All-Optical Switching Demonstrated with Photoactive Yellow Protein Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. PYP Sample Preparation
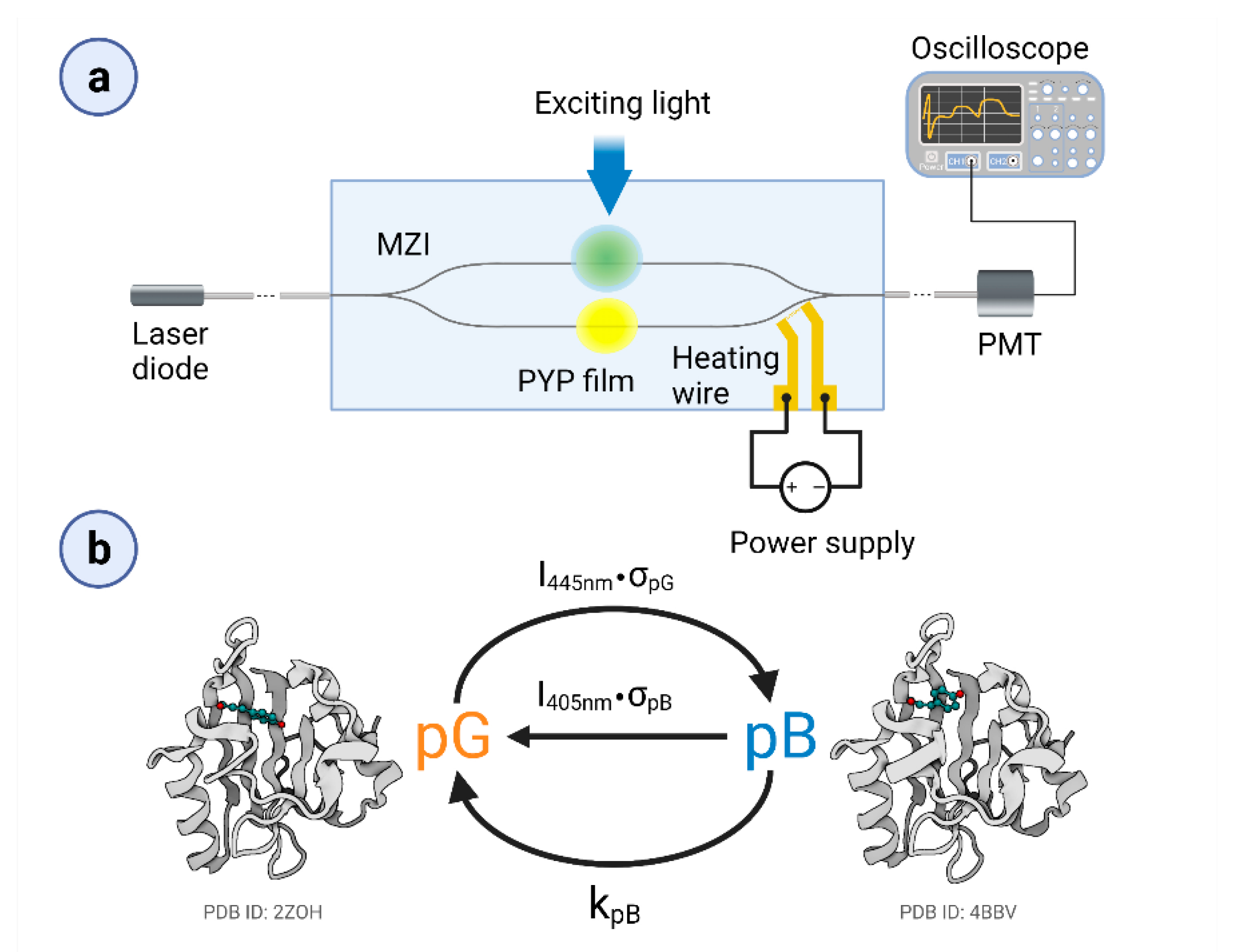
2.2. IO Mach–Zehnder Interferometer Biosensor Fabrication
2.3. Experimental Setup
3. Results
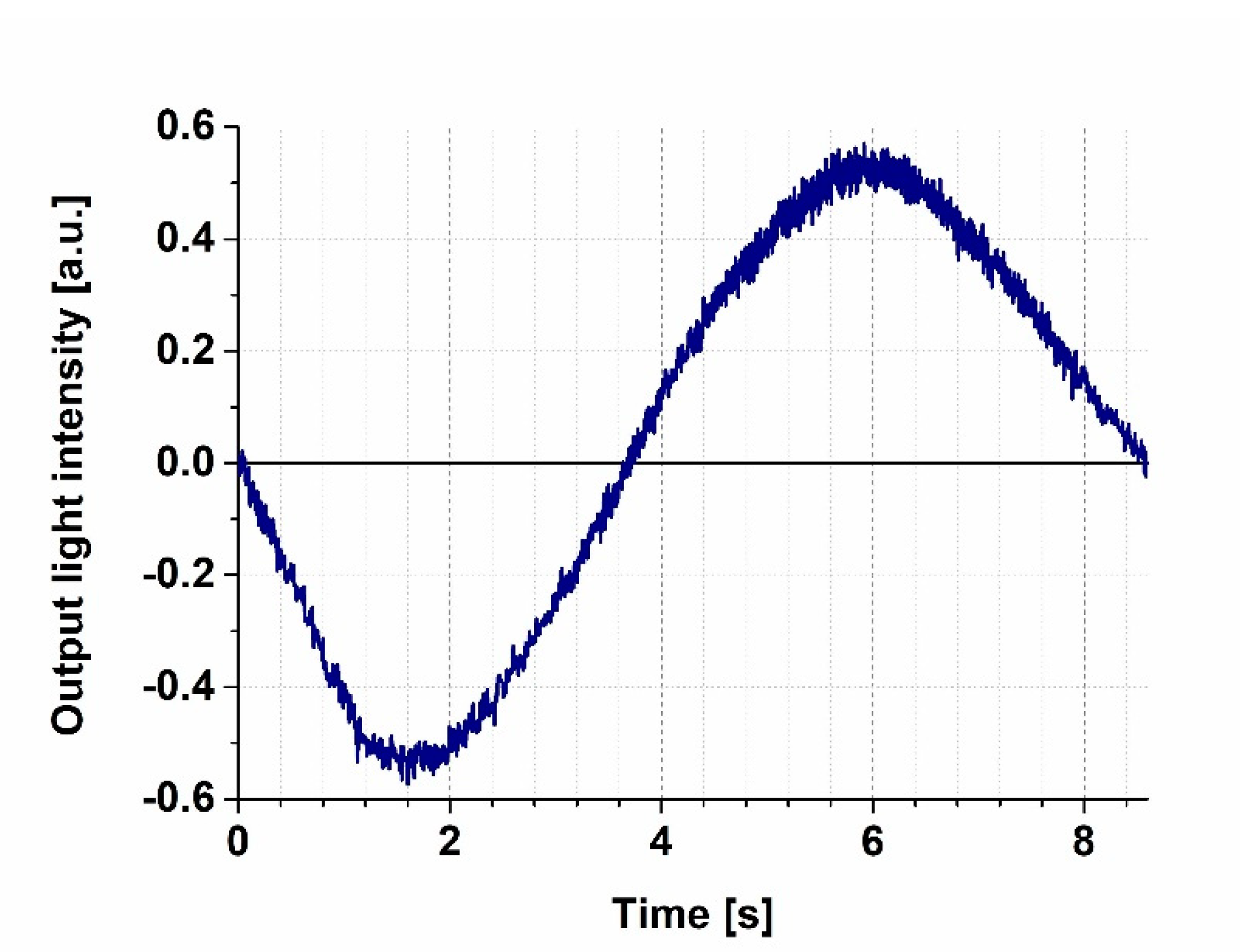
3.1. Calibration of the MZI Bias Point
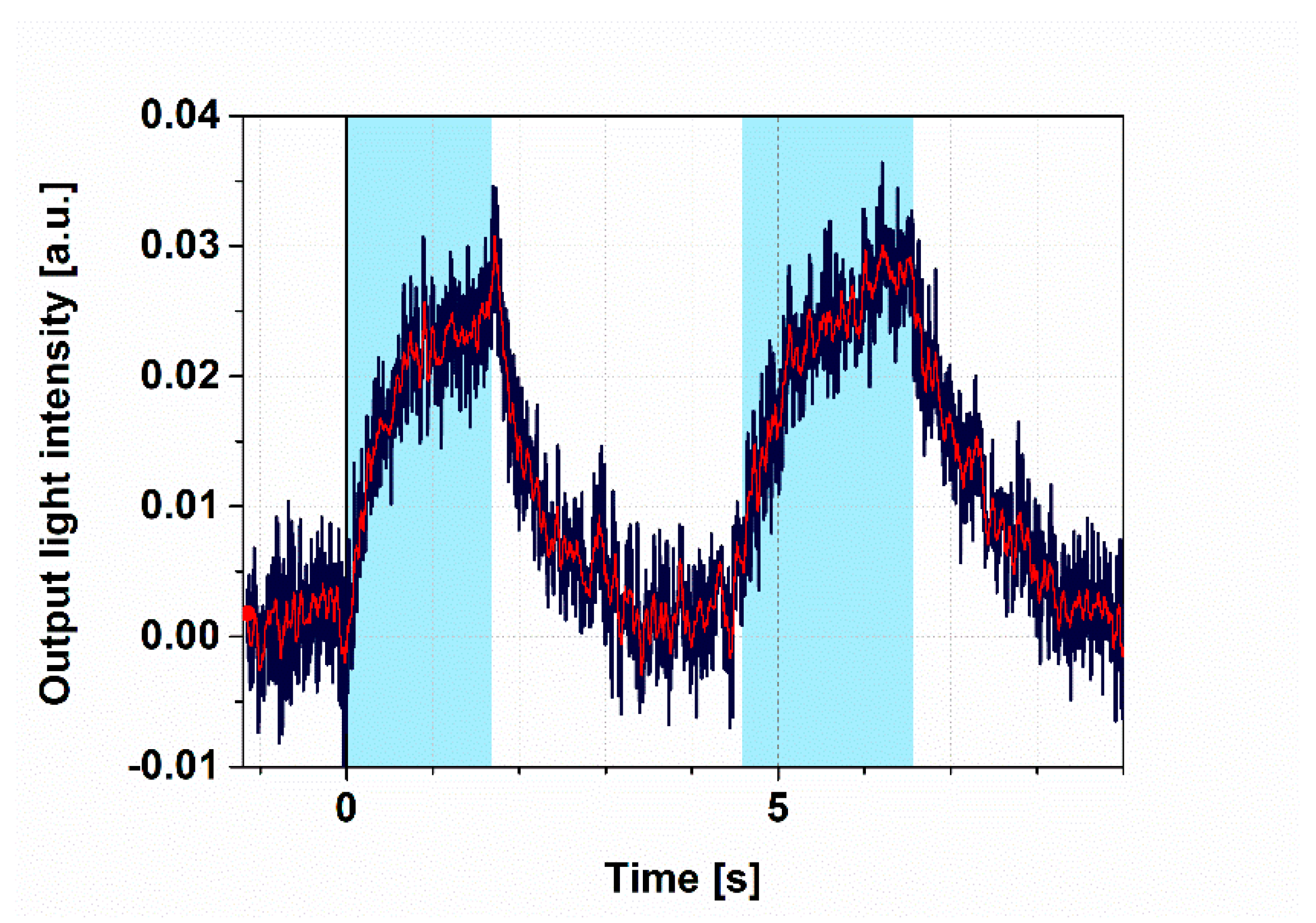
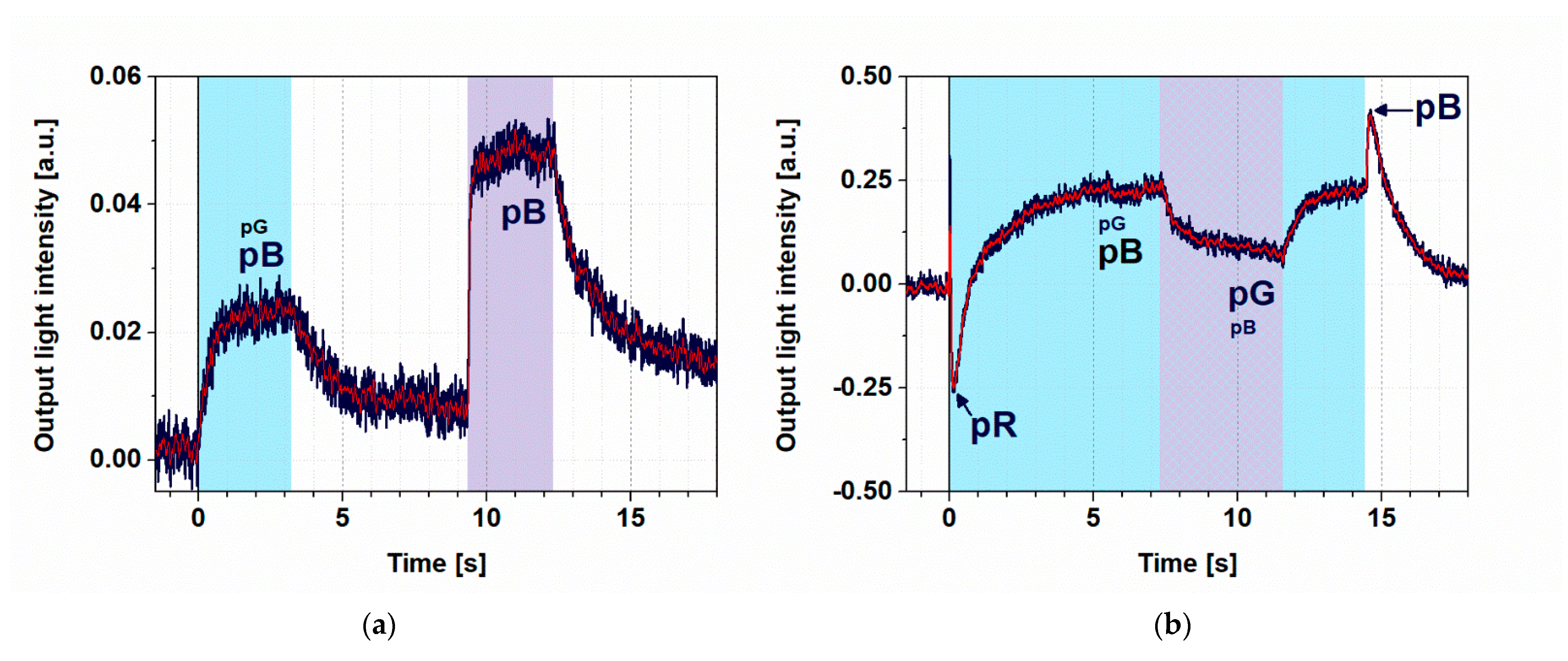
3.2. Demonstration of All-Optical Switching
3.3. Controlling the Photocycle of PYP by Two Excitation Lights
4. Discussion
4.1. Model Calculations
4.2. Evaluation of Kinetics
4.3. On the Opportunity of Increasing Switching Speed
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hales, J.M.; Matichak, J.; Barlow, S.; Ohira, S.; Yesudas, K.; Brédas, J.L.; Perry, J.W.; Marder, S.R. Design of polymethine dyes with large third-order optical nonlinearities and loss figures of merit. Science 2010, 327, 1485–1488. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, P.; Ding, C.; Yang, H.; Gong, Q. Picosecond and low-power all-optical switching based on an organic photonic-bandgap microcavity. Nat. Photonics 2008, 2, 185. [Google Scholar] [CrossRef]
- Fábián, L.; Mathesz, A.; Dér, A. New trends in biophotonics. Acta Biol. Szeged. 2015, 59 (Suppl. S2), 189–202. [Google Scholar]
- Dér, A.; Valkai, S.; Fábián, L.; Ormos, P.; Ramsden, J.J.; Wolff, E.K. Integrated Optical Switching Based on the Protein Bacteriorhodopsin†. Photochem. Photobiol. 2007, 83, 393–396. [Google Scholar] [CrossRef]
- Ormos, P.; Fábián, L.; Oroszi, L.; Wolff, E.K.; Ramsden, J.J.; Dér, A. Protein-based integrated optical switching and modulation. Appl. Phys. Lett. 2002, 80, 4060–4062. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Sethi, P.; Topolancik, J.; Vollmer, F. All-optical reversible logic gates with optically controlled bacteriorhodopsin protein-coated microresonators. Adv. Opt. Technol. 2012, 727206. [Google Scholar] [CrossRef] [Green Version]
- Fábián, L.; Heiner, Z.; Mero, M.; Kiss, M.; Wolff, E.K.; Ormos, P.; Osvay, K.; Dér, A. Protein-based ultrafast photonic switching. Opt. Express 2011, 19, 18861–18870. [Google Scholar] [CrossRef]
- Mathesz, A.; Fábián, L.; Valkai, S.; Alexandre, D.; Marques, P.V.; Ormos, P.; Wolff, E.K.; Dér, A. High-speed integrated optical logic based on the protein bacteriorhodopsin. Biosens. Bioelectron. 2013, 46, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1985, 806, 175–183. [Google Scholar] [CrossRef]
- Meyer, T.E.; Yakali, E.; Cusanovich, M.A.; Tollin, G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry 1987, 26, 418–423. [Google Scholar] [CrossRef]
- Krekic, S.; Zakar, T.; Gombos, Z.; Valkai, S.; Mero, M.; Zimányi, L.; Heiner, Z.; Dér, A. Nonlinear Optical Investigation of Microbial Chromoproteins. Front. Plant Sci. 2020, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- Krekic, S.; Nagy, D.; Taneva, S.G.; Fábián, L.; Zimányi, L.; Dér, A. Spectrokinetic characterization of photoactive yellow protein films for integrated optical applications. Eur. Biophys. J. 2019, 48, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, J.; van Stokkum, I.H.; Crielaard, W.; Hellingwerf, K.J. Kinetics of and intermediates in a photocycle branching reaction of the photoactive yellow protein from Ectothiorhodospira halophila. FEBS Lett. 1999, 458, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Fábián, L.; Wolff, E.K.; Oroszi, L.; Ormos, P.; Dér, A. Fast integrated optical switching by the protein bacteriorhodopsin. Appl. Phys. Lett. 2010, 97, 023305. [Google Scholar] [CrossRef]
- Korposh, S.; James, S.; Partridge, M.; Sichka, M.; Tatam, R. All-optical switching based on optical fibre long period gratings modified bacteriorhodopsin. Opt. Laser Technol. 2018, 101, 162–171. [Google Scholar] [CrossRef]
- Topolancik, J.; Vollmer, F. All-optical switching in the near infrared with bacteriorhodopsin-coated microcavities. Appl. Phys. Lett. 2006, 89, 184103. [Google Scholar] [CrossRef] [Green Version]
- Mathesz, A.; Valkai, S.; Újvárosy, A.; Aekbote, B.; Sipos, O.; Stercz, B.; Kocsis, B.; Szabó, D.; Dér, A. Integrated optical biosensor for rapid detection of bacteria. Optofluid. Microfluid. Nanofluid. 2015, 2, 15–21. [Google Scholar] [CrossRef]
- Jankovics, H.; Kovacs, B.; Saftics, A.; Gerecsei, T.; Tóth, É.; Szekacs, I.; Vonderviszt, F.; Horvath, R. Grating-coupled interferometry reveals binding kinetics and affinities of Ni ions to genetically engineered protein layers. Sci. Rep. 2020, 10, 22253. [Google Scholar] [CrossRef]
- Dér, A.; Valkai, S.; Mathesz, A.; Andó, I.; Wolff, E.K.; Ormos, P. Protein-based all-optical sensor device. Sens. Actuators B: Chem. 2010, 151, 26–29. [Google Scholar] [CrossRef]
- Abdulhalim, I. Model for Photoinduced Defects and Photorefractivity in Optical Fibers. App. Phys. Lett. 1995, 66, 3248–3250. [Google Scholar] [CrossRef]
- Abdulhalim, I. Kinetic model for photoinduced and thermally induced creation and annihilation of metastable defects in hydrogenated amorphous silicon. J. Appl. Phys. 1995, 77, 1897–1901. [Google Scholar] [CrossRef]
- Abdulhalim, I.; Gelbaor, M.; Klebanov, M.; Lyubin, V. Photoinduced phenomena in nano-dimensional glassy As2S3 films. Opt. Mater. Express 2011, 1, 1192–1201. [Google Scholar] [CrossRef]
- Fábián, L.; Krekic, S.; Tóth-Boconádi, R.; Taneva, S.G.; Bálint, A.M.; Nánai, L.; Dér, A. Integrated optical investigation of two light-sensitive proteins. AIP Conf. Proc. 2017, 1796, 040001. [Google Scholar]
- Konold, P.E.; Arik, E.; Weißenborn, J.; Arents, J.C.; Hellingwerf, K.J.; van Stokkum, I.H.; Kennis, J.T.; Groot, M.L. Confinement in crystal lattice alters entire photocycle pathway of the Photoactive Yellow Protein. Nat. Commun. 2020, 11, 4248. [Google Scholar] [CrossRef] [PubMed]
- Khoroshyy, P.; Dér, A.; Zimányi, L. Effect of Hofmeister cosolutes on the photocycle of photoactive yellow protein at moderately alkaline pH. J. Photochem. Photobiol. B Biol. 2013, 120, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Hajdu, K.; Gergely, C.; Martin, M.; Zimányi, L.; Agarwal, V.; Palestino, G.; Hernádi, K.; Németh, Z.; Nagy, L. Light-harvesting bio-nanomaterial using porous silicon and photosynthetic reaction center. Nanoscale Res. Lett. 2012, 7, 400. [Google Scholar] [CrossRef] [Green Version]
- Palestino, G.; Martin, M.; Agarwal, V.; Legros, R.; Cloitre, T.; Zimányi, L.; Gergely, C. Detection and light enhancement of glucose oxidase adsorbed on porous silicon microcavities. Phys. Status Solidi C 2009, 6, 1624–1628. [Google Scholar] [CrossRef]
- Lin, G.R.; Su, S.P.; Wu, C.L.; Lin, Y.H.; Huang, B.J.; Wang, H.Y.; Tsai, C.T.; Wu, C.I.; Chi, Y.C. Si-rich SiNx based Kerr switch enables optical data conversion up to 12 Gbit/s. Sci. Rep. 2015, 5, 9611. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrovszki, D.; Krekic, S.; Valkai, S.; Heiner, Z.; Dér, A. All-Optical Switching Demonstrated with Photoactive Yellow Protein Films. Biosensors 2021, 11, 432. https://doi.org/10.3390/bios11110432
Petrovszki D, Krekic S, Valkai S, Heiner Z, Dér A. All-Optical Switching Demonstrated with Photoactive Yellow Protein Films. Biosensors. 2021; 11(11):432. https://doi.org/10.3390/bios11110432
Chicago/Turabian StylePetrovszki, Dániel, Szilvia Krekic, Sándor Valkai, Zsuzsanna Heiner, and András Dér. 2021. "All-Optical Switching Demonstrated with Photoactive Yellow Protein Films" Biosensors 11, no. 11: 432. https://doi.org/10.3390/bios11110432
APA StylePetrovszki, D., Krekic, S., Valkai, S., Heiner, Z., & Dér, A. (2021). All-Optical Switching Demonstrated with Photoactive Yellow Protein Films. Biosensors, 11(11), 432. https://doi.org/10.3390/bios11110432







