ultrasensitive detection of cancer biomarker matrix metalloproteinase-1. Analyst 2021, 146, 4066–4079. [Google Scholar] [CrossRef] [PubMed]
- Nohle, D.G.; Mandt, R.L.; Couce, M.E.; Parwani, A.V.; Ayers, L.W. Acceptable Weight Ranges for Research Tissue Procurement and Biorepositories, 2015–2017. Biopreservation Biobanking 2018, 16, 463–466. [Google Scholar] [CrossRef]
- Johnson, D.K.; Karanicolas, J. Druggable protein interaction sites are more predisposed to surface pocket formation than the rest of the protein surface. PLoS Comput. Biol. 2013, 9, e1002951. [Google Scholar] [CrossRef]
- Brinckerhoff, C.E.; Rutter, J.L.; Benbow, U. Interstitial collagenases as markers of tumor progression. Clin. Cancer Res. 2000, 6, 4823–4830. [Google Scholar]
- Bendardaf, R.; Buhmeida, A.; Ristamäki, R.; Syrjänen, K.; Pyrhönen, S. MMP-1 (collagenase-1) expression in primary colorectal cancer and its metastases. Scand. J. Gastroenterol. 2007, 42, 1473–1478. [Google Scholar] [CrossRef]

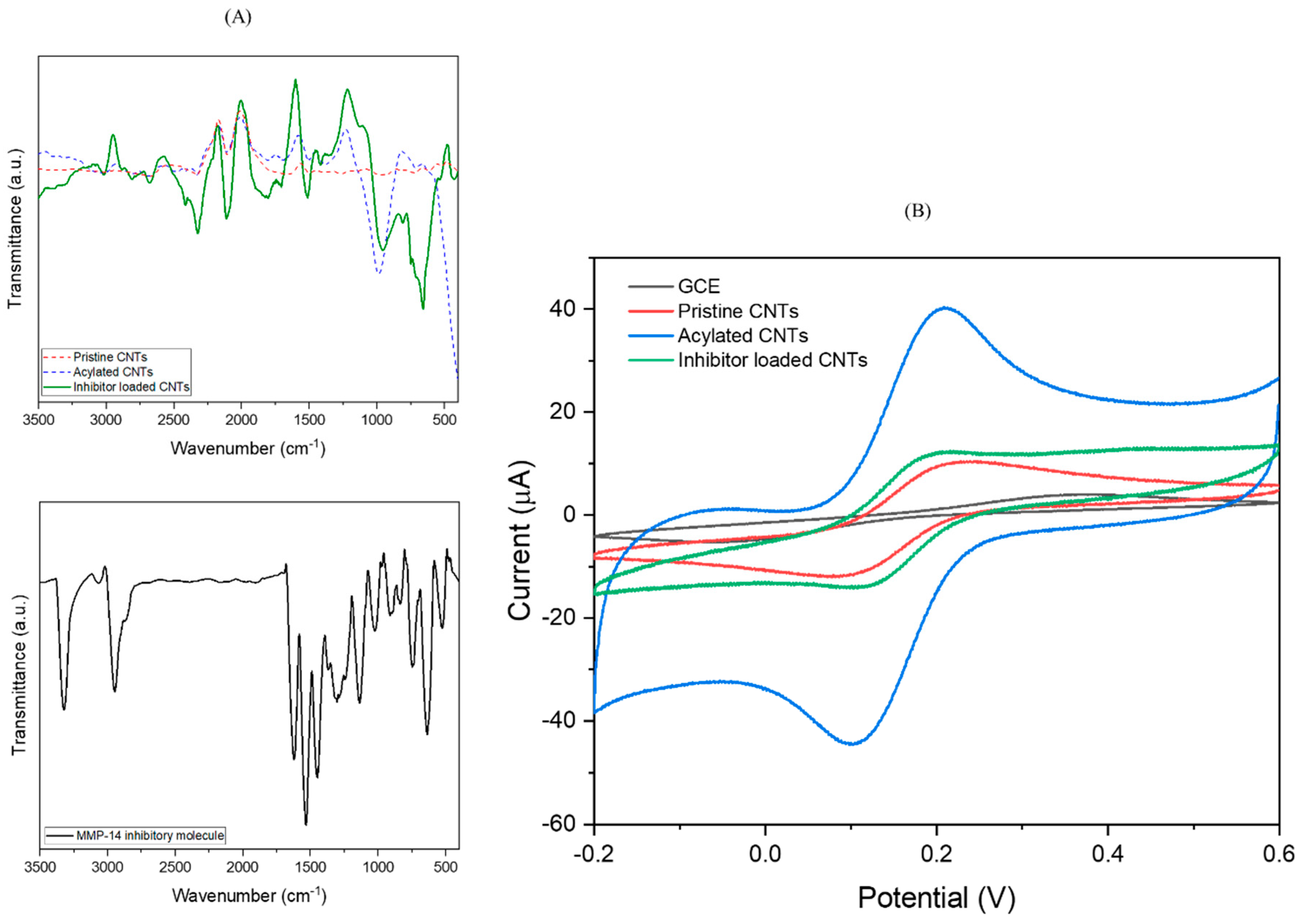
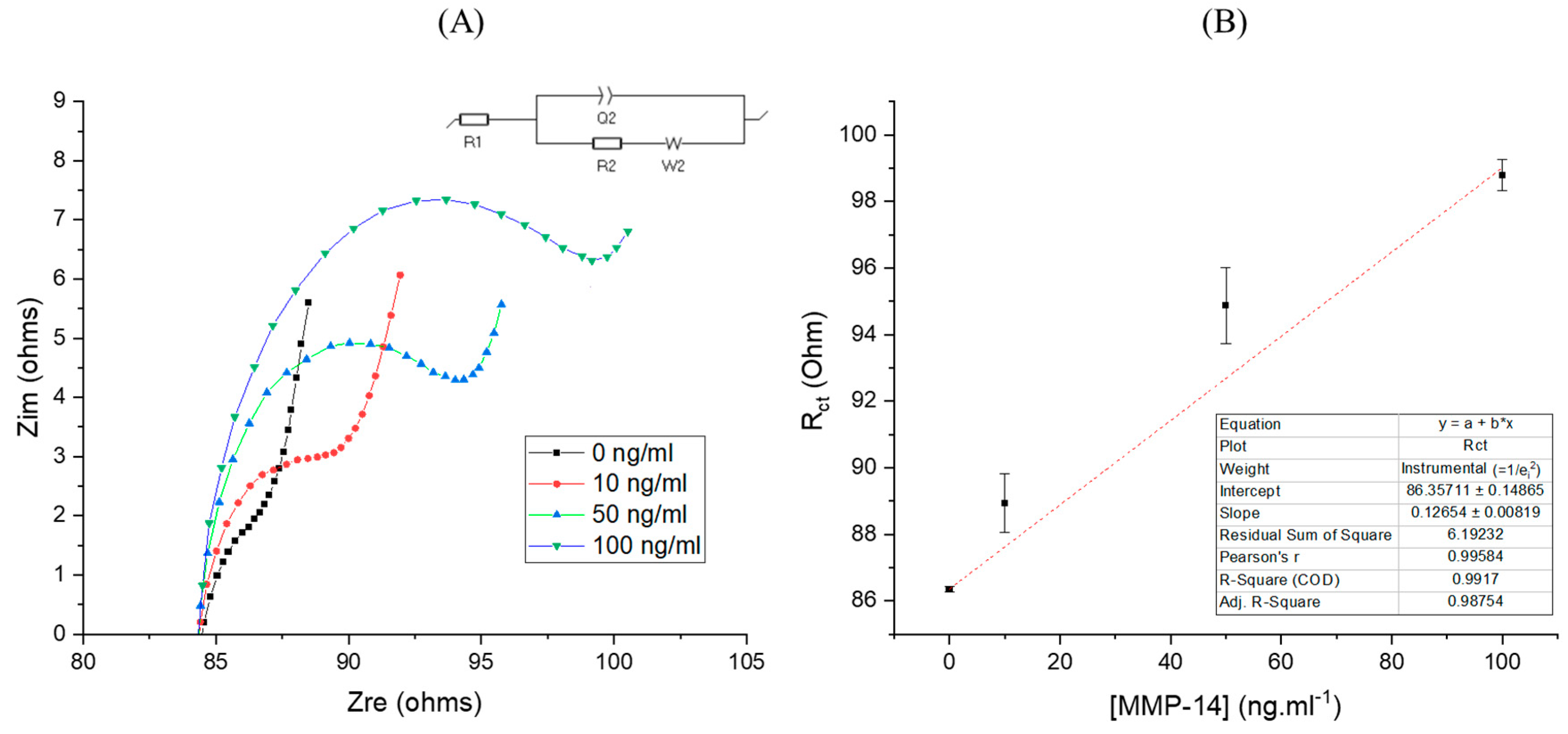
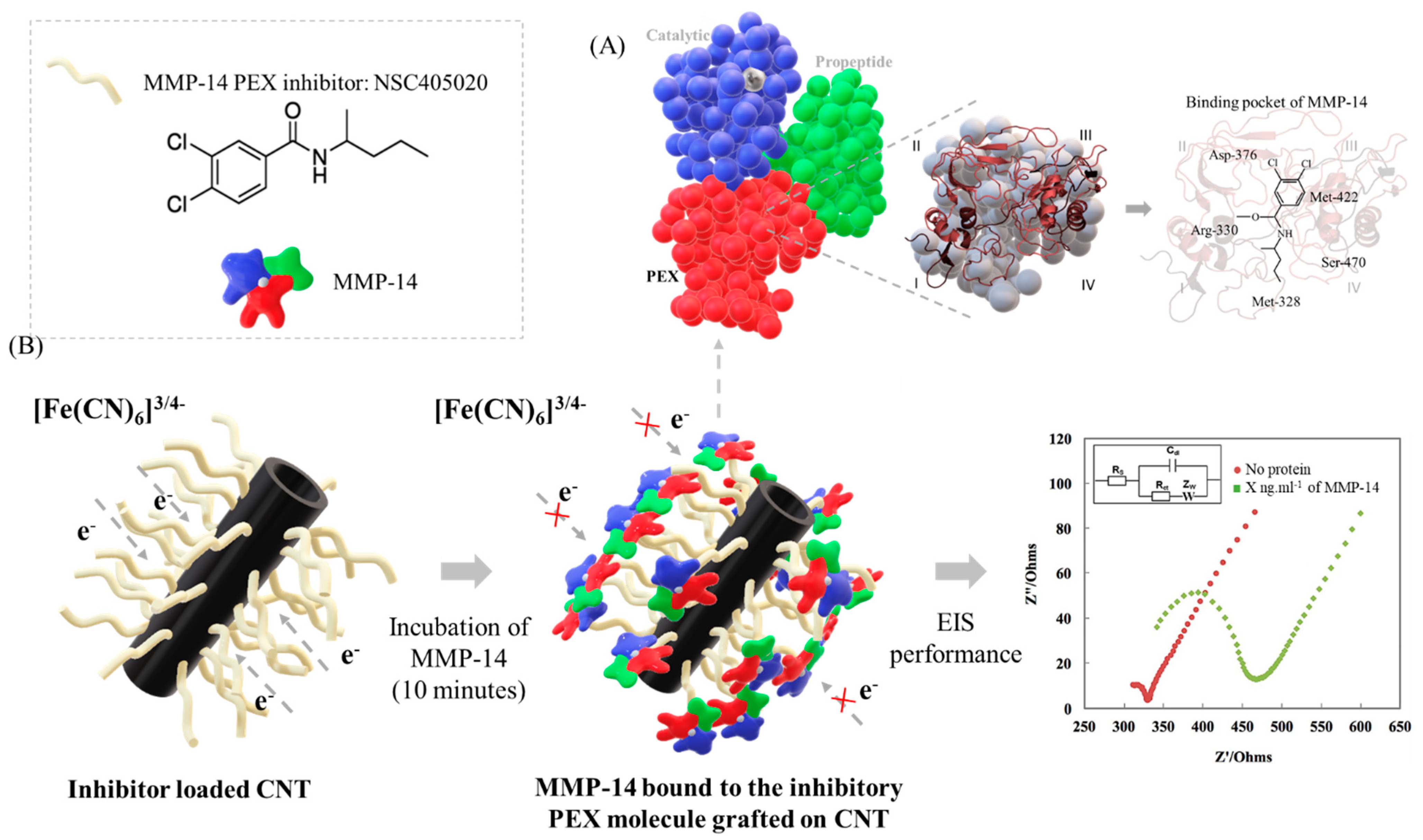
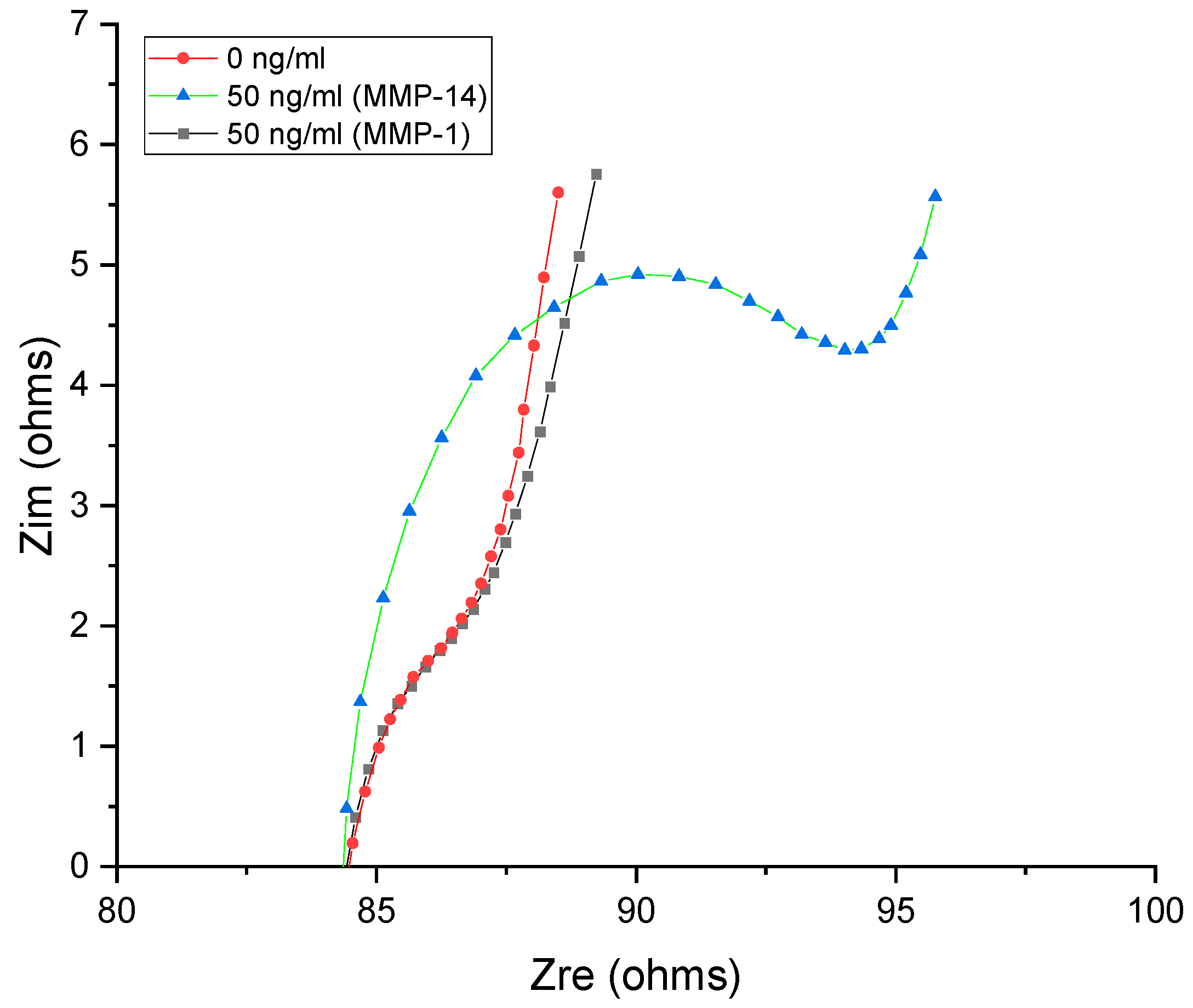
| Type of Cancer | Sample | MMP-14 Level (Healthy) | MMP-14 Level (Non-Healthy) | Ref. |
|---|---|---|---|---|
| Breast | Serum Cell | 8.55 ± 1.66 ng⋅mL−1 - | 16.91 ± 5.87 ng⋅mL−1 ~26.7 ng⋅mg−1 * | [32,33] |
| Head & Neck | Tissue Cell | 0.80 ± 1.1 ng⋅mL−1 - | 5.00 ± 4.3 ng⋅mg−1 ~14.5 ng⋅mg−1 * | [33] |
| Fibrosarcoma | Tissue Cell | <0.01 ng⋅mg−1 * - | ~1.12 ng⋅mg−1 * ~36.1 ng⋅mg−1 * | [33,34] |
| Prostate | Tissue | <0.01 ng⋅mg−1 * | 0.6 ± 0.05 ng⋅mg−1 | [34] |
| Gastric | Tissue | ~1.34 ng⋅mg−1 * | ~3.57 ng⋅mg−1 * | [35] |
| Bladder | Tissue | 7.45 ± 1.18 ng⋅mg−1 | 81.78 ± 9.87 ng⋅mg−1 | [36] |
| Ovarian | Serum | ~6 ng⋅mL−1 * | ~12 ng⋅mL−1 * | [37] |
| Brain | Tissue | ~1 ng⋅mg−1 * | ~10 ng⋅mg−1 * | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, D.; Barralet, J.; Harvey, E.J.; Merle, G. Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs. Biosensors 2022, 12, 884. https://doi.org/10.3390/bios12100884
Vieira D, Barralet J, Harvey EJ, Merle G. Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs. Biosensors. 2022; 12(10):884. https://doi.org/10.3390/bios12100884
Chicago/Turabian StyleVieira, D., J. Barralet, E. J. Harvey, and G. Merle. 2022. "Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs" Biosensors 12, no. 10: 884. https://doi.org/10.3390/bios12100884
APA StyleVieira, D., Barralet, J., Harvey, E. J., & Merle, G. (2022). Detecting the PEX Like Domain of Matrix Metalloproteinase-14 (MMP-14) with Therapeutic Conjugated CNTs. Biosensors, 12(10), 884. https://doi.org/10.3390/bios12100884






