Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes
Abstract
:1. Introduction
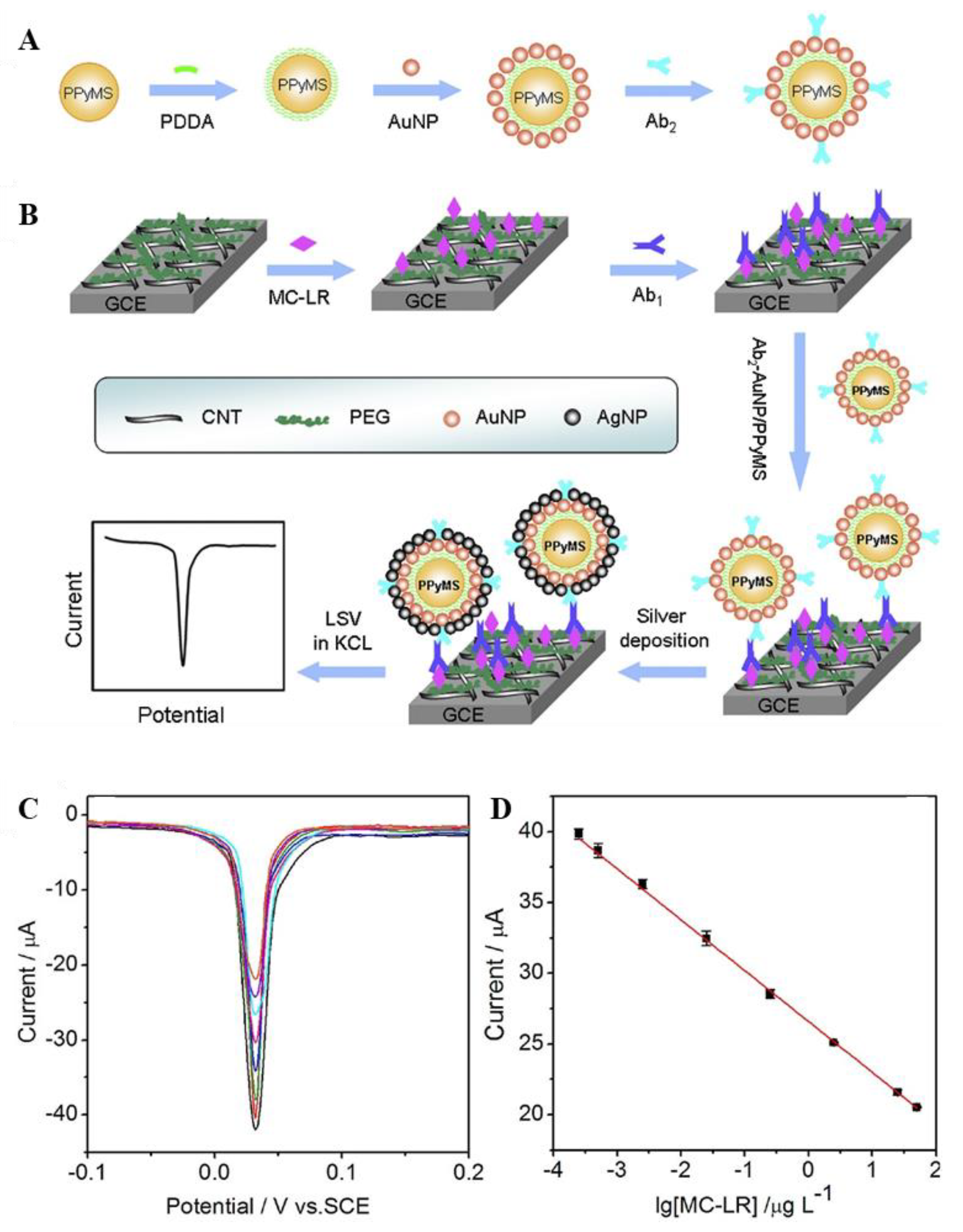
2. Metal Nanoparticle-Based MC Biosensor
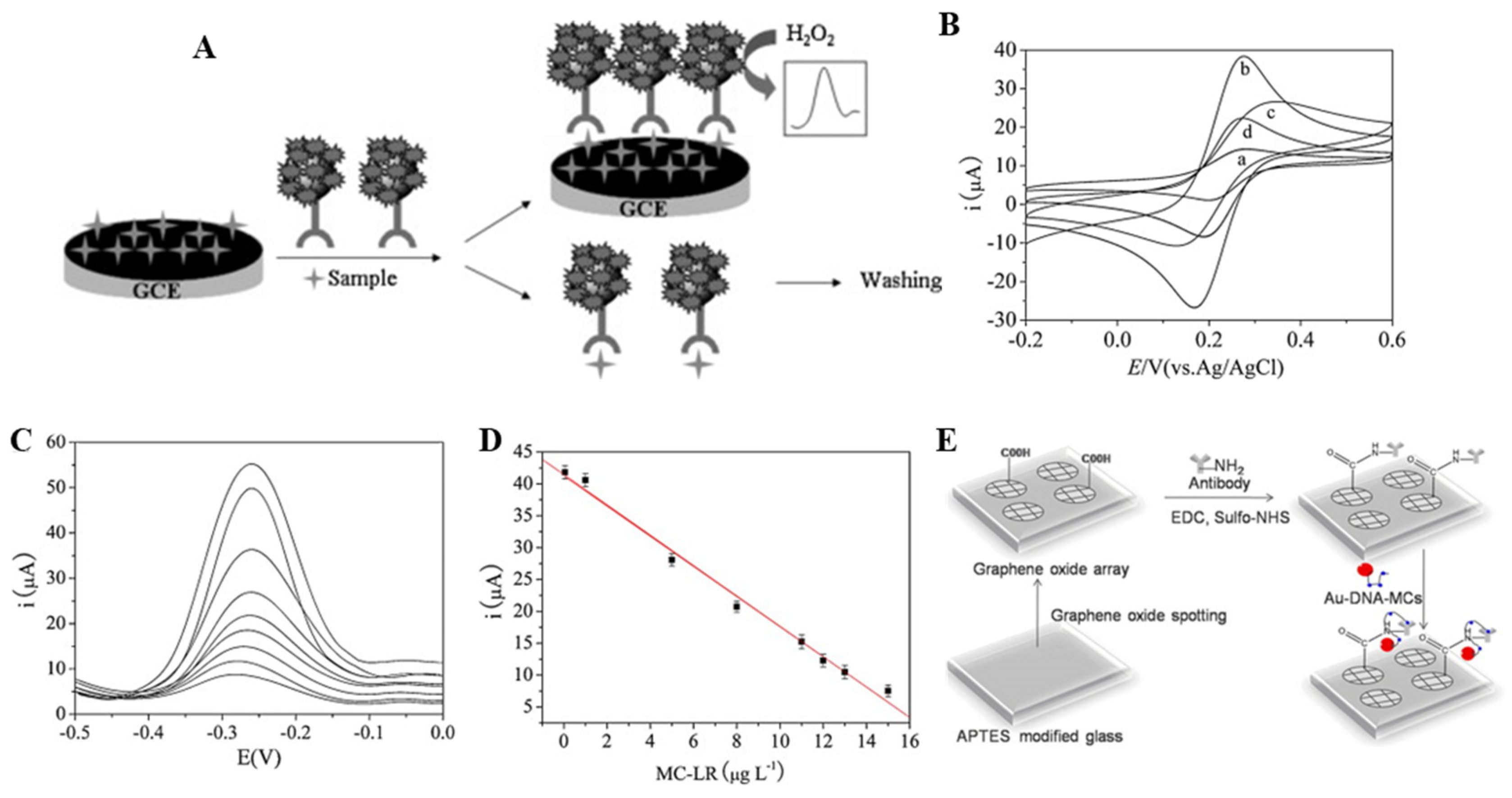
3. Carbon Nanomaterial-Based MC Biosensor
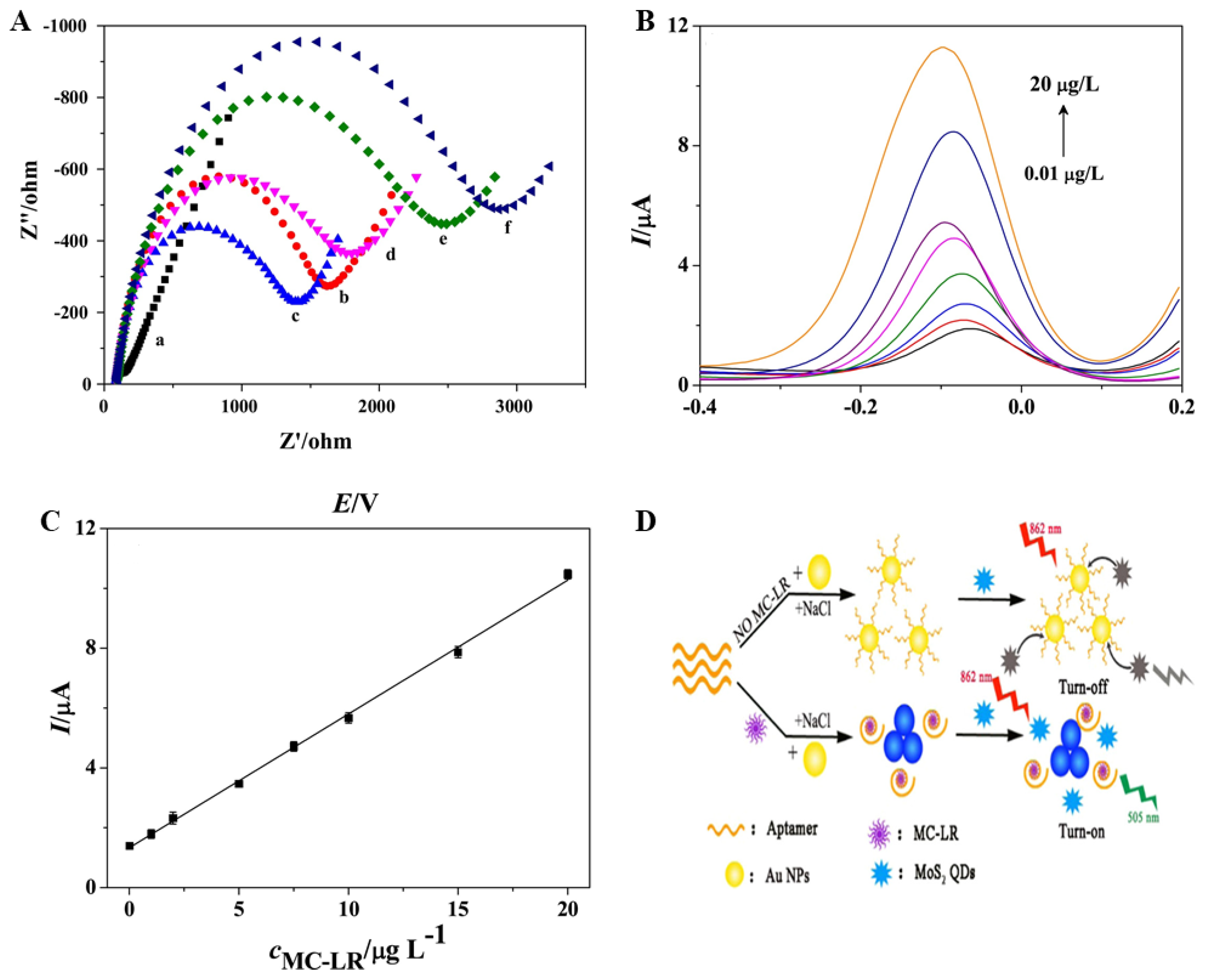
4. Transition Metal Dichalcogenides Nanoparticle-Based MC Biosensor
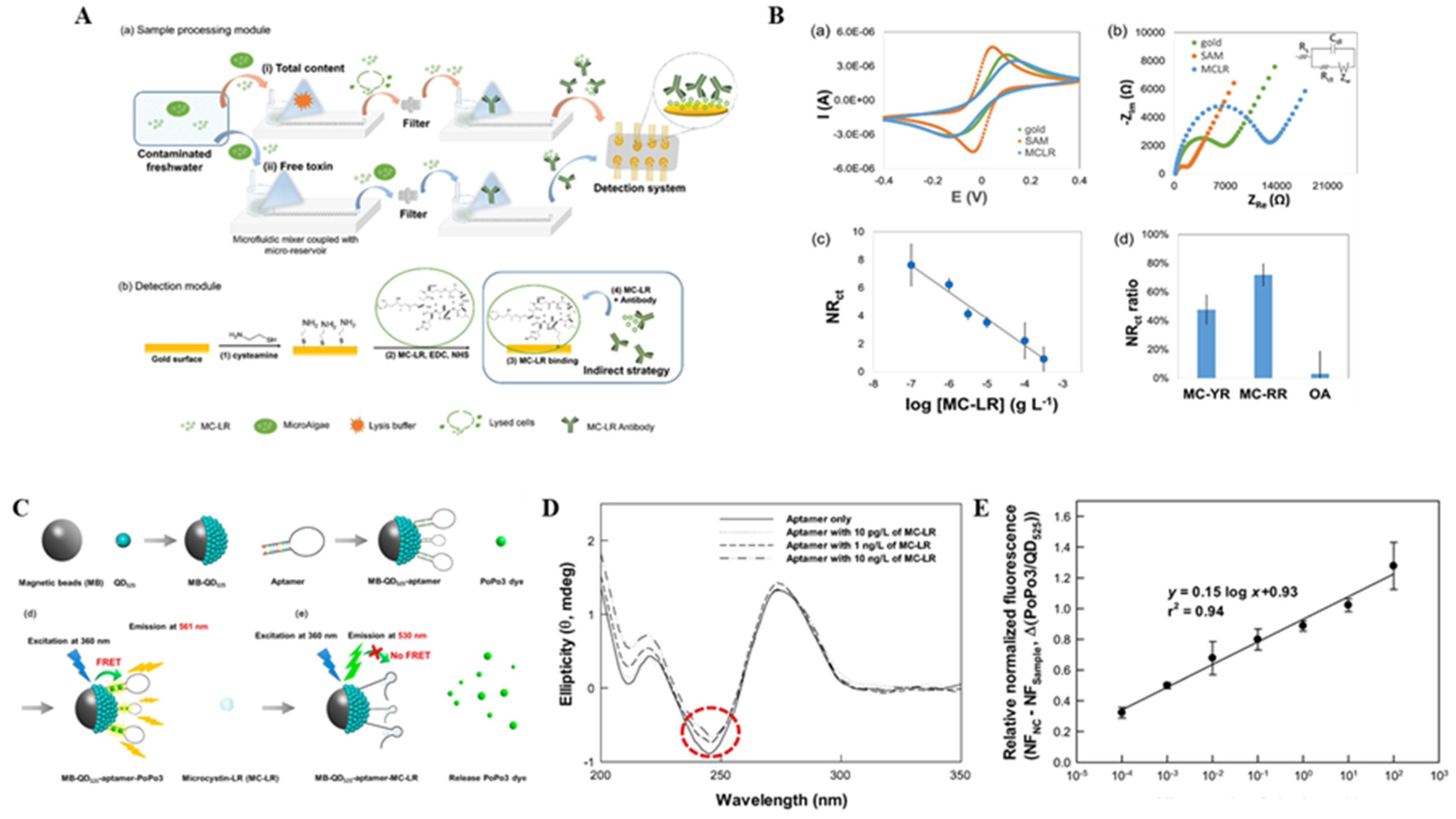
5. MC Biosensors with Other Nanomaterials
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.F.; Van Hassel, W.H.; Andjelkovic, M.; Wilmotte, A.; Rajkovic, A. Cyanotoxins and Food Contamination in Developing Countries: Review of Their Types, Toxicity, Analysis, Occurrence and Mitigation Strategies. Toxins 2021, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Steffen, M.M.; Belisle, B.S.; Watson, S.B.; Boyer, G.L.; Wilhelm, S.W. Status, causes and controls of cyanobacterial blooms in Lake Erie. J. Great Lakes Res. 2014, 40, 215–225. [Google Scholar] [CrossRef]
- Weller, M.G. Immunoassays and biosensors for the detection of cyanobacterial toxins in water. Sensors 2013, 13, 15085–15112. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, V.d.; Marinho, M.M.; Domingos, P.; Oliveira, A.C.; Costa, S.M.; Azevedo, L.d.; Azevedo, S.M. Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brasil, RJ). Toxicon 2003, 42, 289–295. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Choi, B.; Kim, G.; Lee, J. Harmful algal blooms and liver diseases: Focusing on the areas near the four major rivers in South Korea. J. Environ. Sci. Health C 2019, 37, 356–370. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Wu, D.; Li, R.; Zhang, F.; Liu, J. A review on drone-based harmful algae blooms monitoring. Environ. Monit. Assess. 2019, 191, 211. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Biotechnol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Mehinto, A.C.; Smith, J.; Wenger, E.; Stanton, B.; Linville, R.; Brooks, B.W.; Sutula, M.A.; Howard, M.D. Synthesis of ecotoxicological studies on cyanotoxins in freshwater habitats–Evaluating the basis for developing thresholds protective of aquatic life in the United States. Sci. Total Environ. 2021, 795, 148864. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Abbà, A.; Collivignarelli, M.C. CONTROL MEASURES FOR Cyanobacteria AND Cyanotoxins IN DRINKING WATER. Environ. Eng. Manag. J. 2018, 17, 2455–2463. [Google Scholar] [CrossRef]
- Kaur, S.; Srivastava, A.; Ahluwalia, A.S.; Mishra, Y. Cyanobacterial blooms and Cyanotoxins: Occurrence and Detection. In Algae; Springer: Singapore, 2021; pp. 339–352. [Google Scholar]
- Khomutovska, N.; Sandzewicz, M.; Łach, Ł.; Suska-Malawska, M.; Chmielewska, M.; Mazur-Marzec, H.; Cegłowska, M.; Niyatbekov, T.; Wood, S.A.; Puddick, J. Limited microcystin, anatoxin and cylindrospermopsin production by cyanobacteria from microbial mats in cold deserts. Toxins 2020, 12, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, J.S.; Codd, G.A. Cyanobacteria, neurotoxins and water resources: Are there implications for human neurodegenerative disease? Amyotroph. Lat. Scler. 2009, 10, 74–78. [Google Scholar] [CrossRef]
- Catherine, A.; Bernard, C.; Spoof, L.; Bruno, M. Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Lisa Spoof, L., Codd, G.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 107–126. [Google Scholar]
- McElhiney, J.; Lawton, L.A. Detection of the cyanobacterial hepatotoxins microcystins. Toxicol. Appl. Pharmacol. 2005, 203, 219–230. [Google Scholar] [CrossRef]
- Zurawell, R.W.; Chen, H.; Burke, J.M.; Prepas, E.E. Hepatotoxic cyanobacteria: A review of the biological importance of microcystins in freshwater environments. J. Toxicol. Environ. Health Part B 2005, 8, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Srivastava, A.; Oh, H.; Ahn, C.; Choi, G.; Asthana, R.K. Recent trends in development of biosensors for detection of microcystin. Toxicon 2012, 60, 878–894. [Google Scholar] [CrossRef]
- Abe, T.; Lawson, T.; Weyers, J.D.; Codd, G.A. Microcystin-LR inhibits photosynthesis of Phaseolus vulgaris primary leaves: Implications for current spray irrigation practice. New Phytol. 1996, 133, 651–658. [Google Scholar] [CrossRef]
- Pang, P.; Lai, Y.; Zhang, Y.; Wang, H.; Conlan, X.A.; Barrow, C.J.; Yang, W. Recent advancement of biosensor technology for the detection of microcystin-LR. Bull. Chem. Soc. Jpn. 2020, 93, 637–646. [Google Scholar] [CrossRef]
- ISO 20179: 2005 Water Quality-Determination of Microcystins-Method Using Solid Phase extraction (SPE) and High Performance Liquid Chromatography (HPLC) with Ultraviolet (UV) Detection. 2005. Available online: https://www.iso.org/standard/34098.html (accessed on 16 December 2021).
- He, X.; Stanford, B.D.; Adams, C.; Rosenfeldt, E.J.; Wert, E.C. Varied influence of microcystin structural difference on ELISA cross-reactivity and chlorination efficiency of congener mixtures. Water Res. 2017, 126, 515–523. [Google Scholar] [CrossRef]
- Khreich, N.; Lamourette, P.; Renard, P.; Clavé, G.; Fenaille, F.; Créminon, C.; Volland, H. A highly sensitive competitive enzyme immunoassay of broad specificity quantifying microcystins and nodularins in water samples. Toxicon 2009, 53, 551–559. [Google Scholar] [CrossRef]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Vidic, J.; Manzano, M.; Chang, C.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhara, K.; Mahapatra, D.R. Review on electrochemical sensing strategies for C-reactive protein and cardiac troponin I detection. Microchem. J. 2020, 156, 104857. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.; Han, H.; Park, S.; Park, S.Y.; Park, C.; Baek, C.; Lee, T.; Min, J. Fabrication of ultrasensitive electrochemical biosensor for dengue fever viral RNA Based on CRISPR/Cpf1 reaction. Sens. Actuators B Chem. 2021, 326, 128677. [Google Scholar] [CrossRef]
- Raja, I.S.; Vedhanayagam, M.; Preeth, D.R.; Kim, C.; Lee, J.H.; Han, D.W. Development of Two-Dimensional Nanomaterials Based Electrochemical Biosensors on Enhancing the Analysis of Food Toxicants. Int. J. Mol. Sci. 2021, 22, 3277. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Mohammadniaei, M.; Zhang, H.; Yoon, J.; Choi, H.K.; Guo, S.; Guo, P.; Choi, J. Single Functionalized pRNA/Gold Nanoparticle for Ultrasensitive MicroRNA Detection Using Electrochemical Surface-Enhanced Raman Spectroscopy. Adv. Sci. 2020, 7, 1902477. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Minea, A.A. A review on electrical conductivity of nanoparticle-enhanced fluids. Nanomaterials 2019, 9, 1592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senf, B.; Yeo, W.; Kim, J. Recent advances in portable biosensors for biomarker detection in body fluids. Biosensors 2020, 10, 127. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-based biosensors for antibiotic detection: A review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hara, T.O.; Singh, B. Electrochemical Biosensors for Detection of Pesticides and Heavy Metal Toxicants in Water: Recent Trends and Progress. ACS EST Water 2021, 1, 462–478. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S. Sensors and biosensors for the determination of small molecule biological toxins. Sensors 2008, 8, 6045–6054. [Google Scholar] [CrossRef]
- Buckova, M.; Licbinsky, R.; Jandova, V.; Krejci, J.; Pospichalova, J.; Huzlik, J. Fast ecotoxicity detection using biosensors. Water Air Soil Pollut. 2017, 228, 166. [Google Scholar] [CrossRef] [Green Version]
- Cunha, I.; Biltes, R.; Sales, M.G.F.; Vasconcelos, V. Aptamer-based biosensors to detect aquatic phycotoxins and cyanotoxins. Sensors 2018, 18, 2367. [Google Scholar] [CrossRef] [Green Version]
- Bertani, P.; Lu, W. Cyanobacterial toxin biosensors for environmental monitoring and protection. Med. Nov. Technol. Devices 2021, 10, 100059. [Google Scholar] [CrossRef]
- Massey, I.Y.; Wu, P.; Wei, J.; Luo, J.; Ding, P.; Wei, H.; Yang, F. A mini-review on detection methods of microcystins. Toxins 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, V.; Sharma, M.M.M.; Patra, A.; Singh, B.; Mehta, S.; Husen, A. A Review on Biosensors and Nanosensors Application in Agroecosystems. Nanoscale Res. Lett. 2021, 16, 1–24. [Google Scholar] [CrossRef]
- Ma, J.; Du, M.; Wang, C.; Xie, X.; Wang, H.; Zhang, Q. Advances in airborne microorganisms detection using biosensors: A critical review. Front. Environ. Sci. Eng. 2021, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, J.; Li, L.; Zhang, Y.; Xin, S.; Ni, X.; Sun, Y.; Song, K. Recent Progress of the Practical Applications of the Platinum Nanoparticle-Based Electrochemistry Biosensors. Front. Chem. 2021, 9, 677876. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-carbons in biosensor applications: An overview of carbon nanotubes (CNTs) and fullerenes (C 60). Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Jung, J.; Yoon, K.R.; Youn, D.; Park, S.; Kim, I. A high-capacity and long-cycle-life lithium-ion battery anode architecture: Silver nanoparticle-decorated SnO2/NiO nanotubes. ACS Nano 2016, 10, 11317–11326. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 2017, 1, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Cao, X.; Ye, Y.; Liu, S. Gold nanoparticle-based signal amplification for biosensing. Anal. Biochem. 2011, 417, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Zhao, H.; Huang, Y.; Lu, X.; Chen, J.; Jain, R. MXene: An emerging material for sensing and biosensing. Trends Anal. Chem. 2018, 105, 424–435. [Google Scholar] [CrossRef]
- Laurent, S.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Int. J. Mol. Epidemiol. Genet. 2011, 2, 367. [Google Scholar]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-based plasmonic nanoparticles for and their use in biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.; Lee, Y.; Park, S.Y.; Hong, K.; Kim, Y.; Park, C.; Chung, Y.; Lee, M.; Min, J. Fabrication of electrochemical biosensor composed of multi-functional DNA structure/Au nanospike on micro-gap/PCB system for detecting troponin I in human serum. Colloids Surf. B. 2019, 175, 343–350. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Chen, Z. Ultrasensitive electrochemical microcystin-LR immunosensor using gold nanoparticle functional polypyrrole microsphere catalyzed silver deposition for signal amplification. Sens. Actuators B Chem. 2017, 246, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Hutter, E.; Fendler, J.H. Exploitation of localized surface plasmon resonance. Adv Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Willets, K.A.; Van Duyne, R.P. Localized surface plasmon resonance spectroscopy and sensing. Annu. Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.M.; Hafner, J.H. Localized surface plasmon resonance sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ming, H. Review of surface plasmon resonance and localized surface plasmon resonance sensor. Photonic Sens. 2012, 2, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, S.; Lin, M.; Chen, X.; Lin, S.; Du, X.; Li, H.; Ye, H.; Qiu, B.; Lin, Z.; et al. Colorimetric detection of microcystin-LR based on disassembly of orient-aggregated gold nanoparticle dimers. Biosens. Bioelectron. 2015, 68, 475–480. [Google Scholar] [CrossRef]
- Sun, X.; Wu, L.; Ji, J.; Jiang, D.; Zhang, Y.; Li, Z.; Zhang, G.; Zhang, H. Longitudinal surface plasmon resonance assay enhanced by magnetosomes for simultaneous detection of Pefloxacin and Microcystin-LR in seafoods. Biosens. Bioelectron. 2013, 47, 318–323. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haynes, C.L.; McFarland, A.D.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy. Anal. Chem. 2005, 77, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Le Ru, E.; Etchegoin, P. Principles of Surface-Enhanced Raman Spectroscopy: And Related Plasmonic Effects; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Tian, Z.Q. Surface-enhanced Raman spectroscopy: Advancements and applications. J. Raman Spectrosc. 2005, 36, 466–470. [Google Scholar] [CrossRef]
- He, D.; Wu, Z.; Cui, B.; Jin, Z. A novel SERS-based aptasensor for ultrasensitive sensing of microcystin-LR. Food Chem. 2019, 278, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Paidi, S.K.; Sakowski, E.; Preheim, S.; Barman, I. Ultrasensitive detection of hepatotoxic microcystin production from cyanobacteria using surface-enhanced Raman scattering immunosensor. ACS Sens. 2019, 4, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, N. Noble metal nanoparticles-based colorimetric biosensor for visual quantification: A mini review. Chemosensors 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Kanjanawarut, R.; Su, X. Colorimetric detection of DNA using unmodified metallic nanoparticles and peptide nucleic acid probes. Anal. Chem. 2009, 81, 6122–6129. [Google Scholar] [CrossRef]
- Sun, J.; Lu, Y.; He, L.; Pang, J.; Yang, F.; Liu, Y. Colorimetric sensor array based on gold nanoparticles: Design principles and recent advances. TrAC Trends Anal. Chem. 2020, 122, 115754. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Teng, X.; Lai, Y.; Pu, S.; Pang, P.; Wang, H.; Yang, C.; Barrow, C.J.; Yang, W. Enzyme-free fluorescent detection of microcystin-LR using hairpin DNA-templated copper nanoclusters as signal indicator. Talanta 2019, 202, 279–284. [Google Scholar] [CrossRef]
- Ruscito, A.; DeRosa, M.C. Small-molecule binding aptamers: Selection strategies, characterization, and applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ding, J.; Liu, J. A highly specific sodium aptamer probed by 2-aminopurine for robust Na sensing. Nucleic Acids Res. 2016, 44, 10377–10385. [Google Scholar] [CrossRef] [Green Version]
- Alhadrami, H.A.; Chinnappan, R.; Eissa, S.; Rahamn, A.A.; Zourob, M. High affinity truncated DNA aptamers for the development of fluorescence based progesterone biosensors. Anal. Biochem. 2017, 525, 78–84. [Google Scholar] [CrossRef]
- Li, X.; Cheng, R.; Shi, H.; Tang, B.; Xiao, H.; Zhao, G. A simple highly sensitive and selective aptamer-based colorimetric sensor for environmental toxins microcystin-LR in water samples. J. Hazard. Mater. 2016, 304, 474–480. [Google Scholar] [CrossRef]
- He, F.; Liang, L.; Zhou, S.; Xie, W.; He, S.; Wang, Y.; Tlili, C.; Tong, S.; Wang, D. Label-free sensitive detection of microcystin-LR via aptamer-conjugated gold nanoparticles based on solid-state nanopores. Langmuir 2018, 34, 14825–14833. [Google Scholar] [CrossRef]
- Xie, W.; He, S.; Fang, S.; Liang, L.; Shi, B.; Wang, D. Visualizing of AuNPs Protection Aptamer from DNase I enzyme digestion Based on Nanopipette and Its Use for Microcystin-LR Detection. Anal. Chim. Acta 2021, 1173, 338698. [Google Scholar] [CrossRef]
- Liang, X.; Li, N.; Zhang, R.; Yin, P.; Zhang, C.; Yang, N.; Liang, K.; Kong, B. Carbon-based SERS biosensor: From substrate design to sensing and bioapplication. NPG Asia Mater. 2021, 13, 1–36. [Google Scholar] [CrossRef]
- Hwang, H.S.; Jeong, J.W.; Kim, Y.A.; Chang, M. Carbon nanomaterials as versatile platforms for biosensing applications. Micromachines 2020, 11, 814. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A review on graphene-based nanocomposites for electrochemical and fluorescent biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-based biosensors for detection of biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.; Choi, J. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Tian, J.; Quan, X. A graphene and multienzyme functionalized carbon nanosphere-based electrochemical immunosensor for microcystin-LR detection. Colloids Surf. B. 2013, 103, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lei, J.; Pan, R.; Leng, C.; Hu, Z.; Ju, H. In situ assembly of gold nanoparticles on nitrogen-doped carbon nanotubes for sensitive immunosensing of microcystin-LR. Chem. Commun. 2011, 47, 668–670. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Kong, R.; Zhang, X.; Meng, H.; Liu, W.; Tan, W.; Shen, G.; Yu, R. Graphene–DNAzyme based biosensor for amplified fluorescence “turn-on” detection of Pb2 with a high selectivity. Anal. Chem. 2011, 83, 5062–5066. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, W.; Wang, H.; Xie, L.; Liang, Y.; Wei, F.; Idrobo, J.; Pennycook, S.J.; Dai, H. An oxygen reduction electrocatalyst based on carbon nanotube–graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Gilje, S.; Han, S.; Wang, M.; Wang, K.L.; Kaner, R.B. A chemical route to graphene for device applications. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Thin-film particles of graphite oxide 1:: High-yield synthesis and flexibility of the particles. Carbon 2004, 42, 2929–2937. [Google Scholar] [CrossRef]
- Szabó, T.; Szeri, A.; Dékány, I. Composite graphitic nanolayers prepared by self-assembly between finely dispersed graphite oxide and a cationic polymer. Carbon 2005, 43, 87–94. [Google Scholar] [CrossRef]
- Pan, S.; Chen, X.; Li, X.; Cai, M.; Shen, H.; Zhao, Y.; Jin, M. In situ controllable synthesis of graphene oxide-based ternary magnetic molecularly imprinted polymer hybrid for efficient enrichment and detection of eight microcystins. J. Mater. Chem. A. 2015, 3, 23042–23052. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, J.; Sun, Y.; Zhang, Y.; Wen, Z.; Dai, H.; Wang, H.; Li, Z. A graphene oxide based biosensor for microcystins detection by fluorescence resonance energy transfer. Biosens. Bioelectron. 2012, 38, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 1–15. [Google Scholar] [CrossRef]
- Chowdhury, T.; Sadler, E.C.; Kempa, T.J. Progress and Prospects in Transition-Metal Dichalcogenide Research Beyond 2D. Chem. Rev. 2020, 120, 12563–12591. [Google Scholar] [CrossRef]
- Zhang, X.; Teng, S.Y.; Loy, A.C.M.; How, B.S.; Leong, W.D.; Tao, X. Transition metal dichalcogenides for the application of pollution reduction: A review. Nanomaterials 2020, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, S.; Tiwari, U.K.; Deep, A.; Sinha, R.K. Two-dimensional transition metal dichalcogenides assisted biofunctionalized optical fiber SPR biosensor for efficient and rapid detection of bovine serum albumin. Sci. Rep. 2019, 9, 6987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Huang, K.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305–316. [Google Scholar] [CrossRef]
- Gracia-Abad, R.; Sangiao, S.; Bigi, C.; Kumar Chaluvadi, S.; Orgiani, P.; De Teresa, J.M. Omnipresence of Weak Antilocalization (WAL) in Bi2Se3 Thin Films: A Review on Its Origin. Nanomaterials 2021, 11, 1077. [Google Scholar] [CrossRef]
- Yang, M.; Hwang, S.; Jeong, J.; Huh, Y.S.; Choi, B.G. Nitrogen-doped carbon-coated molybdenum disulfide nanosheets for high-performance supercapacitor. Synth. Met. 2015, 209, 528–533. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Yoon, J.; Yi, C.; Lee, T.; Choi, J. Flexible HIV-1 biosensor based on the Au/MoS2 nanoparticles/Au nanolayer on the PET substrate. Nanomaterials 2019, 9, 1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Lee, S.N.; Shin, M.K.; Kim, H.; Choi, H.K.; Lee, T.; Choi, J. Flexible electrochemical glucose biosensor based on GOx/gold/MoS2/gold nanofilm on the polymer electrode. Biosens. Bioelectron. 2019, 140, 111343. [Google Scholar] [CrossRef]
- Barua, S.; Dutta, H.S.; Gogoi, S.; Devi, R.; Khan, R. Nanostructured MoS2-based advanced biosensors: A review. ACS Appl. Nano Mater. 2017, 1, 2–25. [Google Scholar] [CrossRef]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-dimensional layered MoS 2 biosensors enable highly sensitive detection of biomolecules. Sci. Rep. 2014, 4, 7352. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Tang, Y.; Liu, P.; Yang, L.; Li, L.; Zhang, Q.; Zhou, Y.; Khan, M.Z.H. A highly sensitive electrochemical aptasensor for detection of microcystin-LR based on a dual signal amplification strategy. Analyst 2019, 144, 1671–1678. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Li, H.; Yan, F.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. A molybdenum disulfide/gold nanorod composite-based electrochemical immunosensor for sensitive and quantitative detection of microcystin-LR in environmental samples. Sens. Actuators B Chem. 2017, 244, 606–615. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Gao, T.; Zhang, Y.; Ma, D.; Liu, M.; Chen, Y.; Qiao, X.; Tan, P.; Kan, M. Epitaxial monolayer MoS2 on mica with novel photoluminescence. Nano Lett. 2013, 13, 3870–3877. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J. MoS2 quantum dots: Synthesis, properties and biological applications. Mater. Sci. Eng. C 2020, 109, 110511. [Google Scholar] [CrossRef]
- Huang, H.; Du, C.; Shi, H.; Feng, X.; Li, J.; Tan, Y.; Song, W. Water-soluble monolayer molybdenum disulfide quantum dots with upconversion fluorescence. Part. Part. Syst. Charact. 2015, 32, 72–79. [Google Scholar] [CrossRef]
- Cao, H.; Dong, W.; Wang, T.; Shi, W.; Fu, C.; Wu, Y. Aptasensor Based on MoS2 Quantum Dots with Upconversion Fluorescence for Microcystin-LR Detection via the Inner Filter Effect. ACS Sustain. Chem. Eng. 2020, 8, 10939–10946. [Google Scholar] [CrossRef]
- Dos Santos, M.B.; Queirós, R.B.; Geraldes, Á.; Marques, C.; Vilas-Boas, V.; Dieguez, L.; Paz, E.; Ferreira, R.; Morais, J.; Vasconcelos, V. Portable sensing system based on electrochemical impedance spectroscopy for the simultaneous quantification of free and total microcystin-LR in freshwaters. Biosens. Bioelectron. 2019, 142, 111550. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Son, A. Fluorescence resonance energy transfer based quantum dot-Aptasensor for the selective detection of microcystin-LR in eutrophic water. Chem. Eng. J. 2019, 359, 1493–1501. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Zhang, H.; Wang, Z. Simultaneous detection of microcysin-LR and okadaic acid using a dual fluorescence resonance energy transfer aptasensor. Anal. Bioanal. Chem. 2015, 407, 1303–1312. [Google Scholar] [CrossRef]
- Han, C.; Doepke, A.; Cho, W.; Likodimos, V.; de la Cruz, A.A.; Back, T.; Heineman, W.R.; Halsall, H.B.; Shanov, V.N.; Schulz, M.J.; et al. A multiwalled-carbon-nanotube-based biosensor for monitoring microcystin-LR in sources of drinking water supplies. Adv. Funct. Mater. 2013, 23, 1807–1816. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, Y.; Dong, H.; Zhang, X.; Wang, W.; Chen, Z. An electrochemical non-enzymatic immunosensor for ultrasensitive detection of microcystin-LR using carbon nanofibers as the matrix. Sens. Actuators B Chem. 2016, 233, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Zhao, S.; Wu, S.; Wang, Z. Upconversion nanoparticles grafted molybdenum disulfide nanosheets platform for microcystin-LR sensing. Biosens. Bioelectron. 2017, 90, 203–209. [Google Scholar] [CrossRef]
- Pang, P.; Teng, X.; Chen, M.; Zhang, Y.; Wang, H.; Yang, C.; Yang, W.; Barrow, C.J. Ultrasensitive enzyme-free electrochemical immunosensor for microcystin-LR using molybdenum disulfide/gold nanoclusters nanocomposites as platform and Au@ Pt core-shell nanoparticles as signal enhancer. Sens. Actuators B Chem. 2018, 266, 400–407. [Google Scholar] [CrossRef]
- Zhao, C.; Hu, R.; Liu, T.; Liu, Y.; Bai, R.; Zhang, K.; Yang, Y. A non-enzymatic electrochemical immunosensor for microcystin-LR rapid detection based on Ag@ MSN nanoparticles. Colloids Surf. Physicochem. Eng. Asp. 2016, 490, 336–342. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, T.; Hao, Y.; Lu, L.; Cheng, S. Electrochemiluminescent immunosensor based on CdS quantum dots for ultrasensitive detection of microcystin-LR. Sens. Actuators B Chem. 2015, 214, 117–123. [Google Scholar] [CrossRef]
- Raymond, A. Serway. Principles of Physics, 2nd ed.; Saunders College Pub.: Fort Worth, TX, USA; London, UK, 1998; p. 602. [Google Scholar]
- Graphene Fiber, Method for Manufacturing Same and Use There of_(WO2011099761A2). Available online: https://patents.google.com/patent/WO2011099761A2/en (accessed on 16 December 2021).
- Yakovleva, G.E.; Berdinsky, A.S.; Romanenko, A.I.; Khabarov, S.P.; Fedorov, V.E. The conductivity and TEMF of MoS2 with Mo2S3 additive. In Proceedings of the 2015 38th International Convention on Information and Communication Technology, Electronics and Microelectronics (MIPRO), Opatija, Croatia, 25–29 May 2015; IEEE: Manhattan, NY, USA, 2015; pp. 12–14. [Google Scholar]


| Materials | Bioreceptors | Nanoparticle | Detection Method | Linear Range | References | |
|---|---|---|---|---|---|---|
| Metal-NP | Aptamer | AuNS | CV | 0–99.5 | 9.95 × 10−4 | [55] |
| Antibody | AuNP/PPyMS | LSV | 0.25–50 | 0.1 | [56] | |
| Aptamer | AuNP/MNP | SERS | 0.01–200 | 0.002 | [68] | |
| Aptamer | CuNC | Fluorescence | 0.005–1200 | 0.003 | [73] | |
| Aptamer | AuNP | Fluorescence | 0.25–19.90 | 0.83 | [79] | |
| Carbon-NP | Antibody | CNS | DPV | 0.05–15 | 0.016 | [85] |
| Antibody | CNx-MWNT/AuNP | DPV | 0.01–2 | 0.004 | [86] | |
| Antibody | GO/AuNP | FRET | 10−4 × 2.5 | 0.5 | [95] | |
| Antibody | MWCNT | EIS | 0.05–20 | 0.04 | [117] | |
| Antibody | CNF/AuNP | DPV | 0.0025–5 | 0.00168 | [118] | |
| TMD-NP | Aptamer | MoS2 | DPV | 0–199 | 1.99 × 10−3 | [108] |
| Antibody/antibody | MoS2/AuNRs | DPV | 0.01–20 | 0.005 | [109] | |
| Aptamer | MoS2 QD | Fluorescence | 19.90–43.8 × 103 | 9.95 × 10−3 | [113] | |
| Aptamer | MoS2 | Fluorescence | 0.01–50 | 0.02 | [119] | |
| Antibody/antibody | MoS2/AuNCs | DPV | 0.001–1000 | 3 × 10−4 | [120] | |
| Others | Aptamer | - | Fluorescence | 10–100 | 0.110 | [76] |
| Aptamer | fluorescence resonance energy transfer based QD | Fluorescence | 10−4–100 | 10.4 | [114] | |
| Aptamer | UCNP | Fluorescence | 0.1–50 | 25 × 10−6 | [115] | |
| Antibody | Ag@MSN | CA | 0.5–30 × 103 | 0.2 | [121] | |
| Antibody | Cds QD | ECL | 0.01–50 | 0.0028 | [122] |
| Materials | Features | Disadvantages |
|---|---|---|
| Noble metal-based nanomaterial | High stability and durability High conductivity (Ex 1. Ag (6.30 × 107 S/m et 20 °C)) (Ex 2. Au (4.11 × 107 S/m et 20 °C)) [123] | Expensive cost (Ex. Au nanopowder (USD 446 per 1 g)) |
| Carbon-based nanomaterial | Cheap cost Stable semiconducting property (Ex. Carbon nanopowder (USD 11 per 1 g)) | Easy to oxidize Low conductivity (Ex 1. carbon-nanotube) (3 × 107 S/m) (Ex 2. graphene (1.72 × 107 S/m)) [124] |
| Transition metal dichalcogenide-based nanomaterial | Unique conductivity (topological insulator) Unique chemical property | Hard to particulate Low conductivity MoS2 (10−1 to 101 S/m) [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Kim, G.; Seo, Y.; Yoon, Y.; Min, J.; Park, C.; Lee, T. Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes. Biosensors 2021, 11, 525. https://doi.org/10.3390/bios11120525
Park H, Kim G, Seo Y, Yoon Y, Min J, Park C, Lee T. Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes. Biosensors. 2021; 11(12):525. https://doi.org/10.3390/bios11120525
Chicago/Turabian StylePark, Hanbin, Gahyeon Kim, Yoseph Seo, Yejin Yoon, Junhong Min, Chulhwan Park, and Taek Lee. 2021. "Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes" Biosensors 11, no. 12: 525. https://doi.org/10.3390/bios11120525
APA StylePark, H., Kim, G., Seo, Y., Yoon, Y., Min, J., Park, C., & Lee, T. (2021). Improving Biosensors by the Use of Different Nanomaterials: Case Study with Microcystins as Target Analytes. Biosensors, 11(12), 525. https://doi.org/10.3390/bios11120525







