Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enzyme Assay
2.3. Preparation of Biosensors and Electrochemical Measurements
2.4. Enzymatic Synthesis of D-Tagatose
2.5. AFM Measurement
3. Results and Discussion
3.1. Characterization of Electrode Surfaces
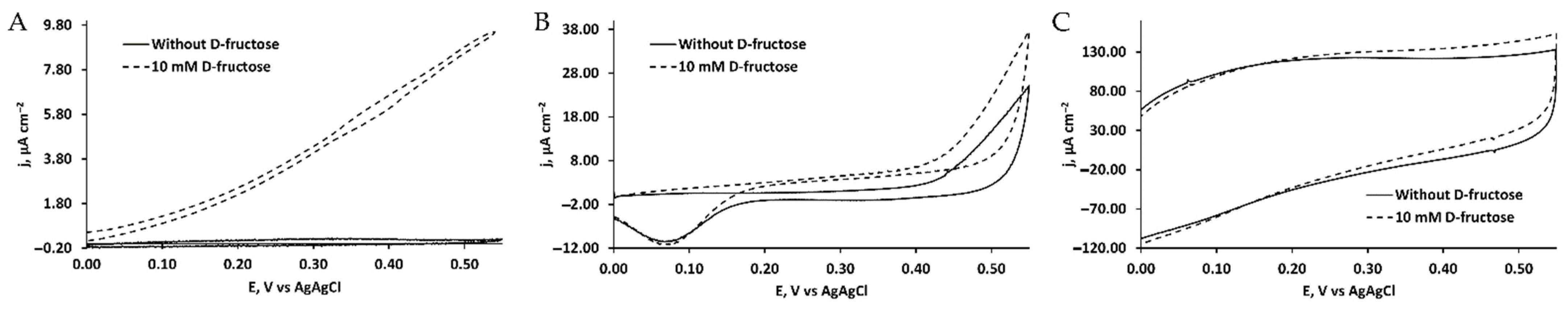
3.2. Bioelectrocatalytic Properties of AuNP/4-MBA/FDH, AuNP/PATP/FDH and TRGO/FDH
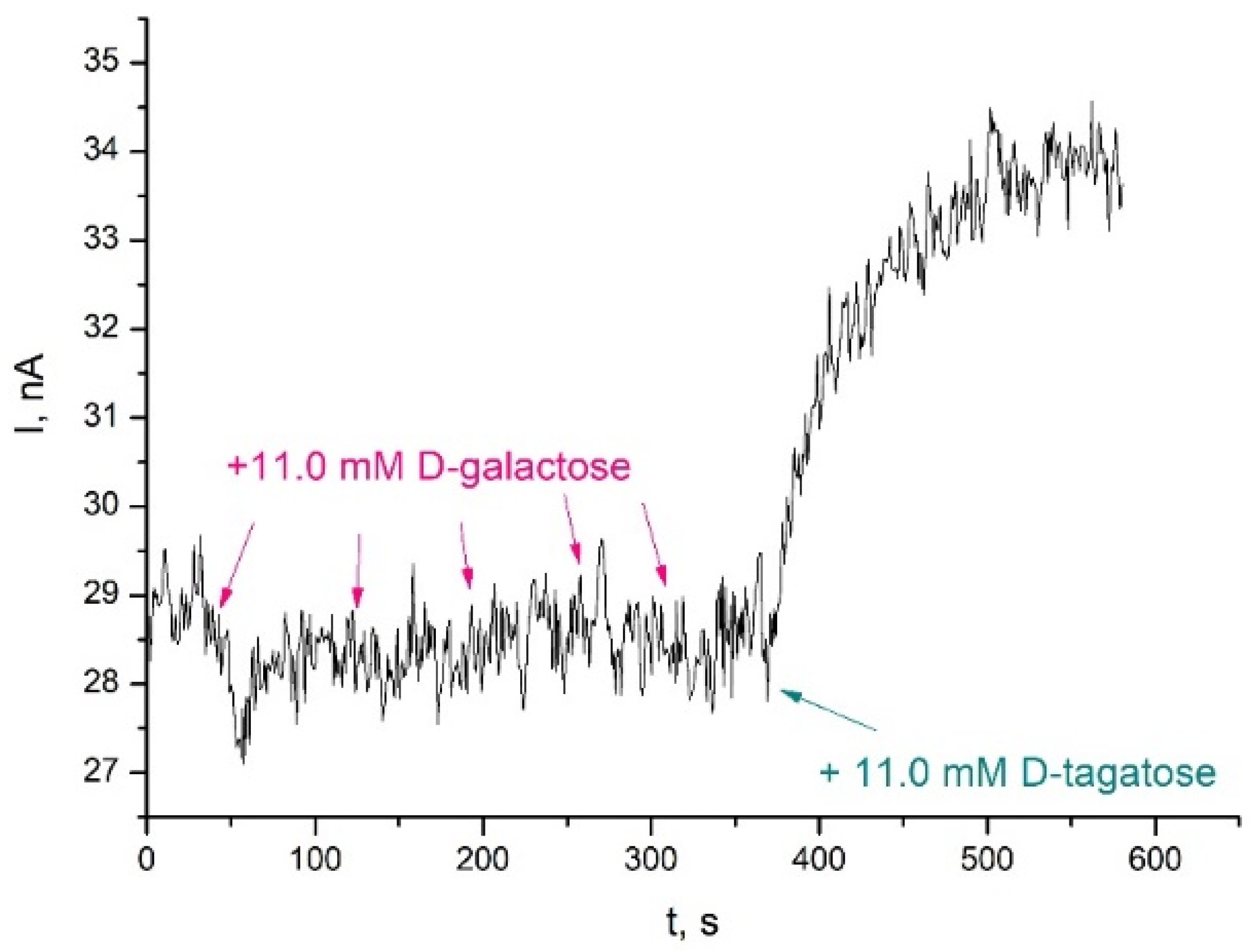
3.3. Analysis of Stability and Selectivity of TRGO/FDH
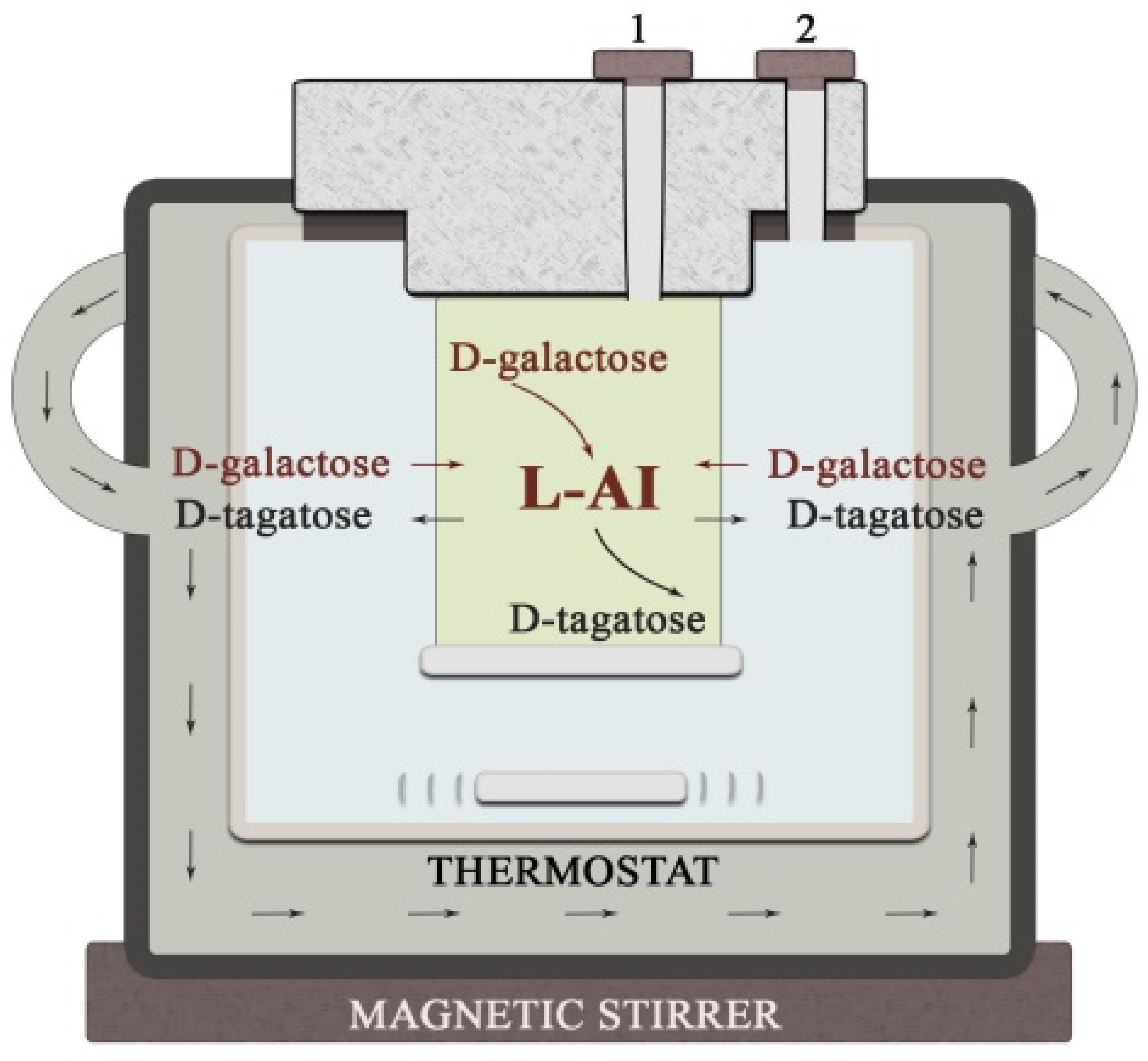
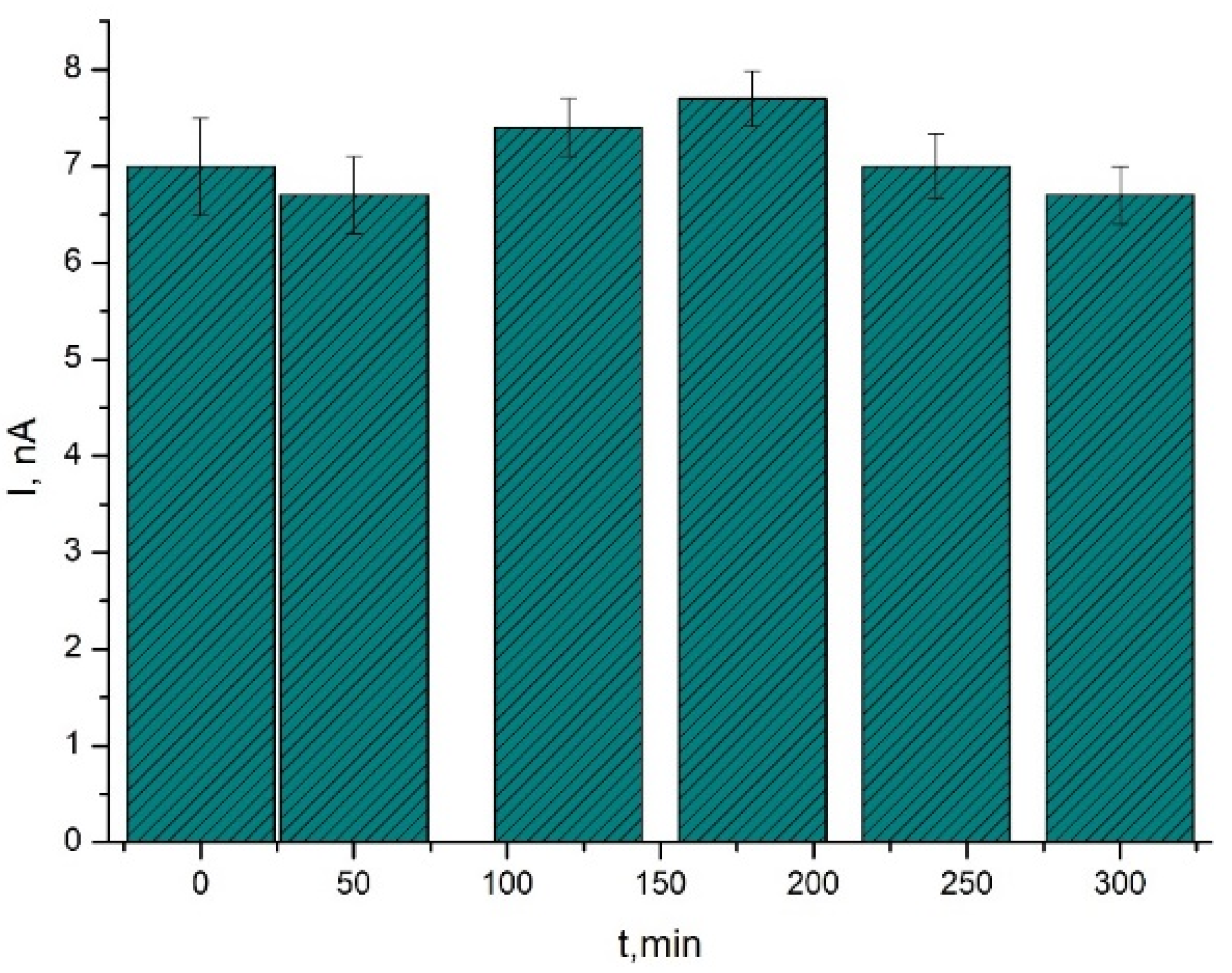
3.4. Application of the Biosensor in D-galactose Bioconversion Reactor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grattieri, M.; Hasan, K.; Minteer, S.D. Bioelectrochemical systems as a multipurpose biosensing tool: Present perspective and future outlook. ChemElectroChem 2017, 4, 834–842. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Dzyadevych, S.V.; Arkhypova, V.N.; Soldatkin, A.P.; El’skaya, A.V.; Martelet, C.; Jaffrezic-Renault, N. Amperometric enzyme biosensors: Past, present and future. ITBM-RBM 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Rinken, T. (Ed.) Biosensors for Environmental Monitoring; IntechOpen: London, UK, 2019. [Google Scholar]
- Jeuken, L.J.C. Structure and modification of electrode materials for protein electrochemistry. In Advances in Biochemical Engineering/Biotechnology; Lars, J.C., Ed.; Springer: Cham, Switzerland, 2016; Volume 158. [Google Scholar]
- Edwards, G.A.; Bergren, A.J.; Porte, M.D. Chemically Modified Electrodes. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 295–327. [Google Scholar]
- MacCarter, D.J.; Lundberg, K.M.; Corstjens, J.P. Porous electrodes: Concept, technology and results. Pacing Clin. Electrophysiol. 1983, 2, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, P. Immobilized biocatalysts. Catalysts 2018, 8, 386. [Google Scholar] [CrossRef] [Green Version]
- Laurila, T.; Sainio, S.; Caro, M.A. Hybrid carbon based nanomaterials for electrochemical detection of biomolecules. Prog. Mater. Sci. 2017, 88, 499–594. [Google Scholar] [CrossRef]
- Ratautas, D.; Dagys, M. Nanocatalysts containing direct electron transfer-capable oxidoreductases: Recent advances and applications. Catalysts 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Vera, C.; Guerrero, C.; Aburto, C.; Cordova, A.; Illanes, A. Conventional and non-conventional applications of β-galactosidases. BBA Proteins Proteom. 2020, 1868, 140271. [Google Scholar] [CrossRef]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed Bioethanol Production: A Process Selection Review on Hydrolysis and Fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef] [Green Version]
- Del Río, P.G.; Gomes-Dias, J.S.; Rocha, C.M.R.; Romaní, A.; Garrote, G.; Domingues, L. Recent trends on seaweed fractionation for liquid biofuels production. Bioresour. Technol. 2020, 299, 122613. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Guo, L.; Wang, K.; Liu, Y.; Xiao, M. β-Galactosidases: A great tool for synthesizing. Biotechnol. Adv. 2020, 39, 107465. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, S.; Fu, F.; Li, G.; Feng, X.; Xu, H.; Ouyang, P. Production of D-tagatose, a functional sweetener, utilizing alginate immobilized Lactobacillus fermentum CGMCC2921 cells. Appl. Biochem. Biotechnol. 2012, 166, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, F.; Hansen, O.C.; Stougaard, P. Enzymatic conversion of D-galactose to D-tagatose: Heterologous expression and characterisation of a thermostable L-arabinose isomerase from Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2004, 64, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Patra, F.; Tomar, S.K.; Arora, S. Technological and functional applications of low-calorie sweeteners from lactic acid bacteria. J. Food Sci. 2009, 74, R16–R23. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, M.; Rollini, M.; Bergomi, S. Biotransformation of D-galactitol to tagatose by acetic acid bacteria. Process Biochem. 2001, 36, 971–977. [Google Scholar] [CrossRef]
- Beadle, J.R.; Saunder, J.P.; Wajada, T.J. Process for Manufacturing Tagatose. Biospherics Inc. U.S. Patent 5002612, 26 March 1991. [Google Scholar]
- Mena, Y.; Zhu, Y.; Zhang, L.; Kang, Z.; Izumori, K.; Sun, Y.; Ma, Y. Enzymatic conversion of D-galactose to D-tagatose: Cloning, overexpression and characterization of L-arabinose isomerase from Pediococcus pentosaceus PC-5. Microbiol. Res. 2014, 169, 171–178. [Google Scholar] [CrossRef]
- Liang, M.; Chen, M.; Liu, X.; Zhai, Y.; Liu, X.W.; Zhang, H.; Xiao, M.; Wang, P. Bioconversion of D-galactose to D-tagatose: Continuous packed bed reaction with an immobilized thermostable L-arabinose isomerase and efficient purification by selective microbial degradation. Appl. Microbiol. Biotechnol. 2012, 93, 1469–1474. [Google Scholar] [CrossRef]
- Rhimia, M.; Chouayekh, H.; Gouillouard, I.; Maguin, E.; Bejar, S. Production of D-tagatose, a low caloric sweetener during milk fermentation using L-arabinose isomerase. Bioresour. Technol. 2011, 102, 3309–3315. [Google Scholar] [CrossRef]
- Oh, H.J.; Kim, P.; Park, Y.C.; Choi, J.H. Bioconversion of D-galactose into D-tagatose by expression of L-arabinose isomerase. Biotechnol. Appl. Biochem. 2000, 31, 1–4. [Google Scholar]
- Bober, J.R.; Nair, N.U. Galactose to tagatose isomerization at moderate temperatures with high conversion and productivity. Nat. Commun. 2019, 10, 4548. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Melo, V.M.M.; Hissa, D.C.; Manzo, R.M.; Mammarella, E.J.; Antunes, A.S.L.M.; García, J.L.; Pessela, B.C.; Gonçalves, L.R.B. One-step immobilization and stabilization of a recombinant Enterococcus faecium DBFIQ E36 L-arabinose isomerase for D-tagatose synthesis. App. Biochem. Biotech. 2019, 188, 310–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.; Zhao, D.; Cheng, S.; Sun, D.; Chen, M.; Gao, Z.; Zhang, C. Towards efficient enzymatic conversion of D-galactose to D-tagatose: Purification and characterization of L-arabinose isomerase from Lactobacillus brevis. Bioproc. Biosyst. Eng. 2019, 42, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Manzo, R.M.; Antunes, A.S.L.M.; Mendes, J.S.; Hissa, D.C.; Gonҫalves, L.R.B.; Mammarella, E.J. Biochemical characterization of heat-tolerant recombinant L-arabinose isomerase from Enterococcus faecium DBFIQ E36 strain with feasible applications in D-tagatose production. Mol. Biotechnol. 2019, 61, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Kulka, R.G. Colorimetric estimation of ketopentoses and ketohexoses. Biochem. J. 1956, 63, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Nagy, G.; Pohl, N.L.B. Complete hexose isomer identification with mass spectrometry. J. Am. Soc. Mass Spectr. 2015, 26, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Idda, I.; Spano, N.; Ciulu, M.; Nurchi, V.M.; Panzanelli, A.; Pilo, M.I.; Sanna, G. Gas chromatography analysis of major free mono- and disaccharides in milk: Method assessment, validation, and application to real samples. J. Sep. Sci. 2016, 39, 4577–4584. [Google Scholar] [CrossRef] [PubMed]
- Montañés, F.; Fornari, T.; Martín-Álvarez, P.J.; Corzo, N.; Olano, A.; Ibáñez, E. Selective recovery of tagatose from mixtures with galactose by direct extraction with supercritical CO2 and different cosolvents. J. Agric. Food Chem. 2006, 54, 8340–8345. [Google Scholar] [CrossRef]
- Bollella, P.; Gorton, L. Enzyme based amperometric biosensors. Curr. Opin. Electrochem. 2018, 10, 157–173. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Development perspective of bioelectrocatalysis-based biosensors. Sensors 2020, 20, 4826. [Google Scholar] [CrossRef]
- Levin, G.V. Tagatose, the new GRAS sweetener and health product. J. Med. Food 2002, 5, 23–36. [Google Scholar] [CrossRef]
- Grant, L.D.; Bell, L.N. Physical and chemical stability of tagatose powder. J. Food Sci. 2012, 77, C308–C313. [Google Scholar] [CrossRef] [PubMed]
- Siepenkoetter, T.; Salaj-Kosla, U.; Magner, E. The Immobilization of fructose dehydrogenase on nanoporous gold electrodes for the detection of fructose. Chem. Electro. Chem. 2017, 4, 905–912. [Google Scholar] [CrossRef]
- Tsujimura, S.; Nishina, A.; Hamano, Y.; Kano, K.; Shiraishi, S. Electrochemical reaction of fructose dehydrogenase on carbon cryogel electrodes with controlled pore sizes. Electrochem. Commun. 2010, 12, 446–449. [Google Scholar] [CrossRef]
- Hamano, Y.; Tsujimura, S.; Shirai, O.; Kano, K. Micro-cubic monolithic carbon cryogel electrode for direct electron transfer reaction of fructose dehydrogenase. Bioelectrochemistry 2012, 88, 114–117. [Google Scholar] [CrossRef]
- Voitechovič, E.; Stankevičiūtė, J.; Vektarienė, A.; Vektaris, G.; Jančienė, R.; Kuisienė, N.; Razumienė, J.; Meškys, R. Bioamperometric systems with fructose dehydrogenase from Gluconobacter japonicus for D-tagatose monitoring. Electroanalysis 2021, 33, 1393–1397. [Google Scholar] [CrossRef]
- Tkac, J.; Svitel, J.; Vostiar, I.; Navratil, M.; Gemeiner, P. Membrane-bound dehydrogenases from Gluconobacter sp.: Interfacial electrochemistry and direct bioelectrocatalysis. Bioelectrochemistry 2009, 76, 53–62. [Google Scholar] [CrossRef]
- Kawai, S.; Yakushi, T.; Matsushita, K.; Kitazumi, Y.; Shirai, O.; Kano, K. Role of a non-ionic surfactant in direct electron transfer-type bioelectrocatalysis by fructose dehydrogenase. Electrochim. Acta 2015, 152, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Šakinytė, I.; Barkauskas, J.; Gaidukevič, J.; Razumienė, J. Thermally reduced graphene oxide: The study and use for reagentless amperometric D-fructose biosensors. Talanta 2015, 144, 1096–1103. [Google Scholar] [CrossRef]
- Malinin, A.S.; Rakhnyanskaya, A.A.; Bacheva, A.V.; Yaroslavov, A.A. Activity of an enzyme immobilized on polyelectrolyte multilayers. Polym. Sci. Ser. A 2011, 53, 52–56. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV−Vis spectra. Anal. Chem. 2007, 79, 4215–4422. [Google Scholar] [CrossRef]
- Dishe, Z.; Broenfreund, E. A new spectrophotometric method for the detection of keto sugars and trioses. J. Biol. Chem. 1951, 192, 583–587. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ivanauskas, F.; Kaunietis, I.; Laurinavičius, V.; Razumienė, J.; Šimkus, R. Apparent Michaelis constant of the enzyme modified porous electrode. J. Math. Chem. 2008, 43, 1516–1526. [Google Scholar] [CrossRef]
- Adachi, T.; Kitazumi, Y.; Shirai, O.; Kano, K. Bioelectrocatalytic Performance of d-Fructose Dehydrogenase. Bioelectrochemistry 2019, 129, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Shirakihara, C.; Taniguchi, I. Direct heterogeneous electron transfer reactions and molecular orientation of fructose dehydrogenase adsorbed onto pyrolytic graphite electrodes. J. Electroanal. Chem. 2007, 610, 1–8. [Google Scholar] [CrossRef]
- Matsumura, H.; Ortiz†, R.; Ludwig, R.; Igarashi, K.; Samejima, M.; Gorton, L. Direct electrochemistry of Phanerochaete chrysosporium cellobiose dehydrogenase covalently attached onto gold nanoparticle modified solid gold electrodes. Langmuir 2012, 28, 10925–10933. [Google Scholar] [CrossRef]
- Tasca, F.; Harreither, W.; Ludwig, R.; Gooding, J.J.; Gorton, L. Cellobiose dehydrogenase aryl diazonium modified single walled carbon nanotubes: Enhanced direct electron transfer through a positively charged surfacefederico. Anal. Chem. 2011, 83, 3042–3049. [Google Scholar] [CrossRef]
- Yan, X.; Ma, S.; Tang, J.; Tanner, D.; Ulstrup, J.; Xiao, X.; Zhang, J. Direct electron transfer of fructose dehydrogenase immobilized on thiol-gold electrodes. Electrochim. Acta 2021, 392, 138946. [Google Scholar] [CrossRef]
- Toyobo Enzymes, D-Fructose Dehydrogenase from Gluconobacter sp. Available online: https://www.toyobo-global.com/seihin/xr/enzyme/pdf_files/201707/FCD_302.pdf (accessed on 7 April 2021).
- Bulska, E. Metrology in Chemistry (Lecture Notes in Chemistry 101), 1st ed.; Springer: Warsaw, Poland, 2018. [Google Scholar]
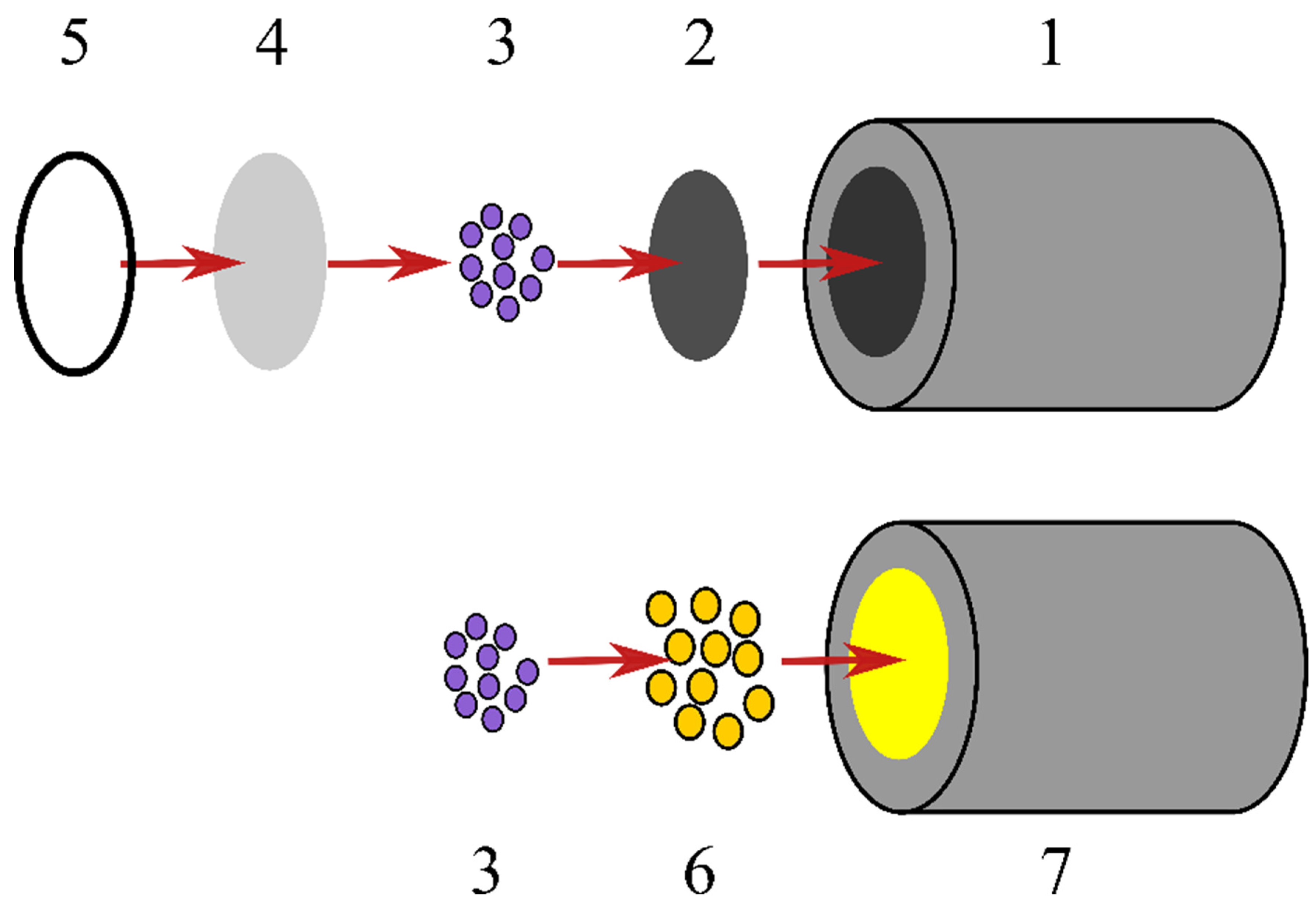



| TRGO/FDH | AuNP/4-MBA/FDH | AuNP/PATF/FDH | |
|---|---|---|---|
| Liner range (D-tagatose), mM | 4.4 *–32.3 | 5.4 *–19.3 | 5.4 *–29.5 |
| Sensitivity (D-tagatose), μA/mMcm2 | 0.030 ± 0.002 | 0.019 ± 0.002 | 0.025 ± 0.001 |
| (D-fructose), mM | 8.1 ± 0.2 | 9.9 ± 0.6 | 24.8 ± 1.5 |
| (D-tagatose), mM | 65 ± 10 | 86 ± 13 | 210 ± 20 |
| Specificity (D-tagatose),% | 0.33 ± 0.08 | 0.68 ± 0.09 | 1.1 ± 0.1 |
| Duration of Bioconversion, h | D-Tagatose Formed, mM | |
|---|---|---|
| Amperometric Biosensor | Spectrophotometric Analysis | |
| 0 | 0.0 | 0.0 |
| 10 | 36.3 ± 1.2 | 36.7 ± 2.8 |
| 20 | 63.6 ± 3.8 | 54.3 ± 2.4 |
| 30 | 81.7 ± 2.8 | 80.0 ± 4.7 |
| 40 | 87.2 ± 6.4 | 92.9 ± 6.5 |
| 50 | 93.5 ± 2.9 | 95.3 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šakinytė, I.; Butkevičius, M.; Gurevičienė, V.; Stankevičiūtė, J.; Meškys, R.; Razumienė, J. Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor. Biosensors 2021, 11, 466. https://doi.org/10.3390/bios11110466
Šakinytė I, Butkevičius M, Gurevičienė V, Stankevičiūtė J, Meškys R, Razumienė J. Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor. Biosensors. 2021; 11(11):466. https://doi.org/10.3390/bios11110466
Chicago/Turabian StyleŠakinytė, Ieva, Marius Butkevičius, Vidutė Gurevičienė, Jonita Stankevičiūtė, Rolandas Meškys, and Julija Razumienė. 2021. "Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor" Biosensors 11, no. 11: 466. https://doi.org/10.3390/bios11110466
APA StyleŠakinytė, I., Butkevičius, M., Gurevičienė, V., Stankevičiūtė, J., Meškys, R., & Razumienė, J. (2021). Reagentless D-Tagatose Biosensors Based on the Oriented Immobilization of Fructose Dehydrogenase onto Coated Gold Nanoparticles- or Reduced Graphene Oxide-Modified Surfaces: Application in a Prototype Bioreactor. Biosensors, 11(11), 466. https://doi.org/10.3390/bios11110466






