Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles
Abstract
1. Introduction
2. Characteristics of Bioparticles
2.1. Nucleotide Chain
2.2. Protein
2.3. Extracellular Vesicle (Exosome)
2.4. Virus
2.5. Bacterial Cells
2.6. Blood Cells
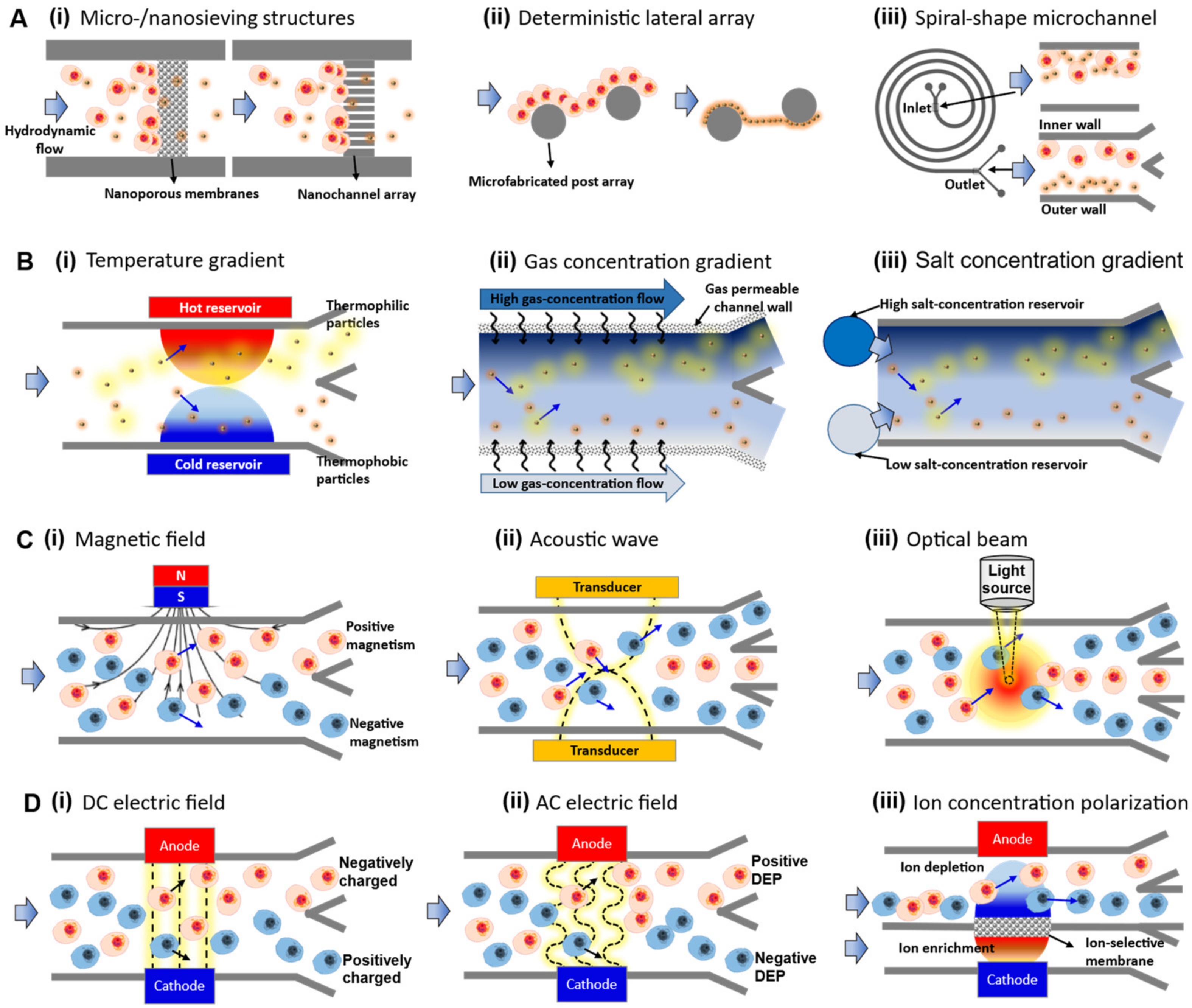
3. Passive Separation Group 1: Hydrodynamics-Based Separation
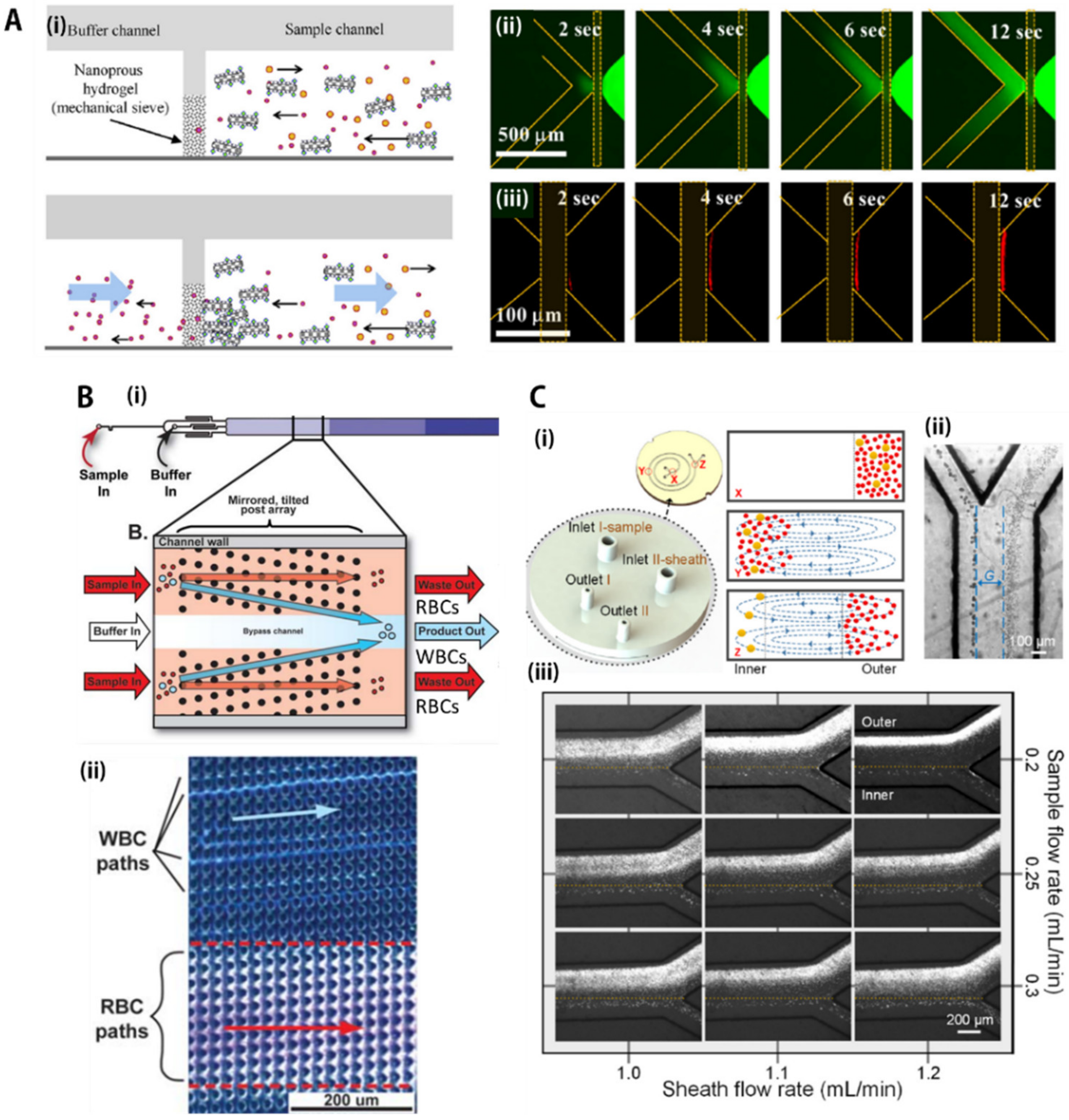
3.1. Sieving/Mechanical Filtration
3.2. Deterministic Lateral Displacement (DLD) Array
3.3. Inertial Focusing
4. Passive Separation Group 2: Gradient-Based Separation
4.1. Temperature Gradient
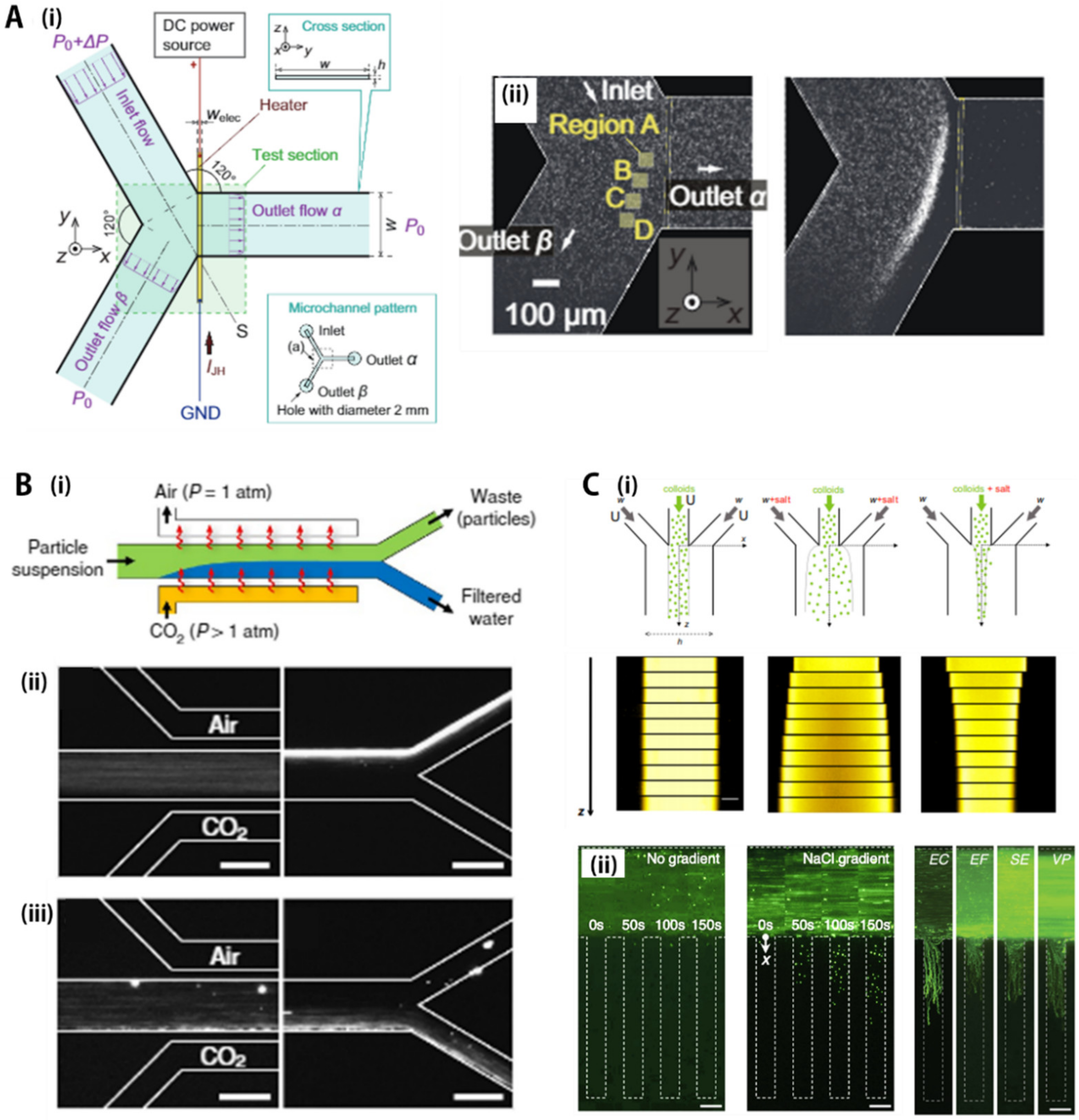
4.2. Gas Concentration Gradient
4.3. Salt Concentration Gradient
5. Active Separation Group 1: Non-Contacting Mechanical Forces
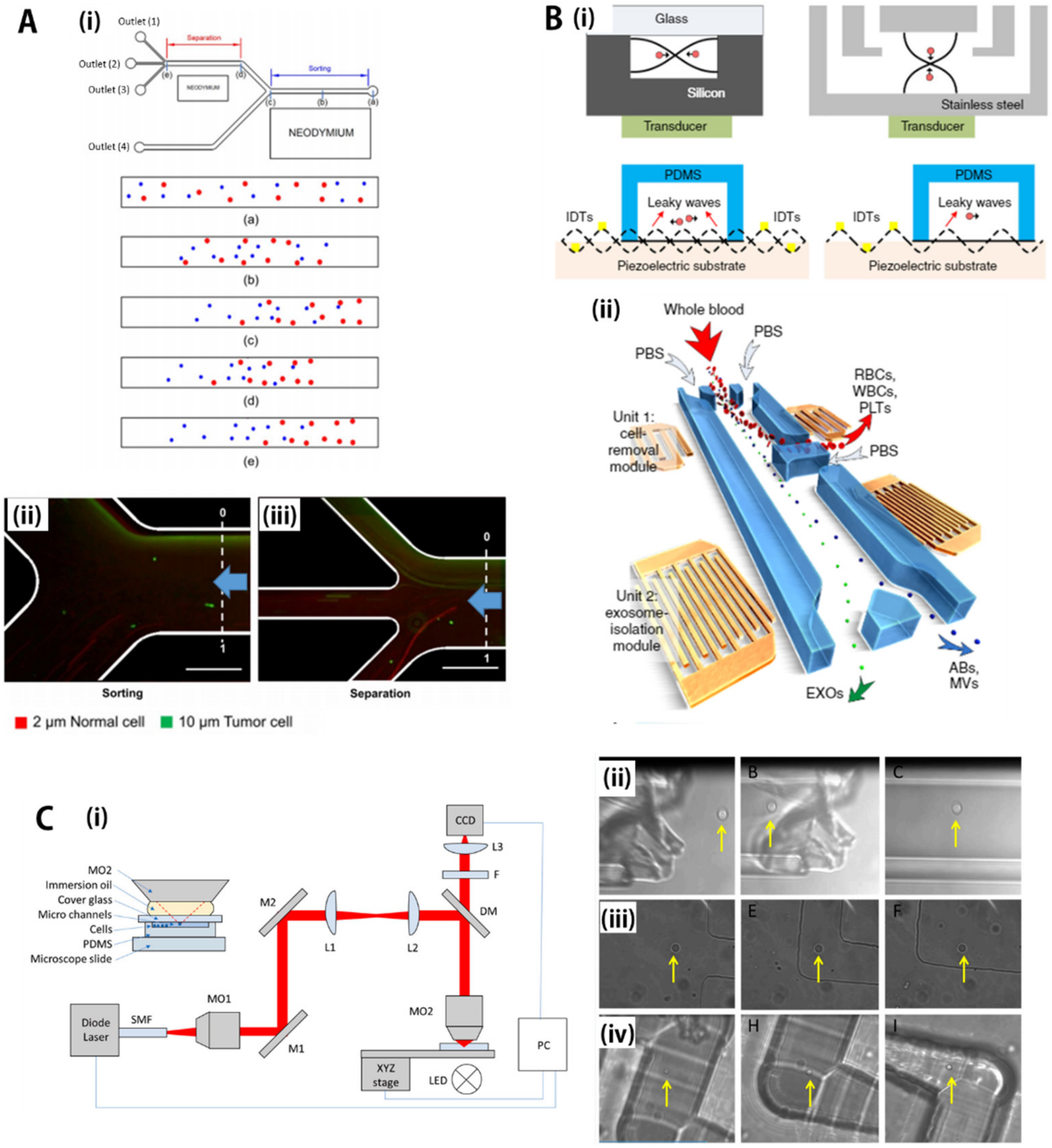
5.1. Magnetic Force (Magnetophoresis)
5.2. Acoustic Force (Accusotophoresis)
5.3. Optical Force (Optophoresis)
6. Active Separation Group 2: Contacting Electrical Forces
6.1. DC Electric Field (Electrophoresis)
6.2. AC Electric Field (Dielectrophoresis)
6.3. DC Electric Field with Perm-Selective Nanojunctions (Ion Concentration Polarization)
7. Discussion: Current Drawbacks and Outlook
7.1. Current Issues in Microfluidic-Based Separation Technology
7.2. Future Research Perspective: Next-Generation Microfluidic Separation Technology
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lin, C.M.; Lai, Y.S.; Liu, H.P.; Chen, C.Y.; Wo, A.M. Trapping of bioparticles via microvortices in a microfluidic device for bioassay applications. Anal. Chem. 2008, 80, 8937–8945. [Google Scholar] [CrossRef]
- Hughes, M.P.; Morgan, H. Dielectrophoretic trapping of single sub-micrometre scale bioparticles. J. Phys. D Appl. Phys. 1998, 31, 2205–2210. [Google Scholar] [CrossRef]
- Chaurey, V.; Rohani, A.; Su, Y.H.; Liao, K.T.; Chou, C.F.; Swami, N.S. Scaling down constriction-based (electrodeless) dielectrophoresis devices for trapping nanoscale bioparticles in physiological media of high-conductivity. Electrophoresis 2013, 34, 1097–1104. [Google Scholar] [CrossRef]
- Lim, E.J.; Ober, T.J.; Edd, J.F.; Desai, S.P.; Neal, D.; Bong, K.W.; Doyle, P.S.; McKinley, G.H.; Toner, M. Inertio-elastic focusing of bioparticles in microchannels at high throughput. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Cheng, I.F.; Chung, C.C.; Chang, H.C. High-throughput electrokinetic bioparticle focusing based on a travelling-wave dielectrophoretic field. Microfluid. Nanofluidics 2011, 10, 649–660. [Google Scholar] [CrossRef]
- Vishwanathan, G.; Juarez, G. Inertial focusing in planar pulsatile flows. J. Fluid Mech. 2021, 921, R1. [Google Scholar] [CrossRef]
- Paranjape, S.R.; Nagendran, T.; Poole, V.; Harris, J.; Taylor, A.M. Compartmentalization of human stem cell-derived neurons within pre-assembled plastic microfluidic chips. Jove-J. Vis. Exp. 2019, 147, e59250. [Google Scholar] [CrossRef] [PubMed]
- Kathrada, A.I.; Wei, S.C.; Xu, Y.; Cheow, L.F.; Chen, C.H. Microfluidic compartmentalization to identify gene biomarkers of infection. Biomicrofluidics 2020, 14, 061502. [Google Scholar] [CrossRef]
- Kim, M.; Lim, J.W.; Lee, S.K.; Kim, T. Nanoscale hydrodynamic film for diffusive mass transport control in compartmentalized microfluidic chambers. Anal. Chem. 2017, 89, 10286–10295. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Bae, J.; Kim, T. Long-term and programmable bacterial subculture in completely automated microchemostats. Anal. Chem. 2017, 89, 9676–9684. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.M.; Smith, K.C.; Dlamini, M.; Pletcher, K.; Yang, J.; Karabacak, M.; Haber, D.A.; Kapur, R.; Toner, M. Continuous flow microfluidic bioparticle concentrator. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, T. Integration of nanoporous membranes into microfluidic devices: Electrokinetic bio-sample pre-concentration. Analyst 2013, 138, 6007–6015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, M.; Jia, M.; Kim, T. Ion concentration polarization in a single and open microchannel induced by a surface-patterned perm-selective film. Analyst 2013, 138, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Wakizaka, Y.; Takemura, Y.; Ohta, R.; Yamada, T.; Yoshida, H. Separation of CTC using microfluidic chip from patients with colorectal cancer. Cancer Sci. 2021, 112, 879. [Google Scholar]
- Su, W.T.; Liang, D.; Tan, M.Q. Microfluidic strategies for sample separation and rapid detection of food allergens. Trends Food Sci. Technol. 2021, 110, 213–225. [Google Scholar] [CrossRef]
- Cho, B.S.; Schuster, T.G.; Zhu, X.Y.; Chang, D.; Smith, G.D.; Takayama, S. Passively driven integrated microfluidic system for separation of motile sperm. Anal. Chem. 2003, 75, 1671–1675. [Google Scholar] [CrossRef]
- Yao, L.; Wang, L.; Huang, F.C.; Cai, G.Z.; Xi, X.G.; Lin, J.H. A microfluidic impedance biosensor based on immunomagnetic separation and urease catalysis for continuous-flow detection of E-coli O157:H7. Sens. Actuators B Chem. 2018, 259, 1013–1021. [Google Scholar] [CrossRef]
- Hou, Y.; Cai, G.Z.; Zheng, L.Y.; Lin, J.H. A microfluidic signal-off biosensor for rapid and sensitive detection of Salmonella using magnetic separation and enzymatic catalysis. Food Control 2019, 103, 186–193. [Google Scholar] [CrossRef]
- Hao, L.; Xue, L.; Huang, F.C.; Cai, G.Z.; Qi, W.Z.; Zhang, M.; Han, Q.A.; Wang, Z.L.; Lin, J.H. A microfluidic biosensor based on magnetic nanoparticle separation, quantum dots labeling and mno2 nanoflower amplification for rapid and sensitive detection of salmonella typhimurium. Micromachines 2020, 11, 281. [Google Scholar] [CrossRef]
- Samiei, E.; Luka, G.S.; Najjaran, H.; Hoorfar, M. Integration of biosensors into digital microfluidics: Impact of hydrophilic surface of biosensors on droplet manipulation. Biosens. Bioelectron. 2016, 81, 480–486. [Google Scholar] [CrossRef]
- Song, Y.L.; Lin, B.Q.; Tian, T.; Xu, X.; Wang, W.; Ruan, Q.Y.; Guo, J.J.; Zhu, Z.; Yang, C.Y. Recent progress in microfluidics-based biosensing. Anal. Chem. 2019, 91, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.K.; Wu, R.G.; Chuang, Y.J.; Khoo, H.S.; Huang, S.H.; Tseng, F.G. Microfluidic systems for biosensing. Sensors 2010, 10, 6623–6661. [Google Scholar] [CrossRef] [PubMed]
- Nys, G.; Fillet, M. Microfluidics contribution to pharmaceutical sciences: From drug discovery to post marketing product management. J. Pharmaceut. Biomed. 2018, 159, 348–362. [Google Scholar] [CrossRef] [PubMed]
- Caffiyar, M.Y.; Lim, K.P.; Basha, I.H.K.; Hamid, N.H.; Cheong, S.C.; Ho, E.T.W. Label-free, high-throughput assay of human dendritic cells from whole-blood samples with microfluidic inertial separation suitable for resource-limited manufacturing. Micromachines 2020, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Syverud, B.C.; Lin, E.; Nagrath, S.; Larkin, L.M. Label-free, high-throughput purification of satellite cells using microfluidic inertial separation. Tissue Eng. Part C Methods 2018, 24, 32–41. [Google Scholar] [CrossRef]
- Guzniczak, E.; Otto, O.; Whyte, G.; Chandra, T.; Robertson, N.A.; Willoughby, N.; Jimenez, M.; Bridle, H. Purifying stem cell-derived red blood cells: A high-throughput label-free downstream processing strategy based on microfluidic spiral inertial separation and membrane filtration. Biotechnol. Bioeng. 2020, 117, 2032–2045. [Google Scholar] [CrossRef]
- Cui, H.C.; Huang, Z.; Dutta, P.; Ivory, C.F. Automated electric valve for electrokinetic separation in a networked microfluidic chip. Anal. Chem. 2007, 79, 1456–1465. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhu, Z.X.; Xiang, N.; Long, F.F.; Ni, Z.H. Automated microfluidic instrument for label-free and high-throughput cell separation. Anal. Chem. 2018, 90, 4212–4220. [Google Scholar] [CrossRef]
- Chen, Q.L.; Ho, H.P.; Cheung, K.L.; Kong, S.K.; Suen, Y.K.; Kwan, Y.W.; Wong, C.K. Design and fabrication of automated sedimentation-based separation and siphon-based extraction for detection of allergic reaction on a centrifugal microfluidic disc. Chin. Opt. Lett. 2010, 8, 957–959. [Google Scholar] [CrossRef][Green Version]
- Mellors, J.S.; Jorabchi, K.; Smith, L.M.; Ramsey, J.M. Integrated microfluidic device for automated single cell analysis using electrophoretic separation and electrospray ionization mass spectrometry. Anal. Chem. 2010, 82, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.F.; Liu, Y.; Xu, C.P.; Lu, H.W.; Wang, C.H. Focusing surface acoustic waves assisted electrochemical detector in microfluidics. Electrophoresis 2020, 41, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Liao, C.S.; Lee, G.B. An integrated microfluidic chip for DNA/RNA amplification, electrophoresis separation and on-line optical detection. Electrophoresis 2006, 27, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Sajeesh, P.; Sen, A.K. Particle separation and sorting in microfluidic devices: A review. Microfluid. Nanofluidics 2014, 17, 1–52. [Google Scholar] [CrossRef]
- Sonker, M.; Sahore, V.; Woolley, A.T. Recent advances in microfluidic sample preparation and separation techniques for molecular biomarker analysis: A critical review. Anal. Chim. Acta 2017, 986, 1–11. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, S.; Lim, G. Electrical force-based continuous cell lysis and sample separation techniques for development of integrated microfluidic cell analysis system: A review. Microelectron. Eng. 2018, 198, 55–72. [Google Scholar] [CrossRef]
- Antfolk, M.; Laurell, T. Continuous flow microfluidic separation and processing of rare cells and bioparticles found in blood—A review. Anal. Chim. Acta 2017, 965, 9–35. [Google Scholar] [CrossRef]
- Bayareh, M. An updated review on particle separation in passive microfluidic devices. Chem. Eng. Process 2020, 153, 107984. [Google Scholar] [CrossRef]
- Zhou, J.; Mukherjee, P.; Gao, H.; Luan, Q.Y.; Papautsky, I. Label-free microfluidic sorting of microparticles. APL Bioeng. 2019, 3, 041504. [Google Scholar] [CrossRef]
- Jeon, H.; Lee, D.H.; Jundi, B.; Pinilla-Vera, M.; Baron, R.M.; Levy, B.D.; Voldman, J.; Han, J. Fully automated, sample-to-answer leukocyte functional assessment platform for continuous sepsis monitoring via microliters of blood. ACS Sens. 2021, 6, 2747–2756. [Google Scholar] [CrossRef]
- Lee, K.; Lee, J.; Ha, D.; Kim, M.; Kim, T. Low-electric-potential-assisted diffusiophoresis for continuous separation of nanoparticles on a chip. Lab Chip 2020, 20, 2735–2747. [Google Scholar] [CrossRef]
- Xiang, N.; Wang, J.; Li, Q.; Han, Y.; Huang, D.; Ni, Z.H. Precise size-based cell separation via the coupling of inertial microfluidics and deterministic lateral displacement. Anal. Chem. 2019, 91, 10328–10334. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Shamloo, A.; Akbari, J. Design of a hybrid inertial and magnetophoretic microfluidic device for CTCs separation from blood. Micromachines 2021, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.; van Asten, A.; Tiggelaar, R.; Gardeniers, H. Microfluidic devices for forensic DNA analysis: A review. Biosensors 2016, 6, 41. [Google Scholar] [CrossRef]
- Obino, D.; Vassalli, M.; Franceschi, A.; Alessandrini, A.; Facci, P.; Viti, F. An overview on microfluidic systems for nucleic acids extraction from human raw samples. Sensors 2021, 21, 3058. [Google Scholar] [CrossRef]
- Tetala, K.K.R.; Vijayalakshmi, M.A. A review on recent developments for biomolecule separation at analytical scale using microfluidic devices. Anal. Chim. Acta 2016, 906, 7–21. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Lonnie, M.; Hooker, E.; Brunstrom, J.M.; Corfe, B.M.; Green, M.A.; Watson, A.W.; Williams, E.A.; Stevenson, E.J.; Penson, S.; Johnstone, A.M. Protein for life: Review of optimal protein intake, sustainable dietary sources and the effect on appetite in ageing adults. Nutrients 2018, 10, 360. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, I.; Babenko, V.; Martinez-Rodriguez, S.; Gavira, J.A. Protein separation under a microfluidic regime. Analyst 2018, 143, 606–619. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.C.; Wu, H.J.; Ugaz, V.M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab Chip 2017, 17, 3558–3577. [Google Scholar] [CrossRef]
- Livshts, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mayya, Y.S. Size distribution of virus laden droplets from expiratory ejecta of infected subjects. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cassedy, A.; Parle-McDermott, A.; O’Kennedy, R. Virus detection: A review of the current and emerging molecular and immunological methods. Front. Mol. Biosci. 2021, 8, 637559. [Google Scholar] [CrossRef]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Dizaj, S.M.; Ardalan, M.; Samiei, M.; Sharifi, S.; Vahed, S.Z.; Huseynova, I.; Khalilov, R.; et al. A comprehensive review of detection methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef]
- Portillo, M.C.; Leff, J.W.; Lauber, C.L.; Fierer, N. Cell size distributions of soil bacterial and archaeal taxa. Appl. Environ. Microb. 2013, 79, 7610–7617. [Google Scholar] [CrossRef]
- Stevens, K.A.; Jaykus, L.A. Bacterial separation and concentration from complex sample matrices: A review. Crit. Rev. Microbiol. 2004, 30, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.F. A review on microdevices for isolating circulating tumor cells. Micromachines 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Kim, S.; Lee, J.; Choi, J.; Kim, R.K.; Lee, S.J.; Sul, O.; Lee, S.B. Clogging-free microfluidics for continuous size-based separation of microparticles. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.Y.; Huang, C.K.; Lu, Y.W. Enhancement of microfluidic particle separation using cross-flow filters with hydrodynamic focusing. Biomicrofluidics 2016, 10, 011906. [Google Scholar] [CrossRef]
- Davies, R.T.; Kim, J.; Jang, S.C.; Choi, E.J.; Gho, Y.S.; Park, J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip 2012, 12, 5202–5210. [Google Scholar] [CrossRef]
- Bolze, H.; Riewe, J.; Bunjes, H.; Dietzel, A.; Burg, T.P. Protective filtration for microfluidic nanoparticle precipitation for pharmaceutical applications. Chem. Eng. Technol. 2021, 44, 457–464. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T. Aptamer-functionalized microtubules for continuous and selective concentration of target analytes. Sens. Actuators B Chem. 2014, 202, 1229–1236. [Google Scholar] [CrossRef]
- Wei, H.B.; Chueh, B.H.; Wu, H.L.; Hall, E.W.; Li, C.W.; Schirhagl, R.; Lin, J.M.; Zare, R.N. Particle sorting using a porous membrane in a microfluidic device. Lab Chip 2011, 11, 238–245. [Google Scholar] [CrossRef]
- Cheng, Y.N.; Ye, X.Y.; Ma, Z.S.; Xie, S.; Wang, W.H. High-throughput and clogging-free microfluidic filtration platform for on-chip cell separation from undiluted whole blood. Biomicrofluidics 2016, 10, 014118. [Google Scholar] [CrossRef]
- Civin, C.I.; Ward, T.; Skelley, A.M.; Gandhi, K.; Lee, Z.P.; Dosier, C.R.; D’Silva, J.L.; Chen, Y.; Kim, M.; Moynihan, J.; et al. Automated leukocyte processing by microfluidic deterministic lateral displacement. Cytom. Part A 2016, 89a, 1073–1083. [Google Scholar] [CrossRef]
- Xiang, N.; Ni, Z.H. Electricity-free hand-held inertial microfluidic sorter for size-based cell sorting. Talanta 2021, 235, 122807. [Google Scholar] [CrossRef]
- Salafi, T.; Zhang, Y.; Zhang, Y. A review on deterministic lateral displacement for particle separation and detection. Nano-Micro Lett. 2019, 11, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhang, H.N.; Li, Y.Y.; Li, X.B.; Wu, J.; Qian, S.Z.; Li, F.C. Dynamic control of particle separation in deterministic lateral displacement separator with viscoelastic fluids. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pariset, E.; Pudda, C.; Boizot, F.; Verplanck, N.; Berthier, J.; Thuaire, A.; Agache, V. Anticipating cutoff diameters in Deterministic Lateral Displacement (DLD) microfluidic devices for an optimized particle separation. Small 2017, 13, 1701901. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Jimenez, M.; Bridle, H. Deterministic lateral displacement for particle separation: A review. Lab Chip 2014, 14, 4139–4158. [Google Scholar] [CrossRef] [PubMed]
- Pariset, E.; Parent, C.; Fouillet, Y.; Francois, B.; Verplanck, N.; Revol-Cavalier, F.; Thuaire, A.; Agache, V. Separation of biological particles in a modular platform of cascaded deterministic lateral displacement modules. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Zeming, K.K.; Salafi, T.; Chen, C.H.; Zhang, Y. Asymmetrical deterministic lateral displacement gaps for dual functions of enhanced separation and throughput of red blood cells. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Kottmeier, J.; Wullenweber, M.; Blahout, S.; Hussong, J.; Kampen, I.; Kwade, A.; Dietzel, A. Accelerated particle separation in a DLD Device at Re > 1 investigated by means of mu PIV. Micromachines 2019, 10, 768. [Google Scholar] [CrossRef]
- Dincau, B.M.; Aghilinejad, A.; Hammersley, T.; Chen, X.L.; Kim, J.H. Deterministic lateral displacement (DLD) in the high Reynolds number regime: High-throughput and dynamic separation characteristics. Microfluid. Nanofluidics 2018, 22, 1–8. [Google Scholar] [CrossRef]
- Khodaee, F.; Movahed, S.; Fatouraee, N.; Daneshmand, F. Numerical simulation of separation of circulating tumor cells from blood stream in Deterministic lateral Displacement (Dld) microfluidic channel. J. Mech. 2016, 32, 463–471. [Google Scholar] [CrossRef]
- Ranjan, S.; Zeming, K.K.; Jureen, R.; Fisher, D.; Zhang, Y. DLD pillar shape design for efficient separation of spherical and non-spherical bioparticles. Lab Chip 2014, 14, 4250–4262. [Google Scholar] [CrossRef]
- Devendra, R.; Drazer, G. Gravity driven deterministic lateral displacement for particle separation in microfluidic devices. Anal. Chem. 2012, 84, 10621–10627. [Google Scholar] [CrossRef]
- Li, N.; Kamei, D.T.; Ho, C.-M. On-chip continuous blood cell subtype separation by Deterministic Lateral Displacement. IEEE-NEMS 2007, 6, 9562776. [Google Scholar]
- Fallahi, H.; Zhang, J.; Nicholls, J.; Phan, H.P.; Nguyen, N.T. Stretchable inertial microfluidic device for tunable particle separation. Anal. Chem. 2020, 92, 12473–12480. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Lin, Y. Inertial focusing and separation of particles in similar curved channels. Sci. Rep. 2019, 9, 1–12. [Google Scholar]
- Lee, D.; Nam, S.M.; Kim, J.A.; Di Carlo, D.; Lee, W. Active control of inertial focusing positions and particle separations enabled by velocity profile tuning with coflow systems. Anal. Chem. 2018, 90, 2902–2911. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.X.; Jia, Y.X.; Wang, P.; Sun, C.K. Progress of inertial microfluidics in principle and application. Sensors 2018, 18, 1762. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Dandy, D.S. High-throughput inertial focusing of micrometer- and sub-micrometer-sized particles separation. Adv. Sci. 2017, 4, 1700153. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.; Jimenez, M.; Bridle, H. Cascading and parallelising curvilinear inertial focusing systems for high volume, wide size distribution, separation and concentration of particles. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Lewis, P.; Balonis, M.; Di Carlo, D.; Sant, G. On the application of inertial microfluidics for the size-based separation of polydisperse cementitious particulates. Front. Mater. 2015, 2, 48. [Google Scholar] [CrossRef]
- Masaeli, M.; Sollier, E.; Amini, H.; Mao, W.B.; Camacho, K.; Doshi, N.; Mitragotri, S.; Alexeev, A.; Di Carlo, D. Continuous inertial focusing and separation of particles by shape. Phys. Rev. X 2012, 2, 031017. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Inertial microfluidics for continuous particle filtration and extraction. Microfluid. Nanofluidics 2009, 7, 217–226. [Google Scholar] [CrossRef]
- Kuntaegowdanahalli, S.S.; Bhagat, A.A.S.; Kumar, G.; Papautsky, I. Inertial microfluidics for continuous particle separation in spiral microchannels. Lab Chip 2009, 9, 2973–2980. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Y.; Zhu, S.; Liu, L.B.; Ni, Z.H.; Xiang, N. Inertial microfluidics for high-throughput cell analysis and detection: A review. Analyst 2021, 146, 6064–6083. [Google Scholar] [CrossRef]
- Xiang, N.; Wang, S.L.; Ni, Z.H. Secondary-flow-aided single-train elastic-inertial focusing in low elasticity viscoelastic fluids. Electrophoresis 2021. [Google Scholar] [CrossRef]
- Zhao, Q.B.; Yuan, D.; Zhang, J.; Li, W.H. A review of secondary flow in inertial microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Li, Q.; Ni, Z.H. Combining inertial microfluidics with cross-flow filtration for high-fold and high-throughput passive volume reduction. Anal. Chem. 2020, 92, 6770–6776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, D.; Sluyter, R.; Yan, S.; Zhao, Q.B.; Xia, H.M.; Tan, S.H.; Nguyen, N.T.; Li, W.H. High-throughput separation of white blood cells from whole blood using inertial microfluidics. IEEE Trans Biomed. Circ. Syst. 2017, 11, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, D.; Lin, J.H.; Wang, M.H.; Xuan, X.C. Simultaneous separation and washing of nonmagnetic particles in an inertial ferrofluid/water coflow. Anal. Chem. 2017, 89, 6915–6920. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, S.; Yuan, D.; Alici, G.; Nguyen, N.T.; Warkiani, M.E.; Li, W.H. Fundamentals and applications of inertial microfluidics: A review. Lab Chip 2016, 16, 10–34. [Google Scholar] [CrossRef]
- Lu, X.Y.; Xuan, X.C. Continuous Microfluidic particle separation via elasto-inertial pinched flow fractionation. Anal. Chem. 2015, 87, 6389–6396. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Y.; Xuan, X.C. Elasto-inertial pinched flow fractionation for continuous shape-based particle separation. Anal. Chem. 2015, 87, 11523–11530. [Google Scholar] [CrossRef]
- Asmolov, E.S.; Dubov, A.L.; Nizkaya, T.V.; Harting, J.; Vinogradova, O.I. Inertial focusing of finite-size particles in microchannels. J. Fluid Mech. 2018, 840, 613–630. [Google Scholar] [CrossRef]
- Erdem, K.; Ahmadi, V.E.; Kosar, A.; Kuddusi, L. Differential sorting of microparticles using spiral microchannels with elliptic configurations. Micromachines 2020, 11, 412. [Google Scholar] [CrossRef]
- Errarte, A.; Martin-Mayor, A.; Aginagalde, M.; Iloro, I.; Gonzalez, E.; Falcon-Perez, J.M.; Elortza, F.; Bou-Ali, M.M. Thermophoresis as a technique for separation of nanoparticle species in microfluidic devices. Int. J. Therm. Sci. 2020, 156, 106435. [Google Scholar] [CrossRef]
- Tsuji, T.; Matsumoto, Y.; Kugimiya, R.; Doi, K.; Kawano, S. Separation of nano- and microparticle flows using thermophoresis in branched microfluidic channels. Micromachines 2019, 10, 321. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Zhu, J.H.; Xia, S.L.; Laminger, T.; Hoflinger, W. Separation of small particles by diffusio- and thermophoresis in a gas-liquid cross-flow array. Chem. Eng. Technol. 2018, 41, 61–69. [Google Scholar] [CrossRef]
- Vigolo, D.; Rusconi, R.; Stone, H.A.; Piazza, R. Thermophoresis: Microfluidics characterization and separation. Soft Matter 2010, 6, 3489–3493. [Google Scholar] [CrossRef]
- So, J.H.; Koo, H.J. Thermophoretic control of particle transport in a microfluidic channel. Korean Chem. Eng. Res. 2019, 57, 730–734. [Google Scholar]
- Niether, D.; Wiegand, S. Thermophoresis of biological and biocompatible compounds in aqueous solution. J. Condens. Matter Phys. 2019, 31, 503003. [Google Scholar] [CrossRef]
- Geelhoed, P.F.; Lindken, R.; Westerweel, J. Thermophoretic separation in microfluidics. Chem. Eng. Res. Des. 2006, 84, 370–373. [Google Scholar] [CrossRef]
- Shin, S.; Shardt, O.; Warren, P.B.; Stone, H.A. Membraneless water filtration using CO2. Nat. Commun. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Abecassis, B.; Cottin-Bizonne, C.; Ybert, C.; Ajdari, A.; Bocquet, L. Osmotic manipulation of particles for microfluidic applications. New J. Phys. 2009, 11, 075022. [Google Scholar] [CrossRef]
- Doan, V.S.; Saingam, P.; Yan, T.; Shin, S. A trace amount of surfactants enables diffusiophoretic swimming of bacteria. ACS Nano 2020, 14, 14219–14227. [Google Scholar] [CrossRef]
- Shimokusu, T.J.; Maybruck, V.G.; Ault, J.T.; Shin, S. Colloid separation by CO2-induced diffusiophoresis. Langmuir 2020, 36, 7032–7038. [Google Scholar] [CrossRef]
- Shim, S.; Stone, H.A. CO2-leakage-driven diffusiophoresis causes spontaneous accumulation of charged materials in channel flow. Proc. Natl. Acad. Sci. USA 2020, 117, 25985–25990. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Chen, Z.; Xiong, G.X.; Zhu, J.H. Trajectory of fine particles removal with diffusiophoresis and thermophoresis in a gas-liquid cross-flow array. RSC Adv. 2019, 9, 26748–26756. [Google Scholar] [CrossRef]
- Shim, S.; Khodaparast, S.; Lai, C.Y.; Yan, J.; Ault, J.T.; Rallabandi, B.; Shardt, O.; Stone, H.A. CO2-driven diffusiophoresis for maintaining a bacteria-free surface. Soft Matter 2021, 17, 2568–2576. [Google Scholar] [CrossRef] [PubMed]
- Shin, S. Diffusiophoretic separation of colloids in microfluidic flows. Phys. Fluids 2020, 32, 101302. [Google Scholar] [CrossRef]
- Seo, M.; Park, S.; Lee, D.; Lee, H.; Kim, S.J. Continuous and spontaneous nanoparticle separation by diffusiophoresis. Lab Chip 2020, 20, 4118–4127. [Google Scholar] [CrossRef]
- Lee, D.; Kim, S.J. Spontaneous diffusiophoretic separation in paper-based microfluidic device. Micro Nano Syst. Lett. 2020, 8, 1–4. [Google Scholar] [CrossRef]
- Ha, D.; Seo, S.; Lee, K.; Kim, T. Dynamic transport control of colloidal particles by repeatable active switching of solute gradients. ACS Nano 2019, 13, 12939–12948. [Google Scholar] [CrossRef]
- Battat, S.; Ault, J.T.; Shin, S.; Khodaparast, S.; Stone, H.A. Particle entrainment in dead-end pores by diffusiophoresis. Soft Matter 2019, 15, 3879–3885. [Google Scholar] [CrossRef]
- Hsu, J.P.; Hsu, Y.R.; Shang-Hung, H.; Tseng, S. Separation of charge-regulated polyelectrolytes by pH-assisted diffusiophoresis. Phys. Chem. Chem. Phys. 2017, 19, 9059–9063. [Google Scholar] [CrossRef]
- Palacci, J.; Cottin-Bizonne, C.; Ybert, C.; Bocquet, L. Osmotic traps for colloids and macromolecules based on logarithmic sensing in salt taxis. Soft Matter 2012, 8, 980–994. [Google Scholar] [CrossRef]
- Hong, J.; Kim, B.; Shin, H. Mixed-scale poly (methyl methacrylate) channel network-based single-particle manipulation via diffusiophoresis. Nanoscale 2018, 10, 14421–14431. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Yeap, S.P.; Lim, J. Working principle and application of magnetic separation for biomedical diagnostic at high- and low-field gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, F.; Madadi, H.; Villone, M.M.; D’Avino, G.; Cusano, A.M.; Vecchione, R.; Ventre, M.; Maffettone, P.L.; Netti, P.A. Magnetophoresis ‘meets’ viscoelasticity: Deterministic separation of magnetic particles in a modular microfluidic device. Lab Chip 2015, 15, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Modak, N.; Datta, A.; Ganguly, R. Influence of the microchannel geometry on magnetophoretic separation of functionalized magnetic beads in a microfluidic sorter and field flow fractionation device. Magnetohydrodynamics 2013, 49, 391–396. [Google Scholar] [CrossRef]
- Plouffe, B.D.; Lewis, L.H.; Murthy, S.K. Computational design optimization for microfluidic magnetophoresis. Biomicrofluidics 2011, 5, 013413. [Google Scholar] [CrossRef] [PubMed]
- Iliescu, C.; Xu, G.L.; Barbarini, E.; Avram, M.; Avram, A. Microfluidic device for continuous magnetophoretic separation of white blood cells. Microsyst. Technol. 2009, 15, 1157–1162. [Google Scholar] [CrossRef]
- Alnaimat, F.; Dagher, S.; Mathew, B.; Hilal-Alnqbi, A.; Khashan, S. Microfluidics based magnetophoresis: A review. Chem. Rec. 2018, 18, 1596–1612. [Google Scholar] [CrossRef]
- Alnaimat, F.; Karam, S.; Mathew, B.; Mathew, B. Magnetophoresis and microfluidics a great union. IEEE Nanotechnol. Mag. 2020, 14, 24–41. [Google Scholar] [CrossRef]
- Lee, J.; Sul, O.; Lee, S.B. Enrichment of circulating tumor cells from whole blood using a microfluidic device for sequential physical and magnetophoretic separations. Micromachines 2020, 11, 481. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhang, B.; Gu, J.L.; Li, S.J. Magnetic beads separation characteristics of a microfluidic bioseparation chip based on magnetophoresis with lattice-distributed soft magnets. J. Magn. Magn. Mater. 2020, 501, 166485. [Google Scholar] [CrossRef]
- Descamps, L.; Le Roy, D.; Tomba, C.; Deman, A.L. Magnetic polymers for magnetophoretic separation in microfluidic devices. Magnetochemistry 2021, 7, 100. [Google Scholar] [CrossRef]
- Kye, H.G.; Park, B.S.; Lee, J.M.; Song, M.G.; Song, H.G.; Ahrberg, C.D.; Chung, B.G. Dual-neodymium magnet-based microfluidic separation device. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Ozcelik, A.; Rufo, J.; Wang, Z.Y.; Fang, R.; Huang, T.J. Acoustofluidic separation of cells and particles. Microsyst. Nanoeng. 2019, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Ouyang, Y.S.; Wang, Z.Y.; Zhang, R.; Huang, P.H.; Chen, C.Y.; Li, H.; Li, P.; Quinn, D.; Dao, M.; et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc. Natl. Acad. Sci. USA 2017, 114, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Keloth, A.; Anderson, O.; Risbridger, D.; Paterson, L. Single cell isolation using optical tweezers. Micromachines 2018, 9, 434. [Google Scholar]
- Mani, K.; Chen, C.Y. A non-invasive acoustic-trapping of zebrafish microfluidics. Biomicrofluidics 2021, 15, 014109. [Google Scholar] [CrossRef]
- Agostini, M.; Cecchini, M. Ultra-high-frequency (UHF) surface-acoustic-wave (SAW) microfluidics and biosensors. Nanotechnology 2021, 32, 312001. [Google Scholar] [CrossRef]
- Zhang, P.R.; Bachman, H.; Ozcelik, A.; Huang, T.J. Acoustic microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Reboud, J.; Torun, H.; McHale, G.; Dodd, L.E.; Wu, Q.; Tao, K.; Yang, X.; Luo, J.T.; Todryk, S.; et al. Integrating microfluidics and biosensing on a single flexible acoustic device using hybrid modes. Lab Chip 2020, 20, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Kwun, J.; Gu, Y.; Manook, M.; Wu, M.; Yoon, J.; Knechtle, S.; Huang, T.J. Isolation of preformed antibodies from whole blood by integrating acoustics and microfluidics in sensitized animal model. Am. J. Trans. 2020, 20, 645. [Google Scholar]
- Dow, P.; Kotz, K.; Gruszka, S.; Holder, J.; Fiering, J. Acoustic separation in plastic microfluidics for rapid detection of bacteria in blood using engineered bacteriophage. Lab Chip 2018, 18, 923–932. [Google Scholar] [CrossRef]
- Kotz, K.T.; Dubay, R.; Berlin, D.; Fiering, J. Separation of lymphocytes using acoustic microfluidics. Cytotherapy 2017, 19, S20–S21. [Google Scholar] [CrossRef]
- Yeo, L.Y.; Friend, J.R. Surface acoustic wave microfluidics. Annu. Rev. Fluid Mech. 2014, 46, 379–406. [Google Scholar] [CrossRef]
- Leibacher, I.; Reichert, P.; Dual, J. Microfluidic droplet handling by bulk acoustic wave (BAW) acoustophoresis. Lab Chip 2015, 15, 2896–2905. [Google Scholar] [CrossRef]
- Ding, X.Y.; Li, P.; Lin, S.C.S.; Stratton, Z.S.; Nama, N.; Guo, F.; Slotcavage, D.; Mao, X.L.; Shi, J.J.; Costanzo, F.; et al. Surface acoustic wave microfluidics. Lab Chip 2013, 13, 3626–3649. [Google Scholar] [CrossRef] [PubMed]
- Amos, B.; Gill, P. Optical tweezers. Meas. Sci. Technol. 1995, 6, 248. [Google Scholar]
- Sorg, B.S.; Kuo, S.C. Single kinesin molecules twisted by optical tweezers. Mol. Biol. Cell 1995, 6, 905. [Google Scholar]
- Semenov, A.N.; Lugovtsov, A.E.; Shirshin, E.A.; Yakimov, B.P.; Ermolinskiy, P.B.; Bikmulina, P.Y.; Kudryavtsev, D.S.; Timashev, P.S.; Muravyov, A.V.; Wagner, C.; et al. Assessment of fibrinogen macromolecules interaction with red blood cells membrane by means of laser aggregometry, flow cytometry, and optical tweezers combined with microfluidics. Biomolecules 2020, 10, 1448. [Google Scholar] [CrossRef]
- Kumar, P.T.; Decrop, D.; Safdar, S.; Passaris, I.; Kokalj, T.; Puers, R.; Aertsen, A.; Spasic, D.; Lammertyn, J. Digital microfluidics for single bacteria capture and selective retrieval using optical tweezers. Micromachines 2020, 11, 308. [Google Scholar] [CrossRef]
- Neve, N.; Kohles, S.S.; Winn, S.R.; Tretheway, D.C. Manipulation of suspended single cells by microfluidics and optical tweezers. Cell Mol. Bioeng. 2010, 3, 213–228. [Google Scholar] [CrossRef]
- Gross, P.; Farge, G.; Peterman, E.J.G.; Wuite, G.J.L. Combining optical tweezers, single-molecule fluorescence microscopy, and microfluidics for studies of dna-protein interactions. Meth. Enzymol. 2010, 475, 427–453. [Google Scholar]
- Murata, M.; Okamoto, Y.; Park, Y.S.; Kaji, N.; Tokeshi, M.; Baba, Y. Cell separation by the combination of microfluidics and optical trapping force on a microchip. Anal. Bioanal. Chem. 2009, 394, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Overton, G. Optical trapping—Zone-plate optical tweezer can integrate with microfluidics. Laser Focus World 2008, 44, 46. [Google Scholar]
- Wang, X.L.; Chen, S.X.; Kong, M.; Wang, Z.K.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011, 11, 3656–3662. [Google Scholar] [CrossRef]
- Saar, K.L.; Peter, Q.; Muller, T.; Challa, P.K.; Herling, T.W.; Knowles, T.P.J. Rapid two-dimensional characterisation of proteins in solution. Microsyst. Nanoeng. 2019, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Puchberger-Enengl, D.; Podszun, S.; Heinz, H.; Hermann, C.; Vulto, P.; Urban, G.A. Microfluidic concentration of bacteria by on-chip electrophoresis. Biomicrofluidics 2011, 5, 044111. [Google Scholar] [CrossRef]
- Perrin, D.; Fremaux, C.; Shutes, A. Capillary microfluidic electrophoretic mobility shift assays: Application to enzymatic assays in drug discovery. Expert Opin. Drug Dis. 2010, 5, 51–63. [Google Scholar] [CrossRef]
- Zhao, K.; Larasati; Duncker, B.P.; Li, D.Q. Continuous Cell Characterization and Separation by Microfluidic Alternating Current Dielectrophoresis. Anal. Chem. 2019, 91, 6304–6314. [Google Scholar] [CrossRef]
- Papadimitriou, V.A.; Segerink, L.I.; Eijkel, J.C.T. Free flow ion concentration polarization focusing (FF-ICPF). Anal. Chem. 2020, 92, 4866–4874. [Google Scholar] [CrossRef]
- Zhao, W.J.; Zhang, L.Q.; Ye, Y.F.; Li, Y.A.; Luan, X.F.; Liu, J.L.; Cheng, J.; Zhao, Y.; Li, M.X.; Huang, C.J. Microsphere mediated exosome isolation and ultra-sensitive detection on a dielectrophoresis integrated microfluidic device. Analyst 2021, 146, 5962–5972. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, D.; Zhao, Q.B.; Yan, S.; Tang, S.Y.; Tan, S.H.; Guo, J.H.; Xia, H.M.; Nguyen, N.T.; Li, W.H. Tunable particle separation in a hybrid dielectrophoresis (DEP)-inertial microfluidic device. Sens. Actuators B Chem. 2018, 267, 14–25. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Chang, H.L.; Neuzil, P. DEP-on-a-chip: Dielectrophoresis applied to microfluidic platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.V.; Manh, T.L.; Nguyen, T.S.; Le, V.T.; Hieu, N.V. Applied electric field analysis and numerical investigations of the continuous cell separation in a dielectrophoresis-based microfluidic channel. J. Sci.-Adv. Mater. Dev. 2021, 6, 11–18. [Google Scholar] [CrossRef]
- Miled, A.; Auclair, B.; Srasra, A.; Sawan, M. Reconfigurable prototyping microfluidic platform for DEP manipulation and capacitive sensing. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Low, W.S.; Kadri, N.A. Computational analysis of enhanced circulating tumour cell (ctc) separation in a microfluidic system with an integrated dielectrophoretic-magnetophorectic (DEP-MAP) technique. Chemosensors 2016, 4, 14. [Google Scholar] [CrossRef]
- Lee, H.T.; Ciou, Y.J.; Yao, D.J. Using a digital microfluidic system to evaluate the stretch length of a droplet with a L-DEP and varied parameters. Inventions 2020, 5, 21. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, I.K.; Taylor, K.; Richters, K.; Baek, D.H.; Ryu, J.H.; Cho, S.J.; Jung, Y.H.; Park, D.W.; Novello, J.; et al. Single-neuronal cell culture and monitoring platform using a fully transparent microfluidic DEP device. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Derakhshan, R.; Mahboubidoust, A.; Ramiar, A. Design of a novel optimized microfluidic channel for CTCs separation utilizing a combination of TSAWs and DEP methods. Chem. Eng. Process. 2021, 167, 108544. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lai, C.P.K.; Chen, C.C.; Lee, G.B. Isolation and recovery of extracellular vesicles using optically-induced dielectrophoresis on an integrated microfluidic platform. Lab Chip 2021, 21, 1475–1483. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Niu, B.S.; Ji, B.; Fang, F.; Guo, X.L.; Wu, Z.Y. Salty biofluidic sample clean-up and preconcentration with a paper-based ion concentration polarization interface. Anal. Chem. 2021, 93, 10236–10242. [Google Scholar] [CrossRef]
- Gholinejad, M.; Jabari Moghadam, A.; Phan, D.T.; Miri, A.K.; Mousavi Shaegh, S.A. Design and application of ion concentration polarization for preconcentrating charged analytes. Phys. Fluids 2021, 33, 051301. [Google Scholar] [CrossRef]
- Gholinejad, M.; Moghadam, A.J.; Shaegh, S.A.M.; Miri, A.K. Multifactorial analysis of ion concentration polarization for microfluidic preconcentrating applications using response surface method. Phys. Fluids 2020, 32, 072012. [Google Scholar] [CrossRef]
- Han, S.I.; Yoo, Y.K.; Lee, J.; Kim, C.; Lee, K.; Lee, T.H.; Kim, H.; Yoon, D.S.; Hwang, K.S.; Kwak, R.; et al. High-ionic-strength pre-concentration via ion concentration polarization for blood-based biofluids. Sens. Actuators B Chem. 2018, 268, 485–493. [Google Scholar] [CrossRef]
- Han, W.B.; Chen, X.Y. A review: Applications of ion transport in micro-nanofluidic systems based on ion concentration polarization. J. Chem. Technol. Biot. 2020, 95, 1622–1631. [Google Scholar] [CrossRef]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of high salinity brine by multi-stage ion concentration polarization desalination. Sci. Rep. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Kim, D.; Ihm, S.; Park, S.; Yu, Y.; Kwak, R. Concentric ion concentration polarization desalination for efficient En-bloc preconcentration and desalination. Desalination 2021, 499, 114810. [Google Scholar] [CrossRef]
- Kim, M.; Wu, L.D.; Kim, B.; Hung, D.T.; Han, J. Continuous and high-throughput electromechanical lysis of bacterial pathogens using ion concentration polarization. Anal. Chem. 2018, 90, 872–880. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, Y.K.; Lee, D.; Kim, C.; Kim, K.H.; Lee, S.; Kwak, S.; Kang, J.Y.; Kim, H.; Yoon, D.S.; et al. Origami paper-based sample preconcentration using sequentially driven ion concentration polarization. Lab Chip 2021, 21, 867–874. [Google Scholar] [CrossRef]
- Kwon, T.; Choi, K.; Han, J. Separation of ultra-high-density cell suspension via elasto-inertial microfluidics. Small 2021, 17, 2101880. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Tay, A.K.P.; Guan, G.F.; Han, J. Membrane-less microfiltration using inertial microfluidics. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
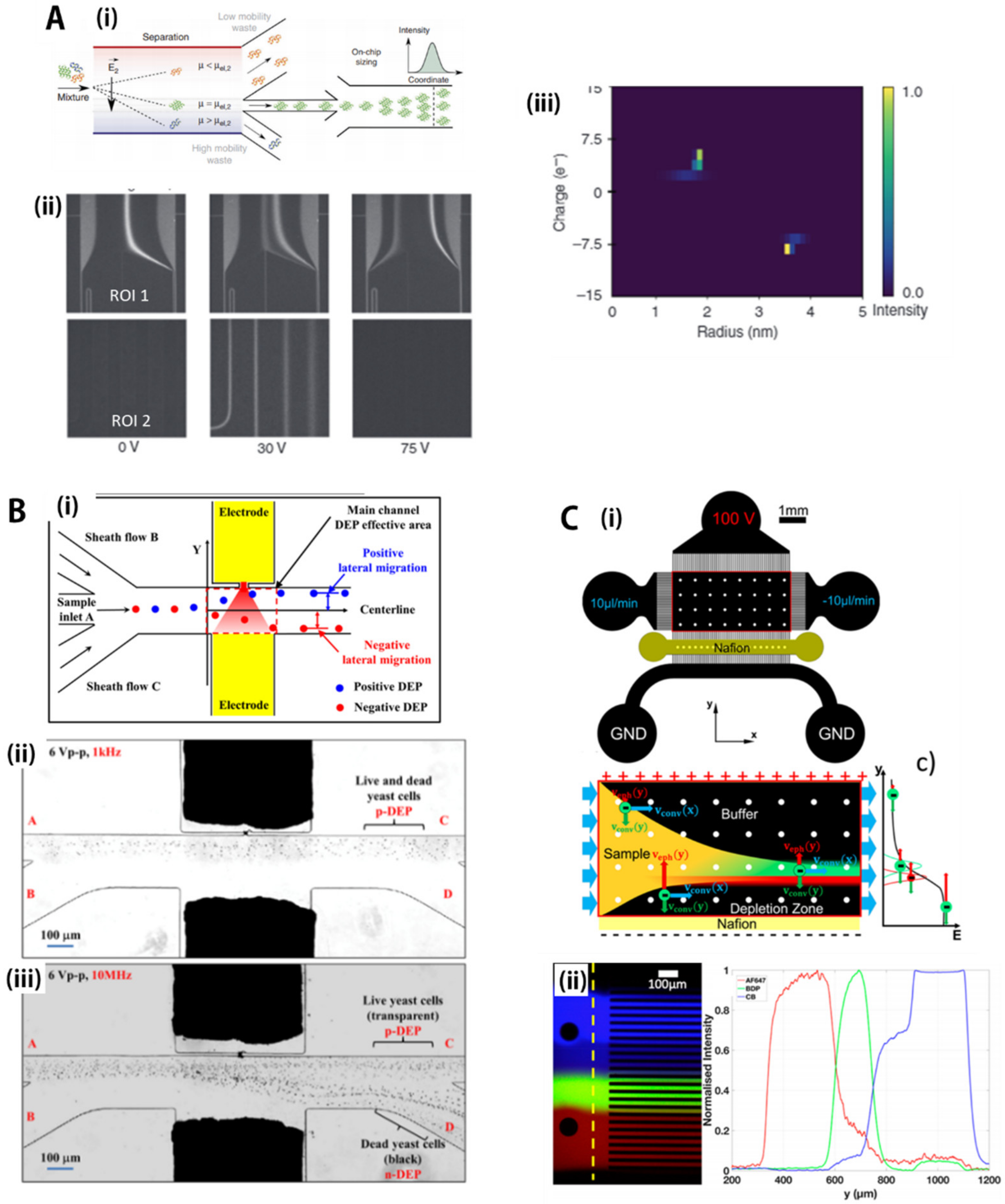
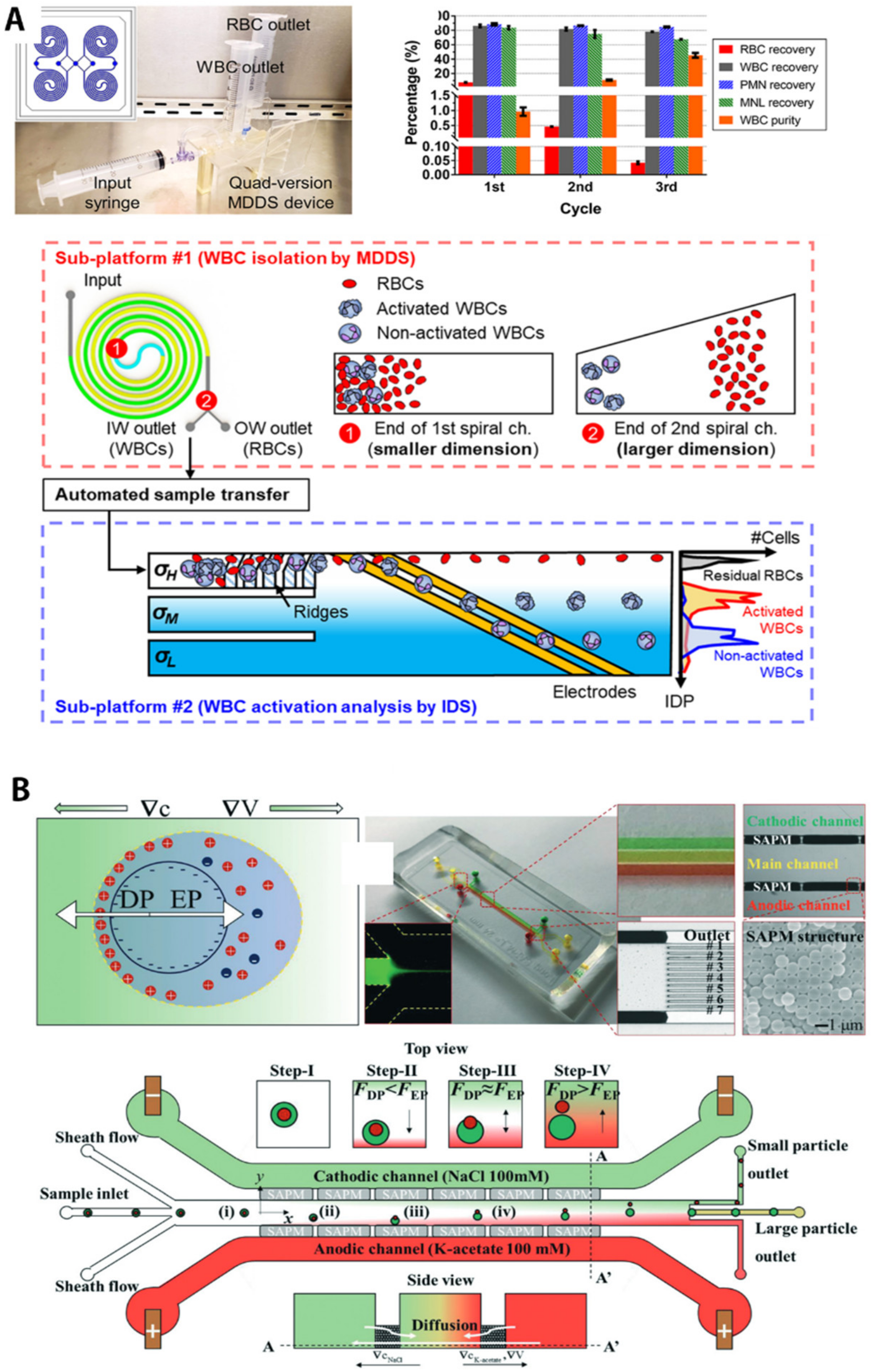
| Separation Criteria | Operational Mechanism | Sample Matrix | Target Bioparticles | Throughput/Recovery Ratio/Others | Reference |
|---|---|---|---|---|---|
| Hydrodynamic flow-based separation | Sieving/mechanical filtration | PBS buffer with BSA proteins | Aptamer-EGFR conjugate bounded on microtubules | 105–106-fold concentration | Kim, M. et al. [62] |
| Deterministic lateral displacement (DLD) array | Human blood sample incubated with fluorochrome-conjugated monoclonal antibodies (mAb) | Human leukocytes (WBCs) | 200 μL during 18 min, 88% target recovery, 99.985% removal of input erythrocytes, >99% of unbound mAb in 18 min | Civin, C.I. et al. [65] | |
| Inertial focusing | Diluted blood spiked with pre-stained tumor cells with a concentration of 104 cells/mL | Tumor cells | 0.2 mL/min, 78.67% rare tumor cell recovery, >96.04% blood cell removal | Xiang, N. et al. [66] | |
| Micro- environmental gradients-based separation | Temperature gradient (thermophoresis) | Tris-HCl aqueous buffer (pH = 8.0) | 0.1 and 1 μm polystyrene particles | Vin = 3.5 µm/s | Tsuji, T. et al. [101] |
| Gas concentration gradient (diffusiophoresis) | Deionized water | Amine-functionalized polystyrene particles | 2 μL/h out of ~2.2 × 107 total particles, only 104 passed during 5 min | Shin, S. et al. [107] | |
| Salt concentration gradient (diffusiophoresis) | 1~100 mM NaCl buffer with 0.1 mM sodium dodecyl sulfate | Gram-positive or -negative, flagellated or nonflagellated bacteria | NA | Doan, V.S. et al. [109] | |
| Non-contacting mechanical force-based separation | Magnetic force (magnetophoresis) | PBS buffer with poly(ethylene oxide) | Glioblastoma cancer cells and neural stem cells | 5–13 µL/min, 97 ± 0.8% for 15 μm microparticles | Kye, H.G. et al. [132] |
| Acoustic force (accusotophoresis) | Blood or extracellular vesicle mixture solution | Exosomes | 4 μL/min, 98.4% purity, > 99.999% blood cell removal rate | Wu, M.X. et al. [134] | |
| Optical force (optophoresis) | Water, media, or buffer solution | Yeast cells (S. cerevisiae) and bacteria (B. subtilis and E. coli) | Vp = 200–300 µm/s | Keloth, A. et al. [135] | |
| Contacting electrical forces-based separation | DC electric field (electrophoresis) | 10 mM sodium phosphate buffer at pH 7.4 | BSA and human lysozyme proteins | 3 μL during 7 min for two-dimensional protein mapping | Saar, K.L. et al. [155] |
| AC electric field (dielectrophoresis) | DI water and 0.4–4.8 mM K2HPO4 solution | Yeast cells (standard lab yeast strain, Saccharomyces cerevisiae S288c) | 3.75 × 10−3 μL/s | Zhao, K. et al. [158] | |
| DC electric field with permselective nanojunctions (ion concentration polarization) | 0.1×PBS buffer and human blood plasma with 3.2% sodium acetate | BODIPY disulfonate | 15 μL/min, ~10-fold concentration factor | Papadimitriou, V.A. et al. [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, S.-w.; Kim, B.; Kim, M. Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles. Biosensors 2021, 11, 464. https://doi.org/10.3390/bios11110464
Choe S-w, Kim B, Kim M. Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles. Biosensors. 2021; 11(11):464. https://doi.org/10.3390/bios11110464
Chicago/Turabian StyleChoe, Se-woon, Bumjoo Kim, and Minseok Kim. 2021. "Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles" Biosensors 11, no. 11: 464. https://doi.org/10.3390/bios11110464
APA StyleChoe, S.-w., Kim, B., & Kim, M. (2021). Progress of Microfluidic Continuous Separation Techniques for Micro-/Nanoscale Bioparticles. Biosensors, 11(11), 464. https://doi.org/10.3390/bios11110464







