MoS2 QDs/8-Armed Poly(Ethylene Glycol) Fluorescence Sensor for Three Nitrotoluenes (TNT) Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Acryloyl Chloride Modified 8-Armed Polyethylene Glycol (PEGOA)
2.3. Preparation of Ammonium Tetrathiomolybdate Solution
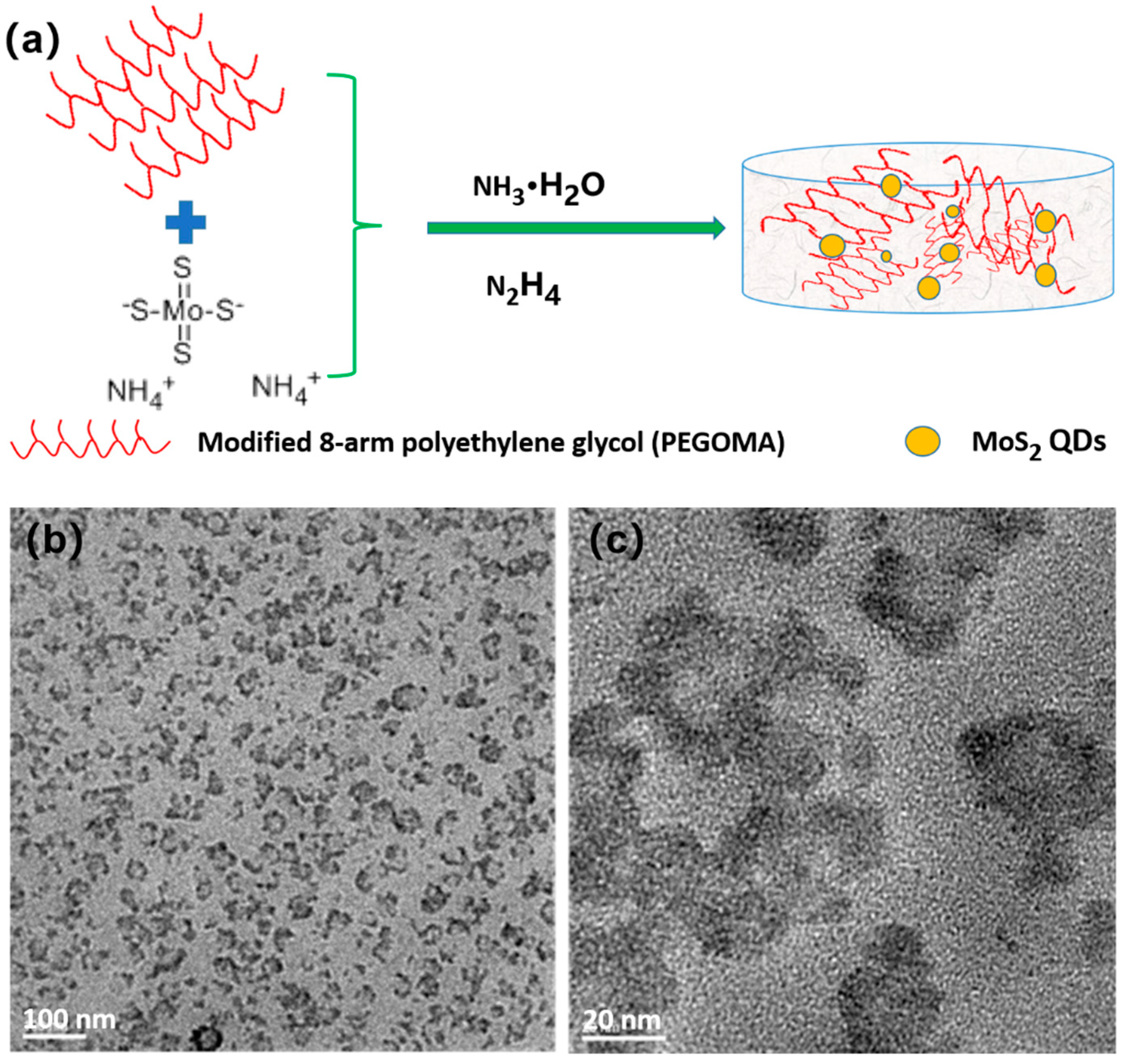
2.4. Synthesis of MoS2 QDs by 8-Armed Polyethylene Glycol Hydrogel Template Method
2.5. Instruments and Measurements
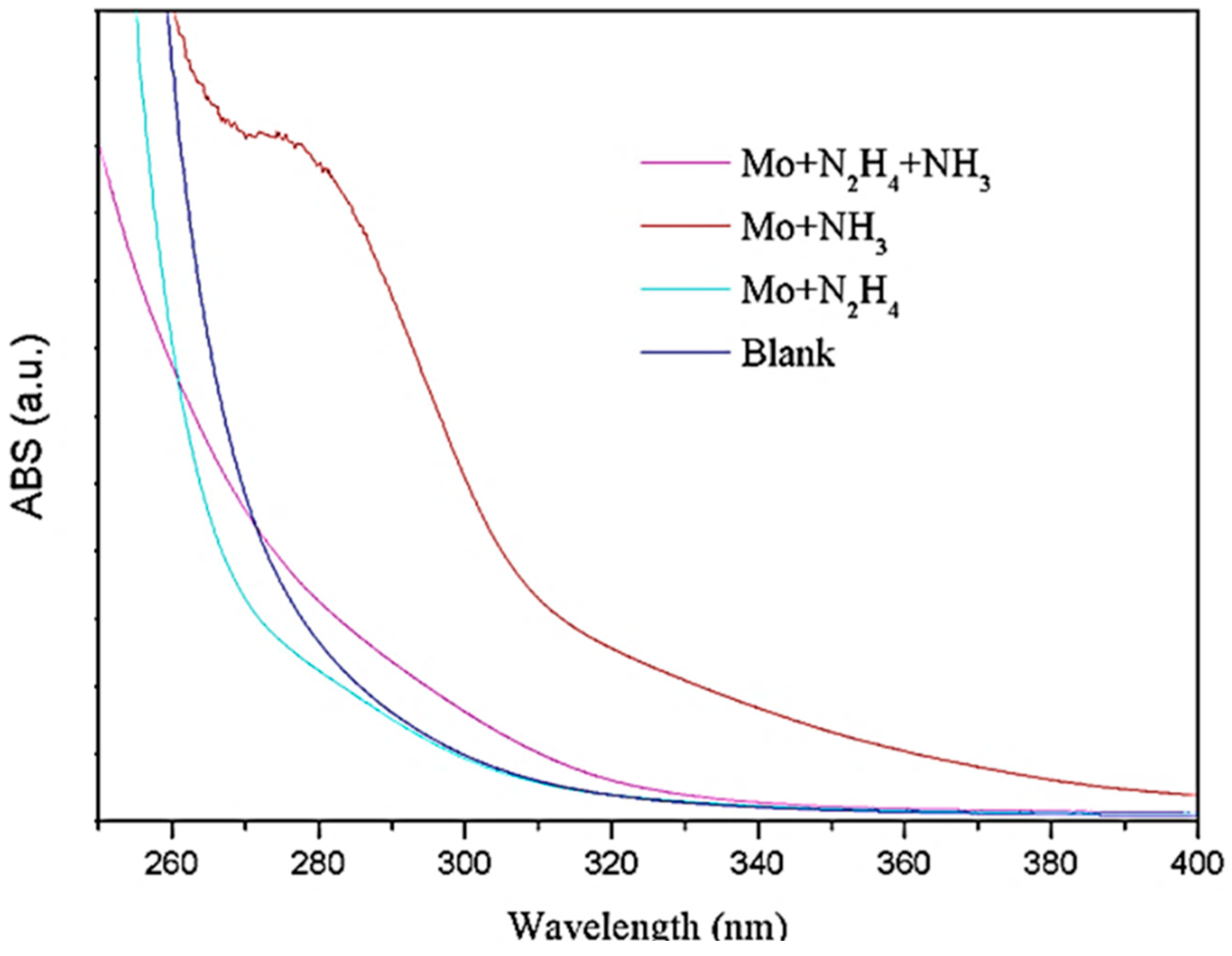
2.5.1. UV-Vis Spectroscopic Testing
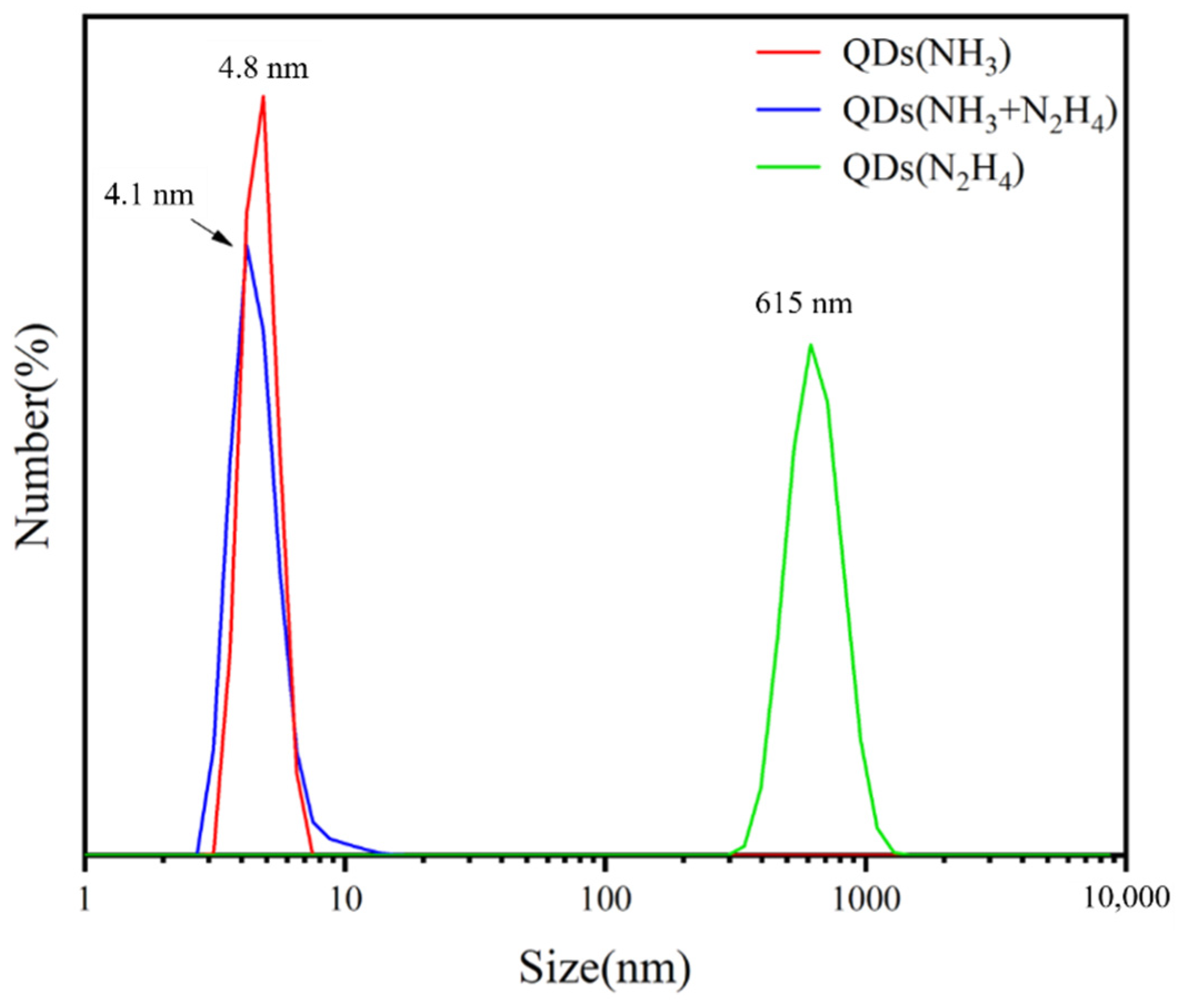
2.5.2. Dynamic Light Scattering (DLS) Testing
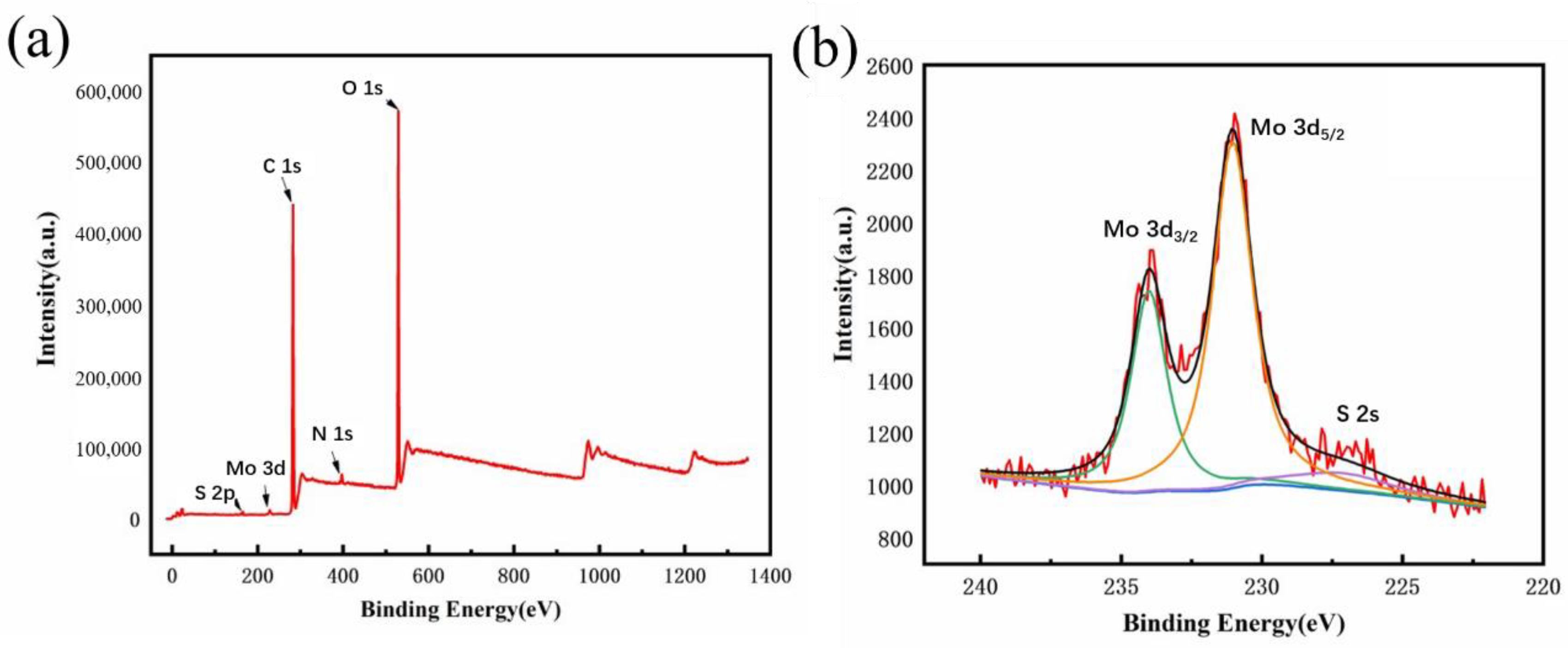
2.5.3. X-ray Photoelectron Spectroscopy (XPS)
2.5.4. Fluorescence Emission Spectra
2.5.5. High-Resolution Transmission Electron Microscopy (HR-TEM) Measurement
2.5.6. Fluorescence Detection of TNT
3. Results and Discussion
3.1. MoS2 QDs Fabrication with the PEGOA Template Method
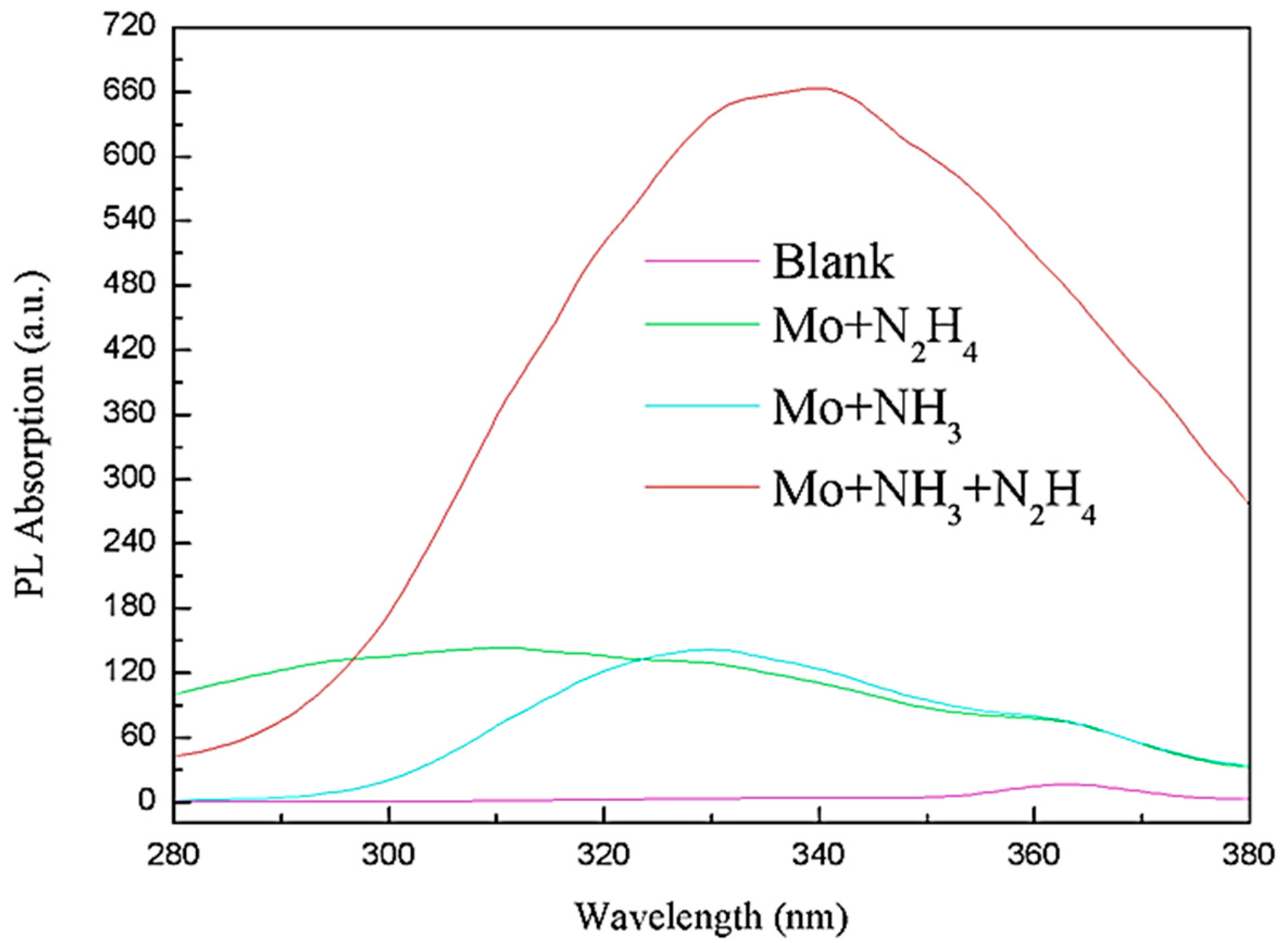
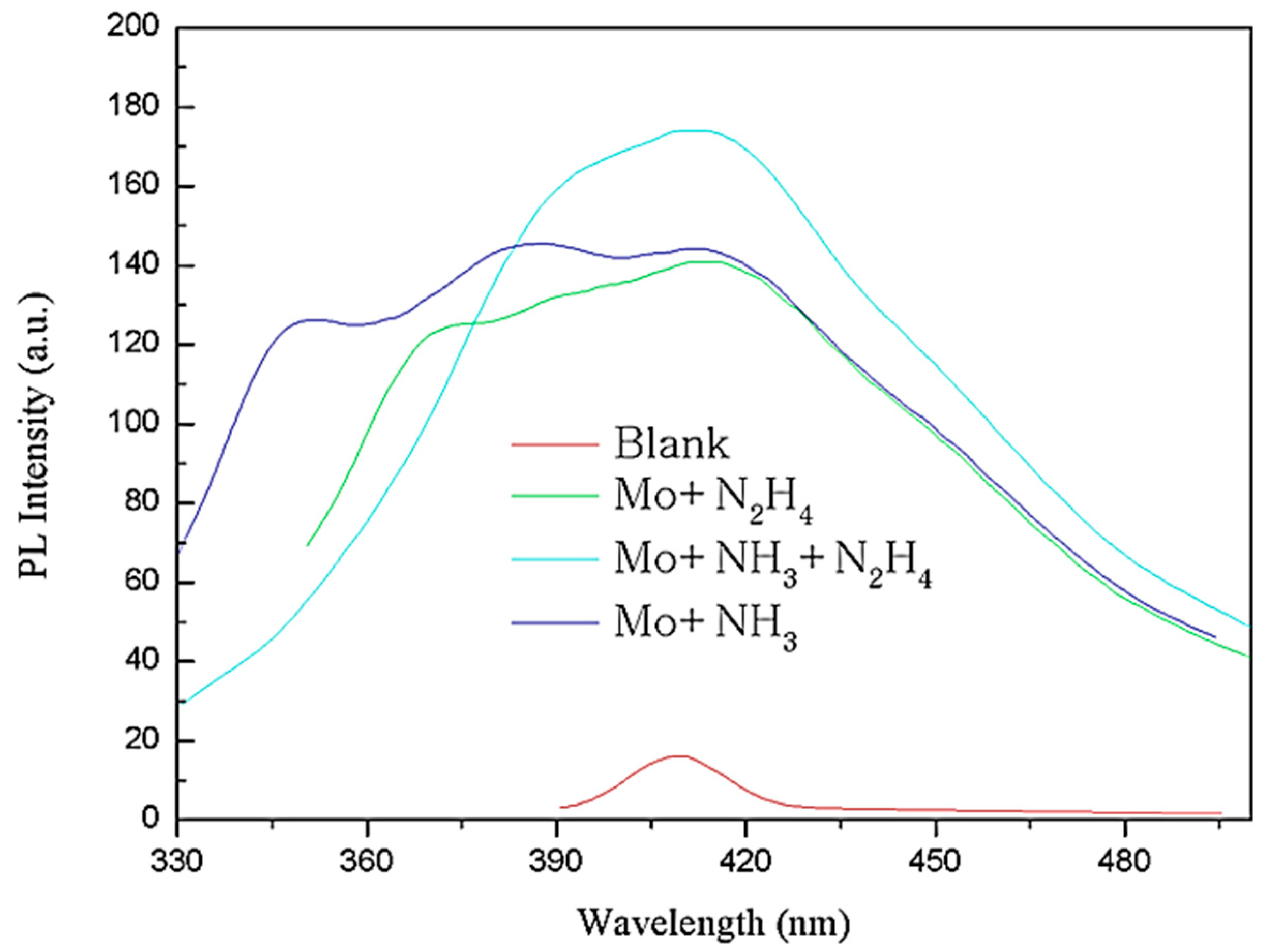
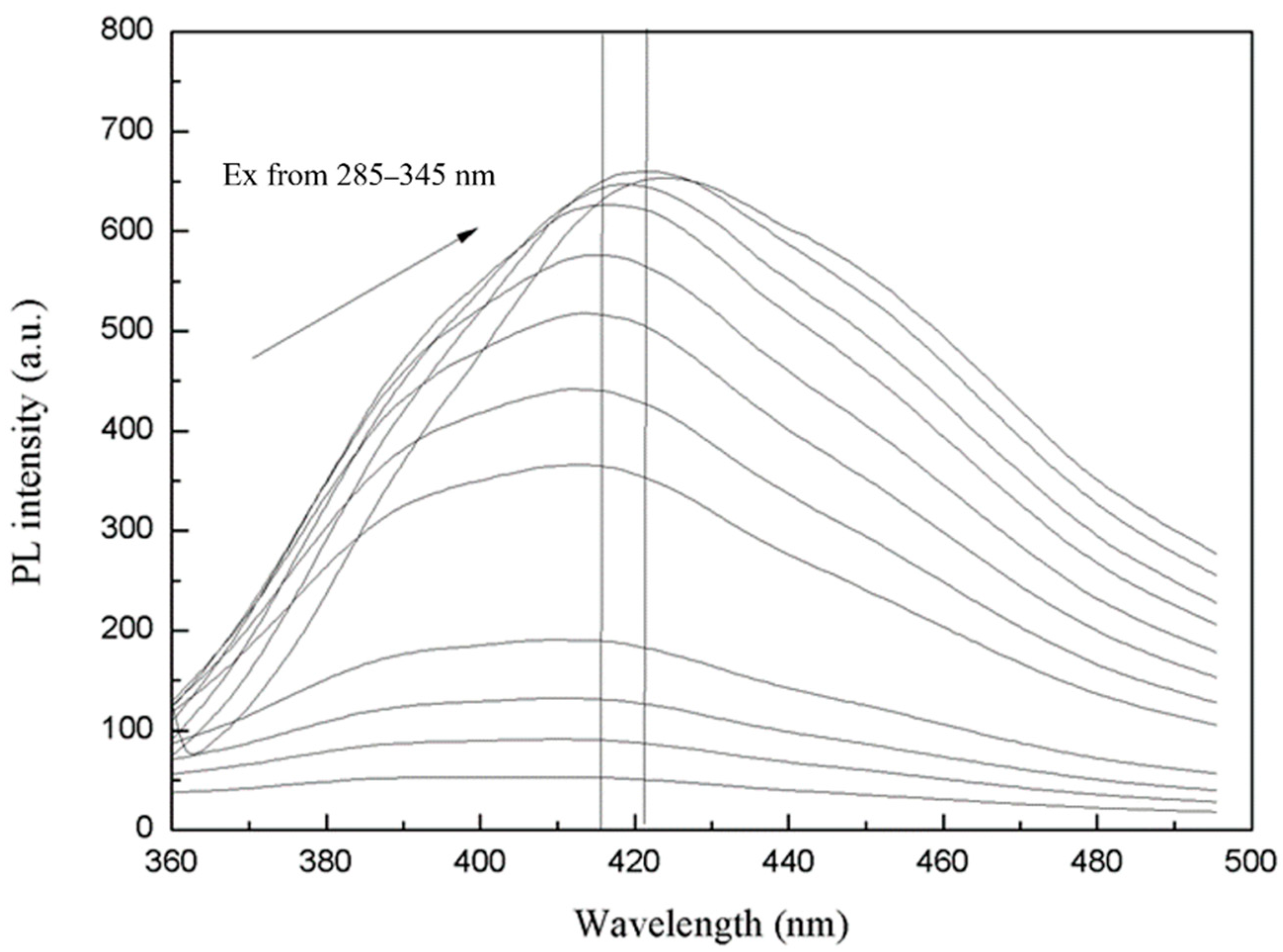
3.2. Photoluminescence Performance Analysis of MoS2 QDs
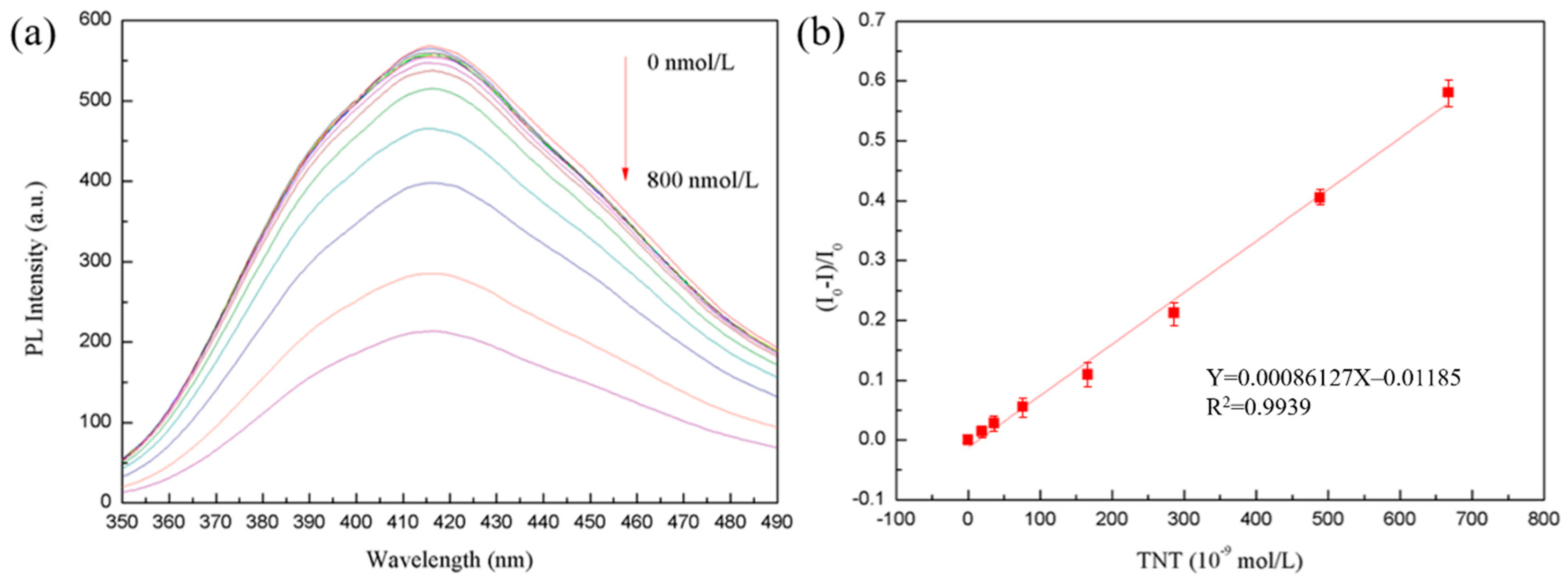
3.3. MoS2 QDs for Fluorescence Detection of TNT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopeček, J. Hydrogel Biomaterials: A Smart Future? Biomaterials 2007, 34, 5185–5192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takuro, M.; Sakai, T.; Akagi, Y.; Chung, U.; Shibayama, M. Sans and Sls Studies on Tetra-Arm Peg Gels in as-Prepared and Swollen States. Macromolecules 2009, 16, 6245–6252. [Google Scholar]
- Novoselov, K.S.; McCann, E.; Morozov, S.V.; Fal’ko, V.I.; Katsnelson, M.I.; Zeitler, U.; Jiang, D.; Schedin, F.; Geim, A.K. Unconventional Quantum Hall Effect and Berry’s Phase of 2π in Bilayer Graphene. Nat. Phys. 2006, 2, 177–180. [Google Scholar] [CrossRef]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Munoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.C.; et al. Graphene and Graphite Nanoribbons: Morphology, Properties, Synthesis, Defects and Applications. Nano Today 2010, 4, 351–372. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication Technologies and Sensing Applications of Graphene-Based Composite Films: Advances and Challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Zhang, L.; Li, J.; Su, Z.; Wei, G. Sequence-Designed Peptide Nanofibers Bridged Conjugation of Graphene Quantum Dots with Graphene Oxide for High Performance Electrochemical Hydrogen Peroxide Biosensor. Adv. Mater. Interfaces 2017, 3, 1600895. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When Biomolecules Meet Graphene: From Molecular Level Interactions to Material Design and Applications. Nanoscale 2016, 47, 19491–19509. [Google Scholar] [CrossRef]
- Tzalenchuk, A.; Lara-Avila, S.; Kalaboukhov, A.; Paolillo, S.; Syväjärvi, M.; Yakimova, R.; Kazakova, O.; Janssen, T.J.; Fal’Ko, V.; Kubatkin, S. Towards a Quantum Resistance Standard Based on Epitaxial Graphene. Nat. Nanotechnol. 2010, 3, 186–189. [Google Scholar] [CrossRef]
- Gutiérrez, H.R.; Perea-López, N.; Elías, A.L.; Berkdemir, A.; Wang, B.; Lv, R.; López-Urías, F.; Crespi, V.H.; Terrones, H.; Terrones, M. Extraordinary Room-Temperature Photoluminescence in Triangular WS2 Monolayers. Nano Lett. 2013, 8, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Ho, W.; Yu, J.C.; Lin, J.; Yu, J.; Li, P. Preparation and Photocatalytic Behavior of MoS2 and WS2 Nanocluster Sensitized TiO2. Langmuir 2004, 14, 5865–5869. [Google Scholar] [CrossRef]
- Splendiani, A.; Sun, L.; Zhang, Y.; Li, T.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 4, 1271–1275. [Google Scholar] [CrossRef]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 12, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 6017, 568–571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Štengl, V.; Henych, J. Strongly Luminescent Monolayered MoS2 Prepared by Effective Ultrasound Exfoliation. Nanoscale 2013, 8, 3387–3394. [Google Scholar] [CrossRef]
- Lin, T.W.; Dhenadhayalan, N.; Lee, H.L.; Lin, Y.T.; Lin, K.C.; Chang, A.H. Fluorescence Turn-on Chemosensors Based on Surface-Functionalized MoS2 Quantum Dots. Sens. Actuators B Chem. 2019, 281, 659–669. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, X.; Jiang, Y.; Sun, W.; Wang, C.; Yang, M.; Zhang, C. MoS2-Qd-Based Dual-Model Photoluminescence Sensing Platform for Effective Determination of Al3+ and Fe3+ Simultaneously in Various Environment. ChemistrySelect 2018, 8, 2326–2331. [Google Scholar] [CrossRef]
- Roy, S.; Bobde, Y.; Ghosh, B.; Chakraborty, C. Targeted Bioimaging of Cancer Cells Using Free Folic Acid-Sensitive Molybdenum Disulfide Quantum Dots through Fluorescence “Turn-Off”. ACS Appl. Bio Mater. 2021, 3, 2839–2849. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, Y.; Gao, T.; Zhang, Y.; Ma, D.; Liu, M.; Chen, Y.; Qiao, X.; Tan, P.; Kan, M.; et al. Epitaxial Monolayer MoS2 on Mica with Novel Photoluminescence. Nano Lett. 2013, 8, 3870–3877. [Google Scholar] [CrossRef]
- Lu, X.; Utama, M.I.B.; Lin, J.; Gong, X.; Zhang, J.; Zhao, Y.; Pantelides, S.T.; Wang, J.; Dong, Z.; Liu, Z.; et al. Large-Area Synthesis of Monolayer and Few-Layer MoSe2 Films on SiO2 Substrates. Nano Lett. 2014, 5, 2419–2425. [Google Scholar] [CrossRef]
- Haldar, D.; Dinda, D.; Saha, S.K. High Selectivity in Water Soluble MoS2 Quantum Dots for Sensing Nitro Explosives. J. Mater. Chem. C 2016, 26, 6321–6326. [Google Scholar] [CrossRef]
- Hakey, P.; Ouellette, W.; Zubieta, J.; Korter, T. Redetermination of Cyclo-Trimethylenetrinitramine. Acta Crystallogr. Sect. E 2008, 8, o1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyman, J.F.; Serve, M.P.; Hobson, D.W.; Lee, L.H.; Uddin, D.E. Acute Toxicity, Distribution, and Metabolism of 2,4,6-Trinitrophenol (Picric Acid) in Fischer 344 Rats. J. Toxicol. Environ. Health 1992, 2, 313–327. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 12, 8208–8271. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wei, G.; Su, Z. One-Pot, In-Situ Synthesis of 8-Armed Poly(Ethylene Glycol)-Coated Ag Nanoclusters as a Fluorescent Sensor for Selective Detection of Cu2+. Biosensors 2020, 10, 131. [Google Scholar] [CrossRef]
- Zhang, Z.; Loebus, A.; de Vicente, G.; Ren, F.; Arafeh, M.; Ouyang, Z.; Lensen, M.C. Synthesis of Poly(Ethylene Glycol)-Based Hydrogels Via Amine-Michael Type Addition with Tunable Stiffness and Postgelation Chemical Functionality. Chem. Mater. 2014, 12, 3624–3630. [Google Scholar] [CrossRef]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Functional Peg-Hydrogels Convey Gold Nanoparticles from Silicon and Aid Cell Adhesion onto the Nanocomposites. Chem. Mater. 2017, 5, 2008–2015. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, Y. Molybdenum Disulfide Quantum Dots as a Photoluminescence Sensing Platform for 2,4,6-Trinitrophenol Detection. Anal. Chem. 2014, 15, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, H. Mesoporous structured MIPs@CDs fluorescence sensor for highly sensitive detection of TNT. Biosens. Bioelectron. 2016, 15, 7463–7470. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, H. Ratiometric fluorescence and mesoporous structure dual signal amplification for sensitive and selective detection of TNT based on MIP@QD fluorescence sensors. Chem. Commun. 2015, 51, 3200–3203. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Su, Z. MoS2 QDs/8-Armed Poly(Ethylene Glycol) Fluorescence Sensor for Three Nitrotoluenes (TNT) Detection. Biosensors 2021, 11, 475. https://doi.org/10.3390/bios11120475
Zhang X, Su Z. MoS2 QDs/8-Armed Poly(Ethylene Glycol) Fluorescence Sensor for Three Nitrotoluenes (TNT) Detection. Biosensors. 2021; 11(12):475. https://doi.org/10.3390/bios11120475
Chicago/Turabian StyleZhang, Xiaoyuan, and Zhiqiang Su. 2021. "MoS2 QDs/8-Armed Poly(Ethylene Glycol) Fluorescence Sensor for Three Nitrotoluenes (TNT) Detection" Biosensors 11, no. 12: 475. https://doi.org/10.3390/bios11120475
APA StyleZhang, X., & Su, Z. (2021). MoS2 QDs/8-Armed Poly(Ethylene Glycol) Fluorescence Sensor for Three Nitrotoluenes (TNT) Detection. Biosensors, 11(12), 475. https://doi.org/10.3390/bios11120475





