Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen
Abstract
1. Introduction
2. Experimental
2.1. Equipment
2.2. Solvents and Chemicals
2.3. Synthesis of Ni-Co Layered Double Hydroxide Hollow Nanostructures
2.4. Preparation of the Ni-Co LDH/SPE Sensor
2.5. Real Samples Preparation
3. Results and Discussion
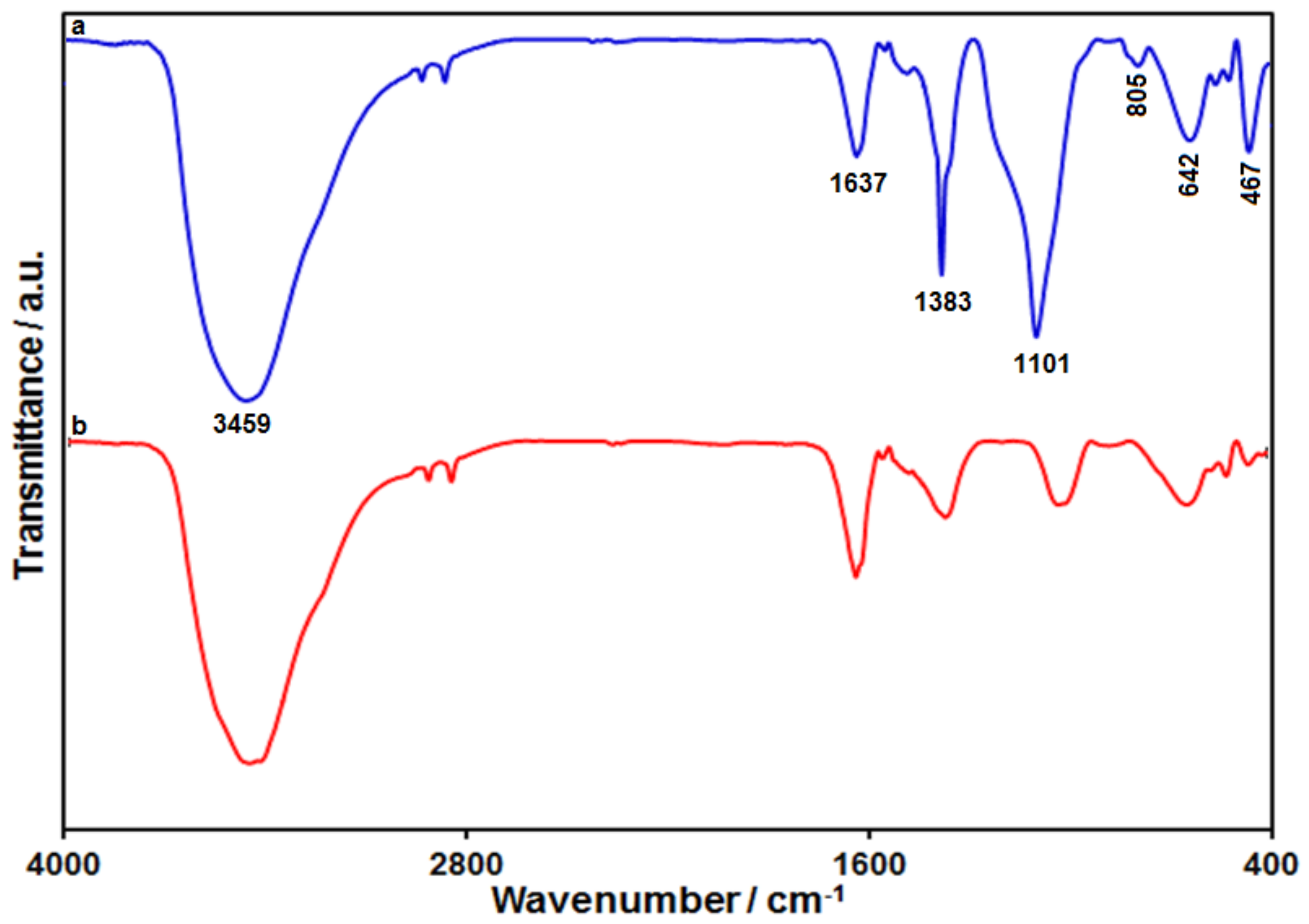
3.1. Characterization of Ni-Co Layered Double Hydroxide Hollow Nanostructures
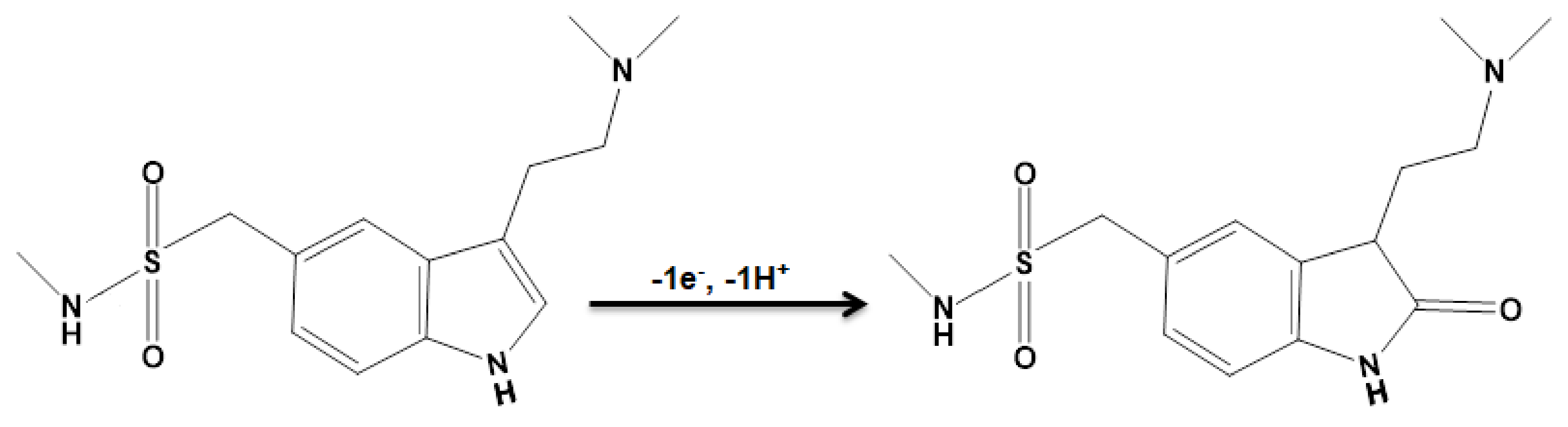
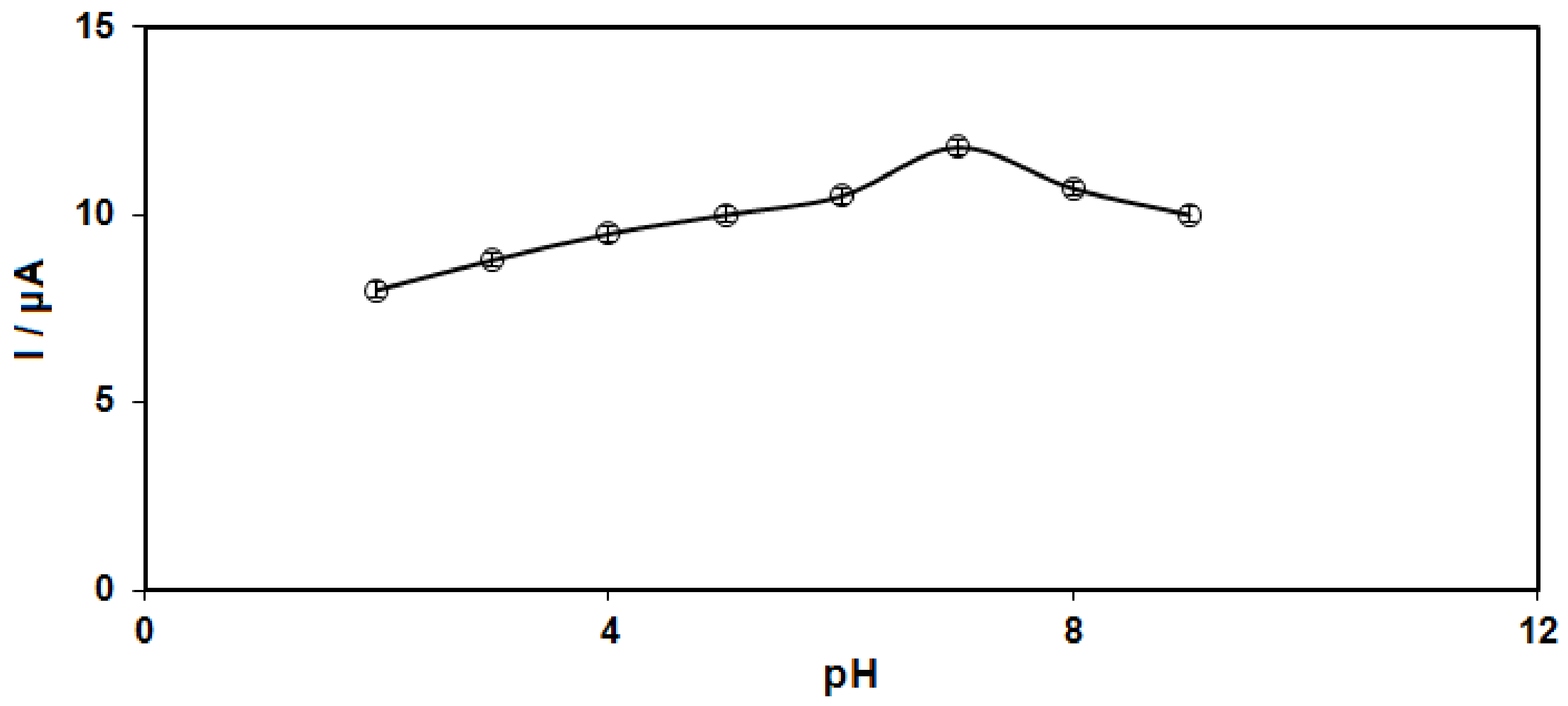
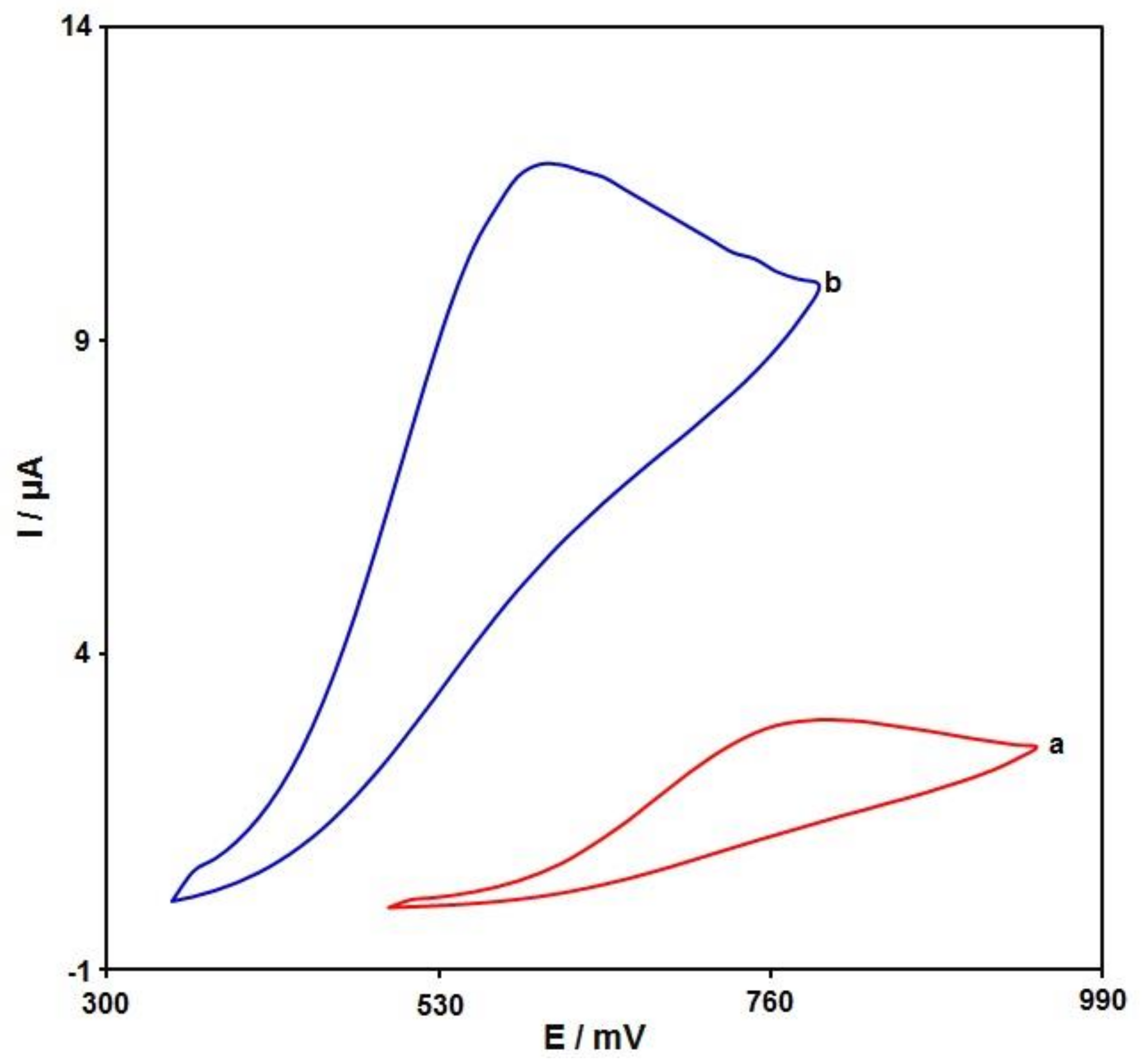
3.2. Studying the Influence on the Structures on Voltammetric Detection of Sumatriptan Oxidation
3.3. Effect of Scan Rate
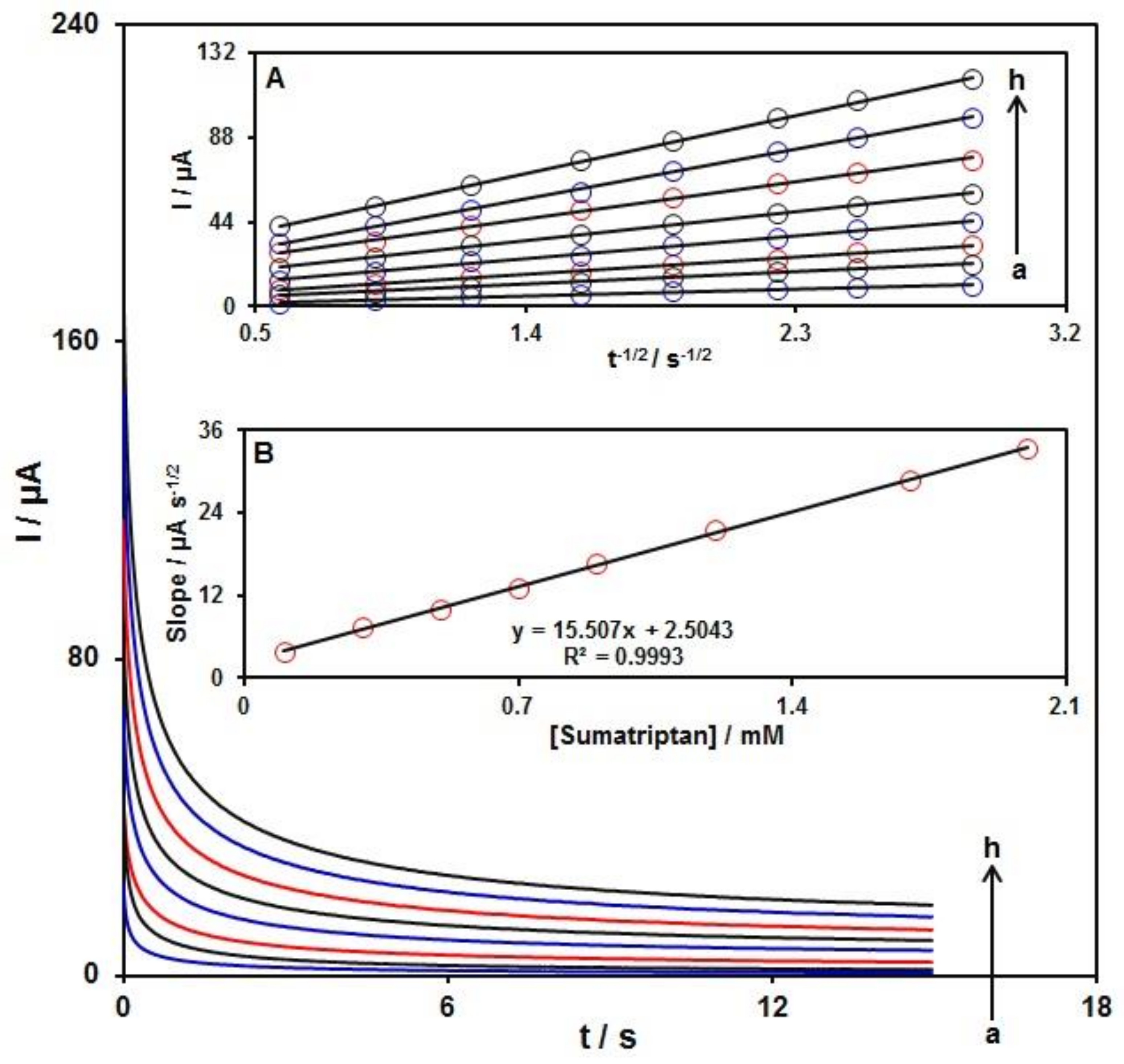
3.4. Chronoamperometric Analysis
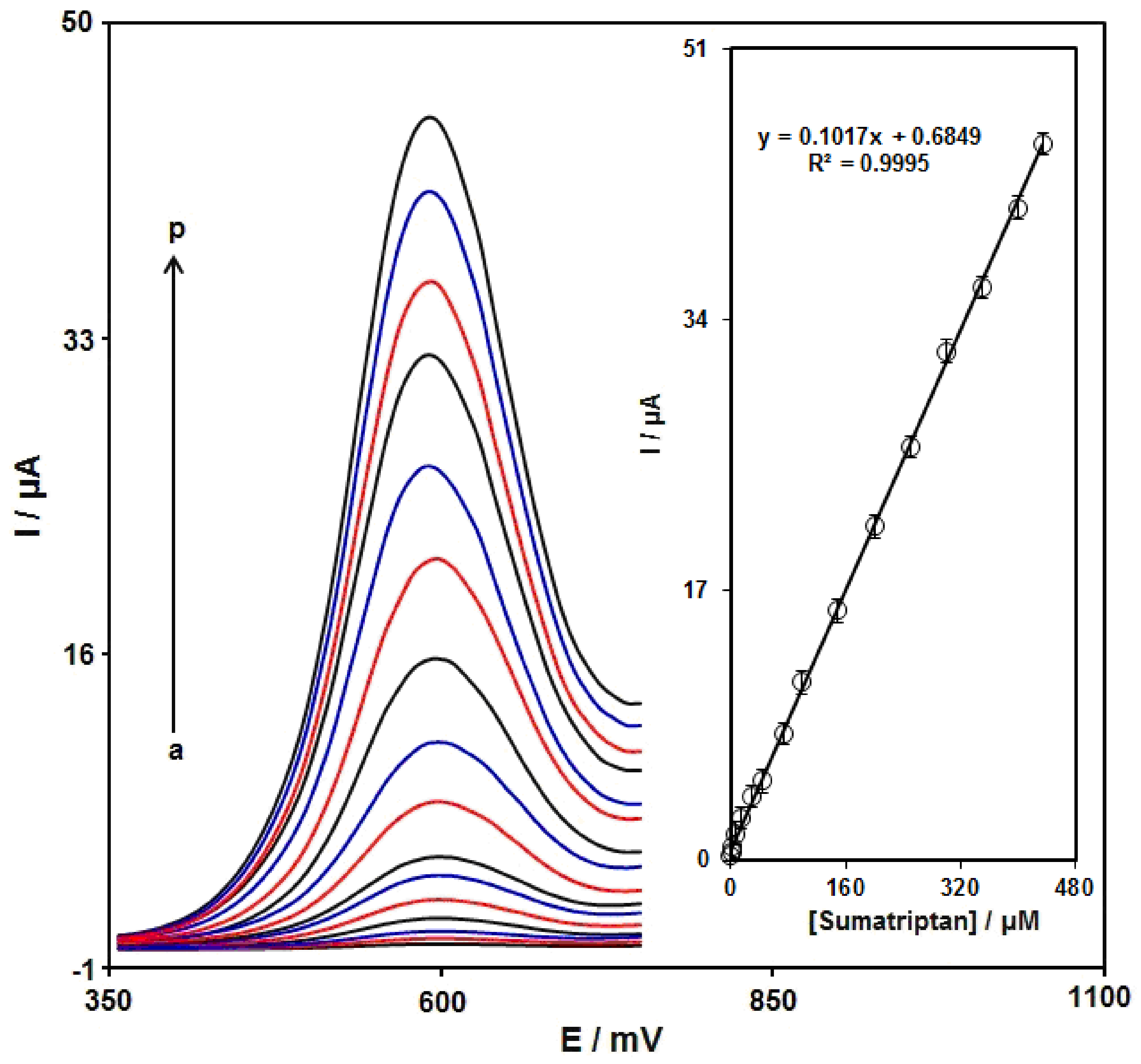
3.5. DPV Analysis of Sumatriptan
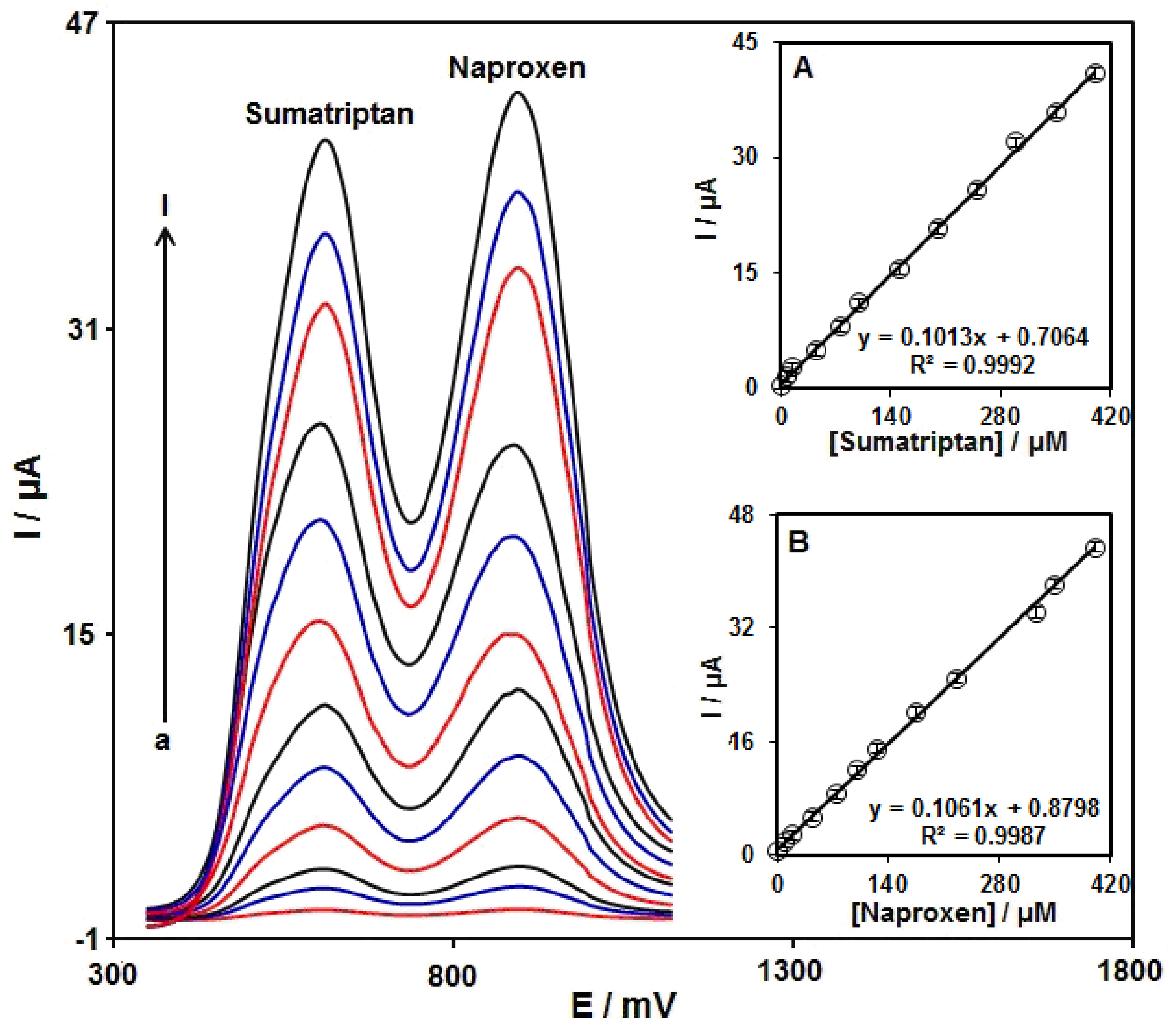
3.6. DPV Analysis of Sumatriptan in the Presence of Naproxen
3.7. Repeatability, Reproducibility, and Stability
3.8. Selectivity Studies
3.9. Analysis of Real Specimens
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coukell, A.J.; Lamb, H.M. Sumatriptan. Pharmacoeconomics 1997, 11, 473–490. [Google Scholar] [CrossRef] [PubMed]
- Kerry, L.D.; Stephen, P.C. Sumatriptan-a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs 1992, 43, 776–791. [Google Scholar]
- Wichitnithad, W.; Nantaphol, S.; Vicheantawatchai, P.; Kiatkumjorn, T.; Wangkangwan, W.; Rojsitthisak, P. Development and validation of liquid chromatography-tandem mass spectrometry method for simple analysis of sumatriptan and its application in bioequivalence study. Pharmaceuticals 2020, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, A.M.; Khalifa, M.E.; Munshi, A.M.; Shah, R.; Keshk, A.A.; Saad, F.; El-Metwaly, N.M. Copper oxide based disposable sensors for sensitive voltammetric assay of sumatriptan. Int. J. Electrochem. Sci. 2021, 16, 210540. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Lipton, R.B.; Ferrari, M.D. Migraine-current understanding and treatment. N. Engl. J. Med. 2002, 346, 257–270. [Google Scholar] [CrossRef]
- Dodick, D.W.; Lipton, R.B.; Goadsby, P.J.; Tfelt-Hansen, P.; Ferrari, M.D.; Diener, H.C.; Parsons, B. Predictors of migraine headache recurrence: A pooled analysis from the eletriptan database. Headache 2008, 48, 184–193. [Google Scholar] [CrossRef]
- Todd, P.A.; Clissold, S.P. Naproxen. A reappraisal of its pharmacology, and therapeutic use in rheumatic diseases and pain states. Drugs 1990, 40, 91–137. [Google Scholar] [CrossRef]
- Doomra, R.; Goyal, A. NSAIDs and self-medication: A serious concern. J. Family Med. Prim. Care 2020, 9, 2183. [Google Scholar] [CrossRef]
- Sarhangzadeh, K. Application of multi wall carbon nanotube–graphene hybrid for voltammetric determination of naproxen. J. Iran. Chem. Soc. 2015, 12, 2133–2140. [Google Scholar] [CrossRef]
- Smith, T.R.; Sunshine, A.; Stark, S.R.; Littlefield, D.E.; Spruill, S.E.; Alexander, W.J. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache 2005, 45, 983–991. [Google Scholar] [CrossRef]
- Solanki, S.D.; Patel, P.U.; Suhagiya, B.N. Development and validation of spectrophotometric method for simultaneous estimation of sumatriptan succinate and naproxen sodium in pharmaceutical dosage form. J. Pharm. Sci. Biosci. Res. 2011, 1, 50–53. [Google Scholar]
- Holzbecher, M.; Ellenberger, H.A.; Marsh, J.M.; Boudreau, S. An ultraviolet spectrophotometric procedure for the routine determination of naproxen. Clin. Biochem. 1979, 12, 66–67. [Google Scholar] [CrossRef]
- Altria, K.D.; Filbey, S.D. Quantitative determination of sumatriptan by capillary electrophoresis. Anal. Proc. 1993, 30, 363–365. [Google Scholar]
- Fillet, M.; Fotsing, L.; Bonnard, J.; Crommen, J. Stereoselective determination of S-naproxen in tablets by capillary electrophoresis. J. Pharmaceut. Biomed. Anal. 1998, 18, 799–805. [Google Scholar] [CrossRef]
- Majithiya, R.J.; Majithiya, J.B.; Umrethia, M.L.; Ghosh, P.K.; Murthy, R.S.R. HPLC method for the determination of sumatriptan in plasma and brain tissue. Ars. Pharm. 2006, 47, 199–210. [Google Scholar]
- Muneer, S.; Muhammad, I.N.; Abrar, M.A.; Munir, I.; Kaukab, I.; Sagheer, A.; Sultana, K. High Performance Liquid Chromatographic Determination of Naproxen in Prepared Pharmaceutical Dosage Form and Human Plasma and its Application to Pharmacokinetic Study. J. Chromatogr. Sep. Tech. 2017, 8, 1–5. [Google Scholar] [CrossRef]
- Oxford, J.; Lant, M.S. Development and validation of a liquid chromatographic-mass spectrometric assay for the determination of sumatriptan in plasma. J. Chromatogr. B Biomed. Sci. Appl. 1989, 496, 137–146. [Google Scholar] [CrossRef]
- Damiani, P.C.; Borraccetti, M.D.; Olivieri, A.C. Direct and simultaneous spectrofluorometric determination of naproxen and salicylate in human serum assisted by chemometric analysis. Anal. Chim. Acta 2002, 471, 87–96. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Flow injection chemiluminescence determination of naproxen based on KMnO4–Na2SO3 reaction in neutral aqueous medium. Anal. Chim. Acta 2006, 577, 107–110. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Kamalzadeh, Z.; Saberi, R.S. Glassy carbon electrode modified with a bilayer of multi-walled carbon nanotube and polypyrrole doped with new coccine: Application to the sensitive electrochemical determination of Sumatriptan. Electrochim. Acta 2011, 56, 10032–10038. [Google Scholar] [CrossRef]
- Amiri, M.; Pakdel, Z.; Bezaatpour, A.; Shahrokhian, S. Electrocatalytic determination of sumatriptan on the surface of carbonpaste electrode modified with a composite of cobalt/Schiff-base complex and carbon nanotube. Bioelectrochemistry 2011, 81, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Thiruppathi, A.R.; Elmahdy, R.; van der Zalm, J.; Chen, A. Graphene-oxide-based electrochemical sensors for the sensitive detection of pharmaceutical drug naproxen. Sensors 2020, 20, 1252. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Kamble, B.; Garadkar, K.M.; Sharma, K.K.; Kamble, P.; Tayade, S.; Ajalkar, B.D. Determination of 4-nitrophenol using MoO3 loaded glassy carbon electrode via electrochemical sensing approach. J. Electrochem. Sci. Eng. 2021, 11, 143–159. [Google Scholar]
- Mohanraj, J.; Durgalakshmi, D.; Rakkesh, R.A.; Balakumar, S.; Rajendran, S.; Karimi-Maleh, H. Facile synthesis of paper based graphene electrodes for point of care devices: A double stranded DNA (dsDNA) biosensor. J. Colloid Interface Sci. 2020, 566, 463–472. [Google Scholar] [CrossRef]
- Montazarolmahdi, M.; Masrournia, M.; Nezhadali, A. A new electrochemical approach for the determination of phenylhydrazine in water and wastewater samples using amplified carbon paste electrode. Chem. Methodol. 2020, 4, 732–742. [Google Scholar]
- Eren, T.; Atar, N.; Yola, M.L.; Karimi-Maleh, H. A sensitive molecularly imprinted polymer based quartz crystal microbalance nanosensor for selective determination of lovastatin in red yeast rice. Food Chem. 2015, 185, 430–436. [Google Scholar] [CrossRef]
- Han, S.; Zhang, X.; Sun, H.; Wei, J.; Wang, H.; Wang, S.; Zhang, Z. Electrochemical behavior and voltammetric determination of chloramphenicol and doxycycline using a glassy carbon electrode modified with single-walled carbon nanohorns. Electroanalysis 2022, 34, 735–742. [Google Scholar] [CrossRef]
- Shamsi, A.; Ahour, F. Electrochemical sensing of thioridazine in human serum samples using modified glassy carbon electrode. Adv. J. Chem. A 2020, 4, 22–31. [Google Scholar]
- Elobeid, W.H.; Elbashir, A.A. Development of chemically modified pencil graphite electrode based on benzo-18-crown-6 and multi-walled CNTs for determination of lead in water samples. Prog. Chem. Biochem. Res. 2019, 2, 24–33. [Google Scholar]
- Karimi-Maleh, H.; Khataee, A.; Karimi, F.; Baghayeri, M.; Fu, L.; Rouhi, J.; Boukherroub, R. A green and sensitive guanine-based DNA biosensor for idarubicin anticancer monitoring in biological samples: A simple and fast strategy for control of health quality in chemotherapy procedure confirmed by docking investigation. Chemosphere 2022, 291, 132928. [Google Scholar] [CrossRef] [PubMed]
- Raoof, J.B.; Ojani, R.; Beitollahi, H. Electrocatalytic determination of ascorbic acid at chemically modified carbon paste electrode with 2,7-bis (ferrocenyl ethynyl) fluoren-9-one. Int. J. Electrochem. Sci. 2007, 2, 534–548. [Google Scholar]
- Couto, R.A.S.; Lima, J.L.F.C.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Rama, E.C.; Costa-García, A. Screen-printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J.P. Screen-printed carbon based biosensors and their applications in agri-food safety. TrAC Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.L. Electrochemical affinity biosensors based on disposable screen-printed electrodes for detection of food allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays-A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Garkani Nejad, F.; Aghaei Afshar, A.; Beitollahi, H. Voltammetric determination of isoniazid in the presence of acetaminophen utilizing MoS2-nanosheet-modified screen-printed electrode. Micromachines 2022, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Z.; Wu, G.; Xu, C.; Wu, J.; Zhang, X.; Liu, J. Applications of electrochemical biosensors based on functional antibody modified screen printed electrodes: A review. Anal. Methods 2021, 14, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Garkani Nejad, F.; Dourandish, Z.; Tajik, S. A novel voltammetric amaranth sensor based on screen printed electrode modified with polypyrrole nanotubes. Environ. Res. 2022, 214, 113725. [Google Scholar] [CrossRef] [PubMed]
- Mirbaloochzehi, M.R.; Rezvani, A.; Samimi, A.; Shayesteh, M. Application of a novel surfactant-modified natural nano-zeolite for removal of heavy metals from drinking water. Adv. J. Chem. A 2020, 3, 612–620. [Google Scholar]
- Fang, X.; Cao, J.; Shen, A. Advances in anti-breast cancer drugs and the application of nano-drug delivery systems in breast cancer therapy. J. Drug Deliv. Sci. Technol. 2020, 57, 101662. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Mirabi, A. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass-derived activated carbon frameworks: A promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostruct. Chem. 2022, 12, 429–439. [Google Scholar] [CrossRef]
- Mohammadi, S.S.; Ghasemi, N.; Ramezani, M. Bio-fabrication of silver nanoparticles using naturally available wild herbaceous plant and its antibacterial activity. Eurasian Chem. Commun. 2020, 2, 87–102. [Google Scholar]
- Liu, Y.; Chen, Z.; Li, Z.; Zhao, N.; Xie, Y.; Du, Y.; Yang, Y. CoNi nanoalloy-Co-N4 composite active sites embedded in hierarchical porous carbon as bi-functional catalysts for flexible Zn-air battery. Nano Energy 2022, 99, 107325. [Google Scholar] [CrossRef]
- Khakyzadeh, V.; Rezaei-Vahidian, H.; Sediqi, S.; Azimi, S.; Karimi-Nami, R. Programming adsorptive removal of organic azo dye from aqueous media using magnetic carbon nano-composite. Chem. Methodol. 2021, 5, 324–330. [Google Scholar]
- Mohammadzadeh Jahani, P.; Beitollahi, H.; Garkani Nejad, F.; Dourandish, Z.; Di Bartolomeo, A. Screen-printed graphite electrode modified with Co3O4 nanoparticles and 2D graphitic carbon nitride as an effective electrochemical sensor for 4-aminophenol detection. Nanotechnology 2022, 33, 395702. [Google Scholar] [CrossRef]
- Miraki, M.; Karimi-Maleh, H.; Taher, M.A.; Cheraghi, S.; Karimi, F.; Agarwal, S.; Gupta, V.K. Voltammetric amplified platform based on ionic liquid/NiO nanocomposite for determination of benserazide and levodopa. J. Mol. Liq. 2019, 278, 672–676. [Google Scholar] [CrossRef]
- Sengar, M.; Saxena, S.; Satsangee, S.; Jain, R. Silver nanoparticles decorated functionalized multiwalled carbon nanotubes modified screen printed sensor for the voltammetric determination of butorphanol. J. Appl. Organomet. Chem. 2021, 1, 95–108. [Google Scholar]
- Pyman, H.; Roshanfekr, H.; Ansari, S. DNA-based electrochemical biosensor using chitosan–carbon nanotubes composite film for biodetection of Pirazon. Eurasian Chem. Commun. 2020, 2, 213–225. [Google Scholar]
- Karimi-Maleh, H.; Sheikhshoaie, M.; Sheikhshoaie, I.; Ranjbar, M.; Alizadeh, J.; Maxakato, N.W.; Abbaspourrad, A. A novel electrochemical epinine sensor using amplified CuO nanoparticles and an-hexyl-3-methylimidazolium hexafluorophosphate electrode. New J. Chem. 2019, 43, 2362–2367. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Torkzadeh-Mahani, M. Electrochemical immunosensor for the detection of anti-thyroid peroxidase antibody by gold nanoparticles and ionic liquid-modified carbon paste electrode. J. Nanostruct. Chem. 2022, 12, 581–588. [Google Scholar] [CrossRef]
- Kouadio, K.E.; Kambiré, O.; Koffi, K.S.; Ouattara, L. Electrochemical oxidation of paracetamol on boron-doped diamond electrode: Analytical performance and paracetamol degradation. J. Electrochem. Sci. Eng. 2021, 11, 71–86. [Google Scholar]
- Tajik, S.; Askari, M.B.; Ahmadi, S.A.; Garkani Nejad, F.; Dourandish, Z.; Razavi, R.; Beitollahi, H.; Di Bartolomeo, A. Electrochemical sensor based on ZnFe2O4/RGO nanocomposite for ultrasensitive detection of hydrazine in real samples. Nanomaterials 2022, 12, 491. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Sadeghi, H.; Shahidi, S.A.; Naghizadeh Raeisi, S.; Ghorbani-HasanSaraei, A.; Karimi, F. Electrochemical determination of folic acid in fruit juices samples using electroanalytical sensor amplified with CuO/SWCNTs and 1-butyl-2, 3-dimethylimidazolium hexafluorophosphate. Chem. Methodol. 2020, 4, 743–753. [Google Scholar]
- Moshirian-Farahi, S.S.; Zamani, H.A.; Abedi, M. Nano-molar level determination of isoprenaline in pharmaceutical and clinical samples; A nanostructure electroanalytical strategy. Eurasian Chem. Commun. 2020, 2, 702–711. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Karimi, F.; Orooji, Y.; Mansouri, G.; Razmjou, A.; Aygun, A.; Sen, F. A new nickel-based co-crystal complex electrocatalyst amplified by NiO dope Pt nanostructure hybrid; a highly sensitive approach for determination of cysteamine in the presence of serotonin. Sci. Rep. 2020, 10, 11699. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Darabi, R.; Shabani-Nooshabadi, M.; Baghayeri, M.; Karimi, F.; Rouhi, J.; Karaman, C. Determination of D&C Red 33 and Patent Blue V Azo dyes using an impressive electrochemical sensor based on carbon paste electrode modified with ZIF-8/g-C3N4/Co and ionic liquid in mouthwash and toothpaste as real samples. Food Chem. Toxicol. 2022, 162, 112907. [Google Scholar] [PubMed]
- Garkani Nejad, F.; Sheikhshoaie, I.; Beitollahi, H. Simultaneous detection of carmoisine and tartrazine in food samples using GO-Fe3O4-PAMAM and ionic liquid based electrochemical sensor. Food Chem. Toxicol. 2022, 162, 112864. [Google Scholar] [CrossRef] [PubMed]
- Kanagavalli, P.; Andrew, C.; Veerapandian, M.; Jayakumar, M. In-situ redox-active hybrid graphene platform for label-free electrochemical biosensor: Insights from electrodeposition and electroless deposition. TrAC Trends Anal. Chem. 2021, 143, 116413. [Google Scholar] [CrossRef]
- Kanagavalli, P.; Pandey, G.R.; Murugan, P.; Veerapandian, M. Electrochemical and DFT studies of andrographolide on electrochemically reduced graphene oxide for anti-viral herbaceutical sensor. Anal. Chim. Acta 2022, 1209, 339877. [Google Scholar] [CrossRef]
- Luo, X.; Morrin, A.; Killard, A.J.; Smyth, M.R. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis 2006, 18, 319–326. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Wang, J.; Lee, P.S. Sulfidation of NiMn-layered double hydroxides/graphene oxide composites toward supercapacitor electrodes with enhanced performance. Adv. Energy Mater. 2016, 6, 1501745. [Google Scholar] [CrossRef]
- Yang, J.; Yu, C.; Fan, X.; Qiu, J. 3D architecture materials made of NiCoAl-LDH Nanoplates coupled with NiCo-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors. Adv. Energy Mater. 2014, 4, 1400761. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Z.H.; Wang, Z.G.; Zhang, F.X.; Jin, J. Layered α-Co(OH)2 nanocones as electrode materials for pseudocapacitors: Understanding the effect of interlayer space on electrochemical activity. Adv. Funct. Mater. 2013, 23, 2758–2764. [Google Scholar] [CrossRef]
- Sarfraz, M.; Shakir, I. Recent advances in layered double hydroxides as electrode materials for high-performance electrochemical energy storage devices. J. Energy Storage 2017, 13, 103–122. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Sanati, S.; Abazari, R.; Morsali, A. Enhanced electrochemical oxygen and hydrogen evolution reactions using an NU-1000@NiMn-LDHS composite electrode in alkaline electrolyte. Chem. Commun. 2020, 56, 6652–6655. [Google Scholar] [CrossRef] [PubMed]
- Baig, N.; Sajid, M. Applications of layered double hydroxides based electrochemical sensors for determination of environmental pollutants: A review. Trends Environ. Anal. Chem. 2017, 16, 1–15. [Google Scholar] [CrossRef]
- Varadwaj, G.; Nyamori, V.O. Layered double hydroxide-and graphene-based hierarchical nanocomposites: Synthetic strategies and promising applications in energy conversion and conservation. Nano Res. 2016, 9, 3598–3621. [Google Scholar] [CrossRef]
- Prevot, V.; Tokudome, Y. 3D hierarchical and porous layered double hydroxide structures: An overview of synthesis methods and applications. J. Mater. Sci. 2017, 52, 11229–11250. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, W.; Tian, Y.; Yang, S.; Zhang, Q.; Lei, D.; Zhao, Y. Nanosheet-assembled NiCo-LDH hollow spheres as high-performance electrodes for supercapacitors. J. Colloid Interface Sci. 2022, 606, 1120–1127. [Google Scholar] [CrossRef]
- Li, M.; Yuan, P.; Guo, S.; Liu, F.; Cheng, J.P. Design and synthesis of Ni-Co and Ni-Mn layered double hydroxides hollow microspheres for supercapacitor. Int. J. Hydrog. Energy 2017, 42, 28797–28806. [Google Scholar] [CrossRef]
- Amin, K.M.; Muench, F.; Kunz, U.; Ensinger, W. 3D NiCo-Layered double Hydroxide@ Ni nanotube networks as integrated free-standing electrodes for nonenzymatic glucose sensing. J. Colloid Interface Sci. 2021, 591, 384–395. [Google Scholar] [CrossRef]
- Vilian, A.E.; Ranjith, K.S.; Lee, S.J.; Umapathi, R.; Hwang, S.K.; Oh, C.W.; Han, Y.K. Hierarchical dense Ni− Co layered double hydroxide supported carbon nanofibers for the electrochemical determination of metronidazole in biological samples. Electrochim. Acta 2020, 354, 136723. [Google Scholar] [CrossRef]
- Fang, X.; Zang, J.; Wang, X.; Zheng, M.S.; Zheng, N. A multiple coating route to hollow carbon spheres with foam-like shells and their applications in supercapacitor and confined catalysis. J. Mater. Chem. A 2014, 2, 6191–6197. [Google Scholar] [CrossRef]
- Soltani, R.; Marjani, A.; Shirazian, S. A hierarchical LDH/MOF nanocomposite: Single, simultaneous and consecutive adsorption of a reactive dye and Cr (vi). Dalton Trans. 2020, 49, 5323–5335. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Khan, A.; Kurniawan, T.A.; Soltani, R.; Albadarin, A.B. Synthesis of hierarchical micro-mesoporous LDH/MOF nanocomposite with in situ growth of UiO-66-(NH2)2 MOF on the functionalized NiCo-LDH ultrathin sheets and its application for thallium (I) removal. J. Mol. Liq. 2021, 336, 116189. [Google Scholar] [CrossRef]
- Bai, L.; Duan, S.; Jiang, W.; Liu, M.; Wang, S.; Sang, M.; Xuan, S. High performance polydopamine-functionalized mesoporous silica nanospheres for U (VI) removal. Appl. Surf. Sci. 2017, 426, 1121–1132. [Google Scholar] [CrossRef]
- Palapa, N.R.; Mohadi, R.; Rachmat, A. Adsorption study of malachite green removal from aqueous solution using Cu/M3+ (M3+= Al, Cr) layered double hydroxide. Mediterr. J. Chem. 2020, 10, 33–45. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khanaliloo, S.; Khorablou, Z.; Dorraji, P.S. Signal amplification for sumatriptan sensing based on polymeric surface decorated with Cu nanoparticles. J. Serb. Chem. Soc. 2018, 83, 449–462. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Shahrokhian, S.; Ghorbani-Bidkorbeh, F. Voltammetric studies of sumatriptan on the surface of pyrolytic graphite electrode modified with multi-walled carbon nanotubes decorated with silver nanoparticles. Talanta 2009, 80, 31–38. [Google Scholar] [CrossRef]
- Jalali-Sarvestani, M.R.; Madrakian, T.; Afkhami, A. Voltammetric Determination of Sumatriptan by an Overoxidized Poly (p-aminophenol) Modified Glassy Carbon Electrode. Anal. Bioanal. Chem. Res. 2021, 8, 245–259. [Google Scholar]




| Electrochemical Sensor | Electrochemical Method | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| CuO/SPE | DPV | 0.33–3.54 μM | 0.066 μM | [4] |
| Cu nanoparticles (NPs)/poly-melamine/glassy carbon electrode | DPV | 0.08–0.58 and 0.58–6.5 μM | 0.025 μM | [85] |
| Multiwalled carbon nanotube (MWCNTs)decorated with silver NPs/pyrolytic graphite electrode | CV | 0.08–100.0 μM | 0.04 μM | [86] |
| MWCNTs and cobalt methyl-salophen complex/carbon paste electrode | DPV | 1.0–1000.0 μM | 0.3 μM | [21] |
| MWCNTs and polypyrrole doped with new coccine/glassy carbon electrode | LSV | 0.02–10.0 μM | 0.006 μM | [20] |
| Overoxidized poly(p-aminophenol) modified glassy carbon electrode | Square wave voltammetry | 1.0–100.0 μM | 0.294 μM | [87] |
| Ni-Co LDH/SPE | DPV | 0.01–435.0 μM | 0.002 μM | This work |
| Sample | Spiked (μM) | Found (μM) | Recovery (%) | RSD (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Sumatriptan | Naproxen | Sumatriptan | Naproxen | Sumatriptan | Naproxen | Sumatriptan | Naproxen | |
| Sumatriptan Tablet | 0 | 0 | 4.0 | - | - | - | 3.3 | - |
| 1.0 | 4.0 | 4.9 | 4.1 | 98.0 | 102.5 | 1.9 | 2.3 | |
| 3.0 | 6.0 | 7.1 | 5.8 | 101.4 | 96.7 | 2.8 | 3.0 | |
| Naproxen Tablet | 0 | 0 | - | 5.0 | - | - | - | 2.9 |
| 5.0 | 1.0 | 5.1 | 5.9 | 102.0 | 98.3 | 3.0 | 2.2 | |
| 7.0 | 3.0 | 6.9 | 8.3 | 98.6 | 103.7 | 1.8 | 3.6 | |
| Urine | 0 | 0 | - | - | - | - | - | - |
| 4.5 | 5.5 | 4.6 | 5.3 | 102.2 | 96.4 | 2.5 | 2.8 | |
| 6.5 | 7.5 | 6.3 | 7.6 | 96.9 | 101.3 | 3.1 | 1.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beitollahi, H.; Dourandish, Z.; Tajik, S.; Sharifi, F.; Jahani, P.M. Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen. Biosensors 2022, 12, 872. https://doi.org/10.3390/bios12100872
Beitollahi H, Dourandish Z, Tajik S, Sharifi F, Jahani PM. Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen. Biosensors. 2022; 12(10):872. https://doi.org/10.3390/bios12100872
Chicago/Turabian StyleBeitollahi, Hadi, Zahra Dourandish, Somayeh Tajik, Fatemeh Sharifi, and Peyman Mohammadzadeh Jahani. 2022. "Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen" Biosensors 12, no. 10: 872. https://doi.org/10.3390/bios12100872
APA StyleBeitollahi, H., Dourandish, Z., Tajik, S., Sharifi, F., & Jahani, P. M. (2022). Electrochemical Sensor Based on Ni-Co Layered Double Hydroxide Hollow Nanostructures for Ultrasensitive Detection of Sumatriptan and Naproxen. Biosensors, 12(10), 872. https://doi.org/10.3390/bios12100872






