Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bonding Mechanism
2.3. Contact Angle Measurement
2.4. Bond Strength Analyses
2.5. Bonded PMMA Microdevice as a Passive Micromixer
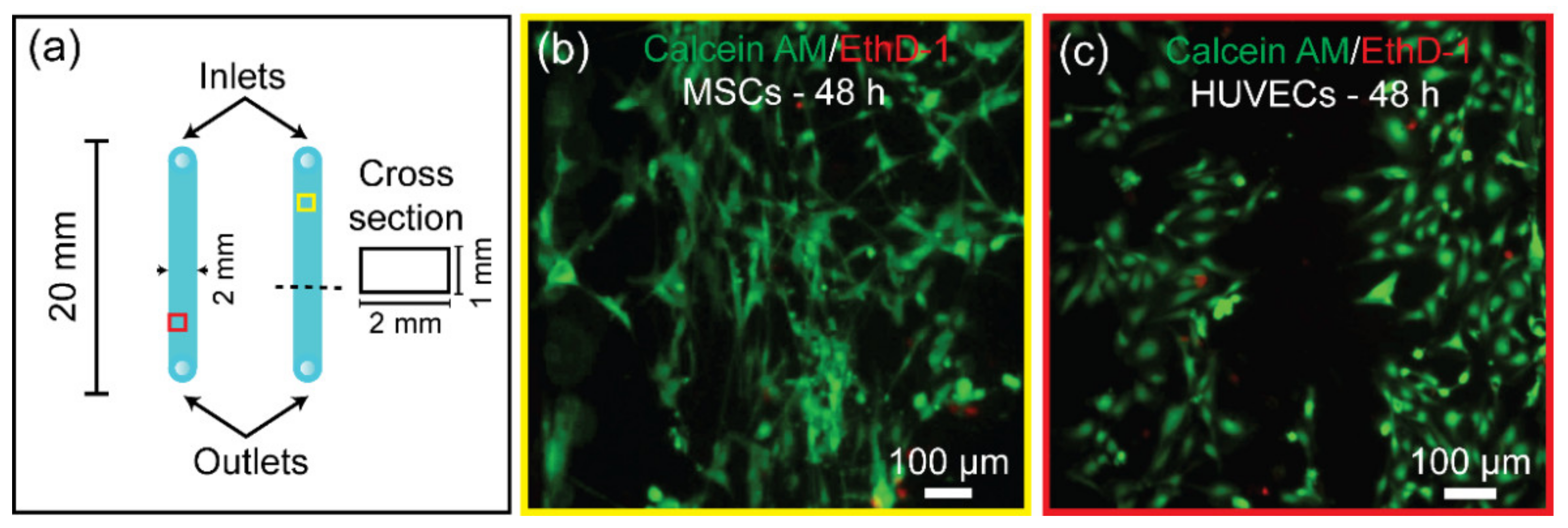
2.6. Bonded PMMA Microdevice as a Platform for Human Cell Culture
3. Results and Discussion
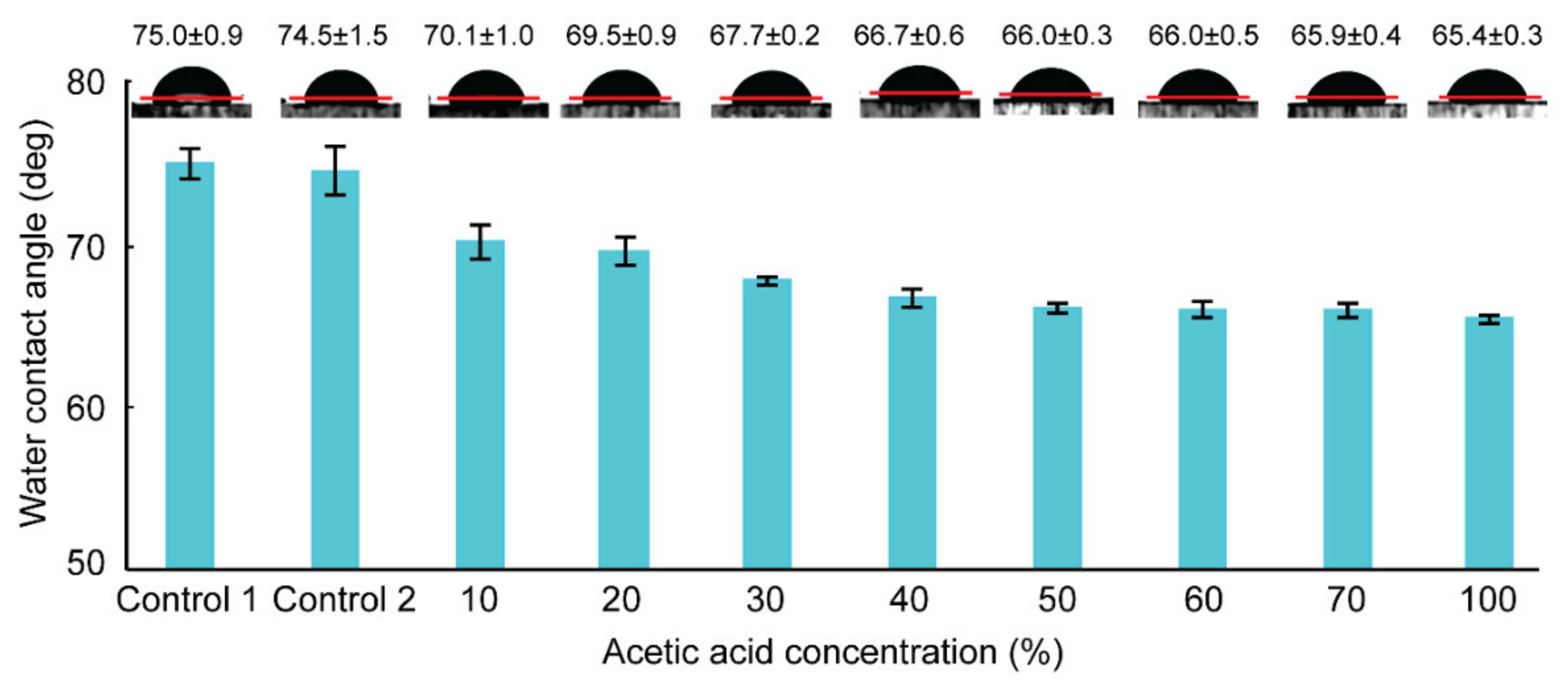
3.1. Contact Angle Measurement
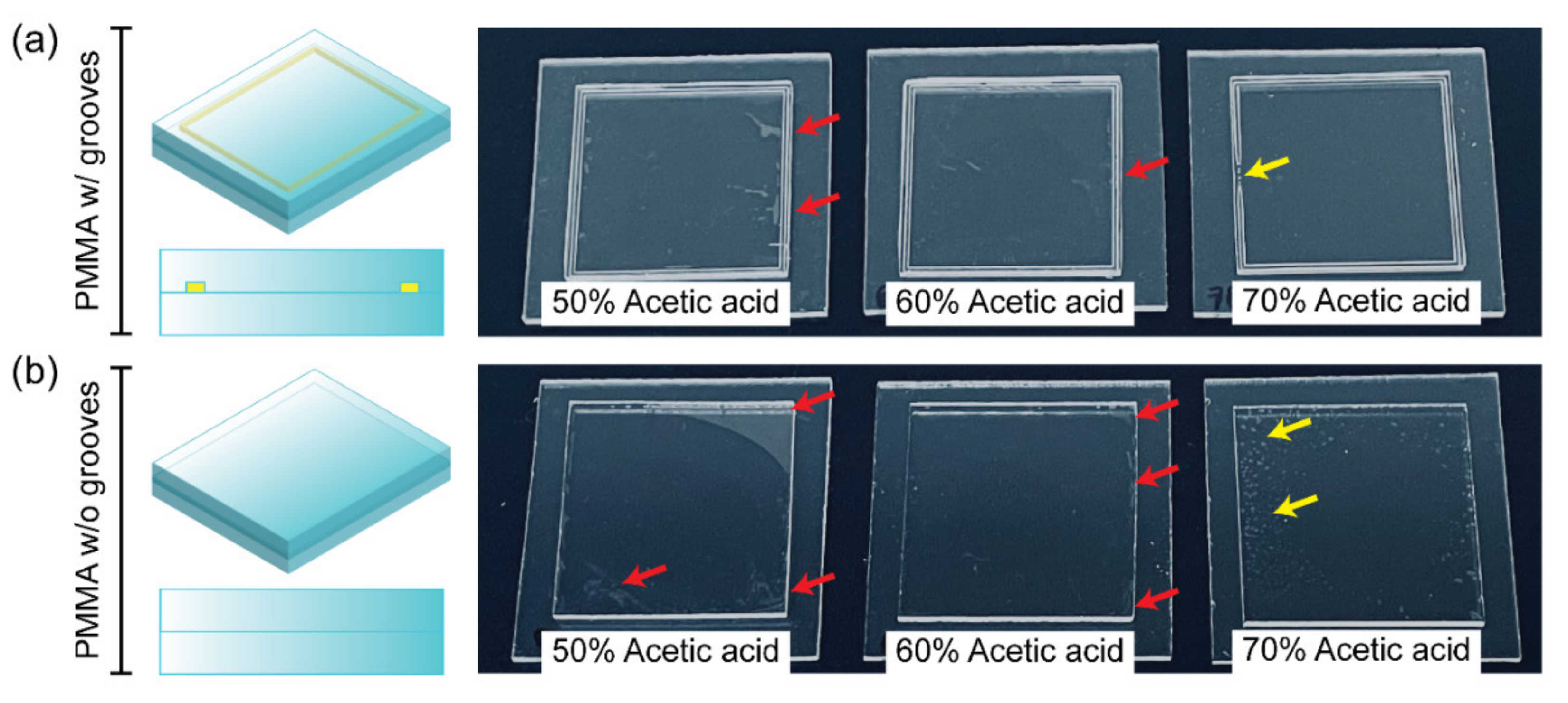
3.2. Bonding Performance: Effect of Acetic Acid Concentrations and Addition of Grooves
3.3. Bond Strength Measurement
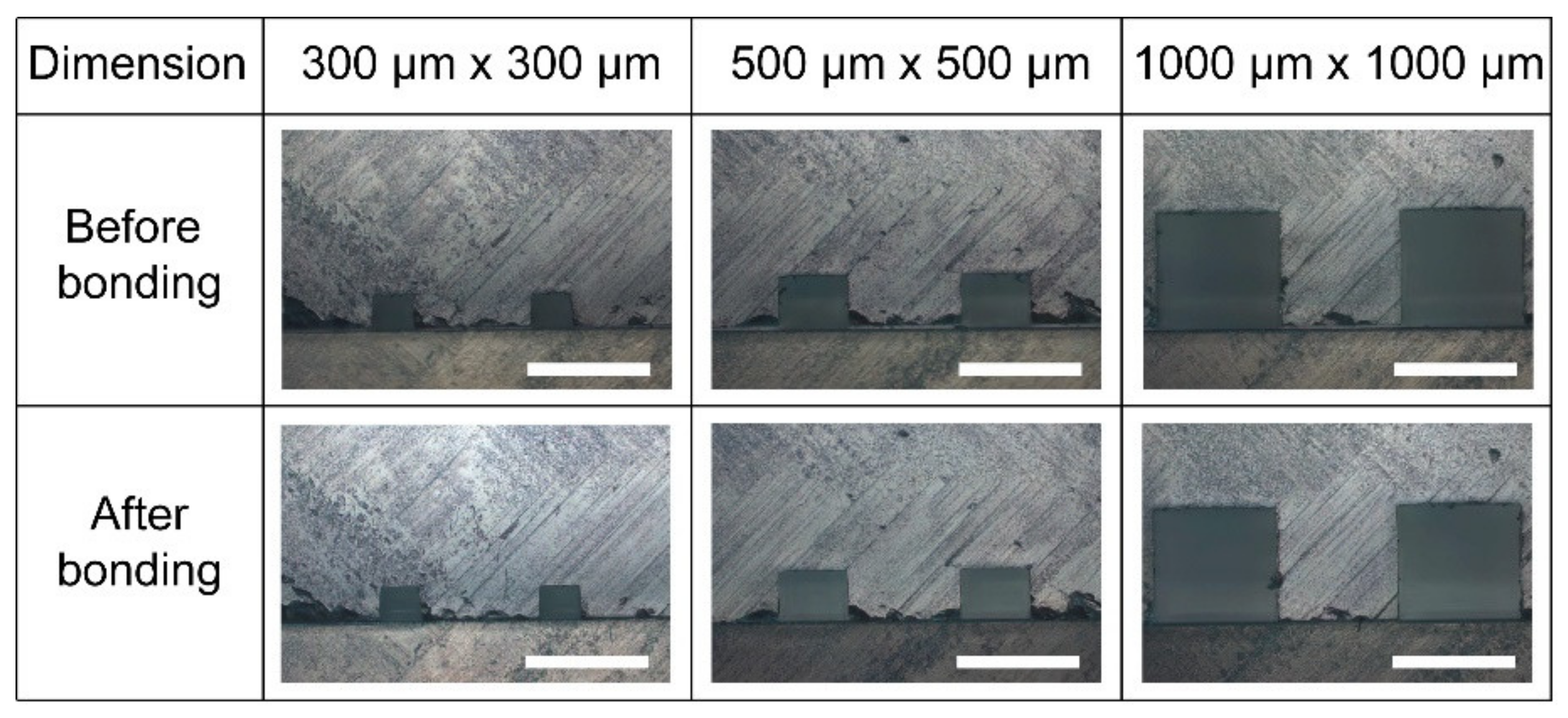
3.4. Microchannel Profile: Cross-Sectional View of the Microchannel
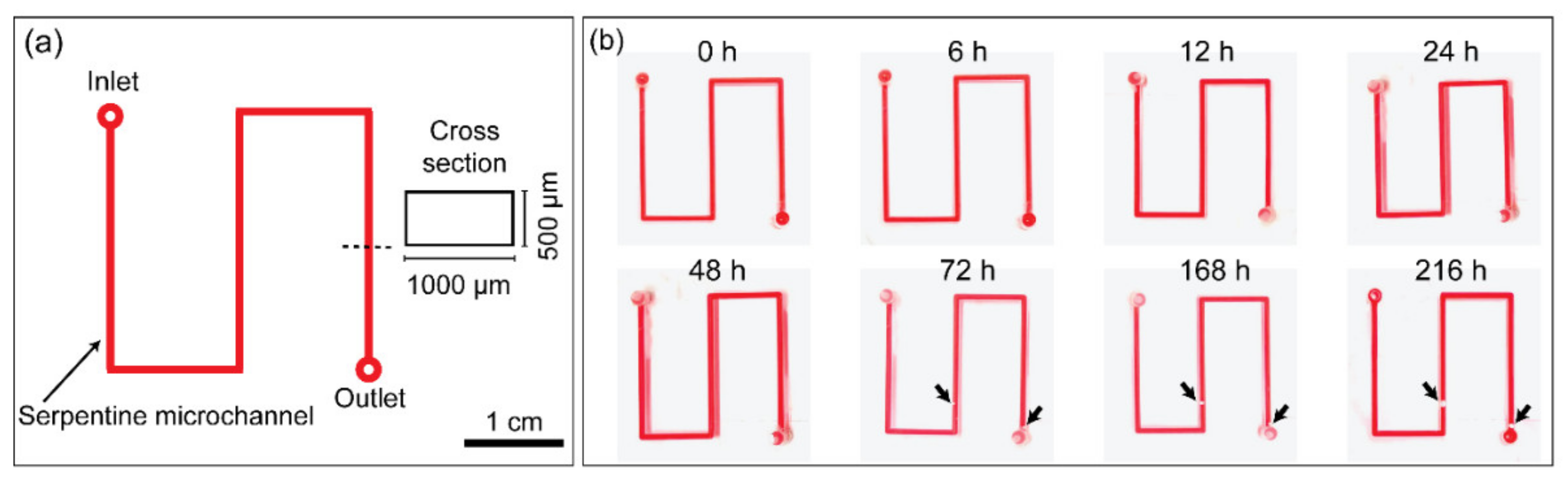
3.5. Leak Test
3.6. Bonded PMMA Microdevice Used as a Planar Passive Micromixer
3.7. Bonded PMMA Microdevice for Human Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef] [PubMed]
- Lafleur, J.P.; Jonsson, A.; Senkbeil, S.; Kutter, J.P. Recent advances in lab-on-a-chip for biosensing applications. Biosens. Bioelectron. 2016, 76, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Tu, Q.; Liu, W.M.; Wang, J.Y. Recent advances in microfluidic technologies for organ-on-a-chip. TrAC-Trends Anal. Chem. 2019, 117, 146–156. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Accounts Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef]
- Ziolkowska, K.; Jedrych, E.; Kwapiszewski, R.; Lopacinska, J.; Skolimowski, M.; Chudy, M. PDMS/glass microfluidic cell culture system for cytotoxicity tests and cells passage. Sens. Actuators B-Chem. 2010, 145, 533–542. [Google Scholar] [CrossRef]
- Temiz, Y.; Lovchik, R.D.; Kaigala, G.V.; Delamarche, E. Lab-on-a-chip devices: How to close and plug the lab? Microelectron. Eng. 2015, 132, 156–175. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. When PDMS isn’t the best. Anal. Chem. 2007, 79, 3248–3253. [Google Scholar] [CrossRef]
- Abgrall, P.; Low, L.N.; Nguyen, N.T. Fabrication of planar nanofluidic channels in a thermoplastic by hot-embossing and thermal bonding. Lab Chip 2007, 7, 520–522. [Google Scholar] [CrossRef]
- Tsao, C.W.; DeVoe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluidics 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Wu, W.; Wu, J.; Kim, J.H.; Lee, N.Y. Instantaneous room temperature bonding of a wide range of non-silicon substrates with poly(dimethylsiloxane) (PDMS) elastomer mediated by a mercaptosilane. Lab Chip 2015, 15, 2819–2825. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Zhang, H.; Kang, D.J.; Kahng, S.H.; Tall, B.; Lee, N.Y. Fabrication of polymerase chain reaction plastic lab-on-a-chip device for rapid molecular diagnoses. Int. Neurourol. J. 2016, 20, S38–S48. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Jung, S.H.; Lee, M.S.; Park, T.E.; Ahn, S.K.; Kang, J.H. Robust chemical bonding of PMMA microfluidic devices to porous PETE membranes for reliable cytotoxicity testing of drugs. Lab Chip 2019, 19, 3706–3713. [Google Scholar] [CrossRef]
- Faghih, M.M.; Sharp, M.K. Solvent-based bonding of PMMA-PMMA for microfluidic applications. Microsyst. Technol. 2019, 25, 3547–3558. [Google Scholar] [CrossRef]
- Chen, P.C.; Duong, L.H. Novel solvent bonding method for thermoplastic microfluidic chips. Sens. Actuators B-Chem. 2016, 237, 556–562. [Google Scholar] [CrossRef]
- Koesdjojo, M.T.; Koch, C.R.; Remcho, V.T. Technique for microfabrication of polymeric-based microchips from an SU-8 master with temperature-assisted vaporized organic solvent bonding. Anal. Chem. 2009, 81, 1652–1659. [Google Scholar] [CrossRef]
- Tran, H.H.; Wu, W.; Lee, N.Y. Ethanol and UV-assisted instantaneous bonding of PMMA assemblies and tuning in bonding reversibility. Sens. Actuators B-Chem. 2013, 181, 955–962. [Google Scholar] [CrossRef]
- Ling, N.; Lee, J.S.; Lee, N.Y. Solvent-assisted low-temperature and low-pressure poly(methylmethacrylate) bonding coupled with selective microchannel hydrophobic coating for reliable sealing. Sens. Actuators A-Phys. 2017, 265, 168–173. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Pham, Q.N.; Lee, N.Y. Clog-free and reliable solvent bonding of poly(methyl methacrylate) microdevice mediated by eco-friendly acetic acid at room temperature and its application for polymerase chain reaction and human cell culture. Sens. Actuators B-Chem. 2019, 282, 1008–1017. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Thai, D.A.; Chae, W.R.; Lee, N.Y. Rapid fabrication of poly(methyl methacrylate) devices for lab-on-a-chip applications using acetic acid and UV treatment. ACS Omega 2020, 5, 17396–17404. [Google Scholar] [CrossRef]
- Holmes, R.J.; McDonagh, C.; McLaughlin, J.A.D.; Mohr, S.; Goddard, N.J.; Fielden, P.R. Microwave bonding of poly(methylmethacrylate) microfluidic devices using a conductive polymer. J. Phys. Chem. Solids 2011, 72, 626–629. [Google Scholar] [CrossRef] [Green Version]
- Mani, K.B.; Hossan, M.R.; Dutta, P. Thermal analysis of microwave assisted bonding of poly(methyl methacrylate) substrates in microfluidic devices. Int. J. Heat Mass Transf. 2013, 58, 229–239. [Google Scholar] [CrossRef]
- Toossi, A.; Moghadas, H.; Daneshmand, M.; Sameoto, D. Bonding PMMA microfluidics using commercial microwave ovens. J. Micromech. Microeng. 2015, 25, 085008. [Google Scholar] [CrossRef]
- Rahbar, M.; Chhina, S.; Sameoto, D.; Parameswaran, M. Microwave-induced, thermally assisted solvent bonding for low-cost PMMA microfluidic devices. J. Micromech. Microeng. 2010, 20, 015026. [Google Scholar] [CrossRef]
- Lei, K.F.; Ahsan, S.; Budraa, N.; Li, W.J.; Mai, J.D. Microwave bonding of polymer-based substrates for potential encapsulated micro/nanofluidic device fabrication. Sens. Actuators A-Phys. 2004, 114, 340–346. [Google Scholar] [CrossRef]
- Wan, A.M.D.; Sadri, A.; Young, E.W.K. Liquid phase solvent bonding of plastic microfluidic devices assisted by retention grooves. Lab Chip 2015, 15, 3785–3792. [Google Scholar] [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Sriram, G.; Alberti, M.; Dancik, Y.; Wu, B.; Wu, R.G.; Feng, Z.X.; Ramasamy, S.; Bigliardi, P.L.; Bigliardi-Qi, M.; Wang, Z.P. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Mater. Today 2018, 21, 326–340. [Google Scholar] [CrossRef]
- Li, L.J.; Chen, Y.; Wang, H.R.; An, G.; Wu, H.K.; Huang, W. A high-throughput, open-space and reusable microfluidic chip for combinational drug screening on tumor spheroids. Lab Chip 2021, 21, 3924–3932. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications. Biosensors 2021, 11, 526. https://doi.org/10.3390/bios11120526
Trinh KTL, Chae WR, Lee NY. Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications. Biosensors. 2021; 11(12):526. https://doi.org/10.3390/bios11120526
Chicago/Turabian StyleTrinh, Kieu The Loan, Woo Ri Chae, and Nae Yoon Lee. 2021. "Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications" Biosensors 11, no. 12: 526. https://doi.org/10.3390/bios11120526
APA StyleTrinh, K. T. L., Chae, W. R., & Lee, N. Y. (2021). Pressure-Free Assembling of Poly(methyl methacrylate) Microdevices via Microwave-Assisted Solvent Bonding and Its Biomedical Applications. Biosensors, 11(12), 526. https://doi.org/10.3390/bios11120526






