Enhanced Molecular Infrared Spectroscopy Employing Bilayer Graphene Acoustic Plasmon Resonator
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Excitation of Bilayer Acoustic Graphene Plasmons
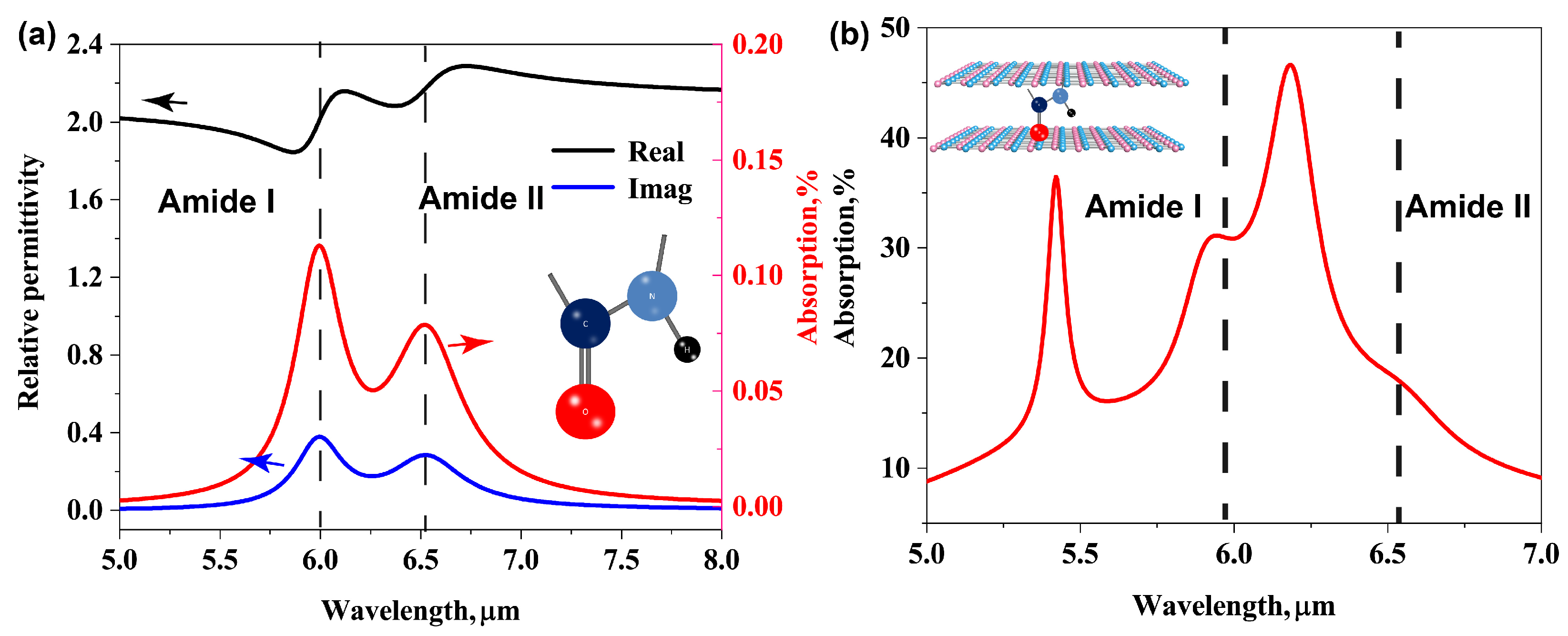
3.2. Protein Molecular Vibrational Fingerprints
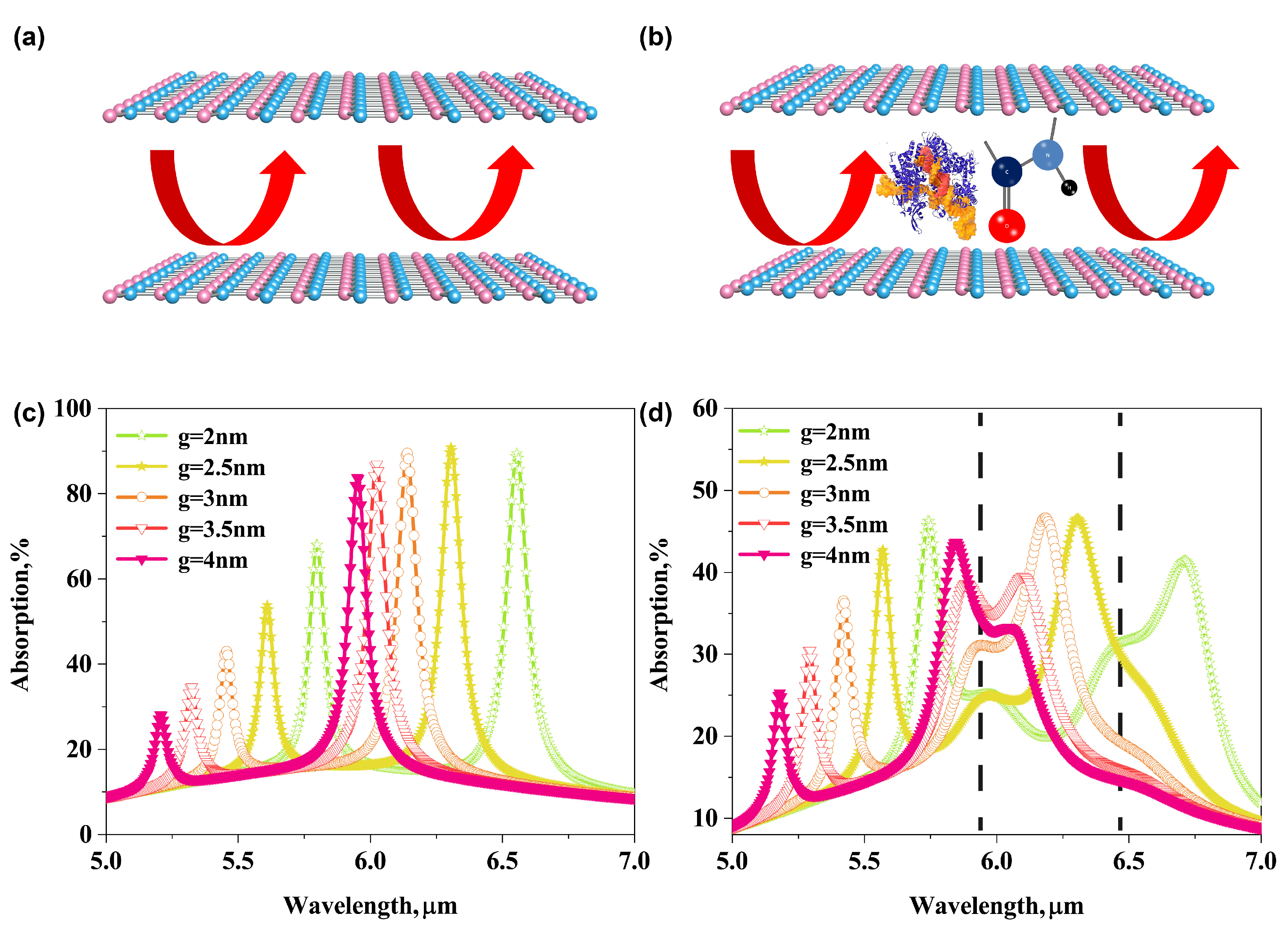
3.3. Enhanced Infrared Absorption Spectroscopy
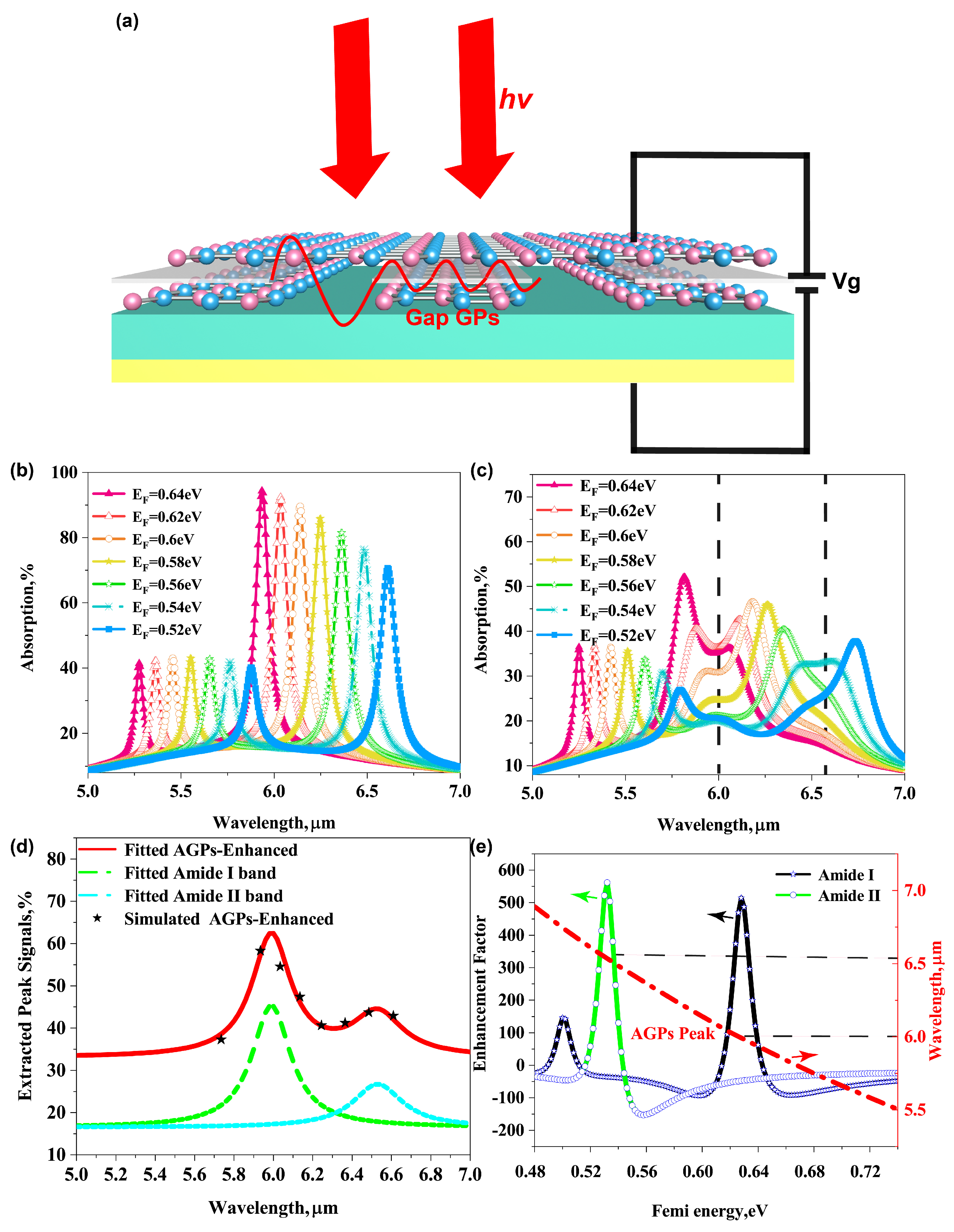
3.4. Detection of Molecular Fingerprints by Tunable Graphene Acoustic Plasmons
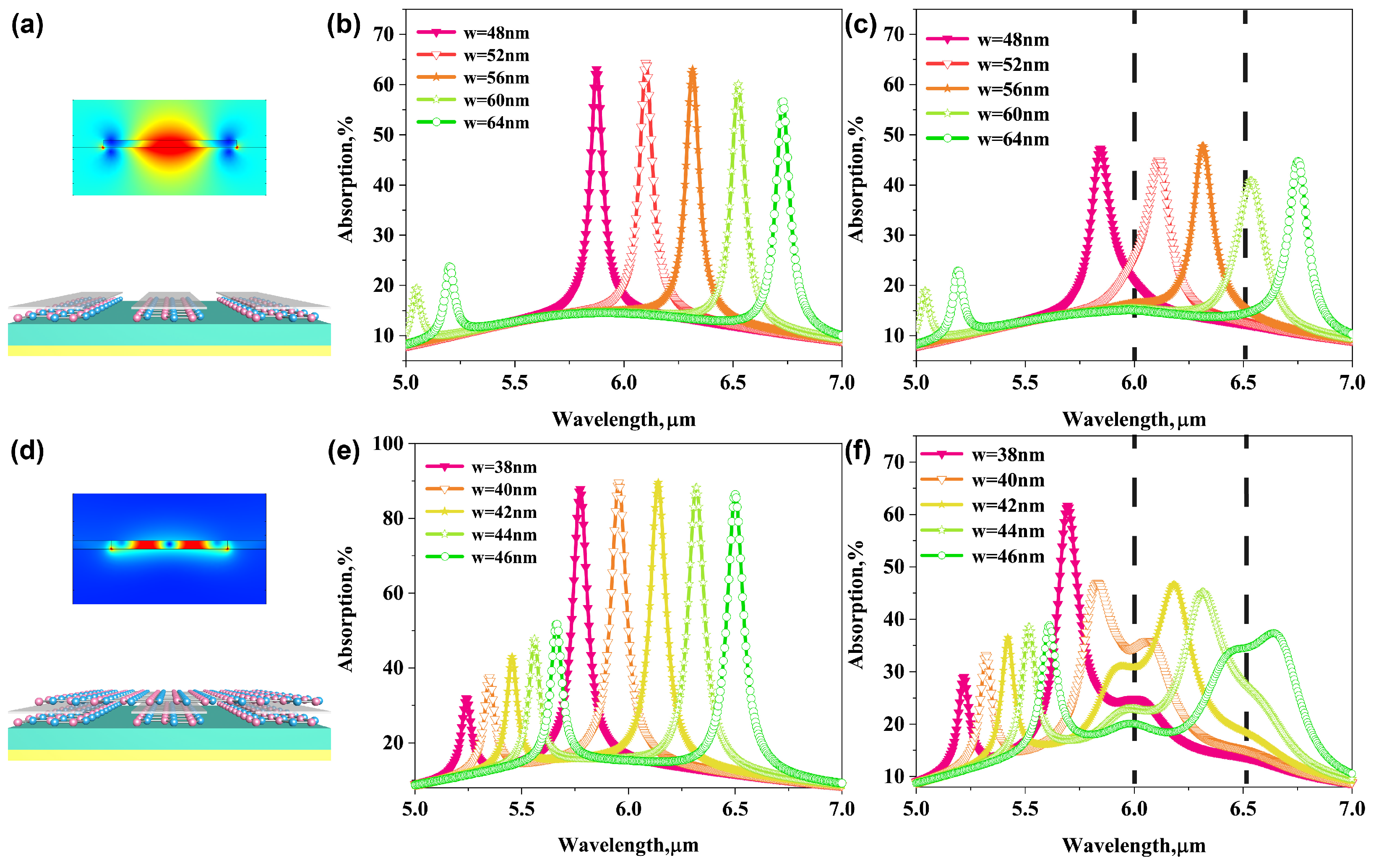
3.5. Comparison between Bilayer AGP Biosensor and GP Biosnesor
3.6. Discussions about the Fabrication of the Proposed Device
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Peak | A | w | ||
|---|---|---|---|---|
| Amide I | 0.165 | 0.109 | 5.990 | 0.241 |
| Amide II | 0.165 | 0.049 | 6.530 | 0.305 |
References
- Yang, X.; Sun, Z.; Low, T.; Hu, H.; Guo, X.; García de Abajo, F.J.; Avouris, P.; Dai, Q. Nanomaterial-Based Plasmon-Enhanced infrared spectroscopy. Adv. Mater. 2018, 30, 1704896. [Google Scholar] [CrossRef]
- Grieco, C.; Kohl, F.R.; Hanes, A.T.; Kohler, B. Probing the heterogeneous structure of eumelanin using ultrafast vibrational fingerprinting. Nat. Commun. 2020, 11, 4569. [Google Scholar] [CrossRef]
- Cai, Y.; Hang, Y.; Zhou, Y.; Zhu, J.; Yang, J.; Wang, X. Graphene-Based Biosensors for Detection of Composite Vibrational Fingerprints in the Mid-Infrared Region. Nanomaterials 2019, 9, 1496. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.; Kepesidis, K.V.; Voronina, L.; Boi, M.; Igman, M. Stability of person-specific blood-based infrared molecular fingerprints opens up prospects for health monitoring. Nat. Commun. 2021, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- Be, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar]
- Xu, S.; Lu, H.; Ference, C.; Qiu, G.; Liang, X. Rapid Nondestructive Detection of Water Content and Granulation in Postharvest “Shatian” Pomelo Using Visible/Near-Infrared Spectroscopy. Biosensors 2020, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querido, W.; Kandel, S.; Pleshko, N. Applications of Vibrational Spectroscopy for Analysis of Connective Tissues. Molecules 2021, 26, 922. [Google Scholar] [CrossRef]
- Hong, Q.; Luo, J.; Wen, C.; Zhang, J.; Yuan, X. Hybrid metal-graphene plasmonic sensor for multi-spectral sensing in both near- and mid-infrared ranges. Opt. Express 2019, 27, 35914. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Z.; Lin, S.; Jiang, S.; Guo, S. Low-cost flexible plasmonic nanobump metasurfaces for label-free sensing of serum tumor marker. Biosens. Bioelectron 2019, 150, 111905. [Google Scholar] [CrossRef]
- Badshah, M.A.; Na, Y.K.; Zia, A.W.; Abbas, N.; Saleem, M.W. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- Chen, J.; Badioli, M.; Alonso-González, P.; Thongrattanasiri, S.; Huth, F.; Osmond, J.; Spasenovi, M.; Centeno, A.; Pesquera, A.; Godignon, P. Optical nano-imaging of gate-tunable graphene plasmons. Nature 2012, 487, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Fei, Z.; Rodin, A.S.; Andreev, G.O.; Bao, W.; McLeod, A.S.; Wagner, M.; Zhang, L.M.; Zhao, Z.; Thiemens, M.; Dominguez, G.; et al. Gate-tuning of graphene plasmons revealed by infrared nano-imaging. Nature 2012, 487, 82–85. [Google Scholar] [CrossRef]
- Koppens, F.H.; Chang, D.E.; García de Abajo, F.J. Graphene plasmonics: A platform for strong light–matter interactions. Nano Lett. 2011, 11, 3370–3377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigo, D.; Limaj, O.; Janner, D.; Etezadi, D.; Javier, G.; de Abajo, F.; Pruneri, V.; Altug, H. Mid-Infrared Plasmonic Biosensing with Graphene. Science 2015, 349, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Yang, X.; Zhai, F.; Hu, D.; Liu, R.; Liu, K.; Sun, Z.; Dai, Q. Far-field nanoscale infrared spectroscopy of vibrational fingerprints of molecules with graphene plasmons. Nat. Commun. 2016, 7, 12334. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Yu, L.; Lei, W. Mid-infrared sensing of molecular vibrational modes with tunable graphene plasmons. Opt. Lett. 2017, 42, 2066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, C.; Ou, J.Y.; Liu, Q.H. Perfect light absorption in graphene by two unpatterned dielectric layers and potential applications. Carbon 2019, 142, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Yang, X.; Guo, X.; Khaliji, K.; Biswas, S.R.; de Abajo, F.J.G.; Low, T.; Sun, Z.; Dai, Q. Gas identification with graphene plasmons. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nong, J.; Tang, L.; Lan, G.; Luo, P.; Wei, W. Combined Visible Plasmons of Ag Nanoparticles and Infrared Plasmons of Graphene Nanoribbons for High-Performance Surface-Enhanced Raman and Infrared Spectroscopies. Small 2020, 17, 2004640. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, B.; Wei, G. Two-Dimensional Material-Based Colorimetric Biosensors: A Review. Biosensors 2021, 11, 259. [Google Scholar] [CrossRef]
- Hwang, E.H.; Sarma, S.D. Plasmon modes of spatially separated double-layer graphene. Phys. Rev. 2009, 80, 205405.1–205405.5. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, D.; Tittl, A.; Limaj, O.; Abajo, F.; Pruneri, V.; Altug, H. Double-layer graphene for enhanced tunable infrared plasmonics. Light Sci. Appl. 2017, 6, e16277. [Google Scholar] [CrossRef] [Green Version]
- Pisarra, M.; Sindona, A.; Riccardi, P.; Silkin, V.M.; Pitarke, J.M. Acoustic plasmons in extrinsic free-standing graphene. New J. Phys. 2014, 16, 083003. [Google Scholar] [CrossRef] [Green Version]
- Alonso-González, P.; Nikitin, A.Y.; Gao, Y.; Woessner, A.; Lundeberg, M.B.; Principi, A.; Forcellini, N.; Yan, W.; Vélez, S.; Huber, A.J. Acoustic terahertz graphene plasmons revealed by photocurrent nanoscopy. Nat. Nanotechnol. 2017, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Epstein, I.; Alcaraz, D.; Huang, Z.; Pusapati, V.V.; Hugonin, J.P.; Kumar, A.; Deputy, X.M.; Khodkov, T.; Rappoport, T.G.; Hong, J.Y. Far-field excitation of single graphene plasmon cavities with ultracompressed mode volumes. Science 2020, 368, 1219. [Google Scholar] [CrossRef] [PubMed]
- Chunchao, W.; Xingqiao, C.; Jianfa, Z.; Wei, X.; Jie, L.; Yingqiu, Z.; Zhihong, Z.; Shiqiao, Q.; Xiaodong, Y. Far-Field Excitation of Acoustic Graphene Plasmons with a Metamaterial Absorber. Adv. Photonics Res. 2020, 2, 2000066. [Google Scholar]
- Alcaraz Iranzo, D.; Nanot, S.; Dias, E.; Epstein, I.; Peng, C.; Efetov, D.K.; Lundeberg, M.B.; Parret, R.; Osmond, J.; Hong, J.Y. Probing the Ultimate Plasmon Confinement Limits with a Van der Waals heterostructure. Science 2018, 360, 291–295. [Google Scholar] [CrossRef] [Green Version]
- Principi, A.; Van Loon, E.; Polini, M.; Katsnelson, M.I. Confining graphene plasmons to the ultimate limit. Phys. Rev. B 2018, 98, 035427. [Google Scholar] [CrossRef] [Green Version]
- Shu, C.; Autore, M.; Jian, L.; Li, P.; Nikitin, A.Y. Acoustic Graphene Plasmon Nanoresonators for Field Enhanced Infrared Molecular Spectroscopy. ACS Photonics 2018, 4, 3089–3097. [Google Scholar]
- Lee, I.H.; Yoo, D.; Avouris, P.; Low, T.; Oh, S.H. Graphene acoustic plasmon resonator for ultrasensitive infrared spectroscopy. Nat. Nanotechnol. 2019, 14, 313. [Google Scholar] [CrossRef] [PubMed]
- Voronin, K.V.; Aguirreche, U.A.; Hillenbrand, R.; Volkov, V.S.; Nikitin, A.Y. Nanofocusing of acoustic graphene plasmon polaritons for enhancing mid-infrared molecular fingerprints. Nanophotonics 2020, 9, 2089–2095. [Google Scholar] [CrossRef]
- Hanson, G.W. Quasi-transverse electromagnetic modes supported by a graphene parallel-plate waveguide. J. Appl. Phys. 2008, 104, 084314. [Google Scholar] [CrossRef]
- Jablan, M.; Buljan, H.; Soljačić, M. Plasmonics in graphene at infrared frequencies. Phys. Rev. B 2009, 80, 245435. [Google Scholar] [CrossRef] [Green Version]
- Nong, J.; Tang, L.; Lan, G.; Luo, P.; Wei, W. Enhanced Graphene Plasmonic Mode Energy for Highly Sensitive Molecular Fingerprint Retrieval. Laser Photonics Rev. 2020, 15, 2000300. [Google Scholar] [CrossRef]
- Tali, S.; Zhou, W. Multiresonant plasmonics with spatial mode overlap: Overview and outlook. Nanophotonics 2019, 8, 1199–1225. [Google Scholar] [CrossRef]
- Lin, I.T.; Liu, J.M. Enhanced graphene plasmon waveguiding in a layered graphene-metal structure. Appl. Phys. Lett. 2014, 105, 081406. [Google Scholar] [CrossRef]
- Hua, L.U.; Gan, X.; Dong, M.; Zhao, J. Graphene-supported manipulation of surface plasmon polaritons in metallic nanowaveguides. Photonics Res. 2017, 5, 162. [Google Scholar]
- Zhang, J.; Hong, Q.; Zou, J.; He, Y.; Qin, S. Fano-Resonance in Hybrid Metal-Graphene Metamaterial and Its Application as Mid-Infrared Plasmonic Sensor. Micromachines 2020, 11, 268. [Google Scholar] [CrossRef] [Green Version]
- Nong, J.; Wei, W.; Lan, G.; Luo, P.; Tang, L. Resolved Infrared Spectroscopy of Aqueous Molecules Employing Tunable Graphene Plasmons in Otto Prism. Anal. Chem. 2020, 92, 15370–15378. [Google Scholar] [CrossRef]
- Mauriz, E.; Lechuga, L.M. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123. [Google Scholar] [CrossRef]
- Neves, M.; Martín-Yerga, D. Advanced Nanoscale Approaches to Single-(Bio)entity Sensing and Imaging. Biosensors 2018, 8, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundeberg, M.B.; Gao, Y.; Asgari, R.; Tan, C.; Van Duppen, B.; Autore, M.; Alonso-González, P.; Woessner, A.; Watanabe, K.; Taniguchi, T.; et al. Tuning quantum nonlocal effects in graphene plasmonics. Science 2017, 357, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonalves, P.; Christensen, T.; Peres, N.; Jauho, A.P.; Mortensen, N.A. Quantum surface-response of metals revealed by acoustic graphene plasmons. Nat. Commun. 2021, 12, 187–190. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, C.; Luo, J.; Xu, W.; Zhu, Z.; Qin, S.; Zhang, J. Enhanced Molecular Infrared Spectroscopy Employing Bilayer Graphene Acoustic Plasmon Resonator. Biosensors 2021, 11, 431. https://doi.org/10.3390/bios11110431
Wen C, Luo J, Xu W, Zhu Z, Qin S, Zhang J. Enhanced Molecular Infrared Spectroscopy Employing Bilayer Graphene Acoustic Plasmon Resonator. Biosensors. 2021; 11(11):431. https://doi.org/10.3390/bios11110431
Chicago/Turabian StyleWen, Chunchao, Jie Luo, Wei Xu, Zhihong Zhu, Shiqiao Qin, and Jianfa Zhang. 2021. "Enhanced Molecular Infrared Spectroscopy Employing Bilayer Graphene Acoustic Plasmon Resonator" Biosensors 11, no. 11: 431. https://doi.org/10.3390/bios11110431
APA StyleWen, C., Luo, J., Xu, W., Zhu, Z., Qin, S., & Zhang, J. (2021). Enhanced Molecular Infrared Spectroscopy Employing Bilayer Graphene Acoustic Plasmon Resonator. Biosensors, 11(11), 431. https://doi.org/10.3390/bios11110431







