Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review
Abstract
1. Introduction
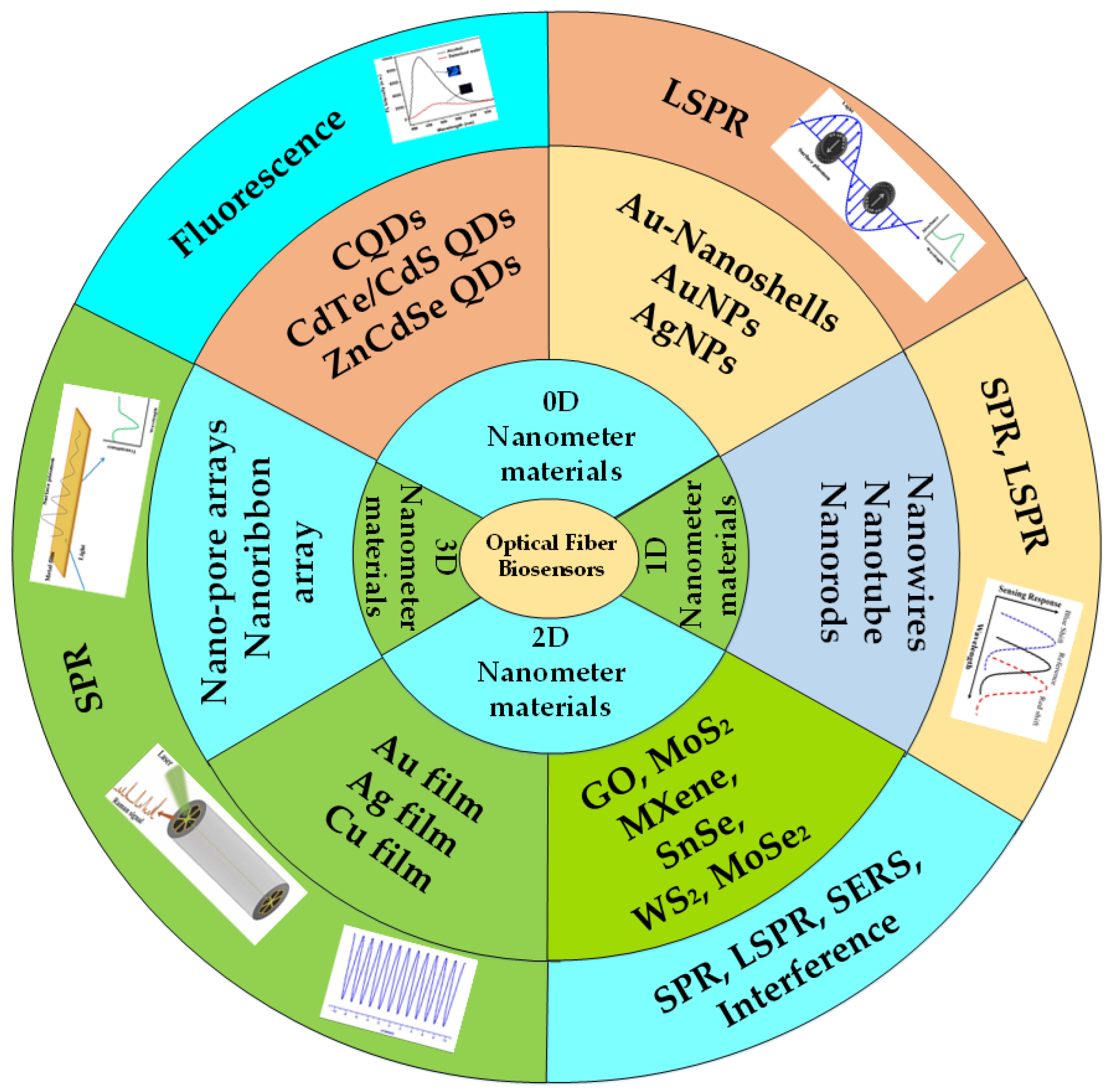
2. Application of 0D Nanomaterials in the Field of Optical Fiber Biosensors
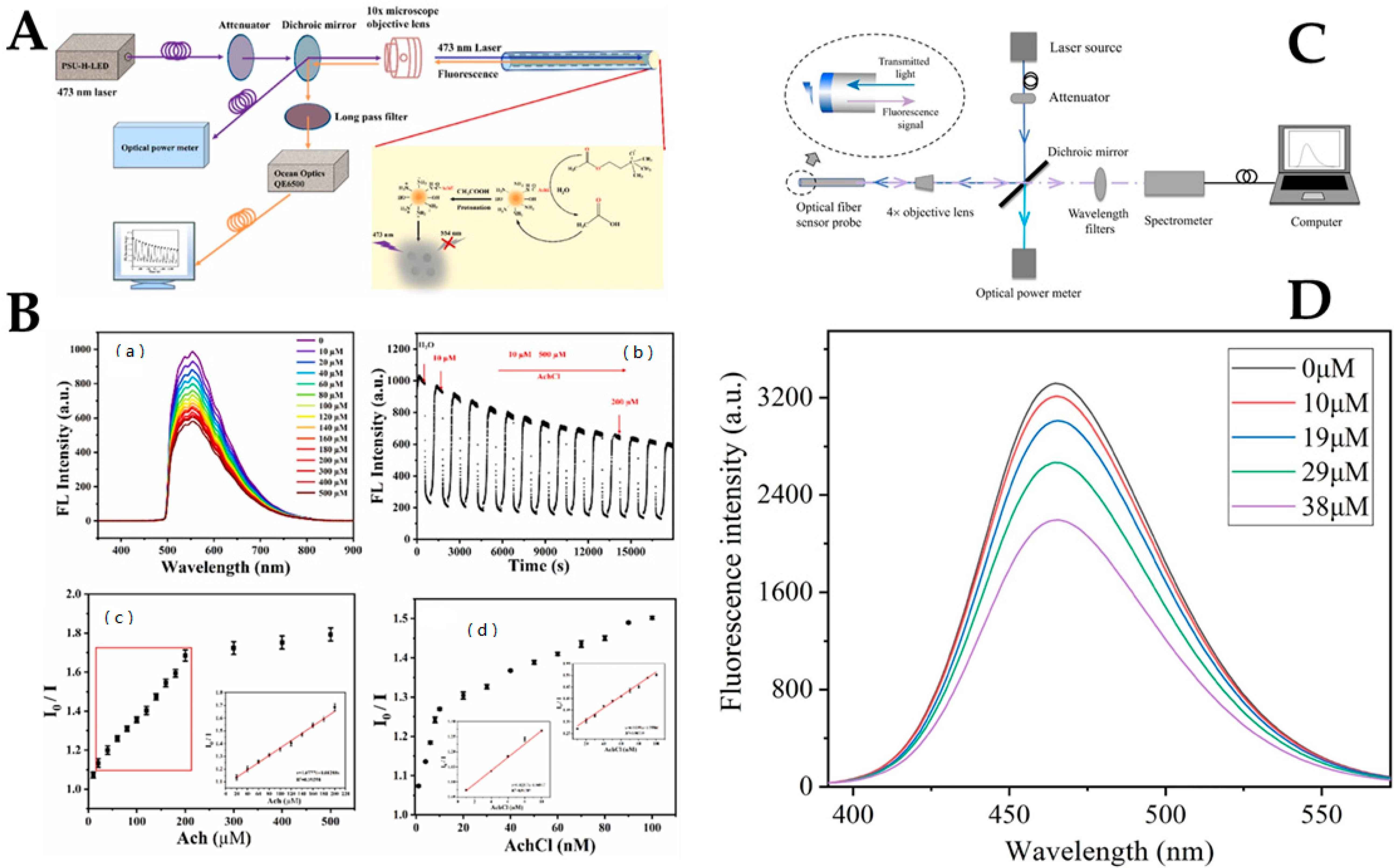
2.1. Quantum Dots
2.2. Metal Nanoparticles
2.3. Other 0D Nanomaterials
3. Application of 1D Nanomaterials in the Field of Optical Fiber Biosensors
3.1. Nanowires
3.2. Nanotubes
3.3. Other 1D Nanomaterials
4. Application of 2D Nanomaterials in the Field of Optical Fiber Biosensors
4.1. Metal Films
4.2. Graphene Oxide
4.3. Molybdenum Disulfide
4.4. MXene
4.5. Other Novel 2D Nanomaterials
5. Application of 3D Nanomaterials in the Field of Optical Fiber Biosensors
6. Future Prospects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Z.; Zhang, Y.; Li, S.; Zhang, Y.; Yang, X.; Zhang, J.; Yuan, L. Specialty optical fibers and 2D materials for sensitivity enhancement of fiber optic SPR sensors: A review. Opt. Laser Technol. 2022, 152, 108167. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kim, K.-H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in surface plasmon resonance–based biosensor technologies for cancer biomarker detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef]
- Chauhan, M.; Kumar Singh, V. Review on recent experimental SPR/LSPR based fiber optic analyte sensors. Opt. Fiber Technol. 2021, 64, 102580. [Google Scholar] [CrossRef]
- Zhang, J.; Mai, X.; Hong, X.; Chen, Y.; Li, X. Optical fiber SPR biosensor with a solid-phase enzymatic reaction device for glucose detection. Sens. Actuators B 2022, 366, 131984. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Q.; Zhou, X.; Zhang, Y.; Zhao, Y. Plug-in optical fiber SPR biosensor for lung cancer gene detection with temperature and pH compensation. Sens. Actuators B 2022, 359, 131596. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Marques, C.; Zhang, B.; Kumar, S. 2D material assisted SMF-MCF-MMF-SMF based LSPR sensor for creatinine detection. Opt. Express 2021, 29, 38150–38167. [Google Scholar] [CrossRef]
- Kim, H.-M.; Park, J.-H.; Lee, S.-K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605. [Google Scholar] [CrossRef]
- Woo-Hu, T.; Yu-Cheng, L.; Jiu-Kai, T.; Yu-Chia, T. Multi-step structure of side-polished fiber sensor to enhance SPR effect. Opt. Laser Technol. 2010, 42, 453–456. [Google Scholar] [CrossRef]
- Xu, Y.; Xiong, M.; Yan, H. A portable optical fiber biosensor for the detection of zearalenone based on the localized surface plasmon resonance. Sens. Actuators B 2021, 336, 129752. [Google Scholar] [CrossRef]
- Liu, S.; Ma, R.; Li, Y.; Zhao, L.; Xia, Y.; Dong, X.; Pang, Y. D-shaped surface plasmon resonance biosensor based on MoS2 in terahertz band. Opt. Fiber Technol. 2021, 66, 102631. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, P.; Jindal, P. Surface plasmon resonance biosensor based on a D-shaped photonic crystal fiber using Ti3C2Tx MXene material. Opt. Mater. 2022, 128, 112397. [Google Scholar] [CrossRef]
- Yu, H.; Chong, Y.; Zhang, P.; Ma, J.; Li, D. A D-shaped fiber SPR sensor with a composite nanostructure of MoS2-graphene for glucose detection. Talanta 2020, 219, 121324. [Google Scholar] [CrossRef]
- Soares, M.S.; Silva, L.C.B.; Vidal, M.; Loyez, M.; Facão, M.; Caucheteur, C.; Segatto, M.E.V.; Costa, F.M.; Leitão, C.; Pereira, S.O.; et al. Label-free plasmonic immunosensor for cortisol detection in a D-shaped optical fiber. Biomed. Opt. Express 2022, 13, 3259–3274. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C.; Wei, Y.; Jiang, T.; Liu, C.; Shi, C.; Zhao, X.; Li, L. Research and application of multi-channel SPR sensor cascaded with fiber U-shaped structure. Optik 2022, 266, 169603. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Soares, M.S.; Marques, C.; Zhang, B.; Kumar, S. Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture. Opt. Express 2022, 30, 13898–13914. [Google Scholar] [CrossRef]
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.; Saha, C.; Kumar, C. A novel periodically tapered structure-based gold nanoparticles and graphene oxide—Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156. [Google Scholar] [CrossRef]
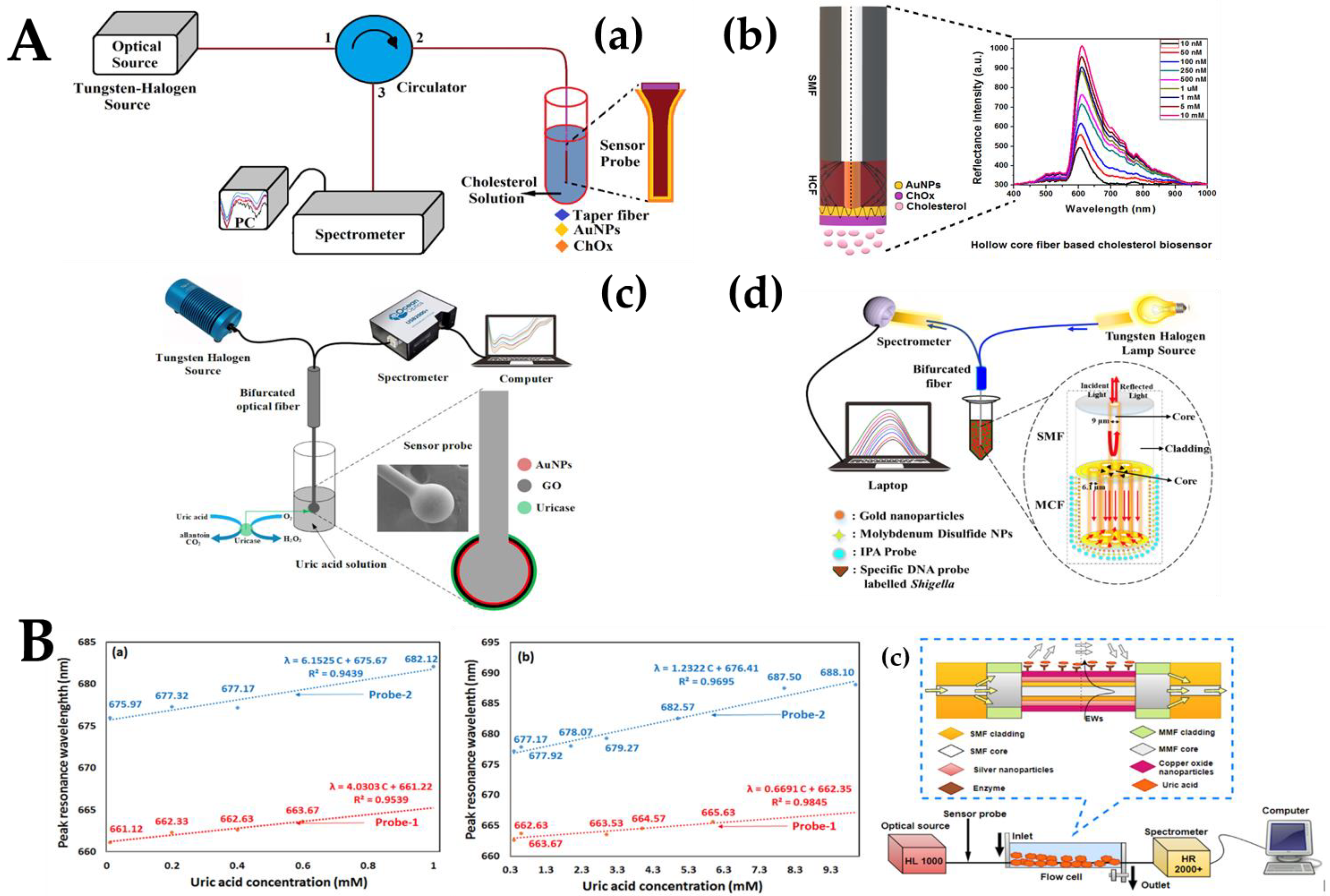
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.K.; Zhang, B.L. SPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160. [Google Scholar] [CrossRef]
- Chen, L.; Leng, Y.-K.; Liu, B.; Liu, J.; Wan, S.-P.; Wu, T.; Yuan, J.; Shao, L.; Gu, G.; Fu, Y.Q.; et al. Ultrahigh-sensitivity label-free optical fiber biosensor based on a tapered singlemode- no core-singlemode coupler for Staphylococcus aureus detection. Sens. Actuators B 2020, 320, 128283. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Zhu, G.; Yang, Q.; Zhang, X.; Cheng, S.; Zhang, B.; Kaushik, B.K.; Liu, F.Z. Development of Uric Acid Biosensor Using Gold Nanoparticles and Graphene Oxide Functionalized Micro-Ball Fiber Sensor Probe. IEEE Trans. NanoBiosci 2020, 19, 173–182. [Google Scholar] [CrossRef]
- Bekmurzayeva, A.; Ashikbayeva, Z.; Myrkhiyeva, Z.; Nugmanova, A.; Shaimerdenova, M.; Ayupova, T.; Tosi, D. Label-free fiber-optic spherical tip biosensor to enable picomolar-level detection of CD44 protein. Sci. Rep. 2021, 11, 19583. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K. Highly sensitive PCF based plasmonic biosensor for hemoglobin concentration detection. Photonic. Nanostruct. 2022, 51, 101040. [Google Scholar] [CrossRef]
- Lv, J.W.; Wang, F.M.; Hu, C.J.; Yang, L.; Fu, H.H.; Zeng, Y.S.; Chu, P.K.; Liu, C. Numerical Analysis of Multifunctional Biosensor with Dual-Channel Photonic Crystal Fibers Based on Localized Surface Plasmon Resonance. Coatings 2022, 12, 742. [Google Scholar] [CrossRef]
- Biplob Hossain, M.; Riazul Islam, S.M.; Tasrif Hossain, K.M.; Faisal Abdulrazak, L.; Nazmus Sakib, M.; Amiri, I.S. High sensitivity hollow core circular shaped PCF surface plasmonic biosensor employing silver coat: A numerical design and analysis with external sensing approach. Results Phys. 2020, 16, 102909. [Google Scholar] [CrossRef]
- Li, J.-X.; Zhang, W.-H.; Tong, Z.-R.; Liu, J.-W. Fiber optic sensor modified by graphene oxide–glucose oxidase for glucose detection. Opt. Commun. 2021, 492, 126983. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Liu, F.; Fu, Q.; Chen, X.; Xu, J.; Zhang, Z.; Huang, Y.; Tang, Y.; Guo, T.; et al. Hydrogen peroxide and glucose concentration measurement using optical fiber grating sensors with corrodible plasmonic nanocoatings. Biomed. Opt. Express 2018, 9, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Z.-H.; Zhao, W.-M.; Wang, L.; Yan, X.; Zhu A-s Qiu, F.-m.; Zhang, K.-K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, X.; Moreno, Y.; Sun, Q.; Yan, Z.; Zhang, L. Sensitivity adjustable biosensor based on graphene oxide coated excessively tilted fiber grating. Sens. Actuators B 2022, 351, 130832. [Google Scholar] [CrossRef]
- Melo, L.B.; Rodrigues, J.M.M.; Farinha, A.S.F.; Marques, C.A.; Bilro, L.; Alberto, N.; Tomé, J.P.C.; Nogueira, R.N. Concentration sensor based on a tilted fiber Bragg grating for anions monitoring. Opt. Fiber Technol. 2014, 20, 422–427. [Google Scholar] [CrossRef]
- Marques, C.A.F.; Bilro, L.B.; Alberto, N.J.; Webb, D.J.; Nogueira, R.N. Narrow bandwidth Bragg gratings imprinted in polymer optical fibers for different spectral windows. Opt. Commun. 2013, 307, 57–61. [Google Scholar] [CrossRef]
- Esposito, F.; Sansone, L.; Srivastava, A.; Cusano, A.M.; Campopiano, S.; Giordano, M.; Iadicicco, A. Label-free detection of vitamin D by optical biosensing based on long period fiber grating. Sens. Actuators B 2021, 347, 130637. [Google Scholar] [CrossRef]
- Wu, P.; Liu, L.; Morgan, S.P.; Correia, R.; Korposh, S. Label-Free Detection of Antibodies Using Functionalised Long Period Grating Optical Fibre Sensors. Results Opt. 2021, 5, 100172. [Google Scholar] [CrossRef]
- Jiang, B.; Bi, Z.; Hao, Z.; Yuan, Q.; Feng, D.; Zhou, K.; Zhang, L.; Gan, X.; Zhao, J. Graphene oxide-deposited tilted fiber grating for ultrafast humidity sensing and human breath monitoring. Sens. Actuators B 2019, 293, 336–341. [Google Scholar] [CrossRef]
- Leitão, C.; Pereira, S.O.; Alberto, N.; Lobry, M.; Loyez, M.; Costa, F.M.; Pinto, J.L.; Caucheteur, C.; Marques, C. Cortisol In-Fiber Ultrasensitive Plasmonic Immunosensing. IEEE Sens. J. 2021, 21, 3028–3034. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Umar, A.A.; Salleh, M.M. Fluorescence Sensor Design for Pesticide Detection using ZnCdSe Quantum Dots. Sains Malays. 2019, 48, 1513–1518. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.Q.; Jian, D.; Li, J.H.; Ding, H.; Yi, D.R.; Liu, F.; Wang, S.Y. Quantitative remote and on-site Hg2+ detection using the handheld smartphone based optical fiber fluorescence sensor (SOFFS). Sens. Actuators B 2019, 301, 127168. [Google Scholar] [CrossRef]
- Cui, J.; Zhou, M.; Li, Y.; Liang, Z.; Li, Y.; Yu, L.; Liu, Y.; Liang, Y.; Chen, L.; Yang, C. A New Optical Fiber Probe-Based Quantum Dots Immunofluorescence Biosensors in the Detection of Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2021, 11, 665241. [Google Scholar] [CrossRef]
- Janus, Ł.; Radwan-Pragłowska, J.; Piątkowski, M.; Bogdał, D. Facile Synthesis of Surface-Modified Carbon Quantum Dots (CQDs) for Biosensing and Bioimaging. Materials 2020, 13, 3313. [Google Scholar] [CrossRef]
- Hussain, E.; Cheng, C.; Li, Y.; Niu, N.; Zhou, H.; Jin, X.; Kong, J.; Yu, C. Benzo[ghi]perylene & coronene as ratiometric reversible optical oxygen nano-sensors. Sens. Actuators B 2019, 287, 27–34. [Google Scholar]
- Zhou, Z.; Yang, Z.; Xia, L.; Zhang, H. Construction of an enzyme-based all-fiber SPR biosensor for detection of enantiomers. Biosens. Bioelectron. 2022, 198, 113836. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.-n.; Zheng, W.; Li, X.; Zhao, Y. Optical fiber SPR biosensor based on gold nanoparticle amplification for DNA hybridization detection. Talanta 2022, 247, 123599. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Semwal, V.; Gupta, B.D. A highly selective LSPR biosensor for the detection of taurine realized on optical fiber substrate and gold nanoparticles. Opt. Fiber Technol. 2019, 52, 101962. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, Y.; He, L.; He, F.; Wu, J.; Huang, Z.; Tian, Y.; Li, Y.; Duan, Y. Development of a rapid and ultra-sensitive cytosensor: Ω-shaped fiber optic LSPR integrated with suitable AuNPs coverage. Sens. Actuators B 2021, 336, 129706. [Google Scholar] [CrossRef]
- Leitão, C.; Pereira, S.O.; Marques, C.; Cennamo, N.; Zeni, L. Cost-Effective Fiber Optic Solutions for Biosensing. Biosensors 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Caucheteur, C.; Villatoro, J.; Liu, F.; Loyez, M.; Guo, T.; Albert, J. Mode-division and spatial-division optical fiber sensors. Adv. Opt. Photonics 2022, 14, 1–86. [Google Scholar] [CrossRef]
- Soares, M.S.; Vidal, M.; Santos, N.F.; Costa, F.M.; Marques, C.; Pereira, S.O.; Leitão, C. Immunosensing Based on Optical Fiber Technology: Recent Advances. Biosensors 2021, 11, 305. [Google Scholar] [CrossRef]
- Sondhi, P.; Maruf, M.H.U.; Stine, K.J. Nanomaterials for Biosensing Lipopolysaccharide. Biosensors 2019, 10, 2. [Google Scholar] [CrossRef]
- Das, R.; Pal, R.; Bej, S.; Mondal, M.; Kundu, K.; Banerjee, P. Recent progress in 0D optical nanoprobes for applications in the sensing of (bio)analytes with the prospect of global health monitoring and detailed mechanistic insights. Mater. Adv. 2022, 3, 4421–4459. [Google Scholar] [CrossRef]
- Oh, S.-H.; Altug, H.; Jin, X.; Low, T.; Koester, S.J.; Ivanov, A.P.; Edel, J.B. Nanophotonic biosensors harnessing van der Waals materials. Nat. Commun. 2021, 12, 3824. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Arsenin, A.V.; Volkov, V.S. SPR analysis of antibody-antigen interactions using graphene oxide linking layers. Mater. Today Proc. 2018, 5, 17442–17446. [Google Scholar] [CrossRef]
- Mao, J.; Yang, X.; Liu, Y.; Wang, Y.; Peng, G.D.; Rao, Y.J.; Gong, Y. Nanomaterial-Enhanced Fiber Optofluidic Laser Biosensor for Sensitive Enzyme Detection. J. Lightwave Technol. 2020, 38, 5205–5211. [Google Scholar] [CrossRef]
- Gajanan, K.; Tijare, S.N. Applications of nanomaterials. Mater. Today Proc. 2018, 5, 1093–1096. [Google Scholar] [CrossRef]
- Dogra, V.; Kaur, G.; Kumar, R.; Kumar, S. Nanomaterials; Applications; Implications and Management. In New Frontiers of Nanomaterials in Environmental Science; Springer: Singapore, 2021; pp. 23–45. [Google Scholar]
- Nikaeen, G.; Abbaszadeh, S.; Yousefinejad, S. Application of nanomaterials in treatment, anti-infection and detection of coronaviruses. Nanomedicine 2020, 15, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Tahir, A.; Wang, H.; Chang, J. Applications of nanotechnology in virus detection, tracking, and infection mechanisms. Wires Nanomed. Nanobiotechnol. 2021, 13, e1700. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.; Lin, H.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Kumar, S.; Singh, R.; Pu, X. Development of Glucose Sensor Using Gold Nanoparticles and Glucose-Oxidase Functionalized Tapered Fiber Structure. Plasmonics 2019, 15, 841–848. [Google Scholar] [CrossRef]
- Tian, L.M.; Jiang, Q.S.; Liu, K.K.; Luan, J.Y.; Naik, R.R.; Singamaneni, S. Bacterial Nanocellulose-Based Flexible Surface Enhanced Raman Scattering Substrate. Adv. Mater. Interfaces 2016, 3, 1600214. [Google Scholar] [CrossRef]
- Fallah, H.; Asadishad, T.; Parsanasab, G.-M.; Harun, S.W.; Mohammed, W.S.; Yasin, M. Optical Fiber Biosensor toward E-coli Bacterial Detection on the Pollutant Water. Eng. J. 2021, 25, 1–8. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, S.; Liu, F.-Z.; Shuang, C.; Zhang, B.; Jha, R.; Kaushik, B.K. Etched multicore fiber sensor using copper oxide and gold nanoparticles decorated graphene oxide structure for cancer cells detection. Biosens. Bioelectron. 2020, 168, 112557. [Google Scholar] [CrossRef]
- Achadu, O.J.; Abe, F.; Hossain, F.; Nasrin, F.; Yamazaki, M.; Suzuki, T.; Park, E.Y. Sulfur-doped carbon dots@polydopamine-functionalized magnetic silver nanocubes for dual-modality detection of norovirus. Biosens. Bioelectron. 2021, 193, 113540. [Google Scholar] [CrossRef]
- Chatterjee, M.; Nath, P.; Kadian, S.; Kumar, A.; Kumar, V.; Roy, P.; Manik, G.; Satapathi, S. Highly sensitive and selective detection of dopamine with boron and sulfur co-doped graphene quantum dots. Sci. Rep. 2022, 12, 9061. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Zhang, B.; Saha, C.; Kumar, C.; Kaushik, B.K.; Kumar, S. Development of Dopamine Sensor Using Silver Nanoparticles and PEG-Functionalized Tapered Optical Fiber Structure. IEEE Trans. Biomed. Eng. 2020, 67, 1542–1547. [Google Scholar] [CrossRef]
- Liu, J.N.; Bu, W.; Shi, J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Xu, L.; Song, J.; Xu, R.; Liu, D.; Dong, B.; Song, H. CdS quantum dots modified CuO inverse opal electrodes for ultrasensitive electrochemical and photoelectrochemical biosensor. Sci. Rep. 2015, 5, 10838. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, L.; Zhang, H.; Wang, P.; Li, H. A new optical fiber biosensor for acetylcholine detection based on pH sensitive fluorescent carbon quantum dots. Sens. Actuators B 2022, 369, 132268. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Zheng, X.; Zhang, H.; Dong, D.; Zhang, Z.; Liu, M.; Zhou, J. An electrochemical biosensor for the detection of epithelial-mesenchymal transition. Nat. Commun. 2020, 11, 192. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M.; Samimi, M.; Shamsipur, M.; Bagher Gholivand, M. A highly sensitive electrochemical biosensor for chlorpyrifos pesticide detection using the adsorbent nanomatrix contain the human serum albumin and the Pd:CdTe quantum dots. Microchem. J. 2022, 179, 107424. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Doong, R.-A. Graphene Quantum Dots Decorated Gold-Polyaniline Nanowire for Impedimetric Detection of Carcinoembryonic Antigen. Sci. Rep. 2019, 9, 7214. [Google Scholar] [CrossRef]
- Lakshmanakumar, M.; Nesakumar, N.; Sethuraman, S.; Rajan, K.S.; Krishnan, U.M.; Rayappan, J.B.B. Functionalized Graphene Quantum Dot Interfaced Electrochemical Detection of Cardiac Troponin I: An Antibody Free Approach. Sci. Rep. 2019, 9, 17348. [Google Scholar] [CrossRef]
- Li, K.; Tu, J.; Zhang, Y.; Jin, D.; Li, T.; Li, J.; Ni, W.; Xiao, M.-M.; Zhang, Z.-Y.; Zhang, G.-J. Ultrasensitive detection of exosomal miRNA with PMO-graphene quantum dots-functionalized field-effect transistor biosensor. iScience 2022, 25, 104522. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Li, Y.; Wang, Y.; Zhang, Q.; Hussain, E.; Yang, M.; Shahzad, S.A.; Yu, D.; Yu, C. Nucleic acid-controlled quantum dots aggregation: A label-free fluorescence turn-on strategy for alkaline phosphatase detection. Talanta 2017, 169, 64–69. [Google Scholar] [CrossRef]
- Wu, W.; Huang, J.; Ding, L.; Lin, H.; Yu, S.; Yuan, F.; Liang, B. A real-time and highly sensitive fiber optic biosensor based on the carbon quantum dots for nitric oxide detection. J. Photochem. Photobiol. A 2021, 405, 112963. [Google Scholar] [CrossRef]
- Ding, L.Y.; Ruan, Y.L.; Li, T.; Huang, J.; Warren-Smith, S.C.; Ebendorff-Heidepriem, H.; Monro, T.M. Nitric oxide optical fiber sensor based on exposed core fibers and CdTe/CdS quantum dots. Sens. Actuators B 2018, 273, 9–17. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen Nk Yang, Q.S.; Zhang, X. LSPR-Based Cholesterol Biosensor Using Hollow Core Fiber Structure. IEEE Sens. J. 2019, 19, 7399–7406. [Google Scholar] [CrossRef]
- Singh, L.; Zhu, G.; Singh, R.; Zhang, B.; Wang, W.; Kaushik, B.K.; Kumar, S. Gold Nanoparticles and Uricase Functionalized Tapered Fiber Sensor for Uric Acid Detection. IEEE Sens. J. 2019, 20, 219–226. [Google Scholar] [CrossRef]
- Eskandari, V.; Sahbafar, H.; Zeinalizad, L.; Mahmoudi, R.; Karimpour, F.; Hadi, A.; Bardania, H. Coating of silver nanoparticles (AgNPs) on glass fibers by a chemical method as plasmonic surface-enhanced Raman spectroscopy (SERS) sensors to detect molecular vibrations of Doxorubicin (DOX) drug in blood plasma. Arab. J. Chem. 2022, 15, 104005. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber optic surface plasmon resonance sensor for detection of E. coli O157:H7 based on antimicrobial peptides and AgNPs-rGO. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar]
- Jiang, Q.; jing, Y.; Ni, Y.; Gao, R.; Zhou, P. Potentiality of carbon quantum dots derived from chitin as a fluorescent sensor for detection of ClO−. Microchem. J. 2020, 157, 105111. [Google Scholar] [CrossRef]
- Zhang, M.; Su, R.; Zhong, J.; Fei, L.; Cai, W.; Guan, Q.; Li, W.; Li, N.; Chen, Y.; Cai, L.; et al. Red/orange dual-emissive carbon dots for pH sensing and cell imaging. Nano Res. 2019, 12, 815–821. [Google Scholar] [CrossRef]
- Sangubotla, R.; Kim, J. Fiber-optic biosensor based on the laccase immobilization on silica-functionalized fluorescent carbon dots for the detection of dopamine and multi-color imaging applications in neuroblastoma cells. Mater. Sci. Eng. C 2021, 122, 111916. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, J.; Hu, Z.; Chen, Y.; Tao, Y.; Wang, L.; Li, L.; Wang, P.; Li, H.-Y.; Zhang, J.; et al. All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens. Bioelectron. 2022, 202, 113974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, X.; Ma, F.; Zhang, C.-y. Advances in quantum dot-based biosensors for DNA-modifying enzymes assay. Coord. Chem. Rev. 2022, 469, 214674. [Google Scholar] [CrossRef]
- Lang, T.; Cao, B.; Shen, C.; Shi, G. Multimode-coreless-multimode fiber biosensor based on surface plasmon resonance. J. Phys. D Appl. Phys. 2019, 52, 195204. [Google Scholar] [CrossRef]
- Zhao, N.; Zhao, D.; Xu, L.-p.; Chen, L.; Wen, Y.; Zhang, X. A Multimode Responsive Aptasensor for Adenosine Detection. J. Nanomater. 2014, 2014, 360347. [Google Scholar] [CrossRef]
- Ding, L.; Fan, C.; Zhong, Y.; Li, T.; Huang, J. A sensitive optic fiber sensor based on CdSe QDs fluorophore for nitric oxide detection. Sens. Actuators B 2013, 185, 70–76. [Google Scholar] [CrossRef]
- Li, Q.; Ding, L.; Zhang, Y.; Wu, T. A Cholesterol Optical Fiber Sensor Based on CQDs-COD/CA Composite. IEEE Sens. J. 2022, 22, 6247–6255. [Google Scholar] [CrossRef]
- Mohsin, D.H.; Mashkour, M.S.; Fatemi, F. Design of aptamer-based sensing platform using gold nanoparticles functionalized reduced graphene oxide for ultrasensitive detection of Hepatitis B virus. Chem. Pap. 2021, 75, 279–295. [Google Scholar] [CrossRef]
- Srisomwat, C.; Yakoh, A.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Amplification-free DNA Sensor for the One-Step Detection of the Hepatitis B Virus Using an Automated Paper-Based Lateral Flow Electrochemical Device. Anal. Chem. 2021, 93, 2879–2887. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.; Chaudhary, S.; Zhang, B.; Kumar, S. 2-D Nanomaterials Assisted LSPR MPM Optical Fiber Sensor Probe for Cardiac Troponin I Detection. IEEE Trans. Instrum. Meas. 2022, 71, 9504609. [Google Scholar] [CrossRef]
- Qiu, G.; Gai, Z.; Tao, Y.; Schmitt, J.; Kullak-Ublick, G.A.; Wang, J. Dual-Functional Plasmonic Photothermal Biosensors for Highly Accurate Severe Acute Respiratory Syndrome Coronavirus 2 Detection. ACS Nano 2020, 14, 5268–5277. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.; Lima, D.; Viana, A.G.; Fujiwara, S.T.; Pessôa, C.A.; Etto, R.M.; Wohnrath, K. A sensitive label-free impedimetric DNA biosensor based on silsesquioxane-functionalized gold nanoparticles for Zika Virus detection. Biosens. Bioelectron. 2019, 141, 111351. [Google Scholar] [CrossRef] [PubMed]
- Cajigas, S.; Alzate, D.; Orozco, J. Gold nanoparticle/DNA-based nanobioconjugate for electrochemical detection of Zika virus. Microchim. Acta 2020, 187, 594. [Google Scholar] [CrossRef] [PubMed]
- Yeter, E.Ç.; Şahin, S.; Caglayan, M.O.; Üstündağ, Z. An electrochemical label-free DNA impedimetric sensor with AuNP-modified glass fiber/carbonaceous electrode for the detection of HIV-1 DNA. Chem. Pap. 2021, 75, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef]
- Lin, W.-P.; Wang, W.-J.; Lee, C.-H.; Jan, F.-J.; Wang, G.-J. A two-in-one immunoassay biosensor for the simultaneous detection of Odontoglossum ringspot virus and Cymbidium mosaic virus. Sens. Actuators B 2022, 350, 130875. [Google Scholar] [CrossRef]
- Layqah, L.A.; Eissa, S. An electrochemical immunosensor for the corona virus associated with the Middle East respiratory syndrome using an array of gold nanoparticle-modified carbon electrodes. Microchim. Acta 2019, 186, 224. [Google Scholar] [CrossRef]
- Eom, G.; Hwang, A.; Lee, D.K.; Guk, K.; Moon, J.; Jeong, J.; Jung, J.; Kim, B.; Lim, E.-K.; Kang, T. Superb Specific, Ultrasensitive, and Rapid Identification of the Oseltamivir-Resistant H1N1 Virus: Naked-Eye and SERS Dual-Mode Assay Using Functional Gold Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 1233–1240. [Google Scholar] [CrossRef]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.-J.; Vilian, A.T.E.; Kim, J.; Lee, S.Y.; Han, Y.-K.; Yoo, S.M.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 10130. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, B.Y.; Haque, F.; Ren, G.; Ou, J.Z. Plasmonic metal oxides and their biological applications. Mater. Horiz. 2022, 9, 2288–2324. [Google Scholar] [CrossRef]
- Agrawal, N.; Saha, C.; Kumar, C.; Singh, R.; Kumar, S. Development of Uric Acid Sensor Using Copper Oxide and Silver Nanoparticles Immobilized SMSMS Fiber Structure-Based Probe. IEEE Trans. Instrum. Meas. 2020, 69, 9097–9104. [Google Scholar] [CrossRef]
- Kumar, S.; Guo, Z.; Singh, R.; Wang, Q.; Zhang, B.; Cheng, S.; Liu, F.; Marques, C.; Kaushik, B.K.; Jha, R. MoS2 Functionalized Multicore Fiber Probes for Selective Detection of Shigella Bacteria based on Localized Plasmon. J. Lightwave Technol. 2020, 39, 4069–4081. [Google Scholar] [CrossRef]
- Bognár, Z.; de Jonge, M.I.; Gyurcsányi, R.E. In situ silver nanoparticle coating of virions for quantification at single virus level. Nanoscale 2022, 14, 2296–2303. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Vasan, A.; Muthupandian, A.; Kanakasabapathy, M.K.; Thirumalaraju, P.; Sreeram, A.; Krishnakumar, S.; Yogesh, V.; Lin, W.; Yu, X.G. Virus detection using nanoparticles and deep neural network-enabled smartphone system. Sci. Adv. 2020, 6, eabd5354. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Miao, P. Silver nanoparticle@DNA tetrahedron-based colorimetric detection of HIV-related DNA with cascade strand displacement amplification. J. Mater. Chem. B 2019, 7, 2608–2612. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, T.; Wang, H.; Huang, Z.; Hu, Z.; Xie, K.; Zhang, W.; Wei, Y.; Wang, G.; Yan, J.; et al. Ultrasensitive Glucose Biosensor Using Micro-Nano Interface of Tilted Fiber Grating Coupled with Biofunctionalized Au Nanoparticles. IEEE Sens. J. 2022, 22, 4122–4134. [Google Scholar] [CrossRef]
- Lu, H.; Liang, T.M.; Wang, H.Y.; Huang, Z.L.; Hu, Z.J.; Xie, K.; Zhang, W.; Wei, Y.Q.; Wang, G.Q.; Yan, J.; et al. Au-NPs signal amplification ultra-sensitivity optical microfiber interferometric biosensor. Opt. Express 2021, 29, 13937–13948. [Google Scholar] [CrossRef]
- Kim, H.-M.; Kim, H.-J.; Park, J.-H.; Lee, S.-K. High-performance biosensor using a sandwich assay via antibody-conjugated gold nanoparticles and fiber-optic localized surface plasmon resonance. Anal. Chim. Acta 2022, 1213, 339960. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, J.; Yang, L. Optical fiber peptide-sensor for ultrasensitive detection of prostate specific antigen. Sens. Actuators B 2022, 369, 132317. [Google Scholar] [CrossRef]
- Samavati, Z.; Samavati, A.; Ismail, A.F.; Rahman, M.A.; Othman, M.H.D.; Yeganeh, F.N. Optical fiber sensor for glycoprotein detection based on localized surface plasmon resonance of discontinuous Ag-deposited nanostructure. Opt. Fiber Technol. 2021, 62, 102476. [Google Scholar] [CrossRef]
- Wang, B.T.; Wang, Q. Sensitivity-Enhanced Optical Fiber Biosensor Based on Coupling Effect Between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Z.; Li, J.; Ma, S.; Fu, G.; Jin, W.; Bi, W.; Dong, Y. Microcavity Fiber SERS Probe Coated with Ag Nanoparticles for Detecting Antibiotic in Milk. IEEE Photonics J. 2021, 13, 6800513. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Kaushik, B.K.; Kumar, S. Localized Surface Plasmon Resonance Based Hetero-Core Optical Fiber Sensor Structure for the Detection of L-Cysteine. IEEE Trans. Nanotechnol. 2020, 19, 201–208. [Google Scholar] [CrossRef]
- Agrawal, N.; Saha, C.; Kumar, C.; Singh, R.; Zhang, B.; Jha, R.; Kumar, S. Detection of L-Cysteine Using Silver Nanoparticles and Graphene Oxide Immobilized Tapered SMS Optical Fiber Structure. IEEE Sens. J. 2020, 20, 11372–11379. [Google Scholar] [CrossRef]
- Kaushik, B.K.; Singh, L.; Singh, R.; Zhu, G.; Zhang, B.; Wang, Q.; Kumar, S. Detection of Collagen-IV Using Highly Reflective Metal Nanoparticles—Immobilized Photosensitive Optical Fiber-Based MZI Structure. IEEE Trans. NanoBiosci. 2020, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tian, J.; Wang, Q.; Xiao, F.; Gao, S.; Shi, W.; Cui, F. Development of CuO coated ceramic hollow fiber membrane for peroxymonosulfate activation: A highly efficient singlet oxygen-dominated oxidation process for bisphenol a degradation. Appl. Catal. B 2019, 256, 117783. [Google Scholar] [CrossRef]
- Chocarro-Ruiz, B.; Fernández-Gavela, A.; Herranz, S.; Lechuga, L.M. Nanophotonic label-free biosensors for environmental monitoring. Curr. Opin. Biotechnol. 2017, 45, 175–183. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Hroncekova, S.; Gajdosova, V.; Jane, E.; Hires, M.; Kasak, P.; Kaman, O.; Sokol, R.; Bella, V.; et al. Advanced impedimetric biosensor configuration and assay protocol for glycoprofiling of a prostate oncomarker using Au nanoshells with a magnetic core. Biosens. Bioelectron. 2019, 131, 24–29. [Google Scholar] [CrossRef]
- Shi, S.H.; Wu, D.C.; Wang, X.; Nie, Q.L.; Liu, Z.J.; Luo, B.B.; Liu, E.H.; Liu, P.; Zhao, M.F. An Immunosensor Based on the Graphene-Oxide-Encapsulated Au-Nanoshell-Coated Long-Period Fiber Grating. Acta Opt. Sin. 2020, 40, 1806001. [Google Scholar]
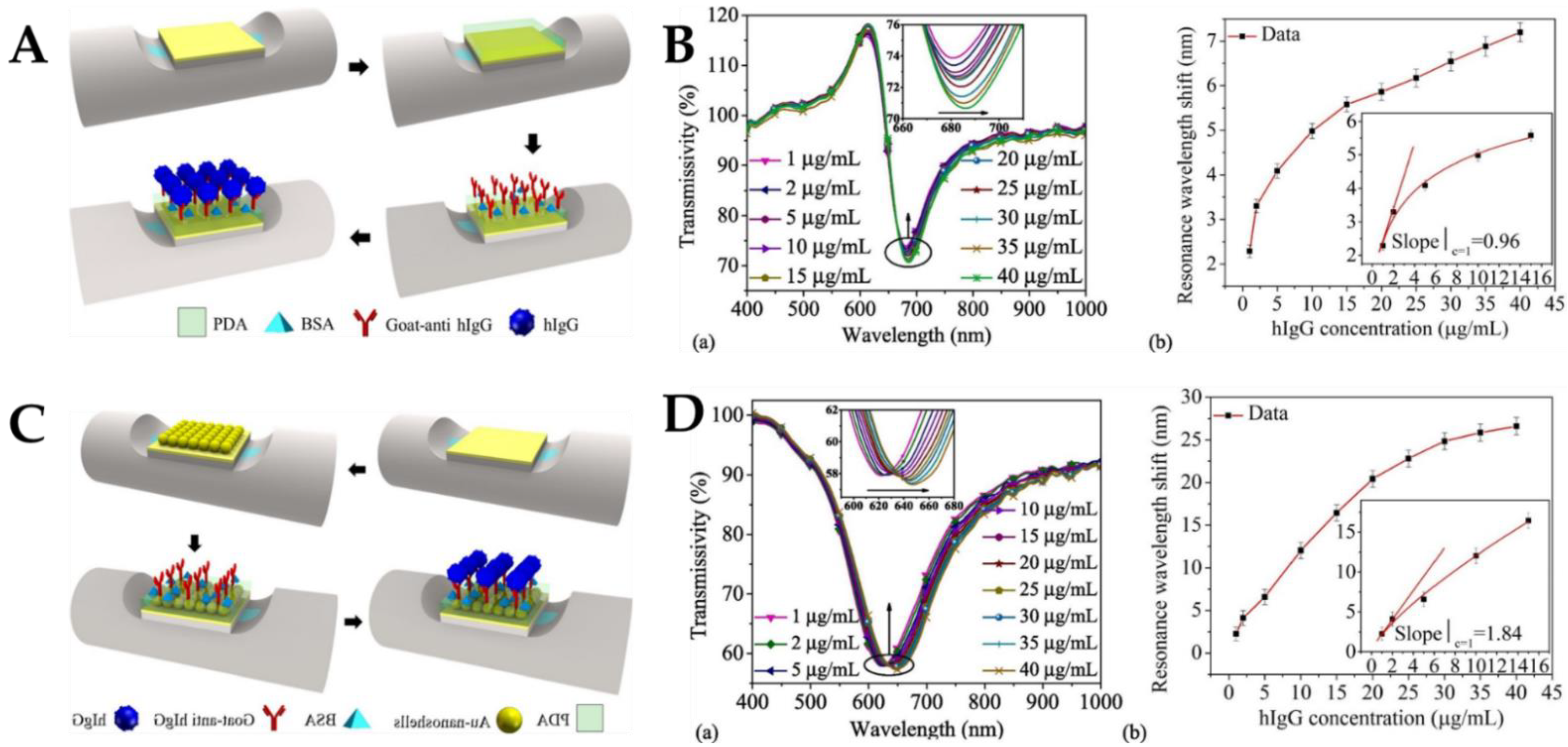
- Cheng, Z.; Wang, Q.; Zhu A-s Qiu F-m Niu, L.-Y.; Jing, J.-Y. Au-nanoshells modified surface field enhanced LRSPR biosensor with low LOD for highly sensitive hIgG sensing. Opt. Laser Technol. 2021, 134, 106656. [Google Scholar] [CrossRef]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.-I.; Kim, K.; Cho, D.-I. Nanowire-Based Biosensors: From Growth to Applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zeng, H.; Liu, J.; Nagashima, K.; Takahashi, T.; Hosomi, T.; Tanaka, W.; Yanagida, T. Nanowire-based sensor electronics for chemical and biological applications. Analyst 2021, 146, 6684–6725. [Google Scholar] [CrossRef] [PubMed]
- Güneş, F.; Aykaç, A.; Erol, M.; Erdem, Ç.; Hano, H.; Uzunbayir, B.; Şen, M.; Erdem, A. Synthesis of hierarchical hetero-composite of graphene foam/α-Fe2O3 nanowires and its application on glucose biosensors. J. Alloys Compd. 2022, 895, 162688. [Google Scholar] [CrossRef]
- Tao, B.; Li, J.; Li, X.; Miao, F. Facile synthesize surfactants and binder-free nanoflake Co(OH)2 on CuCo2S4 nanowire for highly sensitive and selective non-enzymatic glucose sensor. Vacuum 2022, 199, 110953. [Google Scholar] [CrossRef]
- Santos, A.G.; da Rocha, G.O.; de Andrade, J.B. Occurrence of the potent mutagens 2- nitrobenzanthrone and 3-nitrobenzanthrone in fine airborne particles. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Shariati, M.; Sadeghi, M.; Shojaei, S.H.R. Sensory analysis of hepatitis B virus DNA for medicinal clinical diagnostics based on molybdenum doped ZnO nanowires field effect transistor biosensor; a comparative study to PCR test results. Anal. Chim. Acta 2022, 1195, 339442. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Nguyen, H.H.; Mai, A.T. On-chip selective growth of SnO2 nanowires for DNA sensor development. Sens. Actuators A 2020, 312, 112171. [Google Scholar] [CrossRef]
- Selvendran, S.; Raja, A.S.; Yogalakshmi, S. A highly sensitive surface plasmon resonance biosensor using photonic crystal fiber filled with gold nanowire encircled by silicon lining. Optik 2018, 156, 112–120. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H. Optical Properties of ZnO Nanostructures. Small 2006, 2, 944–961. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, W.; Wang, Y.; Zhou, J.; Sun, D.; Zhang, X.; Ruan, S. Highly stabilized and rapid sensing acetone sensor based on Au nanoparticle-decorated flower-like ZnO microstructures. J. Alloys Compd. 2015, 650, 37–44. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Singh, R.; Zhang, W.; Marques, C.; Xie, Y.; Zhang, B.; Kumar, S. Graphene Oxide/Multiwalled Carbon Nanotubes Assisted Serial Quadruple Tapered Structure-Based LSPR Sensor for Glucose Detection. IEEE Sens. J. 2022, 22, 16904–16911. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Ultra-selective fiber optic SPR platform for the sensing of dopamine in synthetic cerebrospinal fluid incorporating permselective nafion membrane and surface imprinted MWCNTs-PPy matrix. Biosens. Bioelectron. 2019, 133, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, Y.; Baryeh, K.; Takalkar, S.; Lund, M.; Zhang, X.; Liu, G. Magnetized carbon nanotubes for visual detection of proteins directly in whole blood. Anal. Chim. Acta 2017, 993, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Feng, Y.; Yao, Q.; He, F. Selection of a new Mycobacterium tuberculosis H37Rv aptamer and its application in the construction of a SWCNT/aptamer/Au-IDE MSPQC H37Rv sensor. Biosens. Bioelectron. 2017, 98, 261–266. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, Y.; Wang, K.; Zhang, Z.; Streit, J.K.; Fagan, J.A.; Tang, J.; Zheng, M.; Yang, C.; Zhu, Z. DNA-directed nanofabrication of high-performance carbon nanotube field-effect transistors. Science 2020, 368, 878–881. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H.; et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Quan, J.; Wang, N.; Zhu, Y. Surface-enhanced Raman scattering activities of carbon nanotubes decorated with silver nanoparticles. Analyst 2016, 141, 5527–5534. [Google Scholar] [CrossRef]
- Pathak, A.; Gupta, B.D. Fiber-Optic Plasmonic Sensor Utilizing CTAB-Functionalized ZnO Nanoparticle-Decorated Carbon Nanotubes on Silver Films for the Detection of Catechol in Wastewater. ACS Appl. Nano Mater. 2020, 3, 2582–2593. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, R.; Xia, F.; Peng, Y. Current status of optical fiber biosensor based on surface plasmon resonance. Biosens. Bioelectron. 2019, 142, 111505. [Google Scholar] [CrossRef]
- Yin, Z.; Jing, X.; Zhang, H.; Wang, C.; Liu, C.; Shao, P. Dual-parameter sensor for simultaneously measuring refractive index and temperature based on no-core fiber and SPR effect. Optik 2022, 262, 169320. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, J.; Wang, B. Highly Sensitive SPR Biosensor Based on Graphene Oxide and Staphylococcal Protein A Co-Modified TFBG for Human IgG Detection. IEEE Trans. Instrum. Meas. 2019, 68, 3350–3357. [Google Scholar] [CrossRef]
- Wang, W.; Mai, Z.; Chen, Y.; Wang, J.; Li, L.; Su, Q.; Li, X.; Hong, X. A label-free fiber optic SPR biosensor for specific detection of C-reactive protein. Sci. Rep. 2017, 7, 16904. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D.; Singh, J.P. Portable fiber-optic SPR platform for the detection of NS1-antigen for dengue diagnosis. Biosens. Bioelectron. 2022, 196, 113720. [Google Scholar] [CrossRef]
- Zheng, W.-L.; Zhang, Y.-N.; Li, L.-K.; Li, X.-G.; Zhao, Y. A plug-and-play optical fiber SPR sensor for simultaneous measurement of glucose and cholesterol concentrations. Biosens. Bioelectron. 2022, 198, 113798. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, B.; Hu, E.T.; Wei, W. Cu/ITO-Coated Uncladded Fiber-Optic Biosensor Based on Surface Plasmon Resonance. IEEE Photonics Technol. Lett. 2019, 31, 1159–1162. [Google Scholar] [CrossRef]
- Samavati, Z.; Borhani, T.N.; Samavati, A. Optical fiber sensor based on magneto-plasmonic features of Ag-Co nanostructure for ppm ammonium detection in aqueous solutions. Opt. Fiber Technol. 2021, 67, 102730. [Google Scholar] [CrossRef]
- Yu, X.; Cheng, H.; Zhang, M.; Zhao, Y.; Qu, L.; Shi, G. Graphene-based smart materials. Nat. Rev. Mater. 2017, 2, 17046. [Google Scholar] [CrossRef]
- Lin, L.P.; Tham, S.-Y.; Loh, H.-S.; Tan, M.T.T. Biocompatible graphene-zirconia nanocomposite as a cyto-safe immunosensor for the rapid detection of carcinoembryonic antigen. Sci. Rep. 2021, 11, 22536. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B. Sensitivity enhanced SPR immunosensor based on graphene oxide and SPA co-modified photonic crystal fiber. Opt. Laser Technol. 2018, 107, 210–215. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, G.; Singh, L.; Wang, Y.; Singh, R.; Zhang, B.; Zhang, X.; Kumar, S. Highly sensitive and selective sensor probe using glucose oxidase/gold nanoparticles/graphene oxide functionalized tapered optical fiber structure for detection of glucose. Optik 2020, 208, 164536. [Google Scholar] [CrossRef]
- Wang, R.; Ren, Z.; Kong, D.; Hu, B.; He, Z. Highly sensitive label-free biosensor based on graphene-oxide functionalized micro-tapered long period fiber grating. Opt. Mater. 2020, 109, 110253. [Google Scholar] [CrossRef]
- Cao, Z.; Yao, B.; Qin, C.; Yang, R.; Guo, Y.; Zhang, Y.; Wu, Y.; Bi, L.; Chen, Y.; Xie, Z.; et al. Biochemical sensing in graphene-enhanced microfiber resonators with individual molecule sensitivity and selectivity. Light Sci. Appl. 2019, 8, 107. [Google Scholar] [CrossRef]
- Xu, B.; Huang, J.; Ding, L.; Cai, J. Graphene oxide-functionalized long period fiber grating for ultrafast label-free glucose biosensor. Mater. Sci. Eng. C 2020, 107, 110329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhou, K.; Wang, C.; Sun, Q.; Yin, G.; Tai, Z.; Wilson, K.; Zhao, J.; Zhang, L. Label-free glucose biosensor based on enzymatic graphene oxide-functionalized tilted fiber grating. Sens. Actuators B 2018, 254, 1033–1039. [Google Scholar] [CrossRef]
- Tong, Z.; Zhao, Y.; Wang, X.; Li, P.; Zhang, W.; Zhang, J. Research on dual-parameter biosensor based on no-core fiber coated by composite film. Optik 2022, 259, 169027. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, B.-T. Surface plasmon resonance biosensor based on graphene oxide/silver coated polymer cladding silica fiber. Sens. Actuators B 2018, 275, 332–338. [Google Scholar] [CrossRef]
- Bazaka, K.; Levchenko, I.; Lim, J.W.M.; Baranov, O.; Corbella, C.; Xu, S.; Keidar, M. MoS2-based nanostructures: Synthesis and applications in medicine. J. Phys. D Appl. Phys. 2019, 52, 183001. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z. 2D MoS2 Nanostructures for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1701158. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Wang, M.; Zhang, Q.; Ma, Q. Distance-dependent plasmon-enhanced electrochemiluminescence biosensor based on MoS2 nanosheets. Biosens. Bioelectron. 2020, 148, 111823. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.J.; Kumar, A.; Patel, S.; Singh, J. 2D layered transition metal dichalcogenides (MoS2): Synthesis, applications and theoretical aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, Z.; Singh, R.; Marques, C.; Wu, Q.; Kaushik, B.K.; Jha, R.; Zhang, B.; Kumar, S. Localized Plasmon-Based Multicore Fiber Biosensor for Acetylcholine Detection. IEEE Trans. Instrum. Meas. 2022, 71, 7000309. [Google Scholar] [CrossRef]
- Geldert, A.; Kenry; Lim, C.T. Paper-based MoS2 nanosheet-mediated FRET aptasensor for rapid malaria diagnosis. Sci. Rep. 2017, 7, 17510. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tiwari, U.K.; Pal, S.S.; Sinha, R.K. Rapid detection of Escherichia coli using fiber optic surface plasmon resonance immunosensor based on biofunctionalized Molybdenum disulfide (MoS2) nanosheets. Biosens. Bioelectron. 2019, 126, 501–509. [Google Scholar] [CrossRef]
- Shariati, M.; Vaezjalali, M.; Sadeghi, M. Ultrasensitive and easily reproducible biosensor based on novel doped MoS2 nanowires field-effect transistor in label-free approach for detection of hepatitis B virus in blood serum. Anal. Chim. Acta 2021, 1156, 338360. [Google Scholar] [CrossRef]
- Singhal, C.; Khanuja, M.; Chaudhary, N.; Pundir, C.S.; Narang, J. Detection of chikungunya virus DNA using two-dimensional MoS2 nanosheets based disposable biosensor. Sci. Rep. 2018, 8, 7734. [Google Scholar] [CrossRef]
- Lu, J.; Chen, M.; Dong, L.; Cai, L.; Zhao, M.; Wang, Q.; Li, J. Molybdenum disulfide nanosheets: From exfoliation preparation to biosensing and cancer therapy applications. Colloids Surf. B 2020, 194, 111162. [Google Scholar] [CrossRef]
- Garkal, A. Molybdenum-based hetero-nanocomposites for cancer therapy, diagnosis and biosensing application: Current advancement and future breakthroughs. J. Control. Release 2021, 330, 257–283. [Google Scholar]
- Sri, S.; Chauhan, D.; Lakshmi, G.B.V.S.; Thakar, A.; Solanki, P.R. MoS2 nanoflower based electrochemical biosensor for TNF alpha detection in cancer patients. Electrochim. Acta 2022, 405, 139736. [Google Scholar] [CrossRef]
- Jandas, P.J.; Luo, J.; Prabakaran, K.; Chen, F.; Fu, Y.Q. Highly stable, love-mode surface acoustic wave biosensor using Au nanoparticle-MoS2-rGO nano-cluster doped polyimide nanocomposite for the selective detection of carcinoembryonic antigen. Mater. Chem. Phys. 2020, 246, 122800. [Google Scholar] [CrossRef]
- Catalán-Gómez, S.; Briones, M.; Cortijo-Campos, S.; García-Mendiola, T.; de Andrés, A.; Garg, S.; Kung, P.; Lorenzo, E.; Pau, J.L.; Redondo-Cubero, A. Breast cancer biomarker detection through the photoluminescence of epitaxial monolayer MoS2 flakes. Sci. Rep. 2020, 10, 16039. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-dimensional Layered MoS2 Biosensors Enable Highly Sensitive Detection of Biomolecules. Sci. Rep. 2014, 4, 7352. [Google Scholar] [CrossRef]
- Kaushik, S.; Tiwari, U.K.; Deep, A.; Sinha, R.K. Two-dimensional transition metal dichalcogenides assisted biofunctionalized optical fiber SPR biosensor for efficient and rapid detection of bovine serum albumin. Sci. Rep. 2019, 9, 6987. [Google Scholar] [CrossRef]
- Gao, L.; Bao, W.; Kuklin, A.V.; Mei, S.; Zhang, H.; Ågren, H. Hetero-MXenes: Theory, Synthesis, and Emerging Applications. Adv. Mater. 2021, 33, 2004129. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, R.; Shao, Y.; Chen, C.; Wu, P.; Wei, Y.; Gao, Y. Detection of GDF11 by using a Ti3C2-MXene-based fiber SPR biosensor. Opt. Express 2021, 29, 36598–36607. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Pan, S.; Guo, X.; Gao, Y.; Zhu, D.; Yang, Q.; Gao, J.; Zhang, C.; Chen, Y. Nb2C MXene-Functionalized Scaffolds Enables Osteosarcoma Phototherapy and Angiogenesis/Osteogenesis of Bone Defects. Nano-Micro Lett. 2021, 13, 30. [Google Scholar] [CrossRef]
- Li, W.; Miao, Y.; Guo, T.; Zhang, K.; Yao, J. Nb2CTx MXene-tilted fiber Bragg grating optofluidic system based on photothermal spectroscopy for pesticide detection. Biomed. Opt. Express 2021, 12, 7051–7063. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Nayak, P.; Xia, C.; Alshareef, H.N. Novel amperometric glucose biosensor based on MXene nanocomposite. Sci. Rep. 2016, 6, 36422. [Google Scholar] [CrossRef]
- Li, M.; Peng, X.; Han, Y.; Fan, L.; Liu, Z.; Guo, Y. Ti3C2 MXenes with intrinsic peroxidase-like activity for label-free and colorimetric sensing of proteins. Microchem. J. 2021, 166, 106238. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, Y.; Jiang, D.; Li, F.; Gan, Y.; Zhu, Y.; Pan, Y.; Wan, H.; Wang, P. Covalently grafting first-generation PAMAM dendrimers onto MXenes with self-adsorbed AuNPs for use as a functional nanoplatform for highly sensitive electrochemical biosensing of cTnT. Microsyst. Nanoeng. 2022, 8, 35. [Google Scholar] [CrossRef]
- Bi, M.; Miao, Y.; Li, W.; Fei, C.; Zhang, K. Ultrasensitive BOD Detection of Fiber Integrated with Nb2CTx MXene for Water Pollution. J. Lightwave Technol. 2022, 40, 2173–2180. [Google Scholar] [CrossRef]
- Yi, D.; Wang, C.; Gao, L.; Chen, Y.; Liu, F.; Geng, Y.; Zhang, H.; Li, X. Ti3CN MXene-based ultra-sensitive optical fiber salinity sensor. Opt. Lett. 2022, 47, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, V.R.; Kumar, S.R.S.; Sankararaman, S. Ultrahigh Sensitivity Surface Plasmon Resonance–Based Fiber-Optic Sensors Using Metal-Graphene Layers with Ti3C2Tx MXene Overlayers. Plasmonics 2020, 15, 457–466. [Google Scholar] [CrossRef]
- Rahman, M.S.; Anower, M.S.; Abdulrazak, L.F. Modeling of a fiber optic SPR biosensor employing Tin Selenide (SnSe) allotropes. Results Phys. 2019, 15, 102623. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.; Jiang, J.; Xu, T.; Wang, S.; Chang, P.; Zhang, Z.; Ma, J.; Liu, T. MoSe2-Au Based Sensitivity Enhanced Optical Fiber Surface Plasmon Resonance Biosensor for Detection of Goat-Anti-Rabbit IgG. IEEE Access 2020, 8, 660–668. [Google Scholar] [CrossRef]
- Zhang, W.L.; Liu, K.; Jiang, J.F.; Xu, T.H.; Wang, S.; Zhang, Z.; Jing, J.Y.; Ma, J.Y.; Liu, T.G. Tungsten Disulfide Modified Tapered Fiber Optic Surface Plasmon Resonance Sensor with Enhanced Sensitivity. Acta Photonica Sin. 2022, 51, 0306002. [Google Scholar]
- Rahman, M.S.; Hasan, M.R.; Rikta, K.A.; Anower, M.S. A novel graphene coated surface plasmon resonance biosensor with tungsten disulfide (WS2) for sensing DNA hybridization. Opt. Mater. 2018, 75, 567–573. [Google Scholar] [CrossRef]
- Cai, Y.; Li, W.; Feng, Y.; Zhao, J.S.; Bai, G.; Xu, J.; Li, J.Z. Sensitivity enhancement of WS2-coated SPR-based optical fiber biosensor for detecting glucose concentration*. Chin. Phys. B 2020, 29, 110701. [Google Scholar] [CrossRef]
- Liang, Y.; Yu, Z.; Li, L.; Xu, T. A self-assembled plasmonic optical fiber nanoprobe for label-free biosensing. Sci. Rep. 2019, 9, 7379. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Zou, X.; Chen, Z.; Liu, H.; Ni, Y. Ultrasensitive Physical, Bio, and Chemical Sensors Derived from 1-, 2-, and 3-D Nanocellulosic Materials. Small 2020, 16, 1906567. [Google Scholar] [CrossRef]
- Vasić, B.; Isić, G.; Gajić, R. Localized surface plasmon resonances in graphene ribbon arrays for sensing of dielectric environment at infrared frequencies. J. Appl. Phys. 2013, 113, 013110. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, C.; Yu, J.; Cao, H.; Li, S.; Jia, W. Design of infrared surface plasmon resonance sensors based on graphene ribbon arrays. Opt. Laser Technol. 2014, 59, 99–103. [Google Scholar] [CrossRef]
- Rodrigo, D.; Limaj, O.; Janner, D.; Etezadi, D.; García de Abajo, F.J.; Pruneri, V.; Altug, H. Mid-infrared plasmonic biosensing with graphene. Science 2015, 349, 165–168. [Google Scholar] [CrossRef] [PubMed]


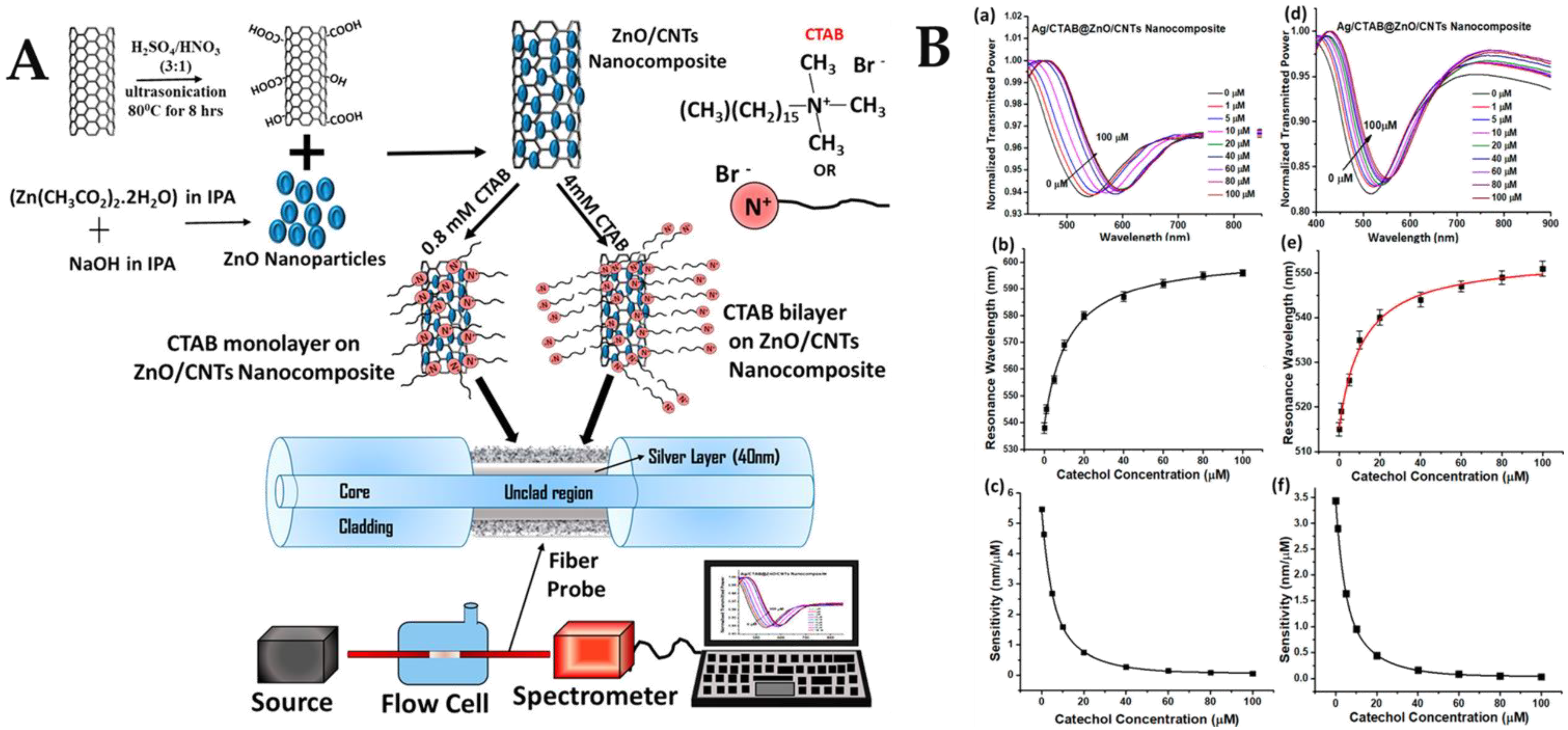
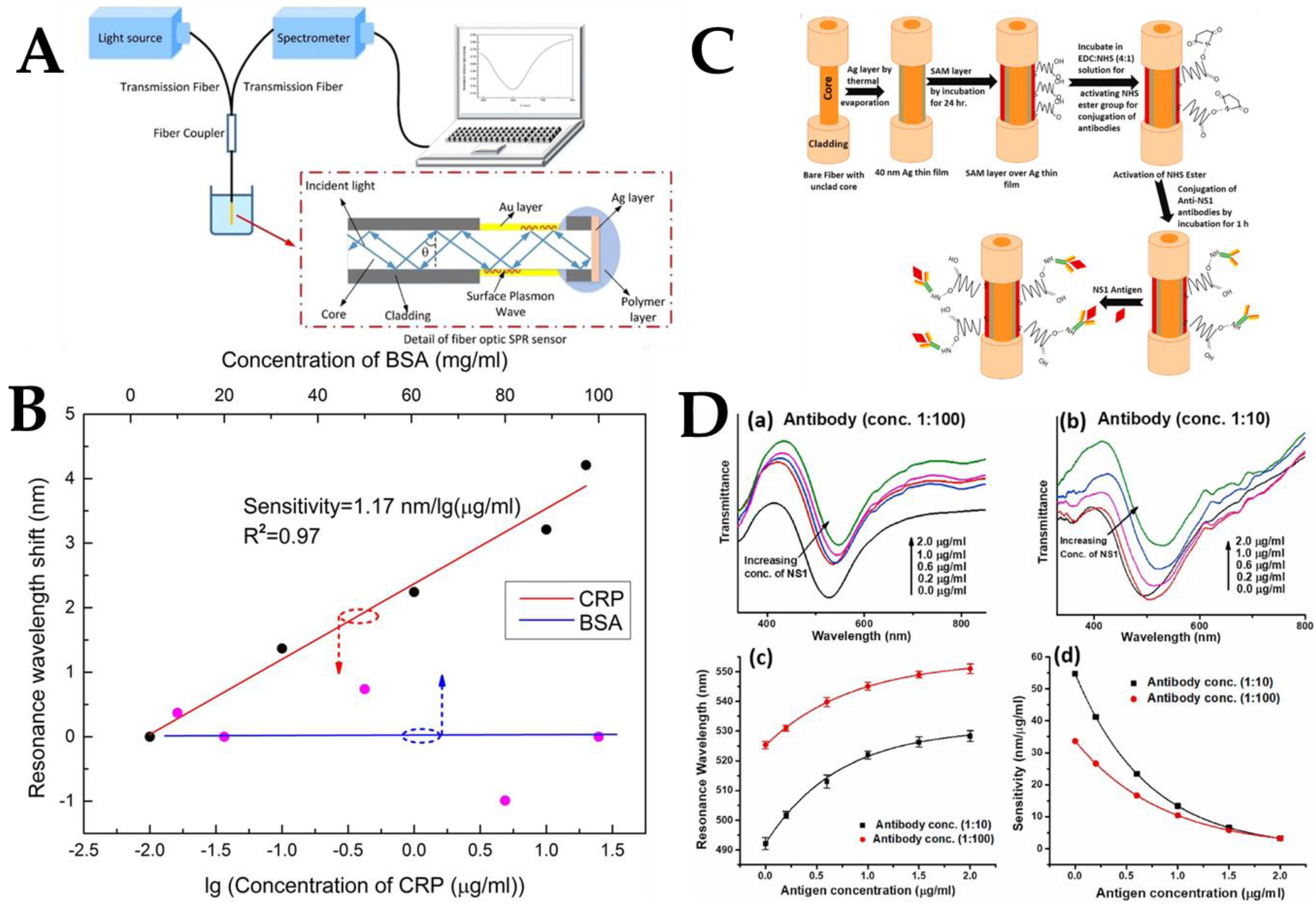

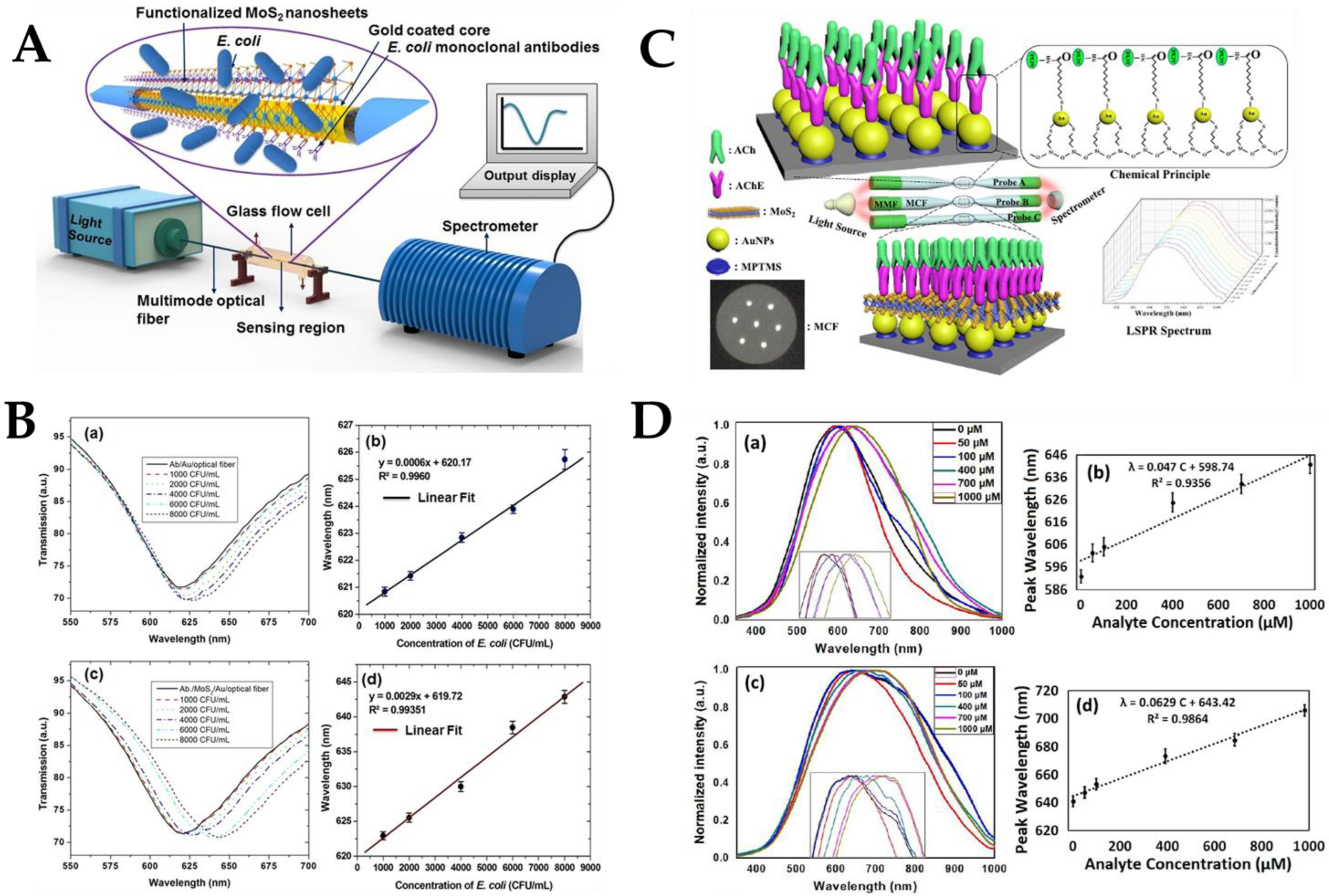
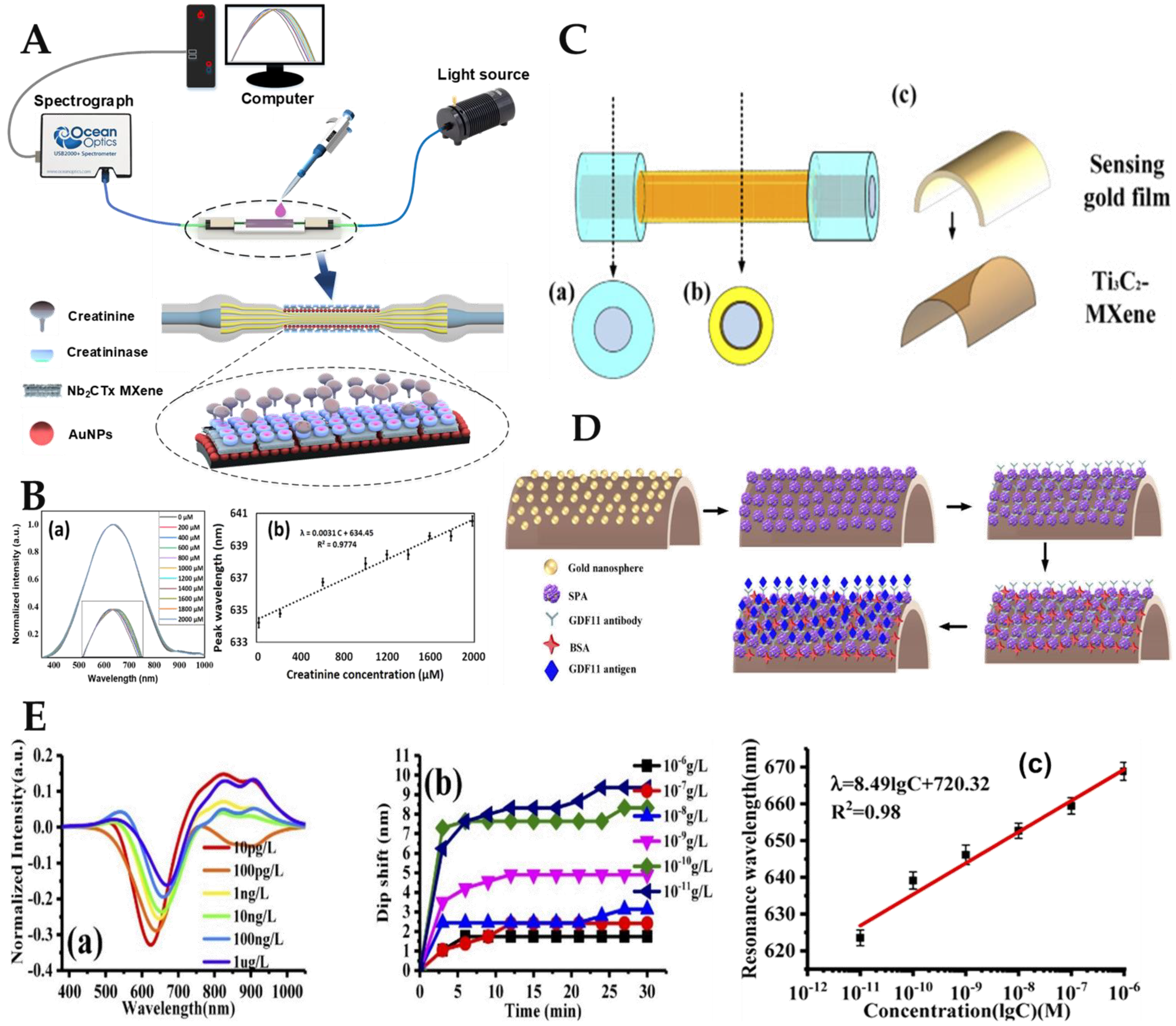
| Optical Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | LoD | Ref. |
|---|---|---|---|---|---|
| Dual-arm fiber optics | Fluorescence | ZnCdSe QDs | Pesticide | 2.5 μg/L | [35] |
| Tapered MMF | Fluorescence | QDs | Staphylococcus aureus | n.r. a | [37] |
| Large-core quartz optical fibers | Fluorescence | CQDs | Glucose | 6.43 μΜ | [56] |
| 25.79 nM | |||||
| Large diameter quartz fiber | Fluorescence | N-CQDs | Acetyl choline | 162.8 μΜ | [66] |
| 2.9 nM | |||||
| Large diameter quartz fiber | Fluorescence | CQDs | NO | 9.12 nM | [73] |
| Exposed core fibers | Fluorescence | CdTe/CdS QDs | NO | 0.01 nM | [74] |
| Tapered SMF | Fluorescence | CQDs | Dopamine | 46.4 nM | [81] |
| Plastic fiber | Fluorescence | CdSe QDs | NO | 0.1 nM | [87] |
| Plastic-clad multimode silica fiber (HPCF) | Fluorescence | CQDs-COD | Cholesterol | 1 μΜ | [88] |
| Optical Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | LoD | Ref. |
|---|---|---|---|---|---|
| Tapered SMF | LSPR | AuNPs | Cholesterol | 53.1 nM | [18] |
| MMF-HCF-MMF | SPR | AuNPs | DNA | 1 pM | [41] |
| Unclad MMF | LSPR | AuNPs | Taurine | 53 μM | [42] |
| Ω-shaped | LSPR | AuNPs | MCF-7 cancer cells | 12 cells/mL | [43] |
| Tapered SMF | LSPR | AuNPs | Glucose | 322 μM | [57] |
| Tapered SMF | LSPR | AgNPs | Dopamine | 0.058 μM | [63] |
| SMF-HCF | LSPR | AuNPs | Cholesterol | 25.5 nM | [75] |
| Tapered SMF | LSPR | AuNPs | Uric acid | 175 μM | [76] |
| SMSMS | LSPR | AgNPs/CuONPs | Uric acid | 0.35 mM | [102] |
| 69.26 μM | |||||
| Ex-TFG | LSPR | AuNPs | Glucose | 2.5 nM | [107] |
| Tapered SMF | LSPR | AuNPs | DNA-c | 1.32 fM | [108] |
| MMF | FO-LSPR | AuNPs | Thyroglobulin | 6.6 fg/mL | [109] |
| SFS | Chemical luminescence | AuNPs | Prostate-specific antigen | 0.3 pg/mL | [110] |
| MMF | LSPR | AgNPs | Glycoprotein | 30.76 nm/ppm | [111] |
| PSF | Coupling effect between SPR and LSPR | AuNPs | Human IgG | 37 ng/mL | [112] |
| MMF | SERS | AgNPs | Antibiotics R6G | 1 nM | [113] |
| SMS | LSPR | PVA-AgNPs | L-Cysteine | 136.6 μM | [114] |
| Tapered SMS | LSPR | AgNPs | L-Cysteine | 63.25 μM | [115] |
| SMPMS | LSPR | AgNPs | Collagen-IV | 126.07 ng/mL | [116] |
| Optical Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | Sensitivity | Ref. |
|---|---|---|---|---|---|
| MMF-SMF-MMF | SPR | Au film | Glucose | 3.10 pm/(mg/dL) | [5] |
| U-shape Fiber | SPR | Au film | Glucose | 0.172 nm/(μg/mL) | [15] |
| Sucrose | 0.738 nm/(mg/mL) | ||||
| Tilted TFBG | SPR | Ag film | Glucose | 0.5 dB/mM | [26] |
| Unclad MMF | SPR | Au film | CRP | 1.17nm/lg (μg/mL) | [144] |
| Unclad fiber | SPR | Ag film | NS1 antigen | 54.7nm/(μg/mL) | [145] |
| HPCF | SPR | Au film | Glucose | 6.6 nm/mM | [146] |
| Cholesterol | 63 pm/nM | ||||
| PCS MMF | SPR | Cu film | BSA | 1.907 nm/(mg/mL) | [147] |
| Unclad Fiber | SPR | Co/Ag film | Ammonium | 0.131 nm/ppm | [148] |
| Optical Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | Sensitivity | Ref. |
|---|---|---|---|---|---|
| SMF-PCF-SMF | SPR | GO | Glucose | 0.1225 nm/(mg/mL) | [25] |
| Ex-TFG | Langmuir adsorption | Monolayer GO | Hemoglobin | 3.83 nm/(mg/mL) | [28] |
| Bilayer GO | 4.33 nm/(mg/mL) | ||||
| Three layers GO | 8.21 nm/(mg/mL) | ||||
| LPG | Interferometer | GO | 25-hydroxyvitamin D3 | 1.0 ng/mL | [31] |
| MMF-HSC-MMF | SPR | GO-AuNRs | Amino acids | n.r. a | [40] |
| MMF-PCF-MMF | SPR | Au film/GO | Human IgG | 0.3 nm/(μg/mL) | [151] |
| Tapered SMF | LSPR | AuNPs/GO | Glucose | 1.06 nm/mM | [152] |
| MTLPG | Optical-tweezer effect | GO nanosheets | Hemoglobin in water | 2 nm/(mg/mL) | [153] |
| Hemoglobin in urea | 1 nm/(mg/mL) | ||||
| Hemoglobin in glucose | 0.73 nm/(mg/mL) | ||||
| MMF-SMF | FRET | GO nanosheets | Dopamine | 0.51 kHz/μM | [154] |
| Nicotine | 0.2 kHz/nM | ||||
| ssDNA | 8.8 kHz/nM | ||||
| LPFG | SPR | GO | Glucose | 0.77 nm/(mg/mL) | [155] |
| TFG | Wavelength modulation | GO | Glucose | 0.25 nm/mM | [156] |
| SMF-NCF-SMF | SPR | GO | Glucose | 0.04 nm/(mg/mL) | [157] |
| PCS | SPR | GO/Ag film | Human IgG | 0.4985 nm/(μg/mL) | [158] |
| Optics Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | LoD | Ref. |
|---|---|---|---|---|---|
| SMF-MCF-MMF-SMF | LSPR | AuNPs/GO/MoS2 | Creatinine | 128.4 μM | [7] |
| D-shaped SMF | SPR | MoS2/GO | Glucose | n.r.a | [13] |
| Etched MPM | LSPR | GO/AuNPs/MoS2 | Cardiac Troponin I | 96.263 ng/mL | [91] |
| SMF-MCF | LSPR | AuNPs/MoS2 | Shigella Bacteria | 1.56 CFU/mL | [103] |
| MMF-Tapered MCF-MMF | LSPR | AuNPs/MoS2 | Acetylcholine | 14.28 μM | [163] |
| MMF-Etched MCF-MMF | 71.30 μM | ||||
| Etched MMF | SPR | MoS2 | E. coli | 97 CFU/mL | [165] |
| Etched MMF | SPR | MoS2 | BSA | 0.29 μg/mL | [174] |
| Optics Fiber Structure | Sensing Mechanism | Nanomaterials | Analyte Biomolecule | LoD | Ref. |
|---|---|---|---|---|---|
| CTC | LSPR | AuNPs/Nb2CTx MXene | Creatinine | 86.12 μM | [16] |
| PTIF | SPR | Ti3C2-MXene | GDF11 | 0.55 pg/L | [176] |
| Tilted fiber Bragg grating | PTS | Nb2CTx MXene | Pesticide | 0.35 ppm | [178] |
| Tapered SMF | SPR | Nb2CTx MXene | BOD | 57 μg/mL | [182] |
| SMF-NCF-TNCF-NCF | SPR | Ti3CN MXene | NaCl | n.r. a | [183] |
| PCS | SPR | Ti3C2-MXene | VOC | n.r. a | [184] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Singh, R.; Wang, Y.; Marques, C.; Zhang, B.; Kumar, S. Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review. Biosensors 2022, 12, 843. https://doi.org/10.3390/bios12100843
Li M, Singh R, Wang Y, Marques C, Zhang B, Kumar S. Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review. Biosensors. 2022; 12(10):843. https://doi.org/10.3390/bios12100843
Chicago/Turabian StyleLi, Muyang, Ragini Singh, Yiran Wang, Carlos Marques, Bingyuan Zhang, and Santosh Kumar. 2022. "Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review" Biosensors 12, no. 10: 843. https://doi.org/10.3390/bios12100843
APA StyleLi, M., Singh, R., Wang, Y., Marques, C., Zhang, B., & Kumar, S. (2022). Advances in Novel Nanomaterial-Based Optical Fiber Biosensors—A Review. Biosensors, 12(10), 843. https://doi.org/10.3390/bios12100843








