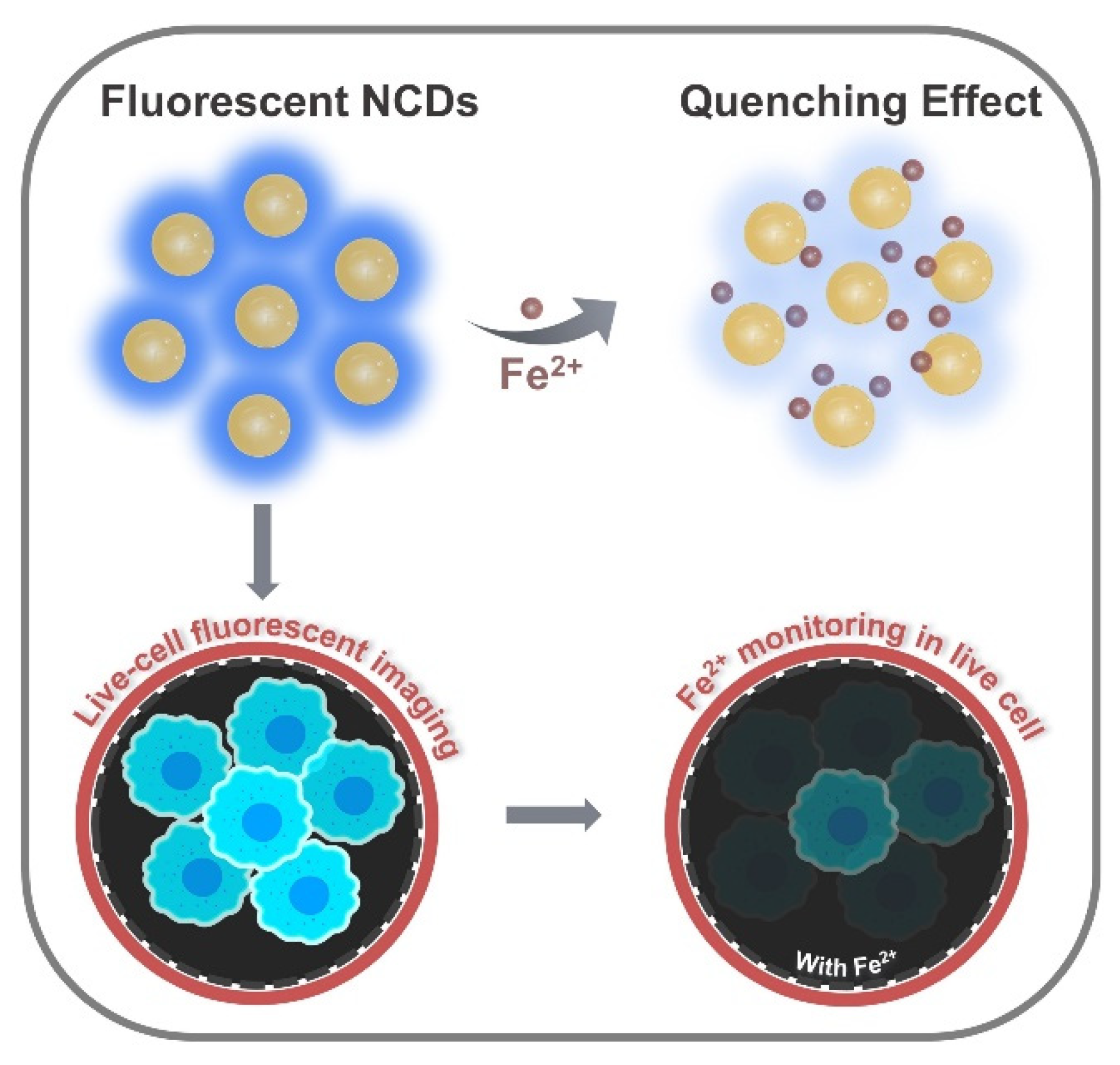
Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of NCDs
2.3. Cell Culture, Cell Cytotoxicity and Cell Uptake
2.4. Turn-Off Fluorescent Probes to Determine Ferrous Ion in Live Cells
3. Results and Discussion
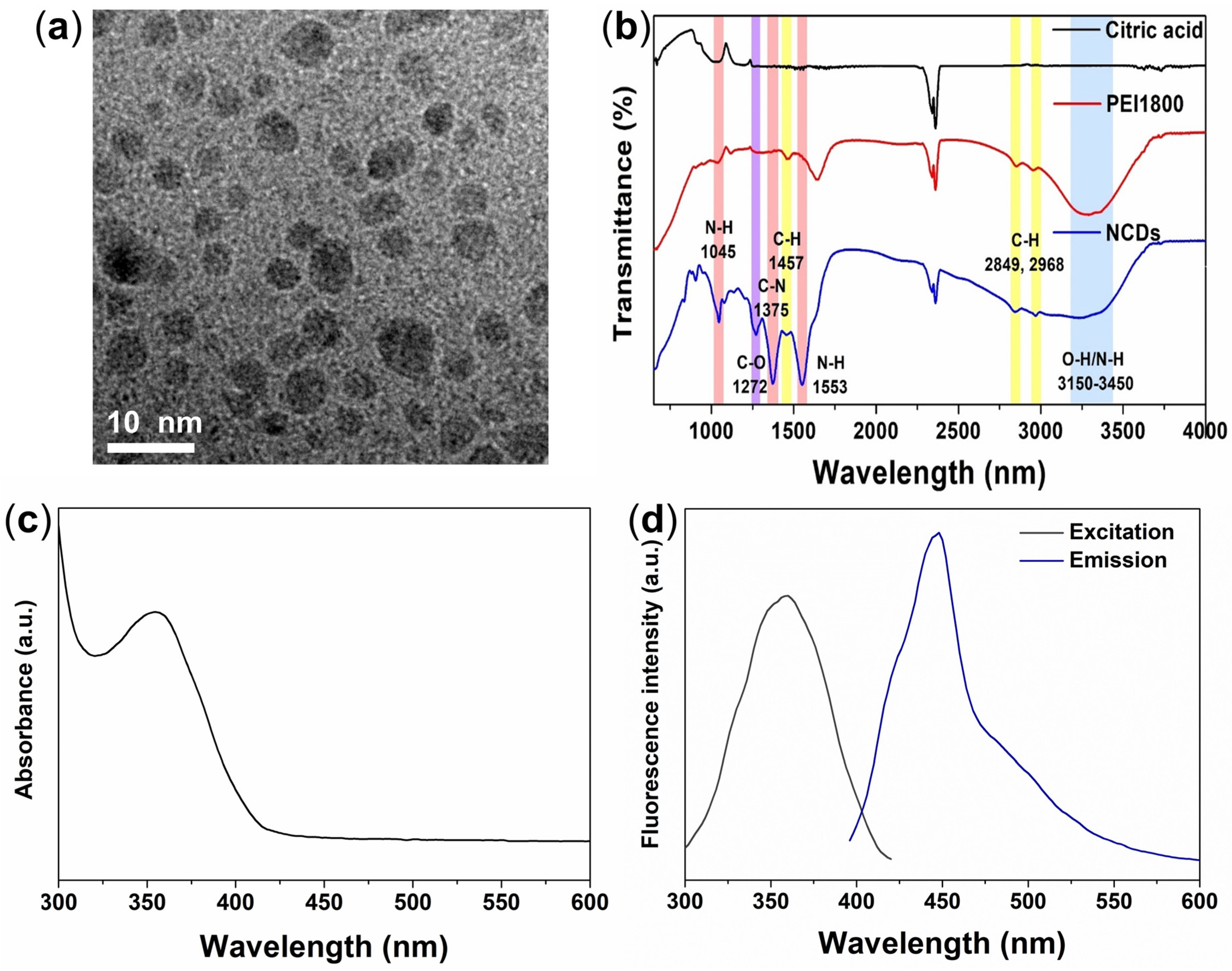
3.1. Physical Characterization of NCDs
3.2. Biocompatibility of NCDs as Fluorescent Nanoprobes and Bioimaging Application
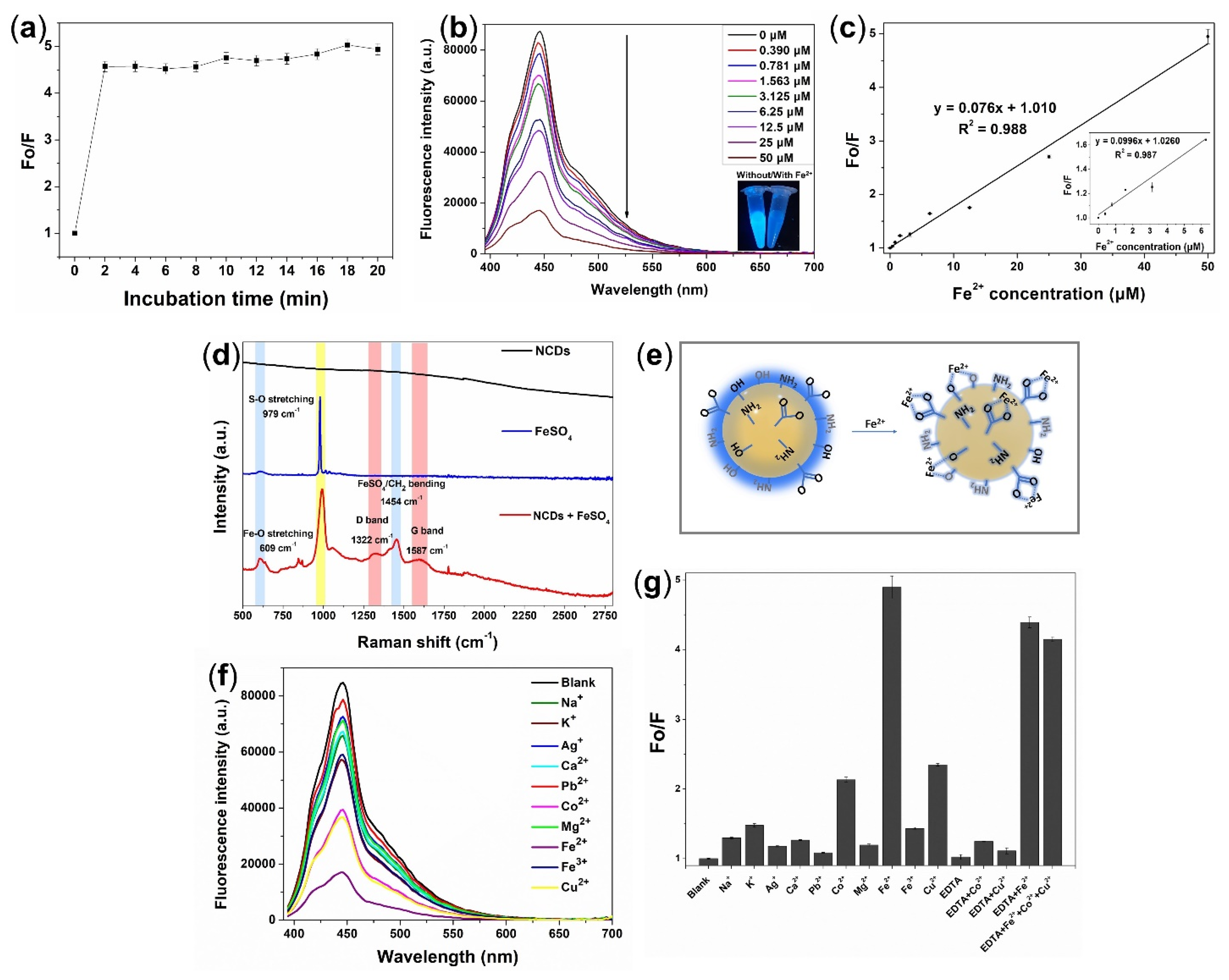
3.3. Rapid Detection of Fe2+ Ion
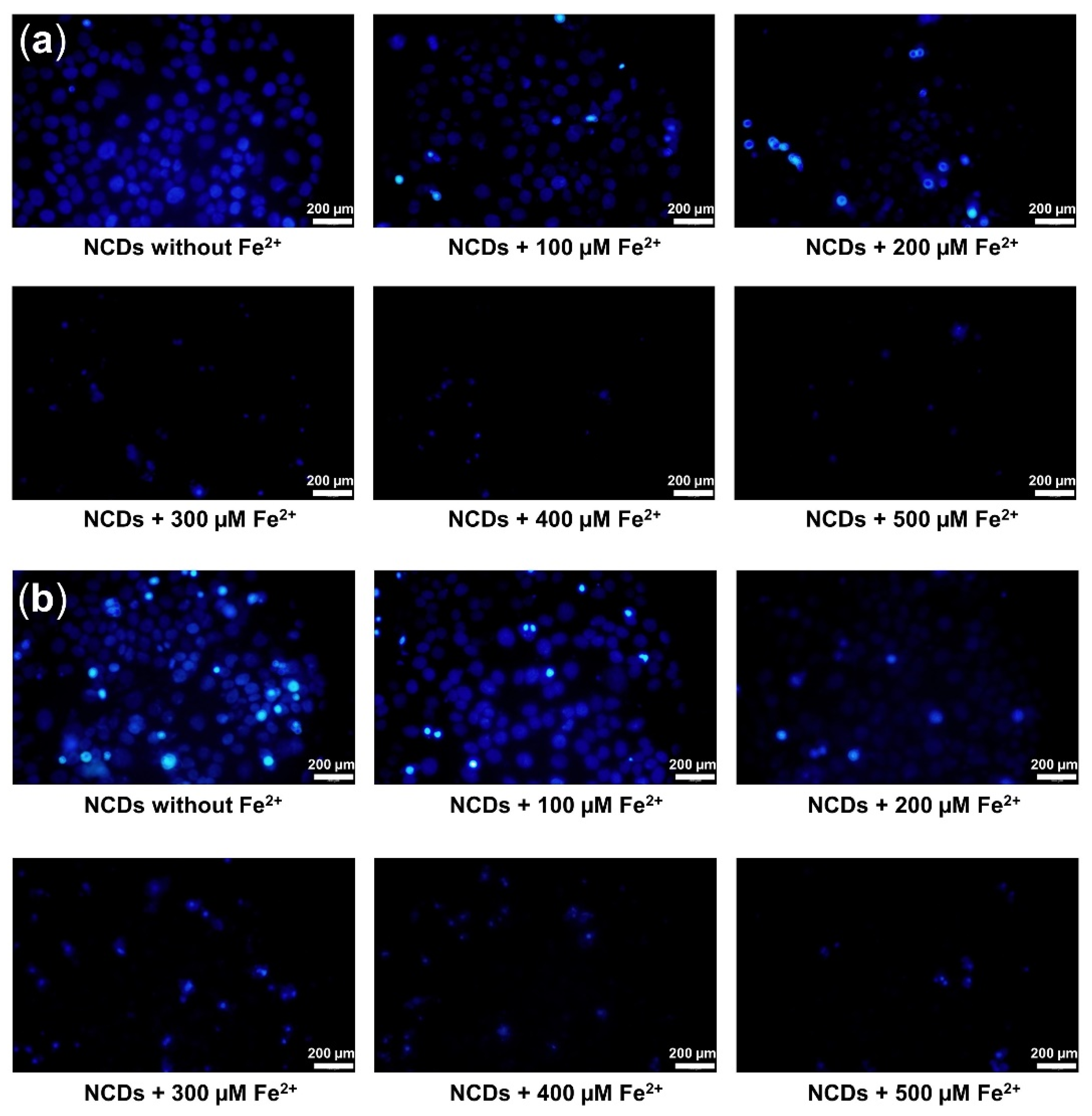
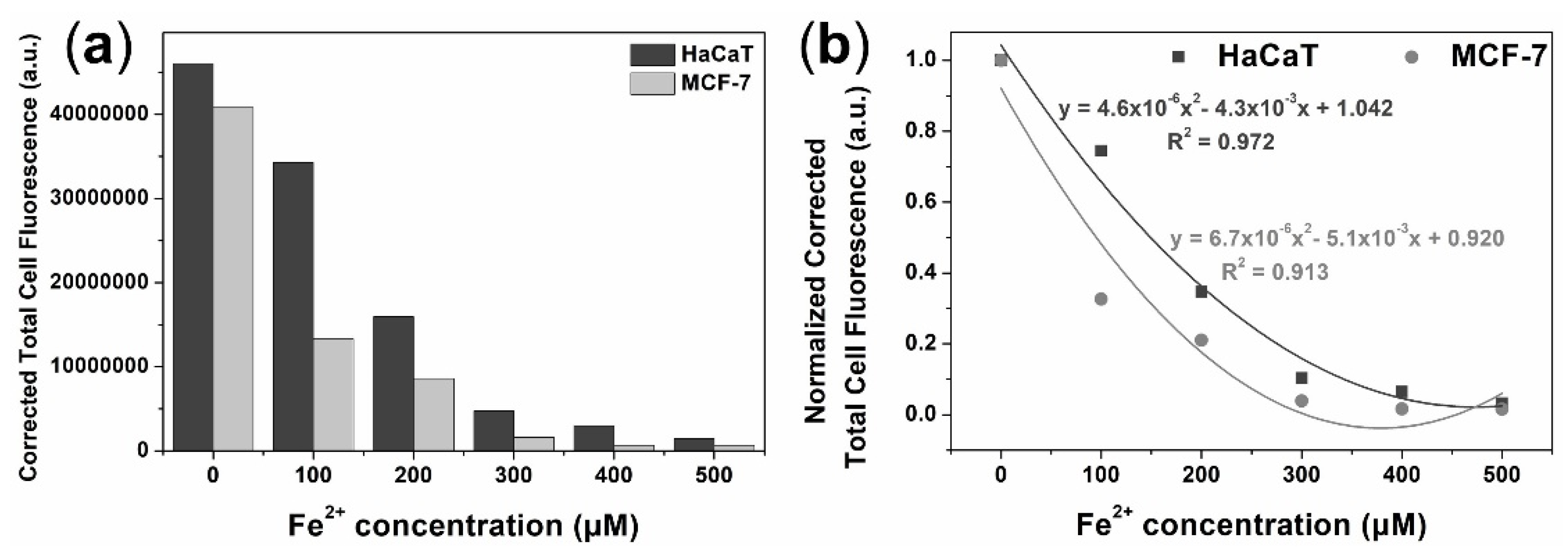
3.4. Turn-Off Fluorescent Probe for Fe2+ Monitoring in Live Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Gozzelino, R.; Arosio, P. Iron homeostasis in health and disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yiannikourides, A.; Latunde-Dada, G.O. A short review of iron metabolism and pathophysiology of iron disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, A.; Jeandriens, J.; Parkes, H.G.; So, P.-W. Iron dyshomeostasis, lipid peroxidation and perturbed expression of cystine/glutamate antiporter in Alzheimer’s disease: Evidence of ferroptosis. Redox Biol. 2020, 32, 101494. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.G.; Connor, J.R.; Meadowcroft, M.D. The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer’s disease: Two sides of the same coin. Neurobiol. Dis. 2015, 81, 49–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Gholam Azad, M.; Dharmasivam, M.; Richardson, V.; Quinn, R.J.; Feng, Y.; Pountney, D.L.; Tonissen, K.F.; Mellick, G.D.; Yanatori, I.; et al. Parkinson’s disease: Alterations in iron and redox biology as a key to unlock therapeutic strategies. Redox Biol. 2021, 41, 101896. [Google Scholar] [CrossRef] [PubMed]
- Riederer, P.; Monoranu, C.; Strobel, S.; Iordache, T.; Sian-Hülsmann, J. Iron as the concert master in the pathogenic orchestra playing in sporadic Parkinson’s disease. J. Neural Transm. 2021, 128, 1577–1598. [Google Scholar] [CrossRef]
- Phan, L.M.T. Fluorescent carbon dots as prospective nanoagents for imaging-assisted biomedical applications. Curr. Med. Chem. 2021. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, S.; Zheng, X.; Li, H.; Chen, Q.; Qin, K.; Ding, Y.; Wei, Y. Carbon dots derived from Fusobacterium nucleatum for intracellular determination of Fe3+ and bioimaging both in vitro and in vivo. Anal. Methods 2021, 13, 1121–1131. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Fe3+-sensitive carbon dots for detection of Fe3+ in aqueous solution and intracellular imaging of Fe3+ inside fungal cells. Front Chem. 2020, 7, 911. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, K.; Yu, J.; Wang, H.; Tu, M.; Wang, X. Nitrogen and chlorine co-doped carbon dots as probe for sensing and imaging in biological samples. R. Soc. Open Sci. 2019, 6, 181557. [Google Scholar] [CrossRef] [Green Version]
- Lesani, P.; Singh, G.; Viray, C.M.; Ramaswamy, Y.; Zhu, D.M.; Kingshott, P.; Lu, Z.; Zreiqat, H. Two-Photon Dual-Emissive Carbon Dot-Based Probe: Deep-tissue imaging and ultrasensitive sensing of intracellular ferric ions. ACS Appl. Mater. Interfaces 2020, 12, 18395–18406. [Google Scholar] [CrossRef]
- Lesani, P.; Ardekani, S.M.; Dehghani, A.; Hassan, M.; Gomes, V.G. Excitation-independent carbon dot probes for exogenous and endogenous Fe3+ sensing in living cells: Fluorescence lifetime and sensing mechanism. Sens. Actuators B Chem. 2019, 285, 145–155. [Google Scholar] [CrossRef]
- Wang, Y.; Man, Y.; Li, S.; Wu, S.; Zhao, X.; Xie, F.; Qu, Q.; Zou, W.-S. Pesticide-derived bright chlorine-doped carbon dots for selective determination and intracellular imaging of Fe(III). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117594. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, L. One-step sonochemical synthesis of versatile nitrogen-doped carbon quantum dots for sensitive detection of Fe2+ ions and temperature in vitro. Mater. Sci. Eng. C 2019, 101, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Tian, Y.; Wang, X.; Gong, D.; Guo, Y.; Iqbal, K.; Wang, Z.; Liu, W.; Qin, W. Carbon dots prepared by solid state method via citric acid and 1,10-phenanthroline for selective and sensing detection of Fe2+ and Fe3+. Sens. Actuators B Chem. 2016, 237, 408–415. [Google Scholar] [CrossRef]
- Liu, G.; Li, B.; Liu, Y.; Feng, Y.; Jia, D.; Zhou, Y. Rapid and high yield synthesis of carbon dots with chelating ability derived from acrylamide/chitosan for selective detection of ferrous ions. Appl. Surf. Sci. 2019, 487, 1167–1175. [Google Scholar] [CrossRef]
- Xiao, S.J.; Chu, Z.J.; Zuo, J.; Zhao, X.J.; Huang, C.Z.; Zhang, L. Fluorescent carbon dots: Facile synthesis at room temperature and its application for Fe2+ sensing. J. Nanopart. Res. 2017, 19, 84. [Google Scholar] [CrossRef]
- Shi, J.; Ni, G.; Tu, J.; Jin, X.; Peng, J. Green synthesis of fluorescent carbon dots for sensitive detection of Fe2+ and hydrogen peroxide. J. Nanopart. Res. 2017, 19, 209. [Google Scholar] [CrossRef]
- Wei, S.; Tan, L.; Yin, X.; Wang, R.; Shan, X.; Chen, Q.; Li, T.; Zhang, X.; Jiang, C.; Sun, G. A sensitive “ON–OFF” fluorescent probe based on carbon dots for Fe2+ detection and cell imaging. Analyst 2020, 145, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, Y.; Wang, W.; Zhou, Z.; Gu, J.-H.; Zhang, Z.; Sun, H.; Liu, F. A paradox: Fe2+-containing agents decreased ROS and apoptosis induced by CoNPs in vascular endothelial cells by inhibiting HIF-1α. Biosci. Rep. 2021, 41, BSR20203456. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Skiada, V.; Barbouti, A. Redox signaling and cancer: The role of “labile” iron. Cancer Lett. 2008, 266, 21–29. [Google Scholar] [CrossRef]
- Molina-Holgado, F.; Hider, R.C.; Gaeta, A.; Williams, R.; Francis, P. Metals ions and neurodegeneration. BioMetals 2007, 20, 639–654. [Google Scholar] [CrossRef]
- Ehrat, F.; Bhattacharyya, S.; Schneider, J.; Löf, A.; Wyrwich, R.; Rogach, A.L.; Stolarczyk, J.K.; Urban, A.S.; Feldmann, J. Tracking the source of carbon dot photoluminescence: Aromatic domains versus molecular fluorophores. Nano Lett. 2017, 17, 7710–7716. [Google Scholar] [CrossRef] [PubMed]
- Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S.R.; Resch-Genger, U.; Voss, T. Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J. Phys. Chem. C 2020, 124, 8894–8904. [Google Scholar] [CrossRef]
- Zhu, S.; Song, Y.; Zhao, X.; Shao, J.; Zhang, J.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Luo, P.; Li, C.; Shi, G. Synthesis of gold@ carbon dots composite nanoparticles for surface enhanced Raman scattering. Phys. Chem. Chem. Phys. 2012, 14, 7360–7366. [Google Scholar] [CrossRef]
- Shah, H.; Xin, Q.; Jia, X.; Gong, J.R. Single precursor-based luminescent nitrogen-doped carbon dots and their application for iron (III) sensing. Arab. J. Chem. 2019, 12, 1083–1091. [Google Scholar] [CrossRef]
- Rouchon, V.; Badet, H.; Belhadj, O.; Bonnerot, O.; Lavédrine, B.; Michard, J.G.; Miska, S. Raman and FTIR spectroscopy applied to the conservation report of paleontological collections: Identification of Raman and FTIR signatures of several iron sulfate species such as ferrinatrite and sideronatrite. J. Raman Spectrosc. 2012, 43, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Chio, C.; Muenow, D. Raman spectroscopic investigation of ferrous sulfate hydrates. In Proceedings of the 37th Annual Lunar and Planetary Science Conference, League City, TX, USA, 13–17 March 2006; p. 1078. [Google Scholar]
- Angel, S.M.; Gomer, N.R.; Sharma, S.K.; McKay, C. Remote Raman spectroscopy for planetary exploration: A review. Appl. Spectrosc. 2012, 66, 137–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, A.; Park, B.; Oh, J.; Sankar, K.V.; Ray, C.; Kim, W.S.; Jun, S.C.J.C. Multicolor emissive carbon dot with solvatochromic behavior across the entire visible spectrum. Carbon 2020, 156, 110–118. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Y.-K.; Sharma, G.; Dong, Y.; Zheng, X.; Li, P.; Johnston, A.; Bappi, G.; Fan, J.Z.; Kung, H.; et al. Bright high-colour-purity deep-blue carbon dot light-emitting diodes via efficient edge amination. Nat. Photonics 2020, 14, 171–176. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Lin, Y.; Periasamy, A.P.; Cang, J.; Chang, H.-T. Parameters affecting the synthesis of carbon dots for quantitation of copper ions. Nanoscale Adv. 2019, 1, 2553–2561. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Ramakrishnan, S.K.; Weisz, K.; Triner, D.; Xie, L.; Attili, D.; Pant, A.; Győrffy, B.; Zhan, M.; Carter-Su, C.; et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016, 24, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhang, F. Iron homeostasis and tumorigenesis: Molecular mechanisms and therapeutic opportunities. Protein Cell. 2015, 6, 88–100. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, L.M.T.; Hoang, T.X.; Cho, S. Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion. Biosensors 2022, 12, 41. https://doi.org/10.3390/bios12010041
Phan LMT, Hoang TX, Cho S. Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion. Biosensors. 2022; 12(1):41. https://doi.org/10.3390/bios12010041
Chicago/Turabian StylePhan, Le Minh Tu, Thi Xoan Hoang, and Sungbo Cho. 2022. "Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion" Biosensors 12, no. 1: 41. https://doi.org/10.3390/bios12010041
APA StylePhan, L. M. T., Hoang, T. X., & Cho, S. (2022). Fluorescent Carbon Dots for Sensitive and Rapid Monitoring of Intracellular Ferrous Ion. Biosensors, 12(1), 41. https://doi.org/10.3390/bios12010041





