The Effect of Haematocrit on Measurement of the Mid-Infrared Refractive Index of Plasma in Whole Blood
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Modification of Haematocrit
2.2.1. Protocol for Reducing Haematocrit
- Measure initial haematocrit (HCT);
- Calculate additional volume of plasma to reduce HCT to desired level using Equation (1):where is the required change in the volume of plasma, and are the initial and target haematocrits and is the volume of the whole blood sample to be modified. Positive requires plasma to be added; negative means plasma should be removed;
- 3.
- Separate plasma from whole blood stock (Eppendorf centrifuge: 3000 rpm, 10 min).
- 4.
- Add plasma to whole blood and gently mix with rotation mixer for 30 min.
2.2.2. Protocol for increasing haematocrit
- Measure initial HCT;
- Calculate the reduction in volume of plasma required for the desired HCT level using Equation (2);
- Gently centrifuge whole blood (1000 rpm, 15 min) to separate plasma and red blood cells;
- Pipette off the difference in plasma;
- Gently mix with rotation mixer for 30 min.
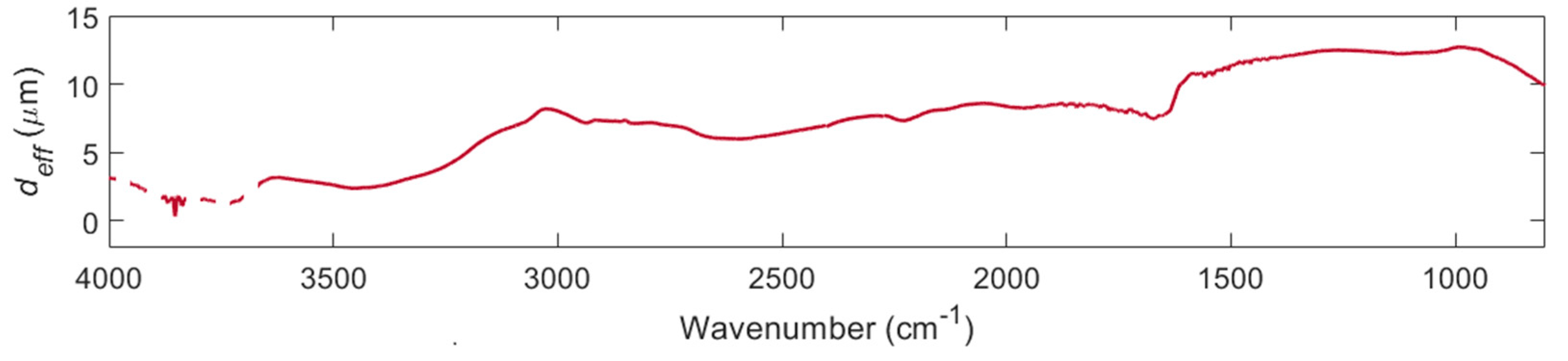
2.3. ATR-FTIR Measurements
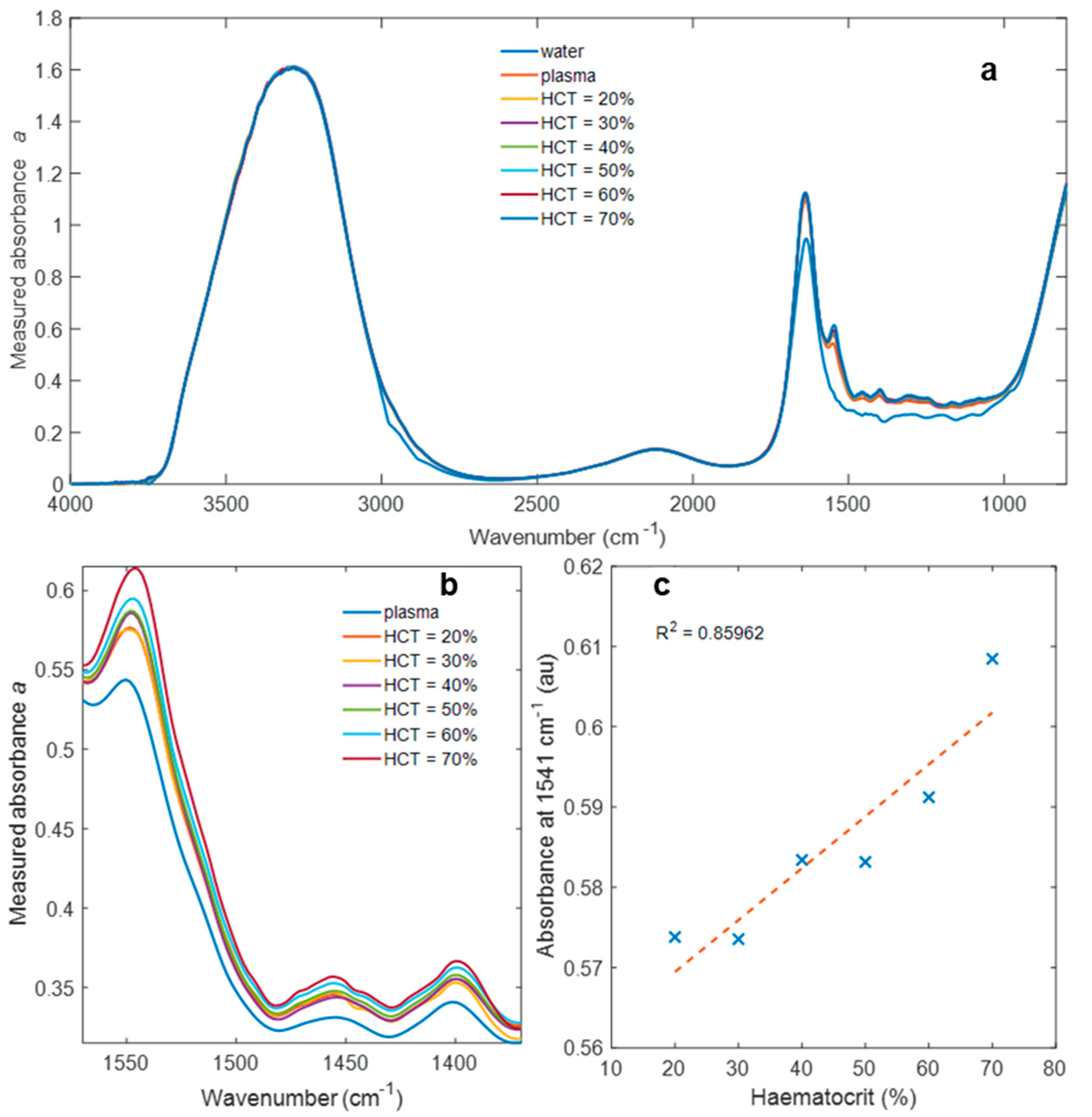
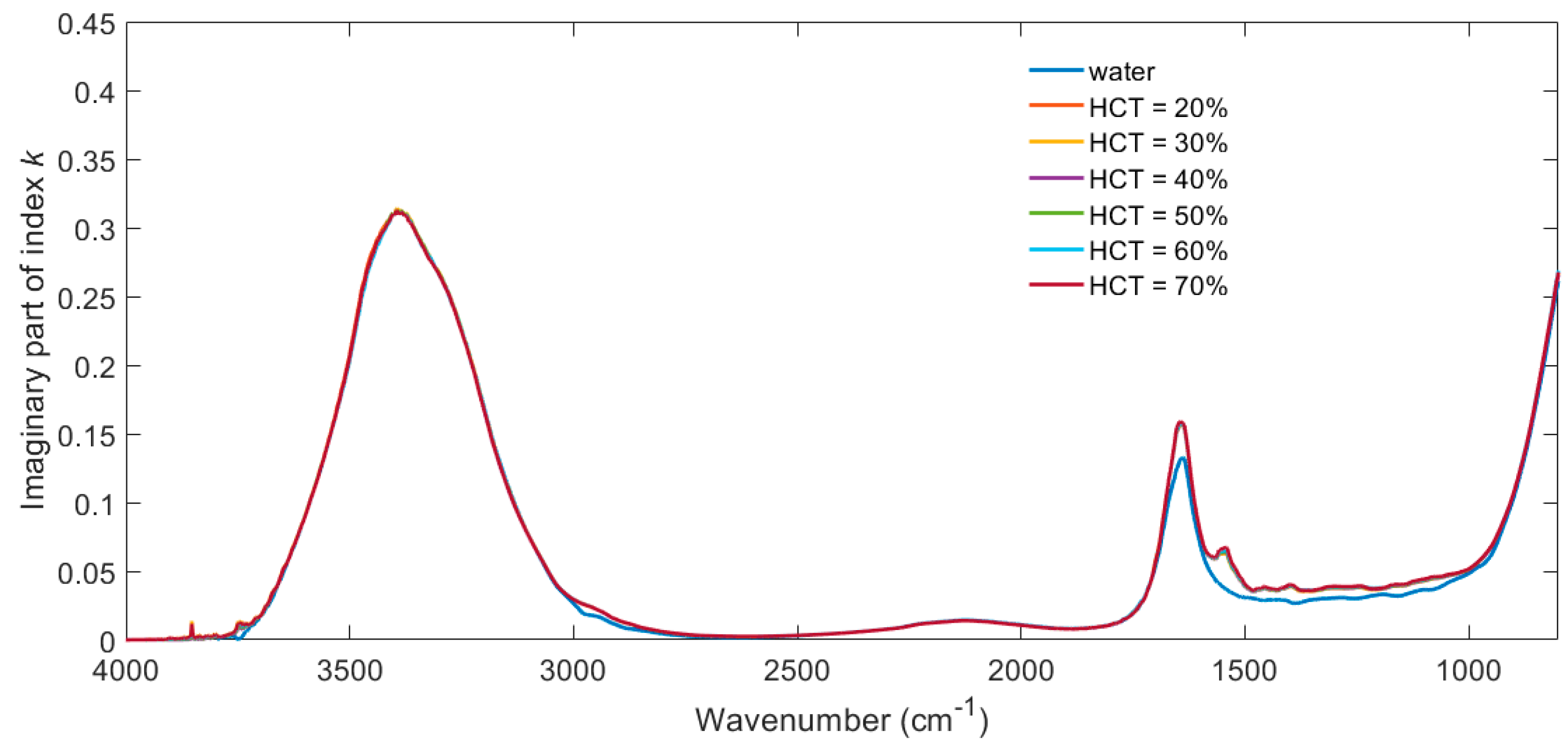
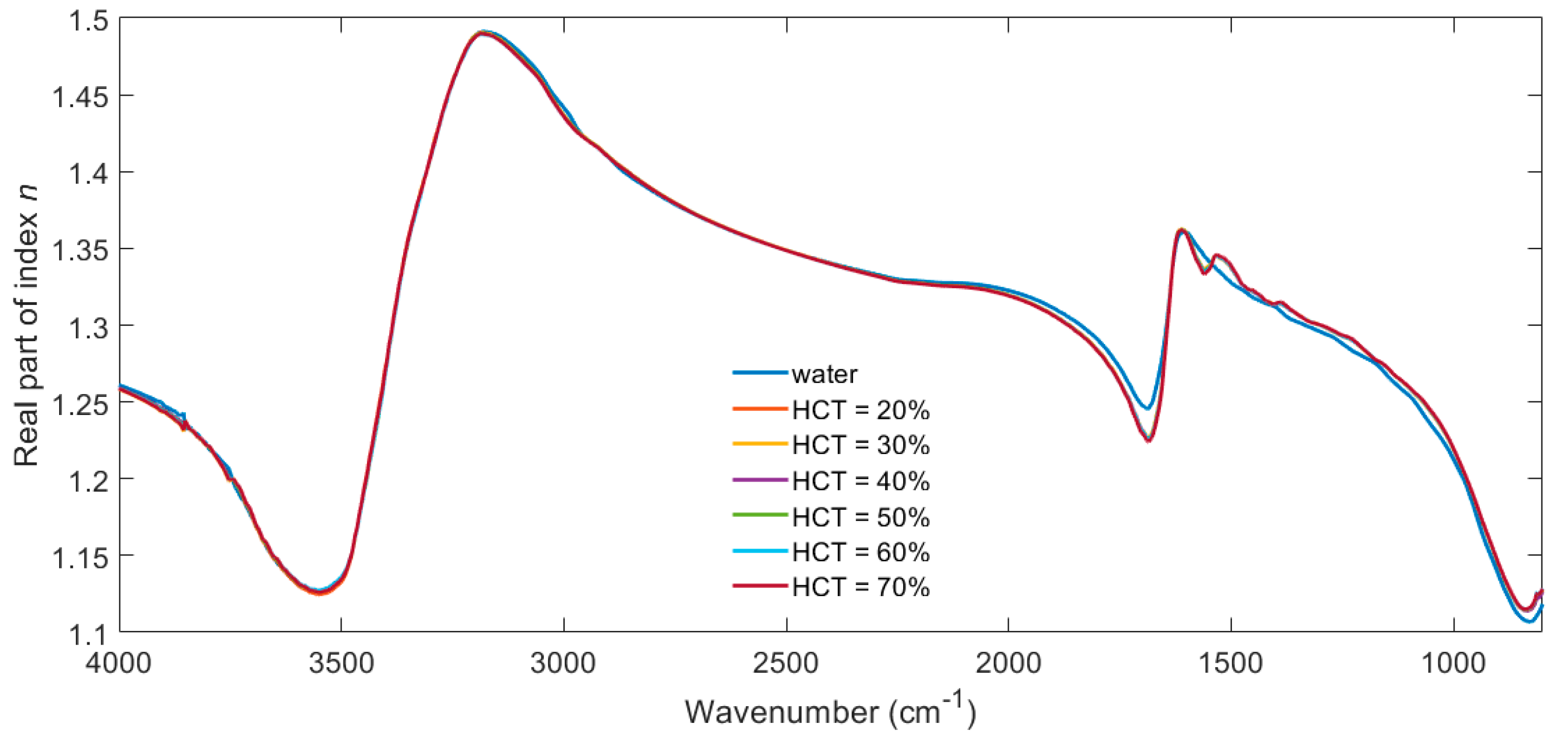
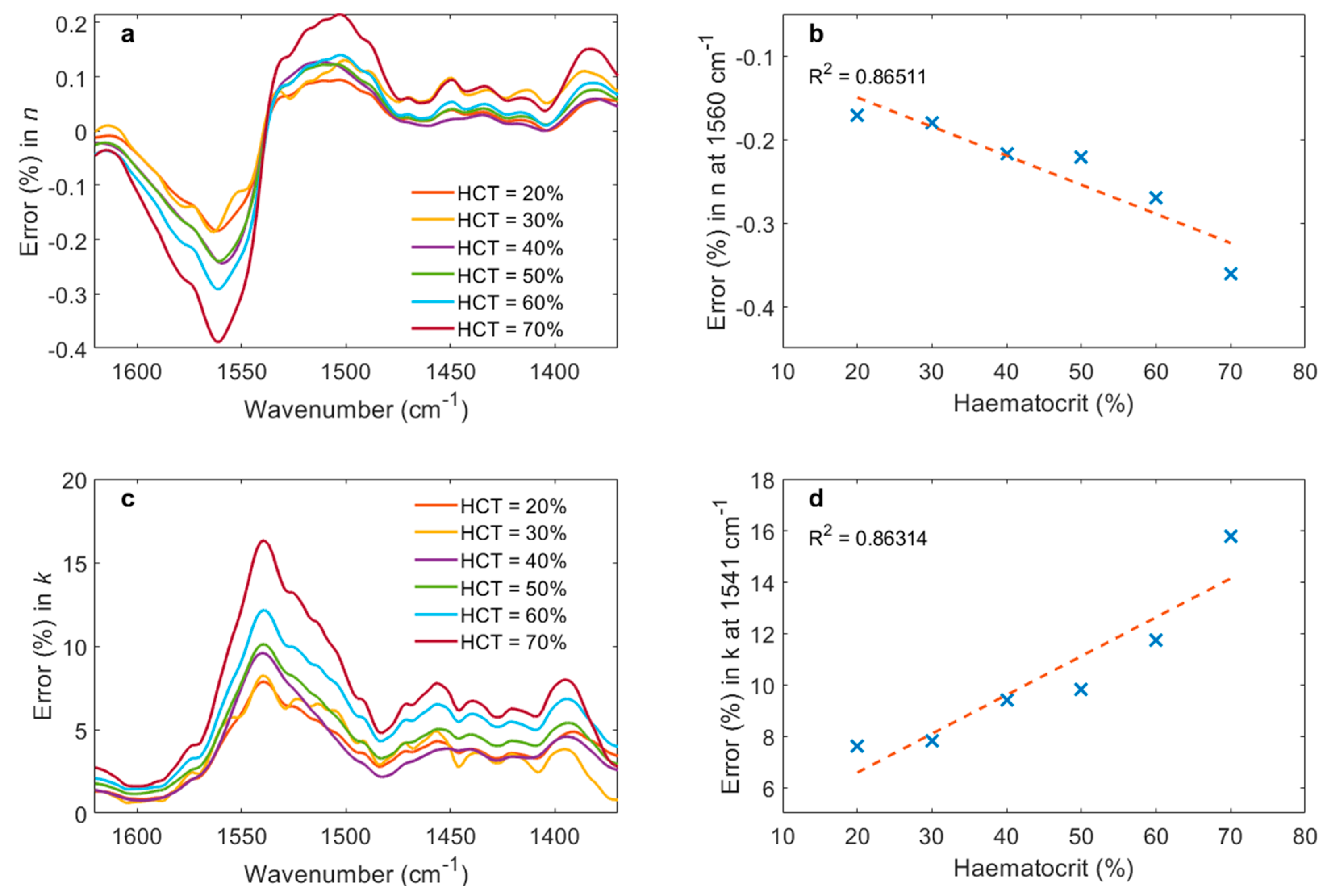
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrick, N.J. Internal Reflection Spectroscopy; Interscience: New York, NY, USA, 1967. [Google Scholar]
- Mirabella, F.M. Internal Reflection Spectroscopy: Theory and Applications; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Tickanen, L.D.; Tejedor-Tejedor, M.I.; Anderson, M.A. Quantitative characterization of aqueous suspensions using variable-angle ATR-FTIR spectroscopy: Determination of optical constants and absorption coefficient spectra. Langmuir 1997, 13, 4829–4836. [Google Scholar] [CrossRef]
- Beullens, K.; Kirsanov, D.; Irudayaraj, J.; Rudnitskaya, A.; Legin, A.; Nicolaï, B.M.; Lammertyn, J. The electronic tongue and ATR–FTIR for rapid detection of sugars and acids in tomatoes. Sens. Actuators B Chem. 2006, 116, 107–115. [Google Scholar] [CrossRef]
- Mittal, V.; Aghajani, A.; Carpenter, L.G.; Gates, J.C.; Butement, J.; Smith, P.G.R.; Wilkinson, J.S.; Murugan, G.S. Fabrication and characterization of high-contrast mid-infrared GeTe4 channel waveguides. Opt. Lett. 2015, 40, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Sieger, M.; Haas, J.; Jetter, M.; Michler, P.; Godejohann, M.; Mizaikoff, B. Mid-Infrared Spectroscopy Platform Based on GaAs/AlGaAs Thin-Film Waveguides and Quantum Cascade Lasers. Anal. Chem. 2016, 88, 2558–2562. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.M.; Vakarin, V.; Gilles, C.; Frigerio, J.; Ballabio, A.; Chaisakul, P.; Roux, X.L.; Alonso-Ramos, C.; Maisons, G.; Vivien, L.; et al. Low-loss Ge-rich Si0.2Ge0.8 waveguides for mid-infrared photonics. Opt. Lett. 2017, 42, 105–108. [Google Scholar] [CrossRef]
- Singh, V.; Lin, P.T.; Patel, N.; Lin, H.; Li, L.; Zou, Y.; Deng, F.; Ni, C.; Hu, J.; Giammarco, J.; et al. Mid-infrared materials and devices on a Si platform for optical sensing. Sci. Technol. Adv. Mater. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Su, P.; Kimerling, L.; Agarwal, A.; Anthony, B.W. Towards on-chip mid infrared photonic aerosol spectroscopy. Appl. Phys. Lett. 2018, 113, 231107. [Google Scholar] [CrossRef]
- Francis, D.; Hodgkinson, J.; Walton, C.; Sizer, J.; Black, P.; Livingstone, B.; Fowler, D.P.; Patel, M.K.; Tatam, R.P. Mid-IR spectroscopic instrumentation for point-of-care diagnosis using a hollow silica waveguide gas cell. In Proceedings of the Progress in Biomedical Optics and Imaging—Proceedings of SPIE, San Francisco, CA, USA, 28 January–2 February 2017. [Google Scholar]
- Ottonello-Briano, F.; Errando-Herranz, C.; Rödjegård, H.; Martin, H.; Sohlström, H.; Gylfason, K.B. Carbon dioxide absorption spectroscopy with a mid-infrared silicon photonic waveguide. Opt. Lett. 2020, 45, 109–112. [Google Scholar] [CrossRef]
- Mittal, V.; Nedeljkovic, M.; Carpenter, L.G.; Khokhar, A.Z.; Chong, H.M.H.; Mashanovich, G.Z.; Bartlett, P.N.; Wilkinson, J.S. Waveguide absorption spectroscopy of bovine serum albumin in the mid-infrared fingerprint region. ACS Sens. 2019, 4, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- López-Lorente, Á.I.; Izquierdo, J.; Kranz, C.; Mizaikoff, B. Boron-doped diamond modified with gold nanoparticles for the characterization of bovine serum albumin protein. Vib. Spectrosc. 2017, 91, 147–156. [Google Scholar] [CrossRef][Green Version]
- Sportelli, M.C.; Tütüncü, E.; Picca, R.A.; Valentini, M.; Valentini, A.; Kranz, C.; Mizaikoff, B.; Barth, H.; Cioffi, N. Inhibiting P. fluorescens biofilms with fluoropolymer-embedded silver nanoparticles: An in-situ spectroscopic study. Sci. Rep. 2017, 7, 11870. [Google Scholar] [CrossRef]
- Heath, C.; Myers, M.; Pejcic, B. The Effect of Pressure and Temperature on Mid-Infrared Sensing of Dissolved Hydrocarbons in Water. Anal. Chem. 2017, 89, 13391–13397. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Müller, A.; Sykora, L.; Mizaikoff, B. Analytical performance of μ-groove silicon attenuated total reflection waveguides. Analyst 2019, 144, 3398–3404. [Google Scholar] [CrossRef]
- Li, W.; Anantha, P.; Lee, K.H.; Qiu, H.D.; Guo, X.; Goh, S.C.K.; Zhang, L.; Wang, H.; Soref, R.A.; Tan, C.S. Spiral Waveguides on Germanium-on-Silicon Nitride Platform for Mid-IR Sensing Applications. IEEE Photonics J. 2018, 10. [Google Scholar] [CrossRef]
- Mittal, V.; Nedeljkovic, M.; Rowe, D.J.; Murugan, G.S.; Wilkinson, J.S. Chalcogenide glass waveguides with paper-based fluidics for mid-infrared absorption spectroscopy. Opt. Lett. 2018, 43, 2913–2916. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.J.; Smith, D.; Wilkinson, J.S. Complex refractive index spectra of whole blood and aqueous solutions of anticoagulants, analgesics and buffers in the mid-infrared. Sci. Rep. 2017, 7, 7356. [Google Scholar] [CrossRef] [PubMed]
- Ferner, R.E.; Dear, J.W.; Bateman, D.N. Management of paracetamol poisoning. BMJ 2011, 342, d2218. [Google Scholar] [CrossRef] [PubMed]
- Dargan, P.; Wallace, C.; Jones, A. An evidence based flowchart to guide the management of acute salicylate (aspirin) overdose. Emerg. Med. J. 2002, 19, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.K.; Meloni, D.; Olivo, F.; Märtlbauer, E.; Dietrich, R.; Niessner, R.; Seidel, M. Validation procedure for multiplex antibiotic immunoassays using flow-based chemiluminescence microarrays. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2017; Volume 1518, pp. 195–212. [Google Scholar]
- Pickering, M.K.; Brown, S.D. Assays for determination of ertapenem for applications in therapeutic drug monitoring, pharmacokinetics and sample stability. Biomed. Chromatogr. 2014, 28, 1525–1531. [Google Scholar] [CrossRef]
- Niessen, W.M.A. Analysis of antibiotics by liquid chromatography-mass spectrometry. J. Chromatogr. A 1998, 812, 53–75. [Google Scholar] [CrossRef]
- Rehm, S.; Rentsch, K.M. LC-MS/MS method for nine different antibiotics. Clin. Chim. Acta 2020, 511, 360–367. [Google Scholar] [CrossRef]
- Mitchell, G.L. Absorption Spectroscopy in Scattering Samples Using Integrated Optics. IEEE J. Quantum Electron. 1977, 13, 173–176. [Google Scholar] [CrossRef]
- Midwinter, J.E. On the use of optical waveguide techniques for internal reflection spectroscopy. IEEE J. Quantum Electron. 1971, 7, 339–344. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 21 October 2021).
- European Medicines Agency. Guideline on Bioanalytical Method Validation. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 25 October 2021).
- Jung, S.M.; Kim, Y.-J.; Ryoo, S.M.; Kim, W.Y. Relationship between low hemoglobin levels and mortality in patients with septic shock. Acute Crit Care 2019, 34, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, R.; Villena, M. Normal hematological values for healthy persons living at 4000 meters in Bolivia. High Alt. Med. Biol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Paediatrics and Child Health. New RCPCH Reference Ranges. Available online: https://www.rcpch.ac.uk/sites/default/files/rcpch/HTWQ/Reference%20ranges%20Jan%2018.pdf (accessed on 30 April 2021).
- Hale, G.M.; Querry, M.R. Optical Constants of Water in the 200-nm to 200-μm Wavelength Region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Ali, L.; Mohammed, M.U.; Khan, M.; Yousuf, A.H.B.; Chowdhury, M.H. High-Quality Optical Ring Resonator-Based Biosensor for Cancer Detection. IEEE Sens. J. 2020, 20, 1867–1875. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Shirkani, H. Highly sensitive mid-infrared SPR biosensor for a wide range of biomolecules and biological cells based on graphene-gold grating. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 114005. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Xu, X.; Zhang, Y.; Li, B. Red-Blood-Cell-Based Microlens: Application to Single-Cell Membrane Imaging and Stretching. ACS Appl. Bio Mater. 2019, 2, 2889–2895. [Google Scholar] [CrossRef]
- Wieneke, S.; Gerhard, C. Tissue optics and laser–tissue interactions. In Lasers in Medical Diagnosis and Therapy; IOP Publishing: Bristol, UK, 2018. [Google Scholar] [CrossRef]
- Smolyanskaya, O.A.; Lazareva, E.N.; Nalegaev, S.S.; Petrov, N.V.; Zaytsev, K.I.; Timoshina, P.A.; Tuchina, D.K.; Toropova, Y.G.; Kornyushin, O.V.; Babenko, A.Y.; et al. Multimodal Optical Diagnostics of Glycated Biological Tissues. Biochemistry 2019, 84, 124–143. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Watach, M.J.; Borovetz, H.S. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin. Hemorheol. Microcirc. 1999, 21, 357–363. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowe, D.J.; Owens, D.R.; Parker, S.L.; Faust, S.N.; Wilkinson, J.S.; Mashanovich, G.Z. The Effect of Haematocrit on Measurement of the Mid-Infrared Refractive Index of Plasma in Whole Blood. Biosensors 2021, 11, 417. https://doi.org/10.3390/bios11110417
Rowe DJ, Owens DR, Parker SL, Faust SN, Wilkinson JS, Mashanovich GZ. The Effect of Haematocrit on Measurement of the Mid-Infrared Refractive Index of Plasma in Whole Blood. Biosensors. 2021; 11(11):417. https://doi.org/10.3390/bios11110417
Chicago/Turabian StyleRowe, David J., Daniel R. Owens, Suzanne L. Parker, Saul N. Faust, James S. Wilkinson, and Goran Z. Mashanovich. 2021. "The Effect of Haematocrit on Measurement of the Mid-Infrared Refractive Index of Plasma in Whole Blood" Biosensors 11, no. 11: 417. https://doi.org/10.3390/bios11110417
APA StyleRowe, D. J., Owens, D. R., Parker, S. L., Faust, S. N., Wilkinson, J. S., & Mashanovich, G. Z. (2021). The Effect of Haematocrit on Measurement of the Mid-Infrared Refractive Index of Plasma in Whole Blood. Biosensors, 11(11), 417. https://doi.org/10.3390/bios11110417



