Stability Assessment of Four Chimeric Proteins for Human Chagas Disease Immunodiagnosis
Abstract
:1. Introduction
2. Materials and Methods
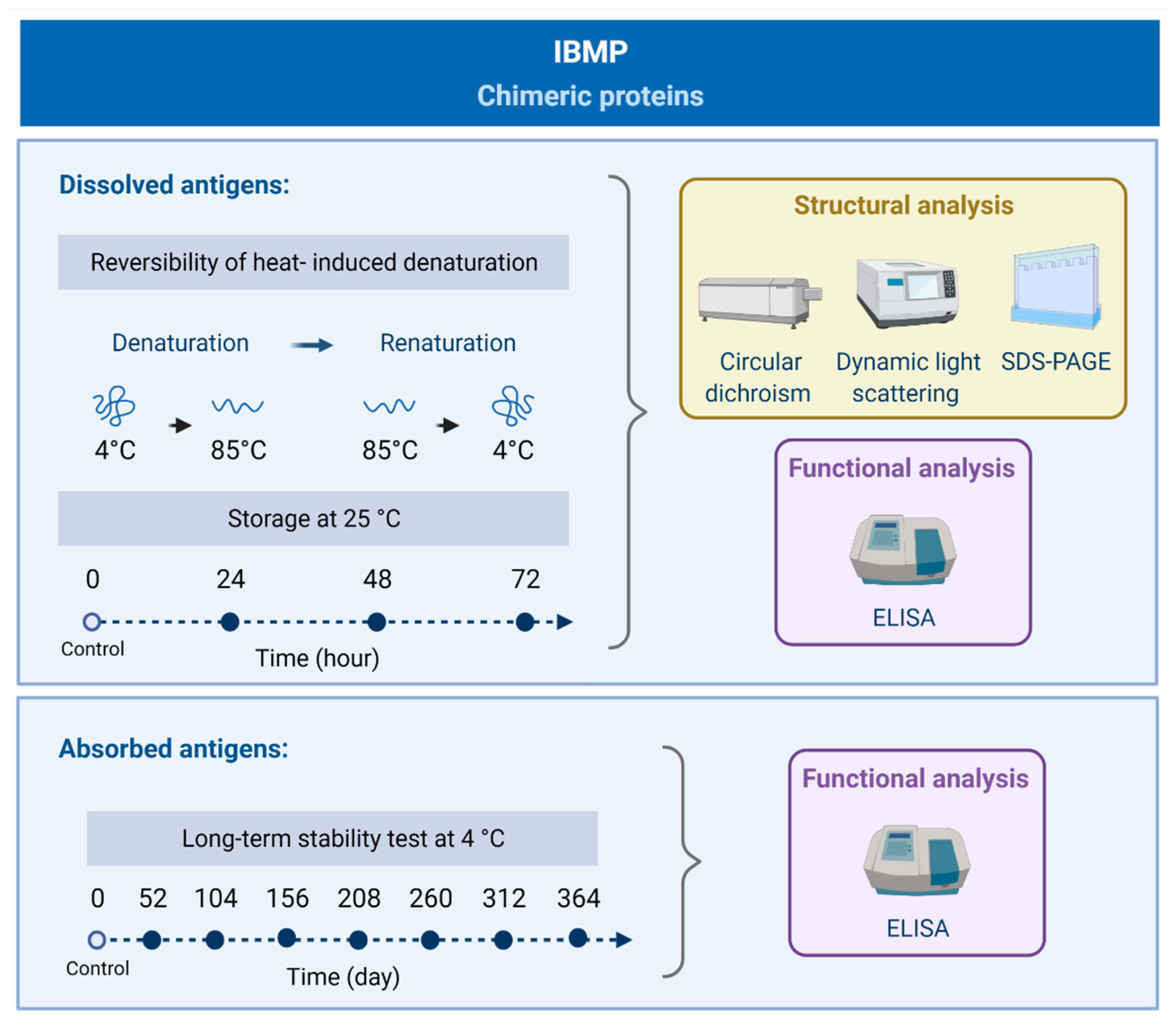
2.1. Study Design
2.2. IBMP Protein Expression and Purification
2.3. Gel Electrophoresis
2.4. Circular Dichroism (CD) Measurements
2.5. Dynamic Light Scattering (DLS)
2.6. Clinical Specimens and Indirect ELISA
2.7. Data Analysis
3. Results
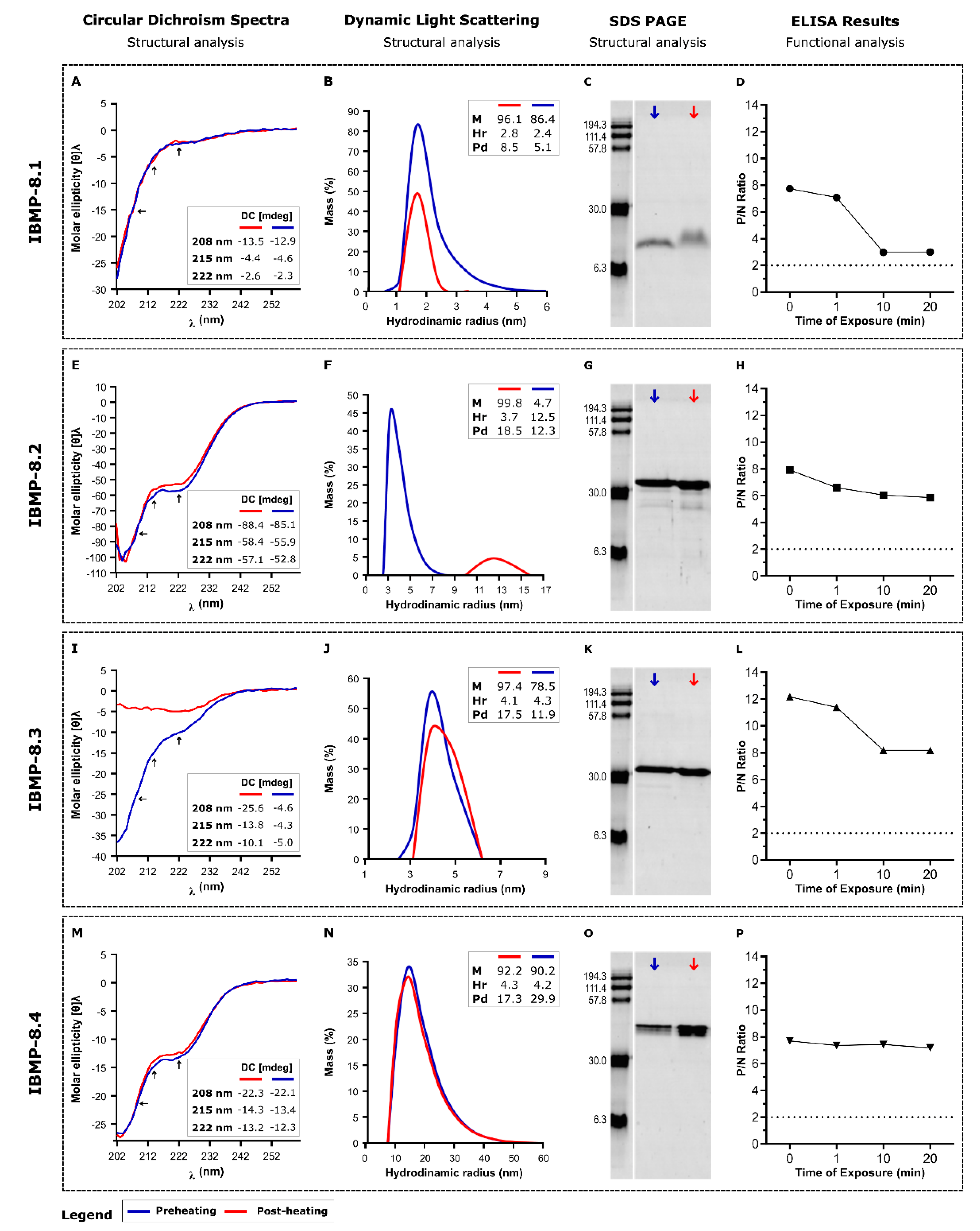
3.1. Reversibility of Heat-Induced Denaturation
3.2. Storage Stability at 25 °C
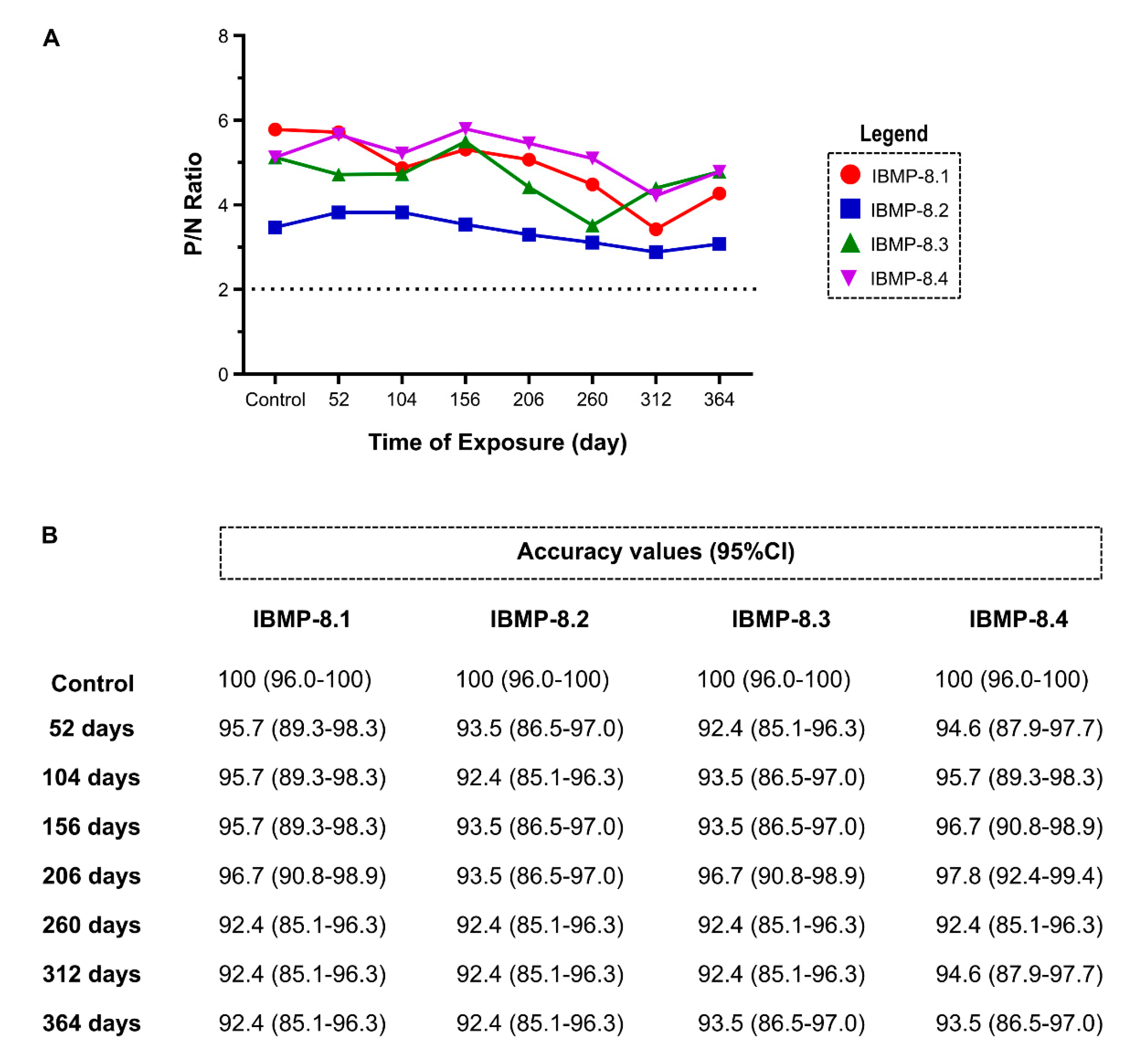
3.3. Long-Term Stability Analysis at 4 °C
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hotez, P.J.; Dumonteil, E.; Woc-Colburn, L.; Serpa, J.A.; Bezek, S.; Edwards, M.S.; Hallmark, C.J.; Musselwhite, L.W.; Flink, B.J.; Bottazzi, M.E. Chagas disease: “The new HIV/AIDS of the Americas”. PLoS Negl. Trop. Dis. 2012, 6, e1498. [Google Scholar] [CrossRef] [PubMed]
- Prata, A. Clinical and epidemiological aspects of Chagas disease. Lancet Infect. Dis. 2001, 1, 92–100. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Souza, W.V.; Barros, M.S.; Nakazawa, M.; Krieger, M.A.; Gomes, Y.M. Chronic Chagas disease diagnosis: A comparative performance of commercial enzyme immunoassay tests. Am. J. Trop. Med. Hyg. 2016, 94, 1034–1039. [Google Scholar] [CrossRef]
- Verani, J.R.; Seitz, A.; Gilman, R.H.; LaFuente, C.; Galdos-Cardenas, G.; Kawai, V.; De Lafuente, E.; Ferrufino, L.; Bowman, N.M.; Pinedo-Cancino, V.; et al. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am. J. Trop. Med. Hyg. 2009, 80, 410–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.L.; Marks, M.; Galdos-Cardenas, G.; Gilman, R.H.; Goodhew, B.; Ferrufino, L.; Halperin, A.; Sanchez, G.; Verastegui, M.; Escalante, P.; et al. Regional variation in the correlation of antibody and T-cell responses to Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 2014, 90, 1074–1081. [Google Scholar] [CrossRef]
- Zingales, B. Trypanosoma cruzi genetic diversity: Something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018, 184, 38–52. [Google Scholar] [CrossRef]
- Leeflang, M.M.G.; Rutjes, A.W.S.; Reitsma, J.B.; Hooft, L.; Bossuyt, P.M.M. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 2013, 185, e537–e544. [Google Scholar] [CrossRef] [Green Version]
- Leeflang, M.M.G.; Bossuyt, P.M.M.; Irwig, L. Diagnostic test accuracy may vary with prevalence: Implications for evidence-based diagnosis. J. Clin. Epidemiol. 2009, 62, 5–12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consultation on International Biological Reference Preparations for Chagas Diagnostic Tests; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Brasil, T.A.C.; Foti, L.; Souza, W.V.; Silva, E.D.; Gomes, Y.M.; Krieger, M.A. Performance assessment of four chimeric Trypanosoma cruzi antigens based on antigen-antibody detection for diagnosis of chronic Chagas disease. PLoS ONE 2016, 11, e0161100. [Google Scholar] [CrossRef]
- Hoft, D.F.; Kim, K.S.; Otsu, K.; Moser, D.R.; Yost, W.J.; Blumin, J.H.; Donelson, J.E.; Kirchhoff, L.V. Trypanosoma cruzi expresses diverse repetitive protein antigens. Infect. Immun. 1989, 57, 1959–1967. [Google Scholar] [CrossRef] [Green Version]
- Camussone, C.; Gonzalez, V.; Belluzo, M.S.; Pujato, N.; Ribone, M.E.; Lagier, C.M.; Marcipar, I.S. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin. Vaccine Immunol. 2009, 16, 899–905. [Google Scholar] [CrossRef] [Green Version]
- Montagnani, F.; Paolis, F.; Beghetto, E.; Gargano, N. Use of recombinant chimeric antigens for the serodiagnosis of Mycoplasma pneumoniae infection. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1377–1386. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Teng, H.; Xu, H.; Qi, S.; He, J.; Gu, D.; Chen, Q.; Ma, H. Chimeric peptide constructs comprising linear B-cell epitopes: Application to the serodiagnosis of infectious diseases. Sci. Rep. 2015, 5, 13364. [Google Scholar] [CrossRef] [Green Version]
- Beghetto, E.; Spadoni, A.; Bruno, L.; Buffolano, W.; Gargano, N. Chimeric antigens of Toxoplasma gondii: Toward standardization of toxoplasmosis serodiagnosis using recombinant products. J. Clin. Microbiol. 2006, 44, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Hollingshead, S.; Jongerius, I.; Exley, R.M.; Johnson, S.; Lea, S.M.; Tang, C.M. Structure-based design of chimeric antigens for multivalent protein vaccines. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Nuccitelli, A.; Cozzi, R.; Gourlay, L.J.; Donnarumma, D.; Necchi, F.; Norais, N.; Telford, J.L.; Rappuoli, R.; Bolognesi, M.; Maione, D.; et al. Structure-based approach to rationally design a chimeric protein for an effective vaccine against Group B Streptococcus infections. Proc. Natl. Acad. Sci. USA 2011, 108, 10278–10283. [Google Scholar] [CrossRef] [Green Version]
- Leony, L.M.; Freitas, N.E.M.; Del-Rei, R.P.; Carneiro, C.M.; Reis, A.B.; Jansen, A.M.; Xavier, S.C.C.; Gomes, Y.M.; Silva, E.D.; Reis, M.G.; et al. Performance of recombinant chimeric proteins in the serological diagnosis of Trypanosoma cruzi infection in dogs. PLoS Negl. Trop. Dis. 2019, 13, e0007545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, T.A.R.; Martins, H.R.; Franco, D.L.; Santos, F.L.N.; Celedon, P.A.F.; Cantuária, V.L.; de Lana, M.; Reis, A.B.; Ferreira, L.F. Impedimetric immunosensor for rapid and simultaneous detection of Chagas and visceral leishmaniasis for point of care diagnosis. Biosens. Bioelectron. 2020, 169, 112573. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.N.; Celedon, P.A.; Zanchin, N.I.; Souza, W.V.; Silva, E.D.; Foti, L.; Krieger, M.A.; Gomes, Y.M. Accuracy of chimeric proteins in the serological diagnosis of chronic Chagas disease—A Phase II study. PLoS Negl. Trop. Dis. 2017, 11, e0005433. [Google Scholar] [CrossRef]
- Del-Rei, R.P.; Leony, L.M.; Celedon, P.A.F.; Zanchin, N.I.T.; Reis, M.G.; Gomes, Y.D.M.; Schijman, A.G.; Longhi, S.A.; Santos, F.L.N. Detection of anti-Trypanosoma cruzi antibodies by chimeric antigens in chronic Chagas disease-individuals from endemic South American countries. PLoS ONE 2019, 14, e0215623. [Google Scholar] [CrossRef] [PubMed]
- Dopico, E.; Del-Rei, R.P.; Espinoza, B.; Ubillos, I.; Zanchin, N.I.T.; Sulleiro, E.; Moure, Z.; Celedon, P.A.F.; Souza, W.V.; Silva, E.D.; et al. Immune reactivity to Trypanosoma cruzi chimeric proteins for Chagas disease diagnosis in immigrants living in a non-endemic setting. BMC Infect Dis. 2019, 19, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.L.N.; Campos, A.C.P.; Amorim, L.D.A.F.; Silva, E.D.; Zanchin, N.I.T.; Celedon, P.A.F.; Del-Rei, R.P.; Krieger, M.A.; Gomes, Y.M. Highly accurate chimeric proteins for the serological diagnosis of chronic Chagas disease: A latent class analysis. Am. J. Trop. Med. Hyg. 2018, 99, 1174–1179. [Google Scholar] [CrossRef]
- Santos, F.L.N.; Celedon, P.A.F.; Zanchin, N.I.T.; Leitolis, A.; Crestani, S.; Foti, L.; Souza, W.V.; Gomes, Y.M.; Krieger, M.A.; de Souza, W.V.; et al. Performance assessment of a Trypanosoma cruzi chimeric antigen in multiplex liquid microarray assays. J. Clin. Microbiol. 2017, 55, 2934–2945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, E.D.; Silva, Â.A.O.; Santos, E.F.; Leony, L.M.; Freitas, N.E.M.; Daltro, R.T.; Ferreira, A.G.P.; Diniz, R.L.; Bernardo, A.R.; Luquetti, A.O.; et al. Development of a new lateral flow assay based on IBMP-8.1 and IBMP-8.4 chimeric antigens to diagnose Chagas disease. Biomed Res. Int. 2020, 2020, 1803515. [Google Scholar] [CrossRef] [PubMed]
- Daltro, R.T.; Leony, L.M.; Freitas, N.E.M.; Silva, Â.A.O.; Santos, E.F.; Del-Rei, R.P.; Brito, M.E.F.; Brandão-Filho, S.P.; Gomes, Y.M.; Silva, M.S.; et al. Cross-reactivity using chimeric Trypanosoma cruzi antigens: Diagnostic performance in settings co-endemic for Chagas disease and American cutaneous or visceral leishmaniasis. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Gaso, M.I.; Villarreal-Gómez, L.J.; Beyssen, D.; Sarry, F.; Reyna, M.A.; Ibarra-Cerdeña, C.N. Biosensors to diagnose Chagas disease: A brief review. Sensors 2017, 17, 2629. [Google Scholar] [CrossRef] [Green Version]
- Janissen, R.; Sahoo, P.K.; Santos, C.A.; da Silva, A.M.; von Zuben, A.A.G.; Souto, D.E.P.; Costa, A.D.T.; Celedon, P.; Zanchin, N.I.T.; Almeida, D.B.; et al. InP nanowire biosensor with tailored biofunctionalization: Ultrasensitive and highly selective disease biomarker detection. Nano Lett. 2017, 17, 5938–5949. [Google Scholar] [CrossRef] [Green Version]

| Chimeric Antigen | Sequence Name | Amino Acid Range | Gene Bank Sequence ID |
|---|---|---|---|
| IBMP-8.1 | Trans-sialidase | 747–774 | XP_820062.1 |
| 60S ribosomal protein L19 | 218–238 | XP_820995.1 | |
| Trans-sialidase | 1435–1449 | XP_813586.1 | |
| Surface antigen 2 (CA-2) | 276–297 | XP_813516.1 | |
| IBMP-8.2 | Antigen, partial | 13–73 | ACM47959.1 |
| Surface antigen 2 (CA-2) | 166–220 | XP_818927.1 | |
| Calpain cysteine peptidase | 31–97 | XP_804989.1 | |
| IBMP-8.3 | Trans-sialidase | 710–754 | XP_813237.1 |
| Flagellar repetitive antigen protein | 15–56 | AAA30177.1 | |
| 60S ribosomal protein L19 | 236–284 | XP_808122.1 | |
| Surface antigen 2 (CA-2) | 279–315 | XP_813516.1 | |
| IBMP-8.4 | Shed-acute-phase-antigen | 681–704 | CAA40511.1 |
| Kinetoplastid membrane protein KMP-11 | 76–92 | XP_810488.1 | |
| Trans-sialidase | 1436–1449 | XP_813586.1 | |
| Flagellar repetitive antigen protein | 20–47 | AAA30177.1 | |
| Trans-sialidase | 740–759 | XP_820062.1 | |
| Surface antigen 2 (CA-2) | 276–298 | XP_813516.1 | |
| Flagellar repetitive antigen protein | 1–68 | AAA30197.1 | |
| 60S ribosomal protein L19 | 218–238 | XP_820995.1 | |
| Microtubule-associated protein | 421–458 | XP_809567.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celedon, P.A.F.; Leony, L.M.; Oliveira, U.D.; Freitas, N.E.M.; Silva, Â.A.O.; Daltro, R.T.; Santos, E.F.; Krieger, M.A.; Zanchin, N.I.T.; Santos, F.L.N. Stability Assessment of Four Chimeric Proteins for Human Chagas Disease Immunodiagnosis. Biosensors 2021, 11, 289. https://doi.org/10.3390/bios11080289
Celedon PAF, Leony LM, Oliveira UD, Freitas NEM, Silva ÂAO, Daltro RT, Santos EF, Krieger MA, Zanchin NIT, Santos FLN. Stability Assessment of Four Chimeric Proteins for Human Chagas Disease Immunodiagnosis. Biosensors. 2021; 11(8):289. https://doi.org/10.3390/bios11080289
Chicago/Turabian StyleCeledon, Paola Alejandra Fiorani, Leonardo Maia Leony, Ueriton Dias Oliveira, Natália Erdens Maron Freitas, Ângelo Antônio Oliveira Silva, Ramona Tavares Daltro, Emily Ferreira Santos, Marco Aurélio Krieger, Nilson Ivo Tonin Zanchin, and Fred Luciano Neves Santos. 2021. "Stability Assessment of Four Chimeric Proteins for Human Chagas Disease Immunodiagnosis" Biosensors 11, no. 8: 289. https://doi.org/10.3390/bios11080289
APA StyleCeledon, P. A. F., Leony, L. M., Oliveira, U. D., Freitas, N. E. M., Silva, Â. A. O., Daltro, R. T., Santos, E. F., Krieger, M. A., Zanchin, N. I. T., & Santos, F. L. N. (2021). Stability Assessment of Four Chimeric Proteins for Human Chagas Disease Immunodiagnosis. Biosensors, 11(8), 289. https://doi.org/10.3390/bios11080289






