Super-Long SERS Active Single Silver Nanowires for Molecular Imaging in 2D and 3D Cell Culture Models
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis of Single AgNWs
2.2. SERS Measurements on Single AgNWs
2.3. Cell Culture
2.4. Principal Component Analysis
2.5. Random Forest and K-Means++ Clustering Algorithm
3. Results and Discussion
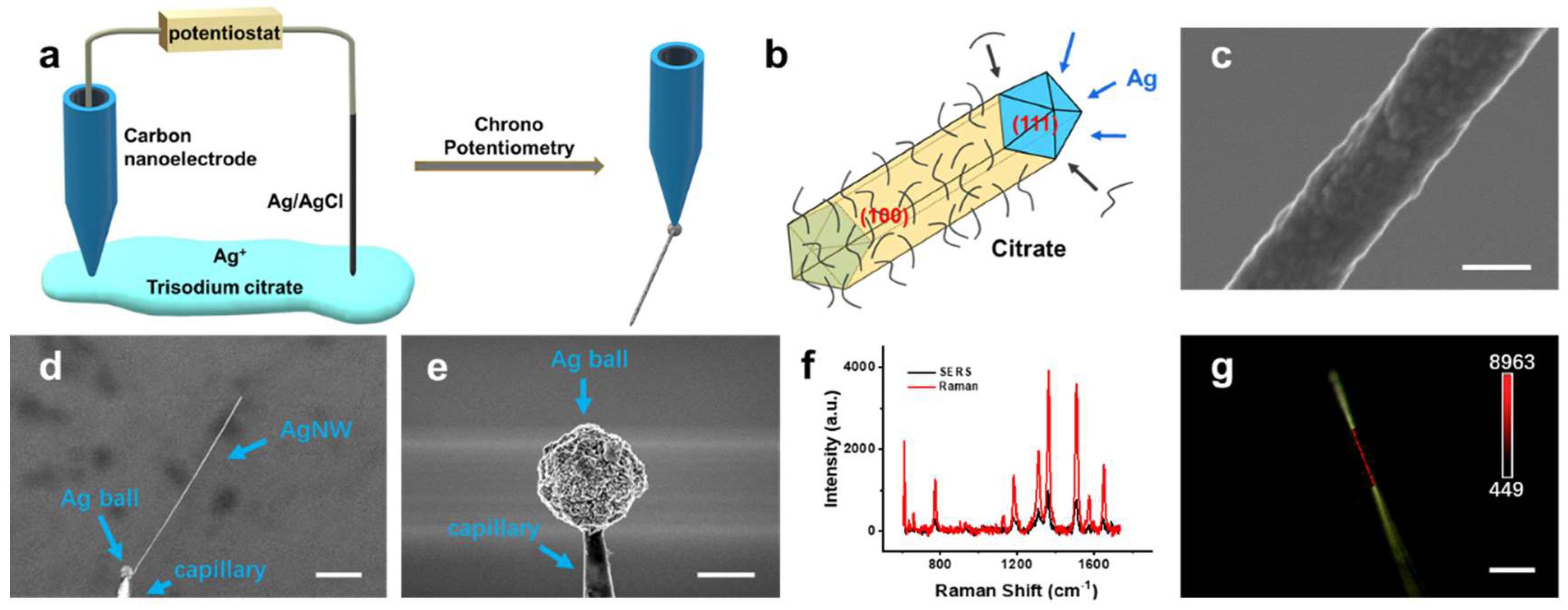
3.1. Synthesis and Characterization of Single AgNWs
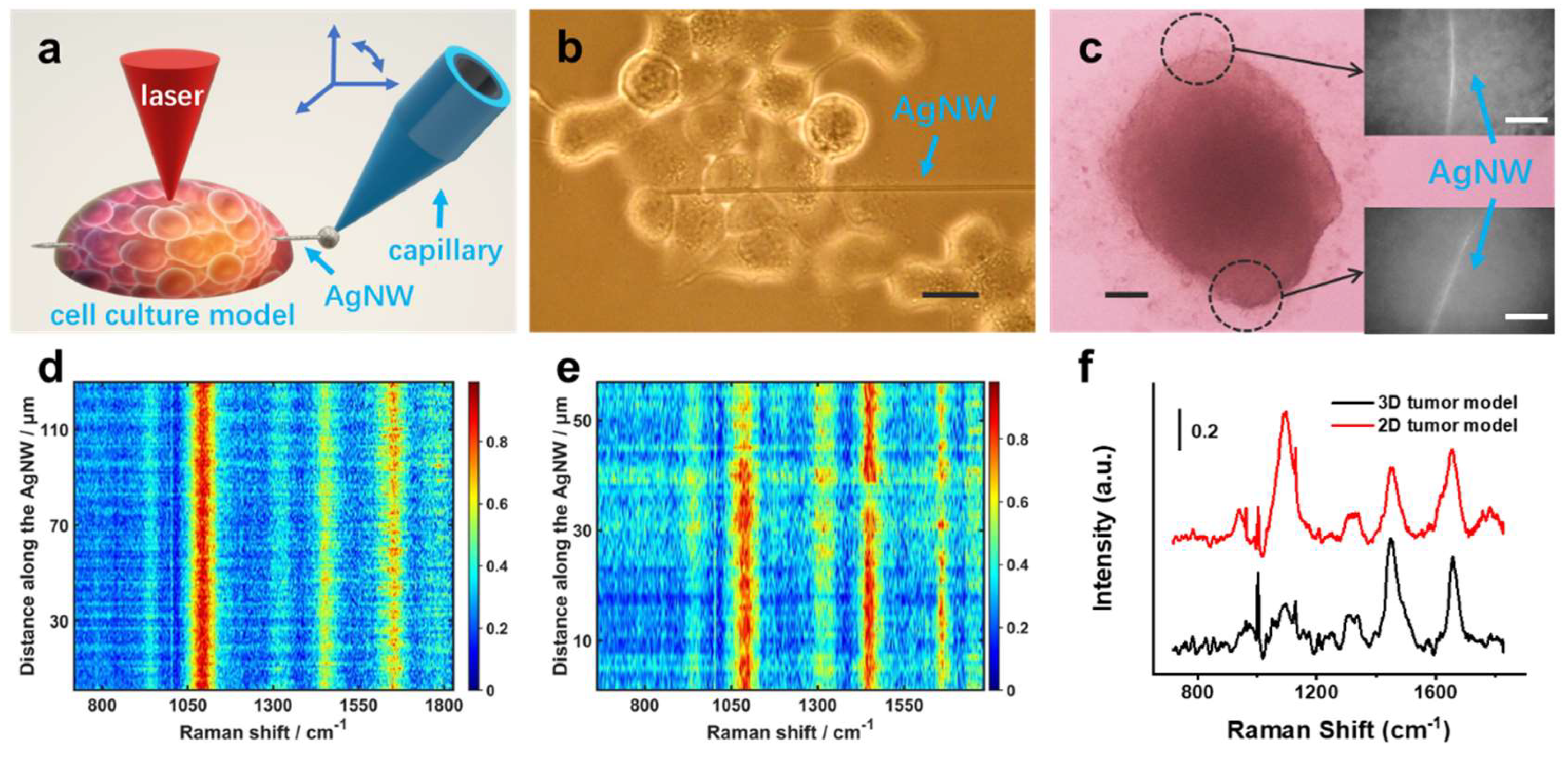
3.2. Raman Spectra Acquisition in 2D and 3D Models
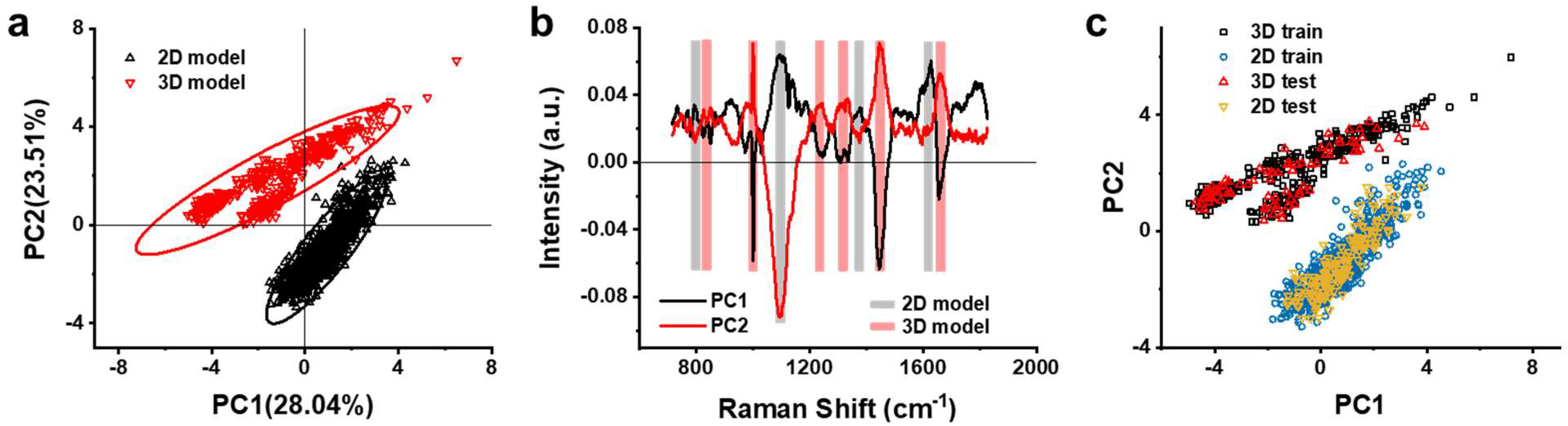
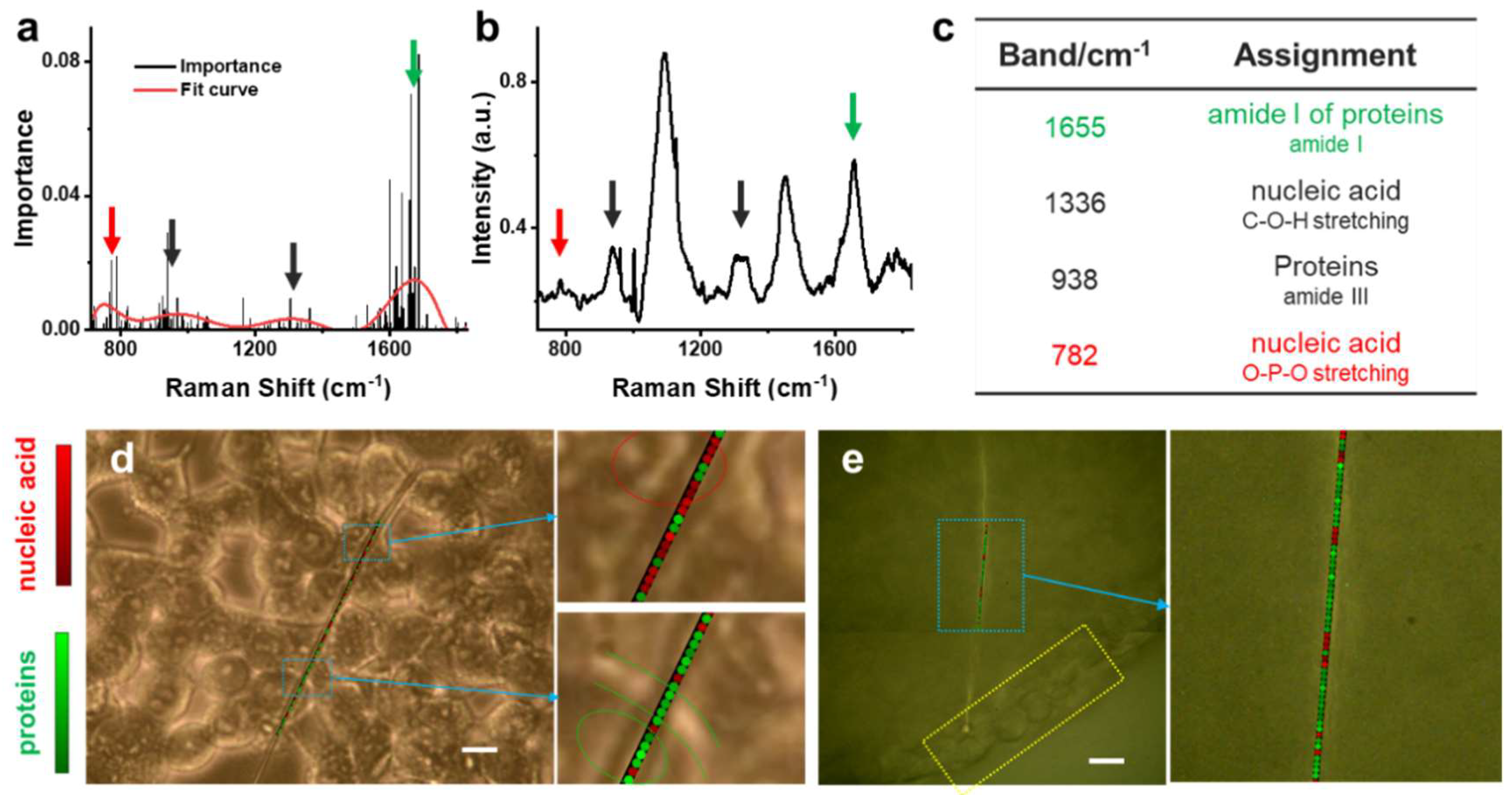
3.3. Multivariate Analysis of Raman Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Associated Content
Conflicts of Interest
References
- Niu, N.; Wang, L. In vitro human cell line models to predict clinical response to anticancer drugs. Pharmacogenomics 2015, 16, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2012, 164, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Osuna de la Peña, D.; Trabulo, S.M.D.; Collin, E.; Liu, Y.; Sharma, S.; Tatari, M.; Behrens, D.; Erkan, M.; Lawlor, R.T.; Scarpa, A.; et al. Bioengineered 3D models of human pancreatic cancer recapitulate in vivo tumour biology. Nat. Commun. 2021, 12, 5623. [Google Scholar] [CrossRef]
- Carter, E.P.; Roozitalab, R.; Gibson, S.V.; Grose, R.P. Tumour microenvironment 3D-modelling: Simplicity to complexity and back again. Trends Cancer 2021, 7, 1033–1046. [Google Scholar] [CrossRef]
- Tuveson, D.; Clevers, H. Cancer modeling meets human organoid technology. Science 2019, 364, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Liu, T.; Liao, J.; Maharjan, S.; Xie, X.; Pérez, M.; Anaya, I.; Wang, S.; Tirado Mayer, A.; Kang, Z.; et al. Reversed-engineered human alveolar lung-on-a-chip model. Proc. Natl. Acad. Sci. USA 2021, 118, e2016146118. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, N.; Sun, X.; Li, Q.; Zeng, Y.; Chen, F.; Sun, S.; Xu, J.; Zhang, J.; Ye, H.; et al. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials 2021, 272, 120770. [Google Scholar] [CrossRef]
- Qin, X.; Sufi, J.; Vlckova, P.; Kyriakidou, P.; Acton, S.E.; Li, V.S.W.; Nitz, M.; Tape, C.J. Cell-type-specific signaling networks in heterocellular organoids. Nat. Methods 2020, 17, 335–342. [Google Scholar] [CrossRef]
- Banh, R.S.; Biancur, D.E.; Yamamoto, K.; Sohn, A.S.W.; Walters, B.; Kuljanin, M.; Gikandi, A.; Wang, H.; Mancias, J.D.; Schneider, R.J.; et al. Neurons release serine to support mRNA translation in pancreatic cancer. Cell 2020, 183, 1202–1218.e25. [Google Scholar] [CrossRef]
- Han, K.; Pierce, S.E.; Li, A.; Spees, K.; Anderson, G.R.; Seoane, J.A.; Lo, Y.-H.; Dubreuil, M.; Olivas, M.; Kamber, R.A.; et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 2020, 580, 136–141. [Google Scholar] [CrossRef]
- Brazovskaja, A.; Treutlein, B.; Camp, J.G. High-throughput single-cell transcriptomics on organoids. Curr. Opin. Biotechnol. 2019, 55, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Krogager, T.P.; Ernst, R.J.; Elliott, T.S.; Calo, L.; Beránek, V.; Ciabatti, E.; Spillantini, M.G.; Tripodi, M.; Hastings, M.H.; Chin, J.W. Labeling and identifying cell-specific proteomes in the mouse brain. Nat. Biotechnol. 2018, 36, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 2016, 165, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Chioni, A.-M.; Grose, R. FGFR1 cleavage and nuclear translocation regulates breast cancer cell behavior. J. Cell Biol. 2012, 197, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Mayr, U.; Boni, A.; Lukonin, I.; Rempfler, M.; Challet Meylan, L.; Stadler, M.B.; Strnad, P.; Papasaikas, P.; Vischi, D.; et al. Self-organization and symmetry breaking in intestinal organoid development. Nature 2019, 569, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Estrada, M.F.; Rebelo, S.P.; Davies, E.J.; Pinto, M.T.; Pereira, H.; Santo, V.E.; Smalley, M.J.; Barry, S.T.; Gualda, E.J.; Alves, P.M.; et al. Modelling the tumor microenvironment in long-term microencapsulated 3D co-cultures recapitulates phenotypic features of disease progression. Biomaterials 2016, 78, 50–61. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Alieva, M.; Wellens, L.M.; Ariese, H.C.R.; Jamieson, P.R.; Vonk, A.M.; Amatngalim, G.D.; Hu, H.; Oost, K.C.; Snippert, H.J.G.; et al. High-resolution 3D imaging of fixed and cleared organoids. Nat. Protoc. 2019, 14, 1756–1771. [Google Scholar] [CrossRef]
- Messal, H.A.; Almagro, J.; Zaw Thin, M.; Tedeschi, A.; Ciccarelli, A.; Blackie, L.; Anderson, K.I.; Miguel-Aliaga, I.; van Rheenen, J.; Behrens, A. Antigen retrieval and clearing for whole-organ immunofluorescence by FLASH. Nat. Protoc. 2021, 16, 239–262. [Google Scholar] [CrossRef]
- Živanović, V.; Seifert, S.; Drescher, D.; Schrade, P.; Werner, S.; Guttmann, P.; Szekeres, G.P.; Bachmann, S.; Schneider, G.; Arenz, C.; et al. Optical nanosensing of lipid accumulation due to enzyme inhibition in live cells. ACS Nano 2019, 13, 9363–9375. [Google Scholar] [CrossRef]
- Živanović, V.; Semini, G.; Laue, M.; Drescher, D.; Aebischer, T.; Kneipp, J. Chemical mapping of leishmania infection in live cells by SERS microscopy. Anal. Chem. 2018, 90, 8154–8161. [Google Scholar] [CrossRef]
- Kallepitis, C.; Bergholt, M.S.; Mazo, M.M.; Leonardo, V.; Skaalure, S.C.; Maynard, S.A.; Stevens, M.M. Quantitative volumetric Raman imaging of three dimensional cell cultures. Nat. Commun. 2017, 8, 14843. [Google Scholar] [CrossRef] [PubMed]
- Aljakouch, K.; Lechtonen, T.; Yosef, H.K.; Hammoud, M.K.; Alsaidi, W.; Kötting, C.; Mügge, C.; Kourist, R.; El-Mashtoly, S.F.; Gerwert, K. Raman microspectroscopic evidence for the metabolism of a tyrosine kinase inhibitor, neratinib, in cancer cells. Angew. Chem. Int. Ed. 2018, 57, 7250–7254. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-enhanced raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Jimenez de Aberasturi, D.; Henriksen-Lacey, M.; Litti, L.; Langer, J.; Liz-Marzán, L.M. Using SERS tags to image the three-dimensional structure of complex cell models. Adv. Funct. Mater. 2020, 30, 1909655. [Google Scholar] [CrossRef]
- Managò, S.; Zito, G.; Rogato, A.; Casalino, M.; Esposito, E.; De Luca, A.C.; De Tommasi, E. Bioderived three-dimensional hierarchical nanostructures as efficient surface-enhanced Raman scattering substrates for cell membrane probing. ACS Appl. Mater. Inter. 2018, 10, 12406–12416. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; García, I.; Charconnet, M.; Astobiza, I.; García-Astrain, C.; Matricardi, C.; Mihi, A.; Carracedo, A.; Liz-Marzán, L.M. Multiplex SERS detection of metabolic alterations in tumor extracellular media. Adv. Funct. Mater. 2020, 30, 1910335. [Google Scholar] [CrossRef]
- Wang, J.; Qi, G.; Qu, X.; Ling, X.; Zhang, Z.; Jin, Y. Molecular profiling of dental pulp stem cells during cell differentiation by surface enhanced Raman spectroscopy. Anal. Chem. 2020, 92, 3735–3741. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-L.; Cao, J.; Hanif, S.; Yuan, C.; Pang, J.; Levicky, R.; Xia, X.-H.; Wang, K. Size-controllable gold nanopores with high SERS Activity. Anal. Chem. 2017, 89, 10407–10413. [Google Scholar] [CrossRef]
- Hanif, S.; Liu, H.; Chen, M.; Muhammad, P.; Zhou, Y.; Cao, J.; Ahmed, S.A.; Xu, J.; Xia, X.; Chen, H.; et al. Organic cyanide decorated SERS active nanopipettes for quantitative detection of hemeproteins and Fe3+ in Single Cells. Anal. Chem. 2017, 89, 2522–2530. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Song, M.S.; Ly, N.H.; Lee, S.Y.; Joo, S.-W. Nanostars on nanopipette tips: A Raman probe for quantifying oxygen levels in hypoxic single cells and tumours. Angew. Chem. Int. Ed. 2019, 58, 2710–2714. [Google Scholar] [CrossRef]
- Liu, J.; He, H.; Xie, D.; Wen, Y.; Liu, Z. Probing low-copy-number proteins in single living cells using single-cell plasmonic immunosandwich assays. Nat. Protoc. 2021, 16, 3522–3546. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.J.; Schrlau, M.G.; Friedman, G.; Gogotsi, Y. Carbon nanotube-tipped endoscope for in situ intracellular surface-enhanced raman spectroscopy. Small 2011, 7, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.-L.; Jiang, H.; Zhang, X.-W.; Qiu, Q.-F.; Tang, Y.; Yang, X.-K.; Liu, Y.-L.; Huang, W.-H. A single nanowire sensor for intracellular glucose detection. Nanoscale 2019, 11, 10702–10708. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Zhang, Y.; Hong, W.; Dong, Y.; Xie, Z.; Quan, Q. Direct Tracking of Amyloid and tau dynamics in neuroblastoma cells using nanoplasmonic fiber tip probes. Nano Lett. 2016, 16, 3989–3994. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.; Zhang, B. Bipolar electrochemical method for dynamic in situ control of single metal nanowire growth. ACS Nano 2015, 9, 2454–2464. [Google Scholar] [CrossRef]
- Li, X.; Yang, T.; Li, S.; Wang, D.; Song, Y.; Zhang, S. Raman spectroscopy combined with principal component analysis and k nearest neighbour analysis for non-invasive detection of colon cancer. LaPhy 2016, 26, 035702. [Google Scholar] [CrossRef]
- Reokrungruang, P.; Chatnuntawech, I.; Dharakul, T.; Bamrungsap, S. A simple paper-based surface enhanced Raman scattering (SERS) platform and magnetic separation for cancer screening. Sensor. Actuat. B Chem. 2019, 285, 462–469. [Google Scholar] [CrossRef]
- Genuer, R.; Poggi, J.-M.; Tuleau-Malot, C. Variable selection using Random Forests. Pattern Recog. Lett. 2010, 31, 2225–2236. [Google Scholar] [CrossRef]
- Arthur, D.; Vassilvitskii, S. k-means++: The advantages of careful seeding. In Proceedings of the Eighteenth Annual ACM-SIAM Symposium on Discrete Algorithms, New Orleans, LA, USA, 7–9 January 2007; pp. 1027–1035. [Google Scholar]
- Pan, X.-T.; Liu, Y.-Y.; Qian, S.-Q.; Yang, J.-M.; Li, Y.; Gao, J.; Liu, C.-G.; Wang, K.; Xia, X.-H. Free-standing single Ag nanowires for multifunctional optical probes. ACS Appl. Mater. Inter. 2021, 13, 19023–19030. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Nam, J.-S.; Shin, H.; Park, J.; Song, H.-I.; Kang, M.; Lim, K.-I.; Choi, Y. Identification of Newly Emerging Influenza Viruses by Detecting the virally infected cells based on surface enhanced Raman spectroscopy and principal component analysis. Anal. Chem. 2019, 91, 5677–5684. [Google Scholar] [CrossRef]
- Bai, C.; Yang, M.; Fan, Z.; Li, S.; Gao, T.; Fang, Z. Associations of chemo- and radio-resistant phenotypes with the gap junction, adhesion and extracellular matrix in a three-dimensional culture model of soft sarcoma. J. Exp. Clin. Cancer Res. 2015, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.-F.; Xu, R. Function of cancer cell-derived extracellular matrix in tumor progression. J. Cancer Metastasis Treat. 2016, 2, 357–364. [Google Scholar] [CrossRef]
| Bands (2D) [cm−1] | Bands (3D) [cm−1] | Tentative Assignment |
|---|---|---|
| 1782 | 1785 | Lipids, C=O stretching |
| 1655 | 1657 | Proteins, amide I |
| 1449 | 1449 | Lipids, -CH2- deformation |
| 1306~1339 | 1306~1338 | Lipids, proteins & nucleic acid |
| 1128 | 1128 | Proteins, C-N and C-C stretching |
| 1097 | 1093 | Phospholipids, C-C stretching |
| 1003 | 1002 | Amino acid, Phe |
| 937 | 938 | Proteins, amide III |
| 782 | 782 | Nucleic acid, O-P-O stretching |
| Bands [cm−1] | 2D Model | 3D Model |
|---|---|---|
| 1662 | Proteins, amide I | |
| 1628 | Lipids, C=C stretching | |
| 1448 | Lipids, -CH2- deformation | |
| 1377 | Lipids, -CH3- deformation | |
| 1336 | Nucleic acid, C-O-H stretching | |
| 1246 | Proteins, amide III | |
| 1096 | Phospholipids, C-C stretching | |
| 1002 | Amino acid, Phe | |
| 851 | Amino acid, Tyr | |
| 794 | Nucleic acid, O-P-O stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, X.-T.; Yang, X.-Y.; Mao, T.-Q.; Liu, K.; Chen, Z.-Z.; Ji, L.-N.; Jiang, D.-C.; Wang, K.; Gu, Z.-Z.; Xia, X.-H. Super-Long SERS Active Single Silver Nanowires for Molecular Imaging in 2D and 3D Cell Culture Models. Biosensors 2022, 12, 875. https://doi.org/10.3390/bios12100875
Pan X-T, Yang X-Y, Mao T-Q, Liu K, Chen Z-Z, Ji L-N, Jiang D-C, Wang K, Gu Z-Z, Xia X-H. Super-Long SERS Active Single Silver Nanowires for Molecular Imaging in 2D and 3D Cell Culture Models. Biosensors. 2022; 12(10):875. https://doi.org/10.3390/bios12100875
Chicago/Turabian StylePan, Xiao-Tong, Xuan-Ye Yang, Tian-Qi Mao, Kang Liu, Zao-Zao Chen, Li-Na Ji, De-Chen Jiang, Kang Wang, Zhong-Ze Gu, and Xing-Hua Xia. 2022. "Super-Long SERS Active Single Silver Nanowires for Molecular Imaging in 2D and 3D Cell Culture Models" Biosensors 12, no. 10: 875. https://doi.org/10.3390/bios12100875
APA StylePan, X.-T., Yang, X.-Y., Mao, T.-Q., Liu, K., Chen, Z.-Z., Ji, L.-N., Jiang, D.-C., Wang, K., Gu, Z.-Z., & Xia, X.-H. (2022). Super-Long SERS Active Single Silver Nanowires for Molecular Imaging in 2D and 3D Cell Culture Models. Biosensors, 12(10), 875. https://doi.org/10.3390/bios12100875






