IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits
Abstract
:1. Introduction
2. Materials and Methods
2.1. IQVISION Hardware Architecture
2.2. IQVISION Algorithm Development
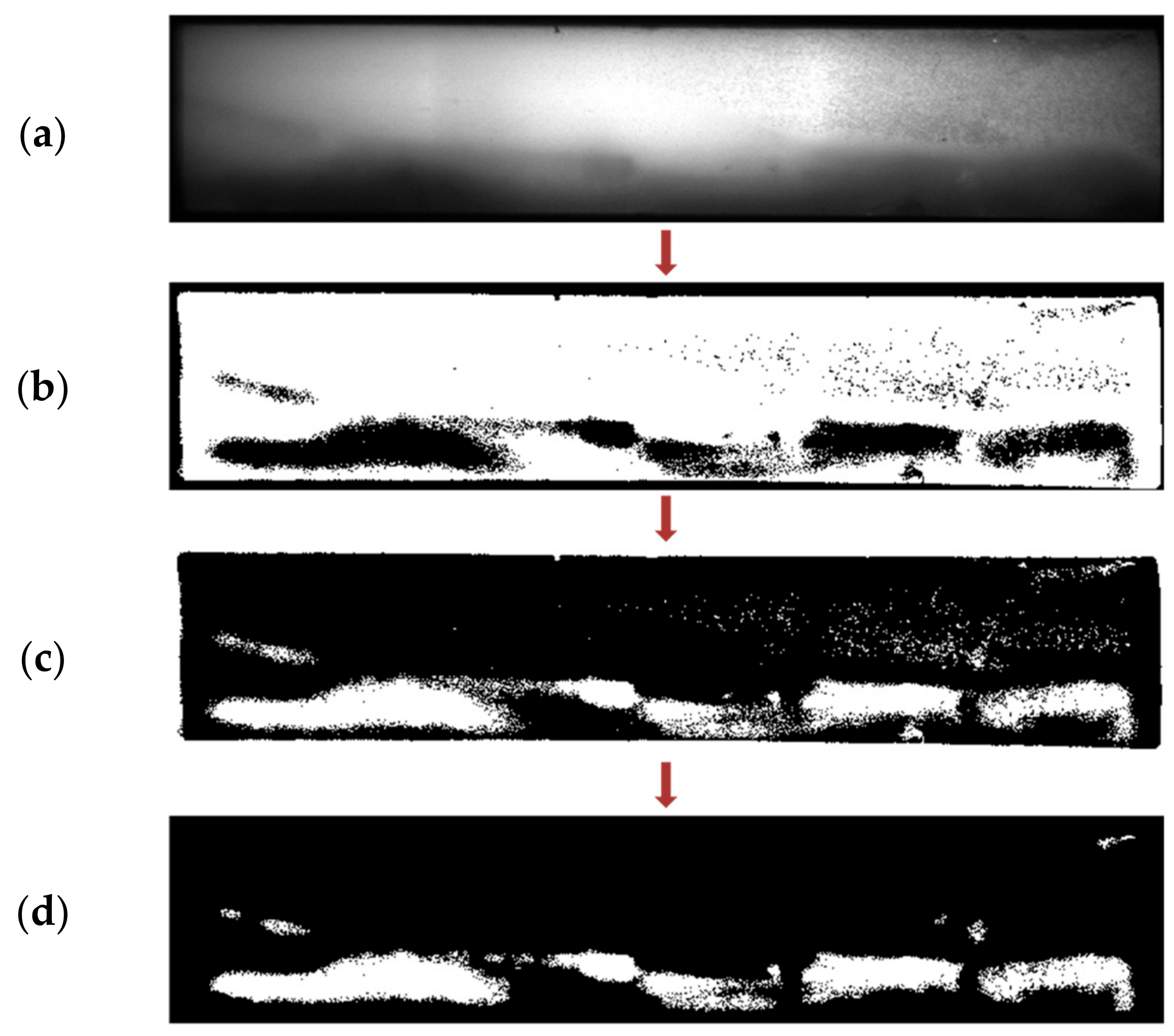
2.2.1. Image Data Acquisition and Noise Removal
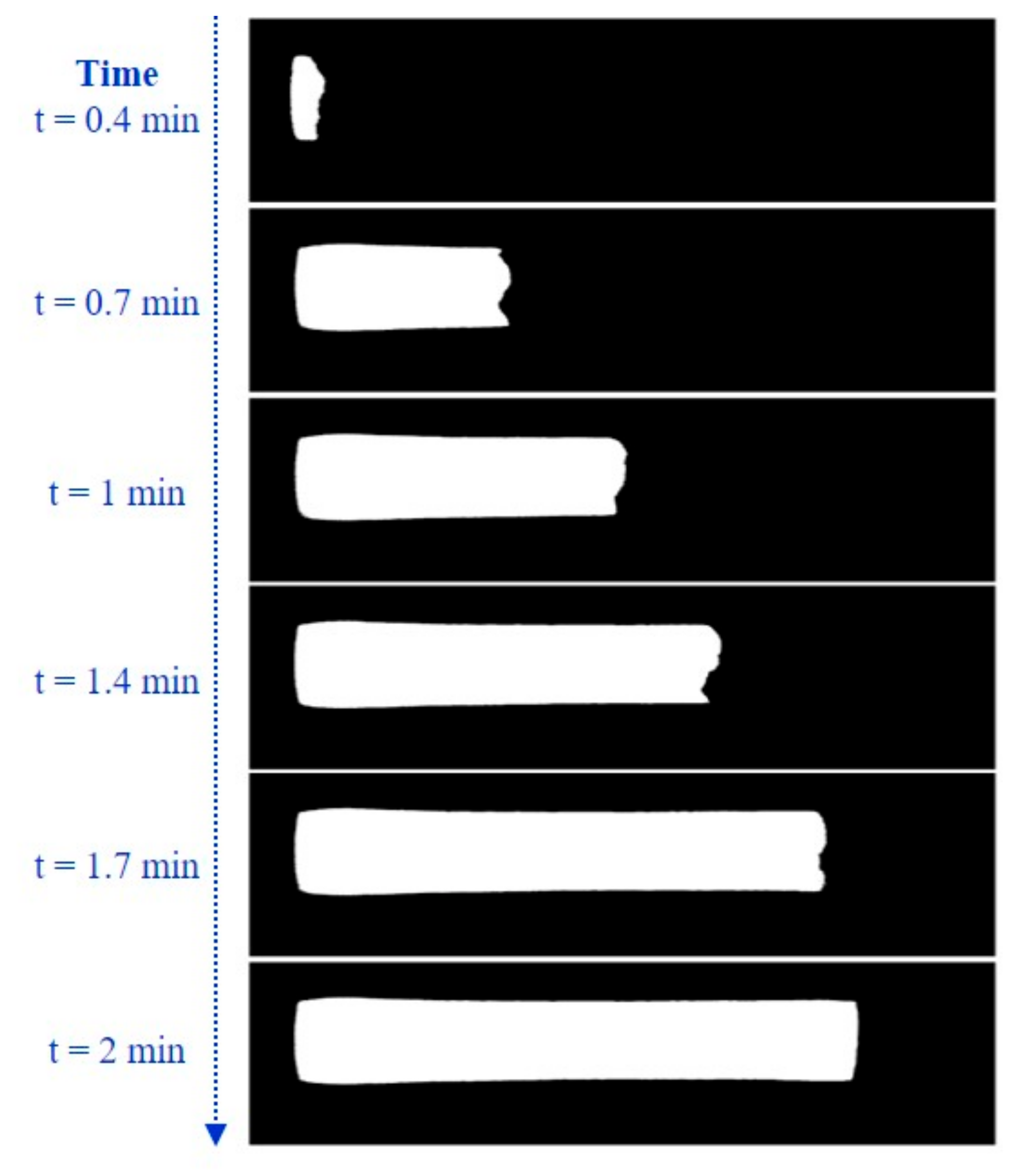
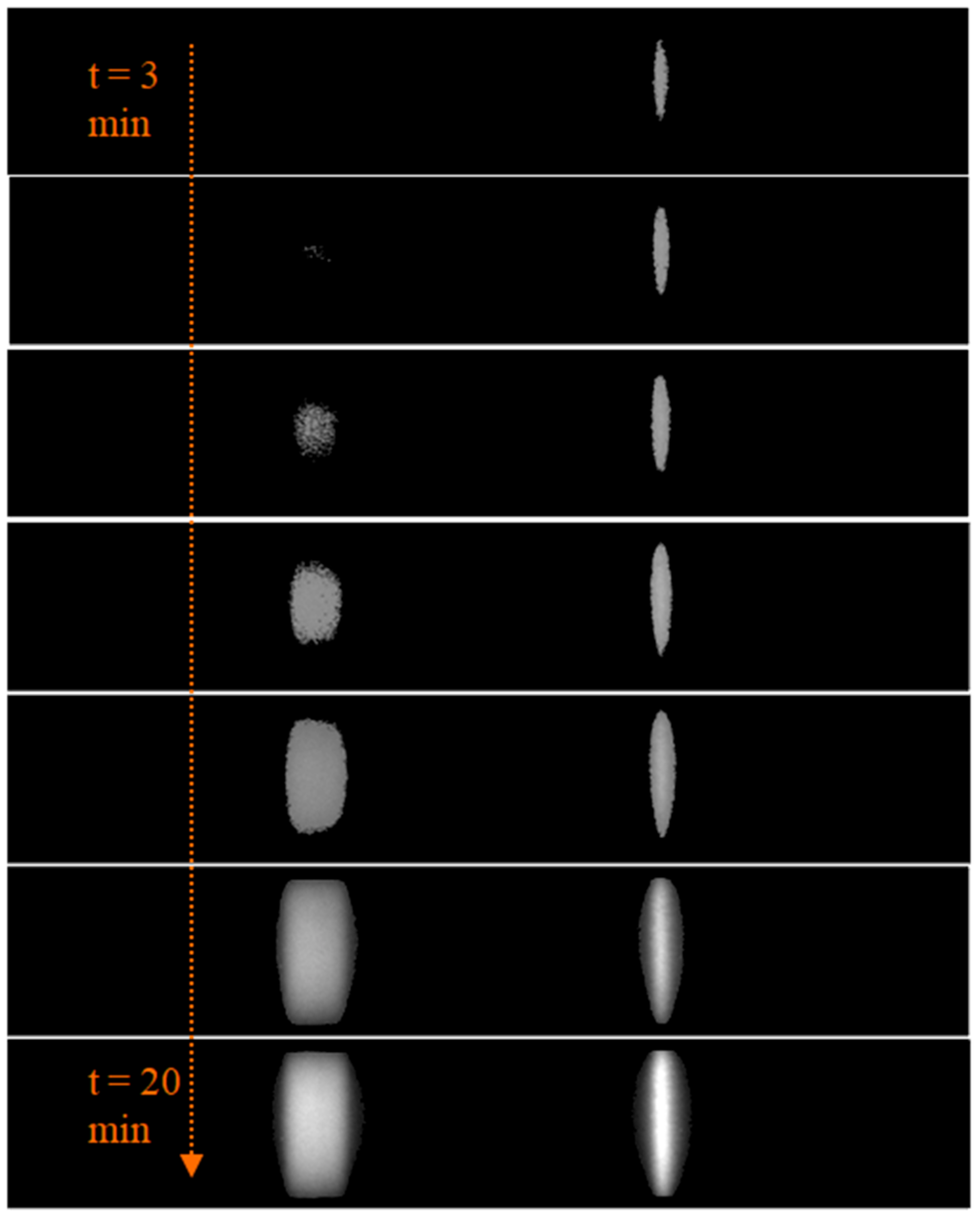
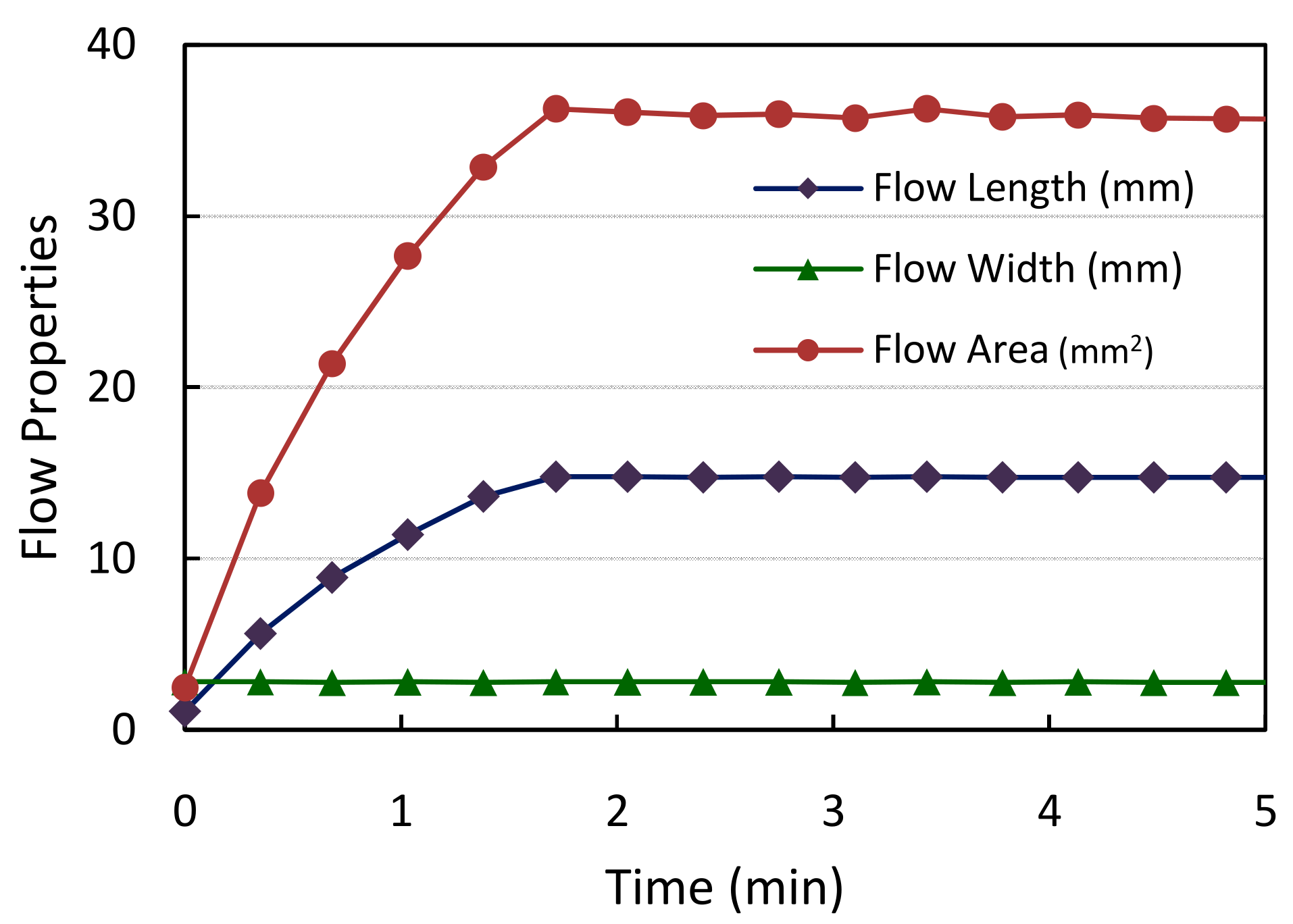
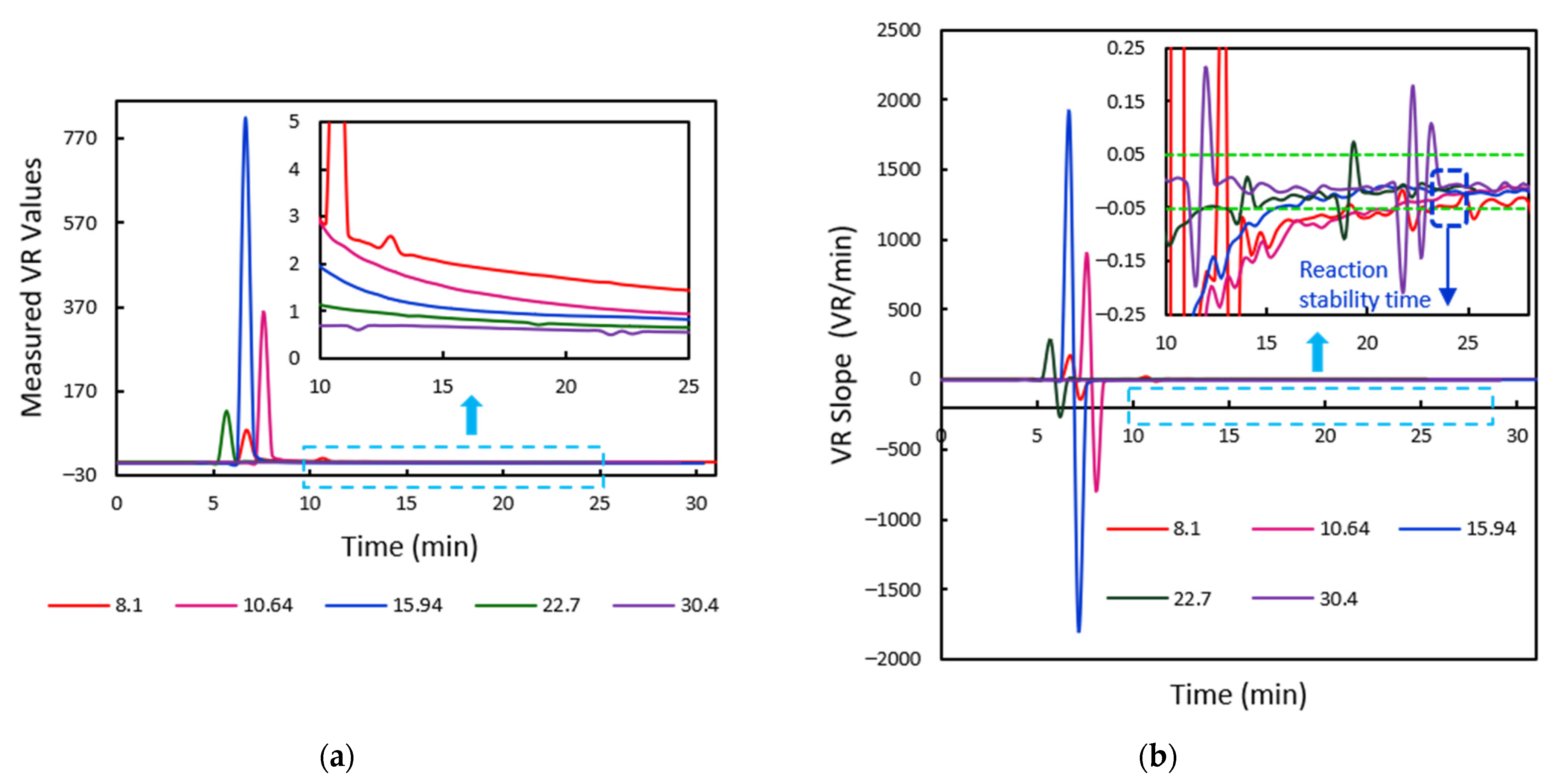
2.2.2. Tracking of Flow Progress
= 0, elsewhere
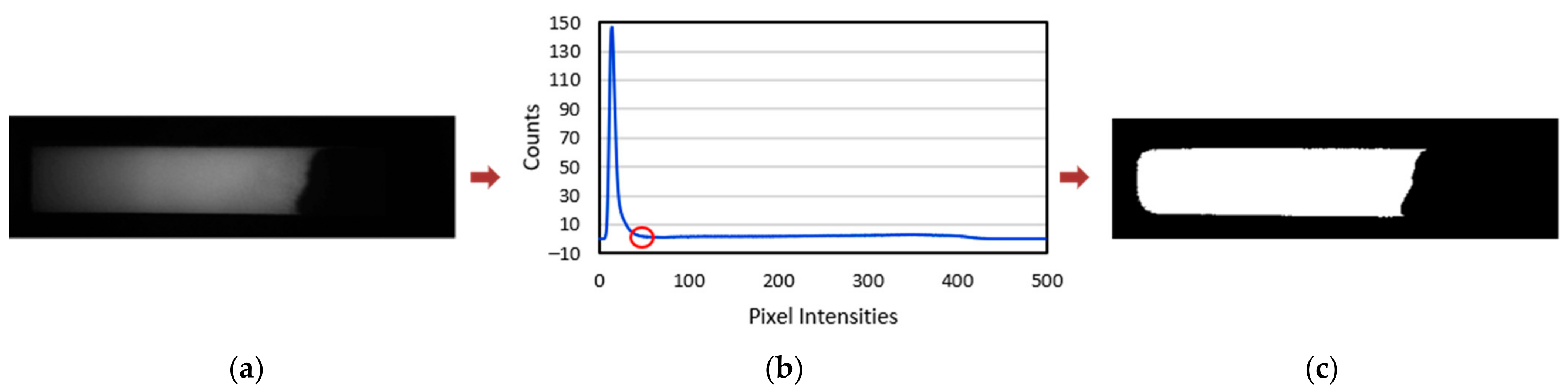
2.2.3. NC Membrane Segmentation
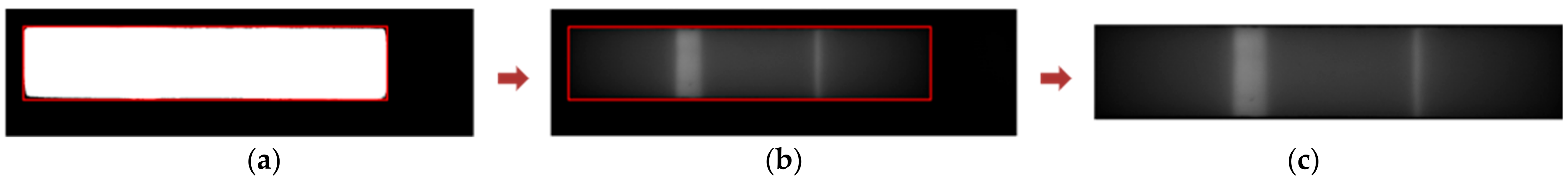
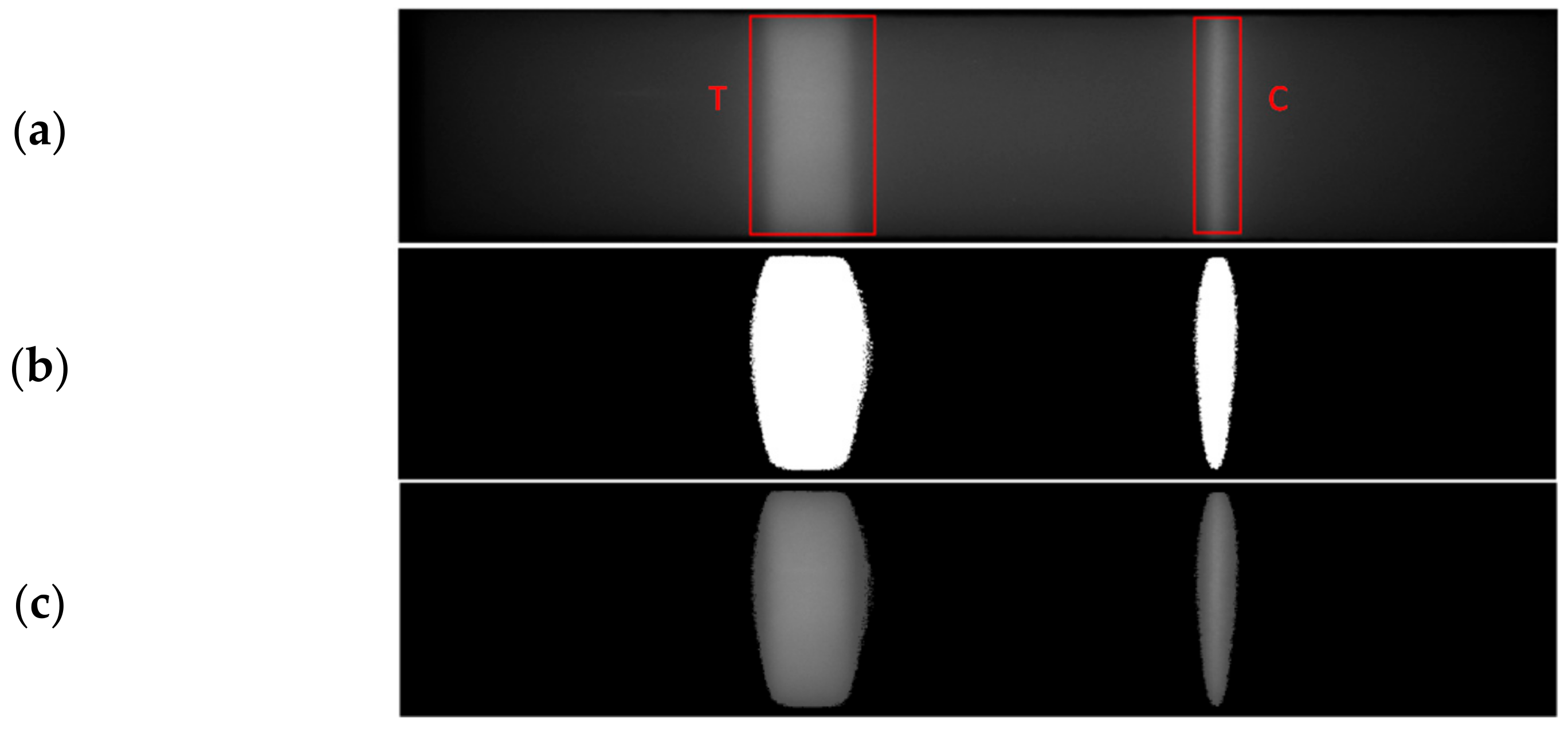
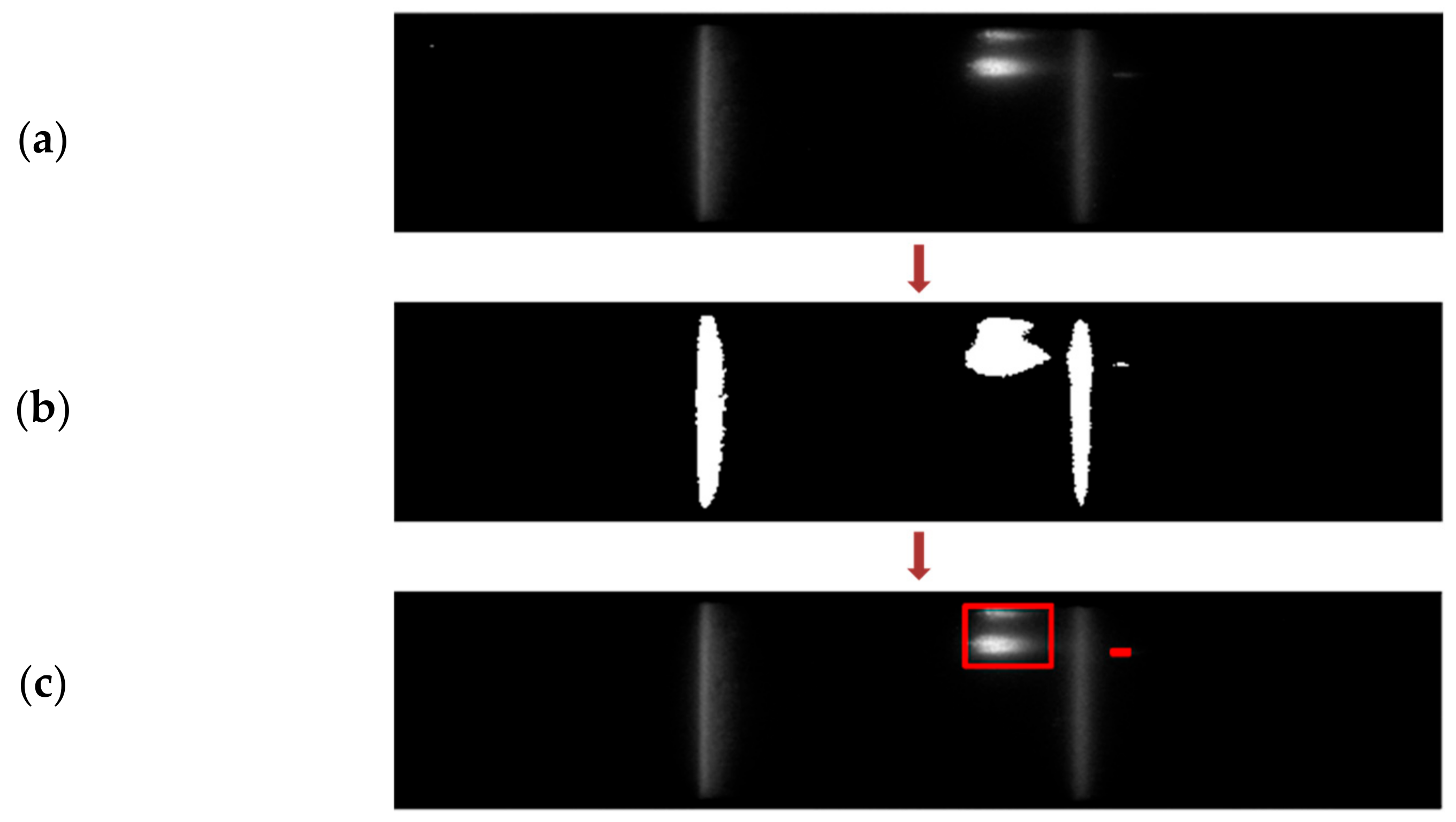
2.2.4. Segmentation of Test and Control Regions
2.2.5. Detection of Abnormalities
- Abnormalities in Sample Flow
- 2.
- Irregularities in Test Cartridge
- 3.
- Presence of Bright/Dark Regions within Test and Control Lines
3. Results and Discussion
3.1. Evaluation of Sample Flow through the Membrane
3.2. Segmentation of Test and Control Lines
3.2.1. Analysis of HbA1C Test Samples
3.2.2. Analysis of Vitamin D Test Samples
3.2.3. Calibration of Sample Cartridges and Performance Analysis
3.2.4. Validation for Abnormality Detection in Test Cartridges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farrell, B.O. Lateral Flow Immunoassay Systems: Evolution from the Current State of the Art to the Next Generation of Highly Sensitive, Quantitative Rapid Assays. Immunoass. Handb. 2013, 89–107. [Google Scholar] [CrossRef]
- Posthuma-Trumpie, G.A.; Korf, J.; Van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.L.; Chen, X.L.; Li, C.H.; Xu, B.; Liu, W.J.; Xu, H.Y.; Xiong, Y.H. Lateral flow immunoassay for quantitative detection of ractopamine in swine urine. Biomed. Environ. Sci. 2014, 27, 134–137. [Google Scholar] [PubMed]
- Sharma, S.; Zapatero-Rodríguez, J.; Estrela, P.; O’Kennedy, R.J. Point-of-Care Diagnostics in Low Resource Settings: Present Status and Future Role of Microfluidics. Biosensors 2015, 5, 577–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gubala, V.; Harris, L.F.; Ricco, A.J.; Tan, M.X.; Williams, D.E. Point of Care Diagnostics: Status and Future. Anal. Chem. 2011, 84, 487–515. [Google Scholar] [CrossRef] [PubMed]
- Ragavendar, M.S.; Anmol, C.M. A mathematical model to predict the optimal test line location and sample volume for lateral flow immunoassays. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 2408–2411. [Google Scholar] [CrossRef]
- Berli, C.L.A.; Kler, P.A. A quantitative model for lateral flow assays. Microfluid. Nanofluidics 2016, 20, 1–9. [Google Scholar] [CrossRef]
- Gasperino, D.J.; Leon, D.; Lutz, B.; Cate, D.M.; Nichols, K.; Bell, D.; Weigl, B.H. Threshold-Based Quantification in a Multiline Lateral Flow Assay via Computationally Designed Capture Efficiency. Anal. Chem. 2018, 90, 6643–6650. [Google Scholar] [CrossRef] [PubMed]
- Bheemavarapu, L.P.; Shah, M.I.; Joseph, J.; Sivaprakasam, M. Image -based Tracking of Immunoassay Reaction Progress in Quantitative Lateral Flow Kits. In Proceedings of the 2019 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Kim, B.C.; Jeong, J.H.; Jeong, D.S.; Choi, E.Y.; Kim, J.H.; Nahm, K.B. Simplified Laser Fluorescence Scanner for Prote-omics Studies and Early Cancer Diagnosis. Proc. SPIE Vol. 2002, 4916, 103–108. [Google Scholar]
- Pilavaki, E.; Parolo, C.; McKendry, R.; Demosthenous, A. Wireless paper-based biosensor reader for the detection of infectious diseases at the point of care. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016; pp. 1–3. [Google Scholar]
- Shah, M.I.; Joseph, J.; Rajagopalan, A.; Sivaprakasam, M. ImageQuant: An image-based quantitative Immunoassay Analyzer. In Proceedings of the 2017 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Istanbul, Turkey, 26–28 June 2019; pp. 420–425. [Google Scholar]
- Jason-Moller, L.; Murphy, M.; Bruno, J. Overview of Biacore Systems and Their Applications. Curr. Protoc. Protein Sci. 2006, 45, 19.13.1–19.13.14. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.M.; Bremer, M.G.; Wichers, J.H.; Van Amerongen, A.; Nielen, M.W. Rapid Antibody Selection Using Surface Plasmon Resonance for High-Speed and Sensitive Hazelnut Lateral Flow Prototypes. Biosensors 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achieving Data-Driven Decisions with Real-Time Interaction Analyses; Technical note, 29270160 AA; GE Healthcare Bio-Sciences AB: Uppsala, Sweden, 2017.
- Farrell, B.O. Evolution in Lateral Flow—Based Immunoassay Systems; Humana Press: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.G.; Nordman, E.S.; Johnson, M.D.; Oldham, M.F. A Low-Cost, High-Performance System for Fluorescence Lateral Flow Assays. Biosensors 2013, 3, 360–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chowdhury, K.B.; Joseph, J.; Reddy, N.; Vasan, J.K.; Sivaprakasam, M. An image based quantitative fluorescence immunoassay reader for HbA1c testing: Calibration & repeatability study. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications, Benevento, Italy, 15–18 May 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Cardullo, R.A.; Hinchcliffe, E.H. Digital Manipulation of Brightfield and Fluorescence Images: Noise Reduction, Contrast Enhancement, and Feature Extraction. Methods Cell Biol. 2007, 81, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Reid, D.B.; Environment, C.; Palo, L.; Alto, P.; Smith, P.L. A Threshold Selection Method from Gray-Level Histo-grams. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar]
- Bradley, D.; Roth, G. Adaptive Thresholding using the Integral Image. J. Graph. Tools 2007, 12, 13–21. [Google Scholar] [CrossRef]
- Viola, P.; Jones, M. Robust Real-Time Face Detection Robust Real-Time Face Detection. Int. J. Comput. Vis. 2014, 2004, 2–3. [Google Scholar]










| Expected HbA1C Concentrations (%) | Measured VR Values | Measured HbA1C Concentrations (%) | Relative Error (%) |
|---|---|---|---|
| 4.5 | 1.1099 | 4.7210 | 4.91 |
| 4.7 | 1.1316 | 4.7454 | 0.97 |
| 5 | 1.2559 | 4.8860 | −2.28 |
| 5.7 | 1.5899 | 5.2657 | −7.62 |
| 6.2 | 2.7497 | 6.6069 | 6.56 |
| 9 | 4.3867 | 8.5598 | −4.89 |
| Expected Vitamin D Concentrations (%) | Measured VR Values | Measured Vitamin D Concentrations (%) | Relative Error (%) |
|---|---|---|---|
| 15.71 | 1.0655 | 16.6800 | 6.16 |
| 17.13 | 1.0501 | 17.0624 | −0.39 |
| 29.9 | 0.6209 | 31.0314 | 3.78 |
| No. of Samples for Flow Abnormality Detection | Expected Outcome | ||
|---|---|---|---|
| Proper | Improper | ||
| Test Outcome | Proper | 10 (TP) | 0 (FP) |
| Improper | 0 (FN) | 6 (TN) | |
| % Flow Sensitivity | 100 | ||
| % Flow Specificity | 100 | ||
| % Flow Accuracy | 100 | ||
| No. of Samples for Detection of Irregularities in NC Membrane | Expected Outcome | ||
|---|---|---|---|
| Proper | Improper | ||
| Test Outcome | Proper | 116 (TP) | 1 (FP) |
| Improper | 4 (FN) | 9 (TN) | |
| % Irregularity Detection Sensitivity | 96 | ||
| % Irregularity Detection Specificity | 90 | ||
| % Irregularity Detection Accuracy | 96 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bheemavarapu, L.P.; Shah, M.I.; Joseph, J.; Sivaprakasam, M. IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits. Biosensors 2021, 11, 211. https://doi.org/10.3390/bios11070211
Bheemavarapu LP, Shah MI, Joseph J, Sivaprakasam M. IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits. Biosensors. 2021; 11(7):211. https://doi.org/10.3390/bios11070211
Chicago/Turabian StyleBheemavarapu, Lalitha Pratyusha, Malay Ilesh Shah, Jayaraj Joseph, and Mohanasankar Sivaprakasam. 2021. "IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits" Biosensors 11, no. 7: 211. https://doi.org/10.3390/bios11070211
APA StyleBheemavarapu, L. P., Shah, M. I., Joseph, J., & Sivaprakasam, M. (2021). IQVision: An Image-Based Evaluation Tool for Quantitative Lateral Flow Immunoassay Kits. Biosensors, 11(7), 211. https://doi.org/10.3390/bios11070211





