A Paper-Based Photoelectrochemical Sensing Platform Based on In Situ Grown ZnO/ZnIn2S4 Heterojunctions onto Paper Fibers for Sensitively Detecting AFP
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
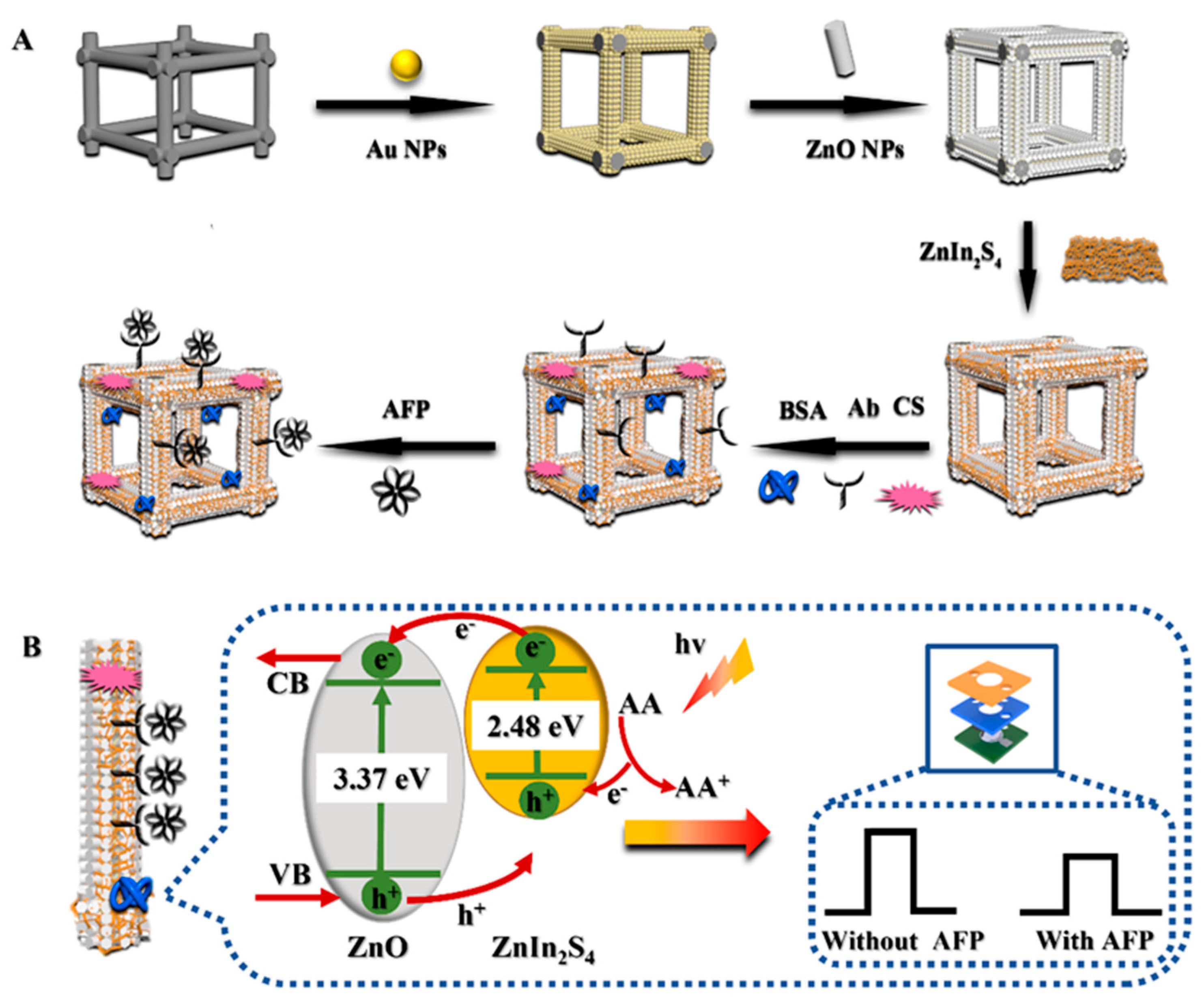
2.3. Sensing Mechanism of μPAD
2.4. Preparation of the Proposed μPAD
2.4.1. Electrodeposition of Paper-Based ZnO
2.4.2. Fabrication of Paper-Based ZnO/ZnIn2S4 Photoelectrode
2.4.3. Practical Structural Design and Analytical Steps of the μPAD
3. Results and Discussion
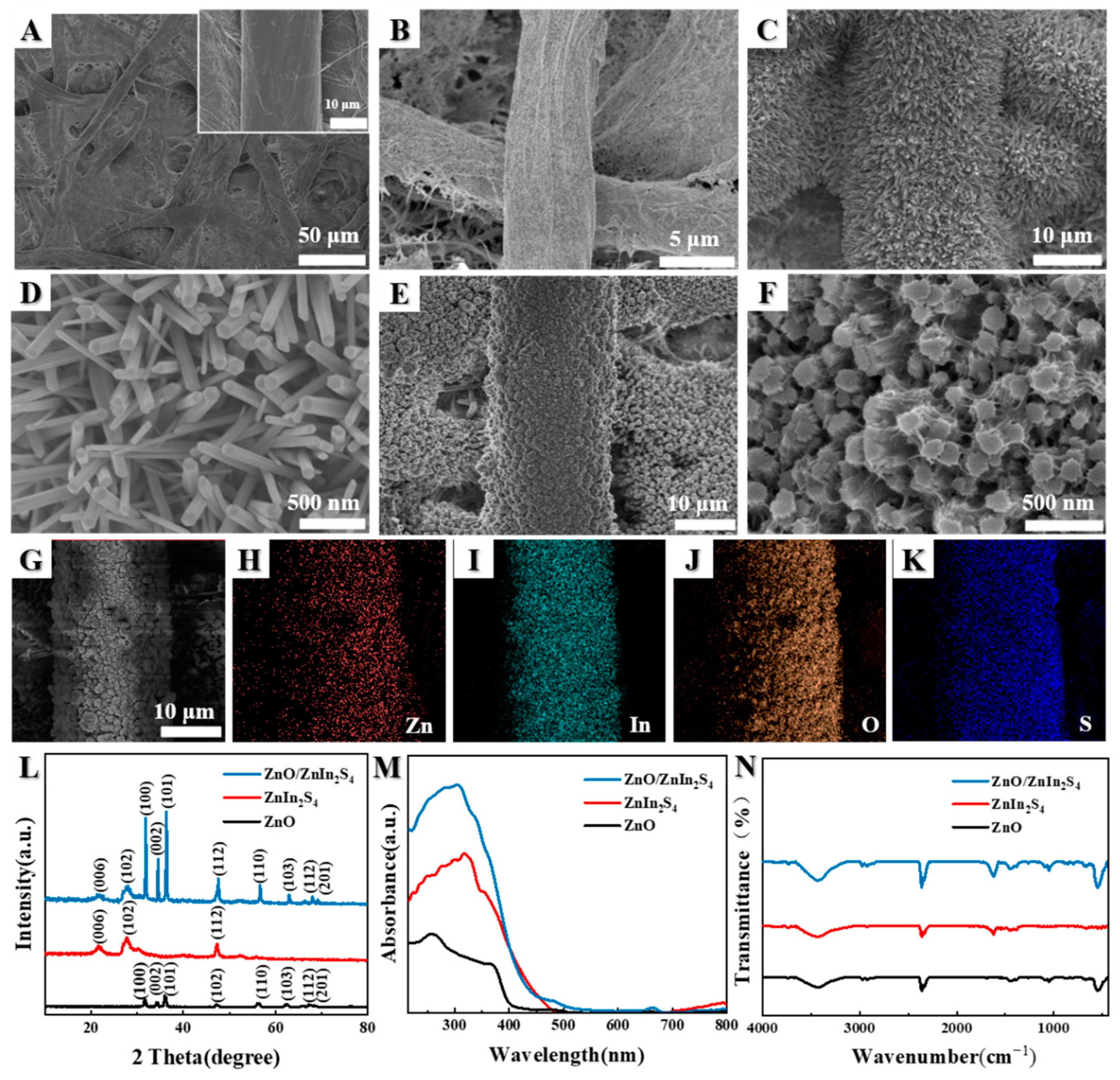
3.1. Characterization of Obtained Samples
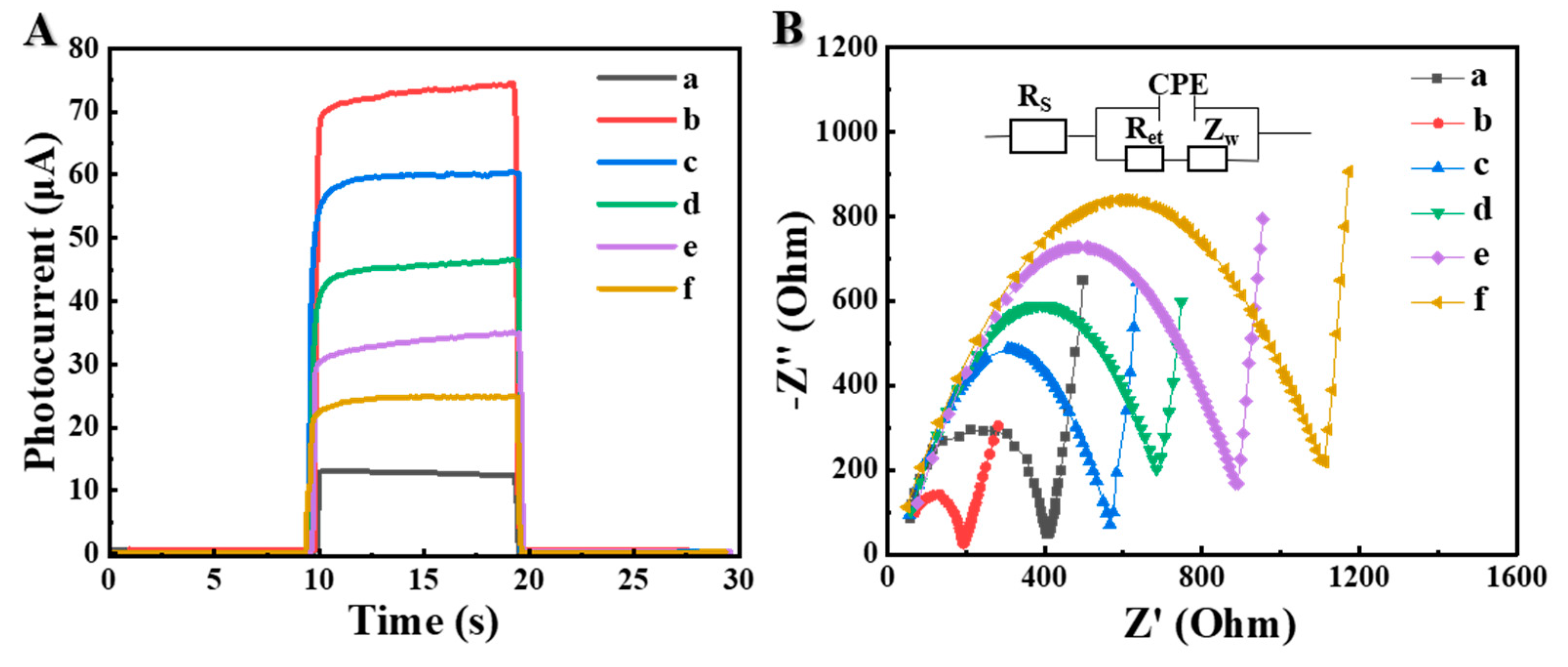
3.2. Characterization of Photoelectric Properties of the Proposed μPAD
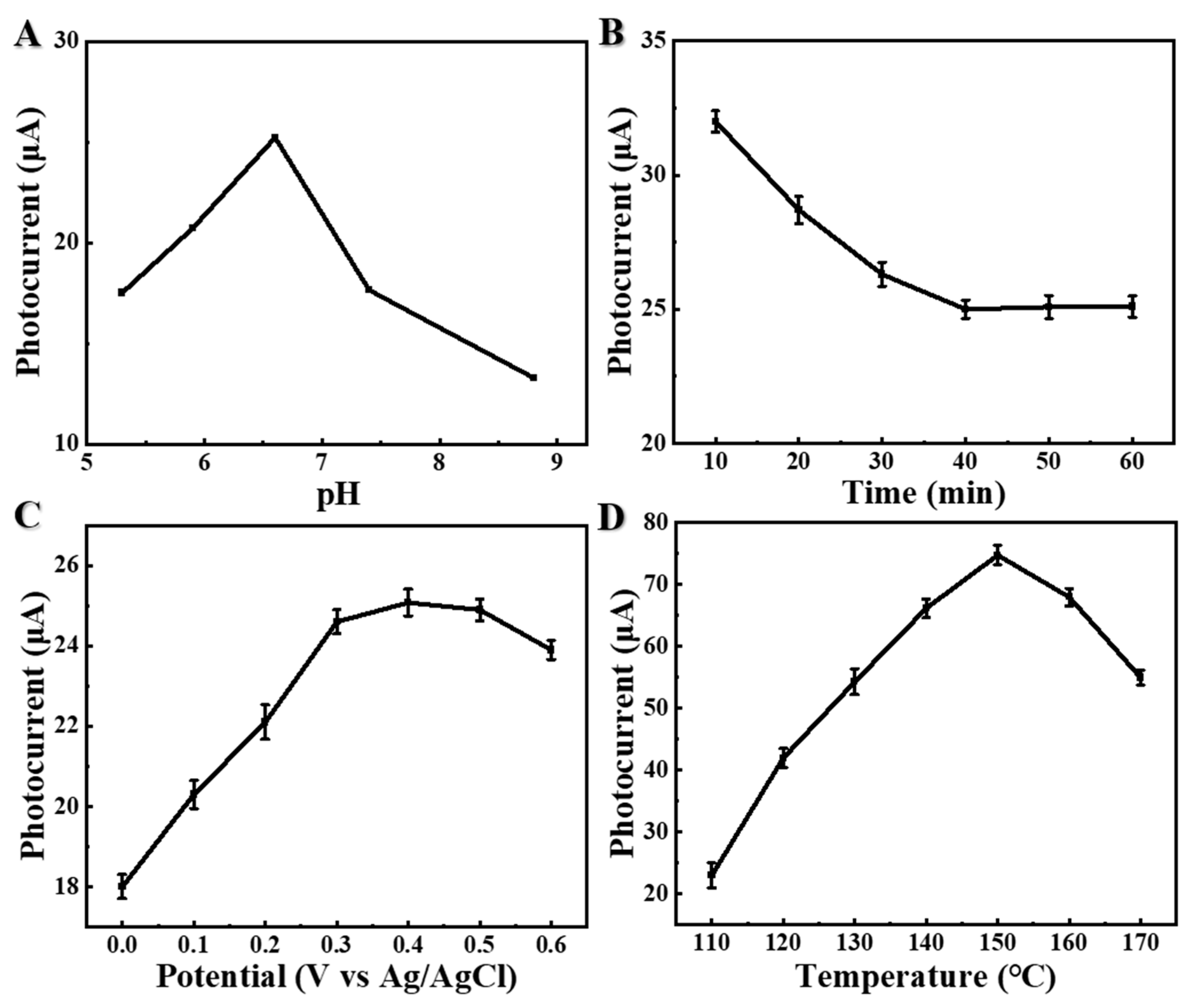
3.3. Optimization of Conditions for the μPAD
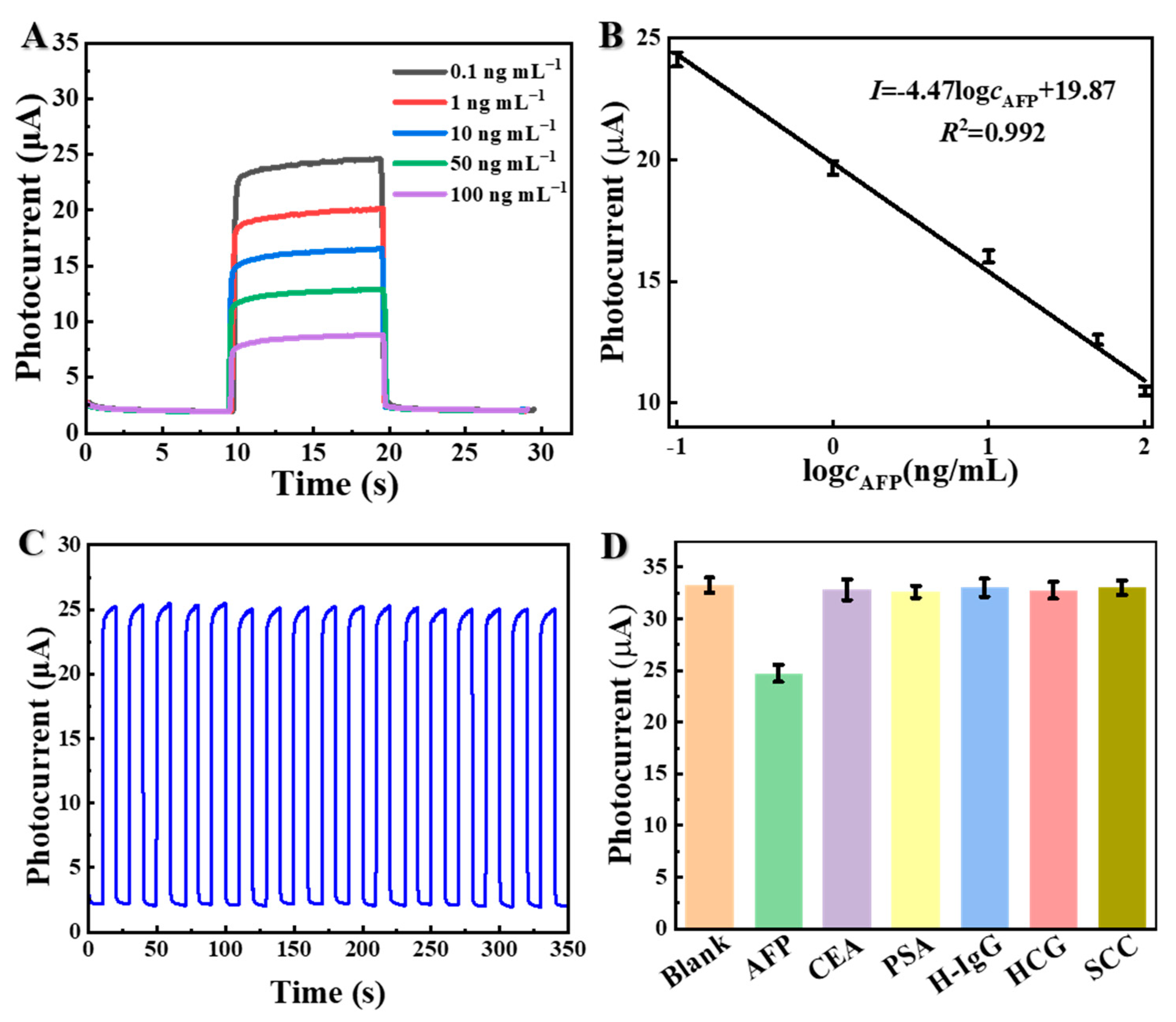
3.4. Specific Performance of the μPAD of PEC for AFP Immunosensing
3.5. Actual Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Luo, Z.; Lv, T.; Zhu, K.; Li, Y.; Wang, L.; Gooding, J.J.; Liu, G.; Liu, B. Paper-Based Ratiometric Fluorescence Analytical Devices Towards Point-of-Care Testing of Human Serum Albumin. Angew. Chem. Int. Ed. 2020, 59, 3131–3136. [Google Scholar] [CrossRef] [PubMed]
- Luan, X.; Pan, Y.; Zhou, D.; He, B.; Liu, X.; Gao, Y.; Yang, J.; Song, Y. Cerium Metal Organic Framework Mediated Molecular Threading for Point-of-Care Colorimetric Assays. Biosens. Bioelectron. 2020, 165, 112406. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic Point-of-Care Tests in Resource-Limited Settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef]
- Pollock, N.R.; Colby, D.; Rolland, J.P. A Point-of-Care Paper-Based Fingerstick Transaminase Test: Toward Low-Cost “Lab-on-a-Chip” Technology for the Developing World. Clin. Gastroenterol. Hepatol. 2013, 11, 478–482. [Google Scholar] [CrossRef]
- Ferguson, B.S.; Buchsbaum, S.F.; Wu, T.T.; Hsieh, K.; Xiao, Y.; Sun, R.; Soh, H.T. Genetic Analysis of H1N1 Influenza Virus from Throat Swab Samples in a Microfluidic System for Point-of-Care Diagnostics. J. Am. Chem. Soc. 2011, 133, 9129–9135. [Google Scholar] [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef]
- Lemarchand, J.; Bridonneau, N.; Battaglini, N.; Carn, F.; Mattana, G.; Piro, B.; Zrig, S.; Noël, V. Challenges, Prospects, and Emerging Applications of Inkjet-Printed Electronics: A Chemist’s Point of View. Angew. Chem. Int. Ed. 2022, 61, e202200166. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. In-Situ Synthesized Polypyrrole-Cellulose Conductive Networks for Potential-Tunable Foldable Power Paper. Nano Energy 2017, 31, 174–182. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Li, L.; Zhang, L.; Hao, S.; Ge, S.; Yu, J. Bi2S3@MoS2 Nanoflowers on Cellulose Fibers Combined with Octahedral CeO2 for Dual-Mode Microfluidic Paper-Based Mirna-141 Sensors. ACS Appl. Mater. Interfaces 2021, 13, 32780–32789. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, F.; Smith, Z.J.; Wei, X. Microfluidic Paper-Based Preconcentration and Retrieval for Rapid Ribonucleic Acid Biomarker Detection and Visualization. Anal. Chem. 2022, 94, 10764–10772. [Google Scholar] [CrossRef]
- Jackson, S.; Lee, S.; Badu-Tawiah, A.K. Automated Immunoassay Performed on a 3D Microfluidic Paper-Based Device for Malaria Detection by Ambient Mass Spectrometry. Anal. Chem. 2022, 94, 5132–5139. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zuo, C.; Zhang, J.; Liu, H.; Fang, X. A Paper-Based Wearable Photodetector for Simultaneous UV Intensity and Dosage Measurement. Adv. Funct. Mater. 2021, 31, 2100026. [Google Scholar] [CrossRef]
- Cheng, F.; Cao, X.; Li, H.; Liu, T.; Xie, X.; Huang, D.; Maharjan, S.; Bei, H.P.; Gomez, A.; Li, J.; et al. Generation of Cost-Effective Paper-Based Tissue Models through Matrix-Assisted Sacrificial 3D Printing. Nano Lett. 2019, 19, 3603–3611. [Google Scholar] [CrossRef]
- Rahbar, M.; Zou, S.; Baharfar, M.; Liu, G. A Customized Microfluidic Paper-Based Platform for Colorimetric Immunosensing: Demonstrated Via hCG Assay for Pregnancy Test. Biosensors 2021, 11, 474. [Google Scholar] [CrossRef]
- Zhu, L.; Lv, X.; Yu, H.; Tan, X.; Rong, Y.; Feng, W.; Zhang, L.; Yu, J.; Zhang, Y. Paper-Based Bipolar Electrode Electrochemiluminescence Platform Combined with Pencil-Drawing Trace for the Detection of M.Sssi Methyltransferase. Anal. Chem. 2022, 94, 8327–8334. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Paper-Based Enzyme Biosensor for One-Step Detection of Hypoxanthine in Fresh and Degraded Fish. ACS Sens. 2020, 5, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Zhou, C.; Zhang, B.; Zhang, L.; Liu, Y.; Hao, S.; Tang, X.; Huang, Y.; Yu, J. Enhanced Catalytic Activity Induced by the Nanostructuring Effect in Pd Decoration onto Doped Ceria Enabling an Origami Paper Analytical Device for High Performance of Amyloid-Beta Bioassay. ACS Appl. Mater. Interfaces 2021, 13, 33937–33947. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zhou, S.; Zhu, L.; Lv, X.; Zhang, J.; Zhang, L.; Zhu, P.; Yu, J. Dnazyme-Triggered Visual and Ratiometric Electrochemiluminescence Dual-Readout Assay for Pb (II) Based on an Assembled Paper Device. Anal. Chem. 2020, 92, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, K.; Kong, Q.; Zhang, L.; Ge, S.; Yu, J. A Self-Powered Origami Paper Analytical Device with a Pop-up Structure for Dual-Mode Electrochemical Sensing of ATP Assisted by Glucose Oxidase-Triggered Reaction. Biosens. Bioelectron. 2020, 148, 111839. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, M.; Kong, J.; Li, Z.; Jiang, J.; Xi, Y.; Li, F. Microfluidic Paper-Based Analytical Device by Using Pt Nanoparticles as Highly Active Peroxidase Mimic for Simultaneous Detection of Glucose and Uric Acid with Use of a Smartphone. Talanta 2022, 237, 122954. [Google Scholar] [CrossRef]
- Tan, X.; Yu, H.; Liang, B.; Han, M.; Ge, S.; Zhang, L.; Li, L.; Li, L.; Yu, J. A Target-Driven Self-Feedback Paper-Based Photoelectrochemical Sensing Platform for Ultrasensitive Detection of Ochratoxin a with an In2S3/WS3 Heterojunction Structure. Anal. Chem. 2022, 94, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Brooks, J.C.; Mace, C.R. Scalable Methods for Device Patterning as an Outstanding Challenge in Translating Paper-Based Microfluidics from the Academic Benchtop to the Point-of-Care. J. Anal. Test. 2019, 3, 50–60. [Google Scholar] [CrossRef]
- Li, Y.; He, R.; Niu, Y.; Li, F. Paper-Based Electrochemical Biosensors for Point-of-Care Testing of Neurotransmitters. J. Anal. Test. 2019, 3, 19–36. [Google Scholar] [CrossRef]
- Yakoh, A.; Mehmeti, E.; Kalcher, K.; Chaiyo, S. Hand-Operated, Paper-Based Rotational Vertical-Flow Immunosensor for the Impedimetric Detection of α-Fetoprotein. Anal. Chem. 2022, 94, 5893–5900. [Google Scholar] [CrossRef]
- Khaliliazar, S.; Toldra, A.; Chondrogiannis, G.; Hamedi, M.M. Electroanalytical Paper-Based Nucleic Acid Amplification Biosensors with Integrated Thread Electrodes. Anal. Chem. 2021, 93, 14187–14195. [Google Scholar] [CrossRef]
- O’Neill, A.F.; Xia, C.; Krailo, M.D.; Shaikh, F.; Pashankar, F.D.; Billmire, D.F.; Olson, T.A.; Amatruda, J.F.; Villaluna, D.; Huang, L.; et al. α-Fetoprotein as a Predictor of Outcome for Children with Germ Cell Tumors: A Report from the Malignant Germ Cell International Consortium. Cancer 2019, 125, 3649–3656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, D.; Su, X.; Hong, Z.; Feng, W.; Xu, M.; Li, F. Two birds with one stone: Amine-functionalized MSNs@Eu(OH)CO3 nanoprobe for efficient dissolution-enhanced afterglow bioassay. Nano Res. 2022, 15, 8360–8366. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, K.; Zhu, L.; Tang, D.; Niessner, R.; Knopp, D. H2-Based Electrochemical Biosensor with Pd Nanowires@Zif-67 Molecular Sieve Bilayered Sensing Interface for Immunoassay. Anal. Chem. 2019, 91, 12055–12062. [Google Scholar] [CrossRef]
- Lorenzo-Gomez, R.; Gonzalez-Robles, D.; Miranda-Castro, R.; de-Los-Santos-Alvarez, N.; Lobo-Castanon, M.J. On the Electrochemical Detection of Alpha-Fetoprotein Using Aptamers: DNA Isothermal Amplification Strategies to Improve the Performance of Weak Aptamers. Biosensors 2020, 10, 46. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, S.Y.; Kim, D.G.; Lee, S.Y.; Kim, J.Y.; Yoo, J.S. Absolute Quantification of N-Glycosylation of Alpha-Fetoprotein Using Parallel Reaction Monitoring with Stable Isotope-Labeled N-Glycopeptide as an Internal Standard. Anal. Chem. 2020, 92, 12588–12595. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Liu, M.; Liu, F.; Bian, S.; Du, S.; Zhu, S.; Wang, H. Biominerized Gold-Hemin@MOF Composites with Peroxidase-Like and Gold Catalysis Activities: A High-Throughput Colorimetric Immunoassay for Alpha-Fetoprotein in Blood by Elisa and Gold-Catalytic Silver Staining. Sens. Actuators B Chem. 2018, 266, 543–552. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, W.; Tan, Q.; Cui, X.; Dai, Z. Electrochemical Assay of the Alpha Fetoprotein-L3 Isoform Ratio to Improve the Diagnostic Accuracy of Hepatocellular Carcinoma. Anal. Chem. 2018, 90, 13051–13058. [Google Scholar] [CrossRef]
- Chen, P.; Jiang, P.; Lin, Q.; Zeng, X.; Liu, T.; Li, M.; Yuan, Y.; Song, S.; Zhang, J.; Huang, J.; et al. Simultaneous Homogeneous Fluorescence Detection of Afp and GPC3 in Hepatocellular Carcinoma Clinical Samples Assisted by Enzyme-Free Catalytic Hairpin Assembly. ACS Appl. Mater. Interfaces 2022, 14, 28697–28705. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, K.; Xiu, M.; Zhou, C.; Li, L.; Zhang, J.; Hao, S.; Zhang, L.; Ge, S.; Huang, Y.; et al. In Situ Growth of WO3/BiVO4 Nanoflowers onto Cellulose Fibers to Construct Photoelectrochemical/Colorimetric Lab-on-Paper Devices for the Ultrasensitive Detection of AFP. J. Mater. Chem. B 2022, 10, 4031–4039. [Google Scholar] [CrossRef]
- Li, Y.J.; Ma, M.J.; Zhu, J.J. Dual-Signal Amplification Strategy for Ultrasensitive Photoelectrochemical Immunosensing of α-Fetoprotein. Anal. Chem. 2012, 84, 10492–10499. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, H.; Cui, K.; Zhang, L.; Ge, S.; Yu, J. Reversible Electron Storage in Tandem Photoelectrochemical Cell for Light Driven Unassisted Overall Water Splitting. Appl. Catal. B Environ. 2020, 275, 119094. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Hu, M.; Zhang, L.; Ge, S.; Cui, K.; Yu, J. Cathode Photoelectrochemical Paper Device for MicroRNA Detection Based on Cascaded Photoactive Structures and Hemin/Pt Nanoparticle-Decorated DNA Dendrimers. ACS Appl. Mater. Interfaces 2020, 12, 17177–17184. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Cui, K.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. Hierarchical Hematite/TiO2 Nanorod Arrays Coupled with Responsive Mesoporous Silica Nanomaterial for Highly Sensitive Photoelectrochemical Sensing. Biosens. Bioelectron. 2018, 117, 515–521. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, H.; Fan, G.C.; Luo, X.; Zhu, J.J. Introduction of an Antifouling Photoelectrode: An Effective Strategy for a High-Performance Photoelectrochemical Cytosensor. J. Mater. Chem. B 2020, 8, 4836–4840. [Google Scholar] [CrossRef]
- Li, L.; Shi, H.; Yu, H.; Tan, X.; Wang, Y.; Ge, S.; Wang, A.; Cui, K.; Zhang, L.; Yu, J. Ultrathin MoSe2 Nanosheet Anchored CdS-ZnO Functional Paper Chip as a Highly Efficient Tandem Z-Scheme Heterojunction Photoanode for Scalable Photoelectrochemical Water Splitting. Appl. Catal. B Environ. 2021, 292, 120184. [Google Scholar] [CrossRef]
- Zhao, W.W.; Xu, J.J.; Chen, H.Y. Photoelectrochemical Bioanalysis: The State of the Art. Chem. Soc. Rev. 2015, 44, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Li, P.P.; Liu, X.P.; Mao, C.J.; Jin, B.K.; Zhu, J.J. Photoelectrochemical DNA Biosensor Based on G-C3N4/MoS2 2D/2D Heterojunction Electrode Matrix and Co-Sensitization Amplification with CdSe QDs for the Sensitive Detection of ssDNA. Anal. Chim. Acta 2019, 1048, 42–49. [Google Scholar] [CrossRef]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xie, X.; Zeng, Y.; Shi, S.; Wang, G.; Yang, L.; Wang, C.-Z.; Lin, S. Highly Efficient and Stable P-Type ZnO Nanowires with Piezotronic Effect for Photoelectrochemical Water Splitting. Nano Energy 2019, 61, 550–558. [Google Scholar] [CrossRef]
- Zhang, X.; Villafuerte, J.; Consonni, V.; Sarigiannidou, E.; Capsal, J.F.; Bruhat, A.; Grinberg, D.; Petit, L.; Cottinet, P.J.; Le, M.Q. Optimization Strategies Used for Boosting Piezoelectric Response of Biosensor Based on Flexible Micro-ZnO Composites. Biosensors 2022, 12, 245. [Google Scholar] [CrossRef]
- Al Fatease, A.; Haque, M.; Umar, A.; Ansari, S.G.; Mahnashi, M.H.; Alhamhoom, Y.; Ansari, Z.A. Fabrication and Characterization of Acute Myocardial Infarction Myoglobin Biomarker Based on Chromium-Doped Zinc Oxide Nanoparticles. Biosensors 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Jin, H.; Huang, F.; Wang, Z.; Wang, F.; Dai, Z. Ultrasensitive determination of intracellular hydrogen peroxide by equipping quantum dots with a sensing layer via self-passivation. Nano Res. 2022, 15, 4350–4356. [Google Scholar]
- Ibupoto, Z.H.; Ali, S.M.; Khun, K.; Chey, C.O.; Nur, O.; Willander, M. ZnO Nanorods Based Enzymatic Biosensor for Selective Determination of Penicillin. Biosensors 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Ma, H.-P.; Yang, J.-H.; Tao, J.-J.; Yuan, K.-P.; Cheng, P.-H.; Huang, W.; Wang, J.-C.; Guo, Q.-X.; Lu, H.-L.; Zhang, D.W. Low-Temperature Epitaxial Growth of High-Quality Gaon Films on ZnO Nanowires for Superior Photoelectrochemical Water Splitting. Nano Energy 2019, 66, 104089. [Google Scholar] [CrossRef]
- Li, J.; Jin, B.; Jiao, Z. Rationally Embedded Zinc Oxide Nanospheres Serving as Electron Transport Channels in Bismuth Vanadate/Zinc Oxide Heterostructures for Improved Photoelectrochemical Efficiency. J. Colloid Interface Sci. 2021, 592, 127–134. [Google Scholar] [CrossRef]
- Shen, Q.; Zhao, X.; Zhou, S.; Hou, W.; Zhu, J.-J. ZnO/CdS Hierarchical Nanospheres for Photoelectrochemical Sensing of Cu2+. J. Phys. Chem. C 2011, 115, 17958–17964. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Guo, S.; Xin, X.; Zhang, Y.; Guo, P.; Tang, S.; Li, X. Sulfur-Deficient ZnIn2S4/Oxygen-Deficient WO3 Hybrids with Carbon Layer Bridges as a Novel Photothermal/ Photocatalytic Integrated System for Z-Scheme Overall Water Splitting. Adv. Energy Mater. 2021, 11, 2102452. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In Situ Irradiated XPS Investigation on S-Scheme TiO2@ ZnIn2S4 Photocatalyst for Efficient Photocatalytic CO2 Reduction. Small 2021, 17, e2103447. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, X.; Qu, Y.; Zhou, F.; Wang, Z.; Wang, W.; Zhao, C.; Wang, H.; Li, L.; Yao, Y.; et al. A hierarchical heterostructure of CdS QDs confined on 3D ZnIn2S4 with boosted charge transfer for photocatalytic CO2 reduction. Nano Res. 2021, 14, 81–90. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, X.; Kang, Z.; Hu, Y.; Zhang, X.; Zhang, Y. Photoelectrochemical Performance Enhancement of ZnO Photoanodes from ZnIn2S4 Nanosheets Coating. Nano Energy 2015, 14, 392–400. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Yan, Z.; Chen, M.; Zhang, L.; Zhao, P.; Yu, J. Ultrasensitive Photoelectrochemical Detection of MicroRNA on Paper by Combining a Cascade Nanozyme-Engineered Biocatalytic Precipitation Reaction and Target-Triggerable DNA Motor. ACS Sens. 2020, 5, 1482–1490. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.F.; Li, X.Y.; Yu, W.B.; Dong, W.D.; Zhao, H.; Hu, Z.Y.; Deng, Z.; Wang, C.; Wu, S.J.; et al. Molybdenum Disulfide Quantum Dots Directing Zinc Indium Sulfide Heterostructures for Enhanced Visible Light Hydrogen Production. J. Colloid Interface Sci. 2019, 551, 111–118. [Google Scholar] [CrossRef]
- Shi, X.M.; Fan, G.C.; Shen, Q.; Zhu, J.J. Photoelectrochemical DNA Biosensor Based on Dual-Signal Amplification Strategy Integrating Inorganic-Organic Nanocomposites Sensitization with Lambda-Exonuclease-Assisted Target Recycling. ACS Appl. Mater. Interfaces 2016, 8, 35091–35098. [Google Scholar] [CrossRef]
- Mohammadi-Aloucheh, R.; Habibi-Yangjeh, A.; Bayrami, A.; Latifi-Navid, S.; Asadi, A. Enhanced Anti-Bacterial Activities of ZnO Nanoparticles and ZnO/CuO Nanocomposites Synthesized Using Vaccinium arctostaphylos L. Fruit Extract. Artif. Cells. Nanomed. Biotechnol. 2018, 46, 1200–1209. [Google Scholar] [CrossRef]
- Niu, L.; Hu, S.; Ma, Y.; Wang, M.; Lv, B.; Meng, S.; Hua, F.; Li, L.; Su, B.; Lei, Z.; et al. Fe-Doped UiO-66 Combined with ZnIn2S4 for Adsorption and Degradation of RhB under Visible Light. Nano 2020, 15, 2050089. [Google Scholar] [CrossRef]
- Zhu, T.; Ye, X.; Zhang, Q.; Hui, Z.; Wang, X.; Chen, S. Efficient Utilization of Photogenerated Electrons and Holes for Photocatalytic Redox Reactions Using Visible Light-Driven Au/ ZnIn2S4 Hybrid. J. Hazard. Mater. 2019, 367, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.-L.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Cocatalyst Decorated ZnIn2S4 Composites for Cooperative Alcohol Conversion and H2 Evolution. Appl. Catal. B Environ. 2021, 298, 120541. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-Unit-Cell ZnIn2S4 Monolayer with Sulfur Vacancies for Photocatalytic Hydrogen Evolution. Appl. Catal. B Environ. 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Poornaprakash, B.; Chalapathi, U.; Subramanyam, K.; Vattikuti, S.V.P.; Park, S.-H. Wurtzite Phase Co-Doped ZnO Nanorods: Morphological, Structural, Optical, Magnetic, and Enhanced Photocatalytic Characteristics. Ceram. Int. 2020, 46, 2931–2939. [Google Scholar] [CrossRef]
- Fan, G.C.; Zhu, H.; Du, D.; Zhang, J.R.; Zhu, J.J.; Lin, Y. Enhanced Photoelectrochemical Immunosensing Platform Based on CdSeTe@CdS:Mn Core-Shell Quantum Dots-Sensitized TiO2 Amplified by CuS Nanocrystals Conjugated Signal Antibodies. Anal. Chem. 2016, 88, 3392–3399. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, K.; Lin, Z.; Tang, D. Novel Photoelectrochemical Immunosensor for Disease-Related Protein Assisted by Hemin/G-quadruplex-Based DNAzyme on Gold Nanoparticles to Enhance Cathodic Photocurrent on p-CuBi2O4 Semiconductor. Biosens. Bioelectron. 2017, 96, 317–323. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Wang, H.; Cao, W.; Du, B.; Wei, Q. Sandwich-Type Electrochemical Immunoassay Based on Co3O4@MnO2-thionine and Pseudo-Elisa Method toward Sensitive Detection of Alpha Fetoprotein. Biosens. Bioelectron. 2018, 106, 179–185. [Google Scholar] [CrossRef]
- Hu, D.; Cui, H.; Wang, X.; Luo, F.; Qiu, B.; Cai, W.; Huang, H.; Wang, J.; Lin, Z. Highly Sensitive and Selective Photoelectrochemical Aptasensors for Cancer Biomarkers Based on MoS2/Au/GaN Photoelectrodes. Anal. Chem. 2021, 93, 7341–7347. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, L.; Chen, J.; Tang, Y.; Xia, B.; Tang, D. Full-Spectrum Responsive Photoelectrochemical Immunoassay Based on Β-In2S3@Carbon Dot Nanoflowers. Electrochim. Acta 2020, 332, 135473. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, L.; Chen, J.; Li, M.; Tang, D. Graded Oxygen-Doped CdS Electrode for Portable Photoelectrochemical Immunoassay of Alpha-Fetoprotein Coupling with a Digital Multimeter Readout. Sens. Actuators B Chem. 2021, 343, 130136. [Google Scholar] [CrossRef]
- Wu, Y.; Su, H.; Yang, J.; Wang, Z.; Li, D.; Sun, H.; Guo, X.; Yin, S. Photoelectrochemical Immunosensor for Sensitive Detection of Alpha-Fetoprotein Based on a Graphene Honeycomb Film. J. Colloid Interface Sci. 2020, 580, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Liang, M.; Xu, H.; Tang, S.; Yang, H.; Song, H. Sensitive fluorescence immunoassay of alpha-fetoprotein through copper ions modulated growth of quantum dots in-situ. Sens. Actuators B Chem. 2017, 247, 408–413. [Google Scholar] [CrossRef]
- Kong, F.; Xu, B.; Du, Y.; Xu, J.; Chen, H. A branched electrode based electrochemical platform: Towards new label-free and reagentless simultaneous detection of two biomarkers. Chem. Commun. 2013, 49, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ren, J. Gold nanoparticles based chemiluminescent resonance energy transfer for immunoassay of alpha fetoprotein cancer marker. Anal. Chim. Acta 2011, 686, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Song, X.; Dong, Z.; Meng, X.; Chen, Y.; Yang, L. Photonic crystal fiberbased immunosensor for high-performance detection of alpha fetoprotein. Biosens. Bioelectron. 2017, 91, 431–435. [Google Scholar] [CrossRef] [PubMed]


| Serum Sample | Added, a (ng mL−1) | Found, (ng mL−1) | Recovery, b % | RSD, % |
|---|---|---|---|---|
| 1 | 0.1 | 10.08 × 10−2 | 100.8 | 5.02 |
| 2 | 0.5 | 4.90 × 10−1 | 98.0 | 5.37 |
| 3 | 1 | 9.77 × 10−1 | 97.7 | 3.29 |
| 4 | 5 | 4.76 | 95.2 | 2.80 |
| 5 | 10 | 9.59 | 95.9 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Li, X.; Xiu, M.; Huang, K.; Cui, K.; Zhang, J.; Ge, S.; Hao, S.; Yu, J.; Huang, Y. A Paper-Based Photoelectrochemical Sensing Platform Based on In Situ Grown ZnO/ZnIn2S4 Heterojunctions onto Paper Fibers for Sensitively Detecting AFP. Biosensors 2022, 12, 818. https://doi.org/10.3390/bios12100818
Huang J, Li X, Xiu M, Huang K, Cui K, Zhang J, Ge S, Hao S, Yu J, Huang Y. A Paper-Based Photoelectrochemical Sensing Platform Based on In Situ Grown ZnO/ZnIn2S4 Heterojunctions onto Paper Fibers for Sensitively Detecting AFP. Biosensors. 2022; 12(10):818. https://doi.org/10.3390/bios12100818
Chicago/Turabian StyleHuang, Jiali, Xu Li, Mingzhen Xiu, Kang Huang, Kang Cui, Jing Zhang, Shenguang Ge, Shiji Hao, Jinghua Yu, and Yizhong Huang. 2022. "A Paper-Based Photoelectrochemical Sensing Platform Based on In Situ Grown ZnO/ZnIn2S4 Heterojunctions onto Paper Fibers for Sensitively Detecting AFP" Biosensors 12, no. 10: 818. https://doi.org/10.3390/bios12100818
APA StyleHuang, J., Li, X., Xiu, M., Huang, K., Cui, K., Zhang, J., Ge, S., Hao, S., Yu, J., & Huang, Y. (2022). A Paper-Based Photoelectrochemical Sensing Platform Based on In Situ Grown ZnO/ZnIn2S4 Heterojunctions onto Paper Fibers for Sensitively Detecting AFP. Biosensors, 12(10), 818. https://doi.org/10.3390/bios12100818







