A Review of Capillary Pressure Control Valves in Microfluidics
Abstract
1. Introduction
2. Categories of CPCV
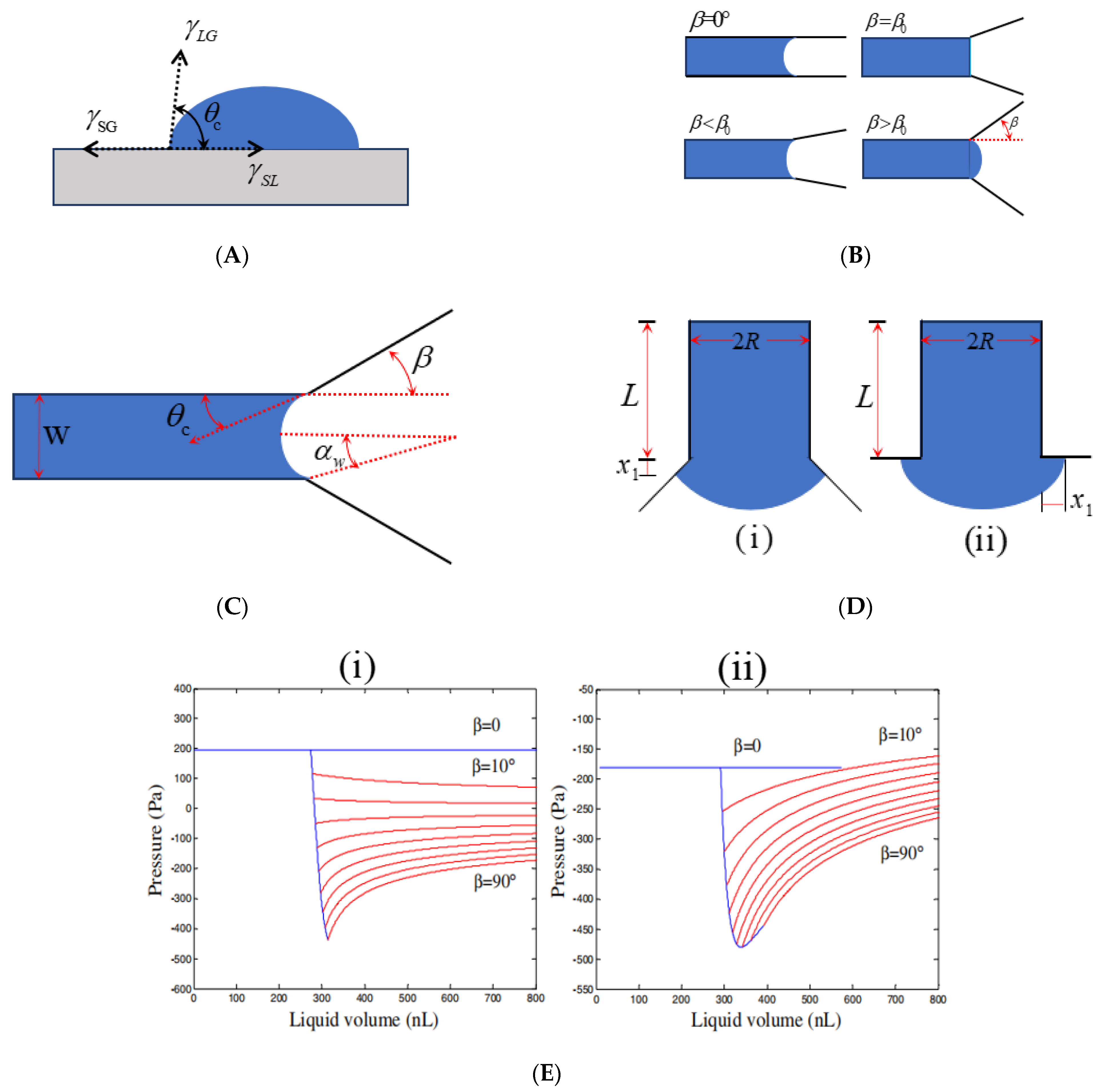
2.1. Surface Tension (γ)
2.2. Contact Angle (θ)
2.2.1. Hydrophobic/Hydrophilic
2.2.2. Material Properties
| Materials | Advantages | Weaknesses | Contact Angles with Water (°) |
|---|---|---|---|
| Silicon | Chemical inertness Smooth surface Mature technology Easy to mass production | Fragile High cost Opaque Complex surface chemistry | Silica (super-hydrophobic): 161 [61] Silica (conventional): 24.5 [61] Silicon (FDTS): 113.7 ± 3.1 [62] Silicon (DDMS):105 [63] |
| Glass | Good electroosmotic Good optical properties Easy for surface treatment | Fragile, high cost Bonding difficult Difficulty in a large aspect ratio | Glass (uncoated): 68.5 [64] Glass (2% APTES): 40 [65] Glass (MSNPs-CVD): 175 ± 2 [66] Glass (MSNPs-Sol): 158 ± 2 [66] |
| Polymer | Variety and low cost Transmission of light Easy to process and form Cheap mass production | Low heat-resistant Low thermal conductivity | PDMS: 113.5 ± 2 [59] PMMA: 97 [60] PDMS (APTES+MA): 60 [67] |
2.2.3. Surface Treatment Methods
2.2.4. Partial Hydrophilic/Hydrophobic Treatment
2.3. Channel Shape
2.3.1. Straight Microchannel
2.3.2. Shape Change Microchannel
2.4. Examples of the CPCV
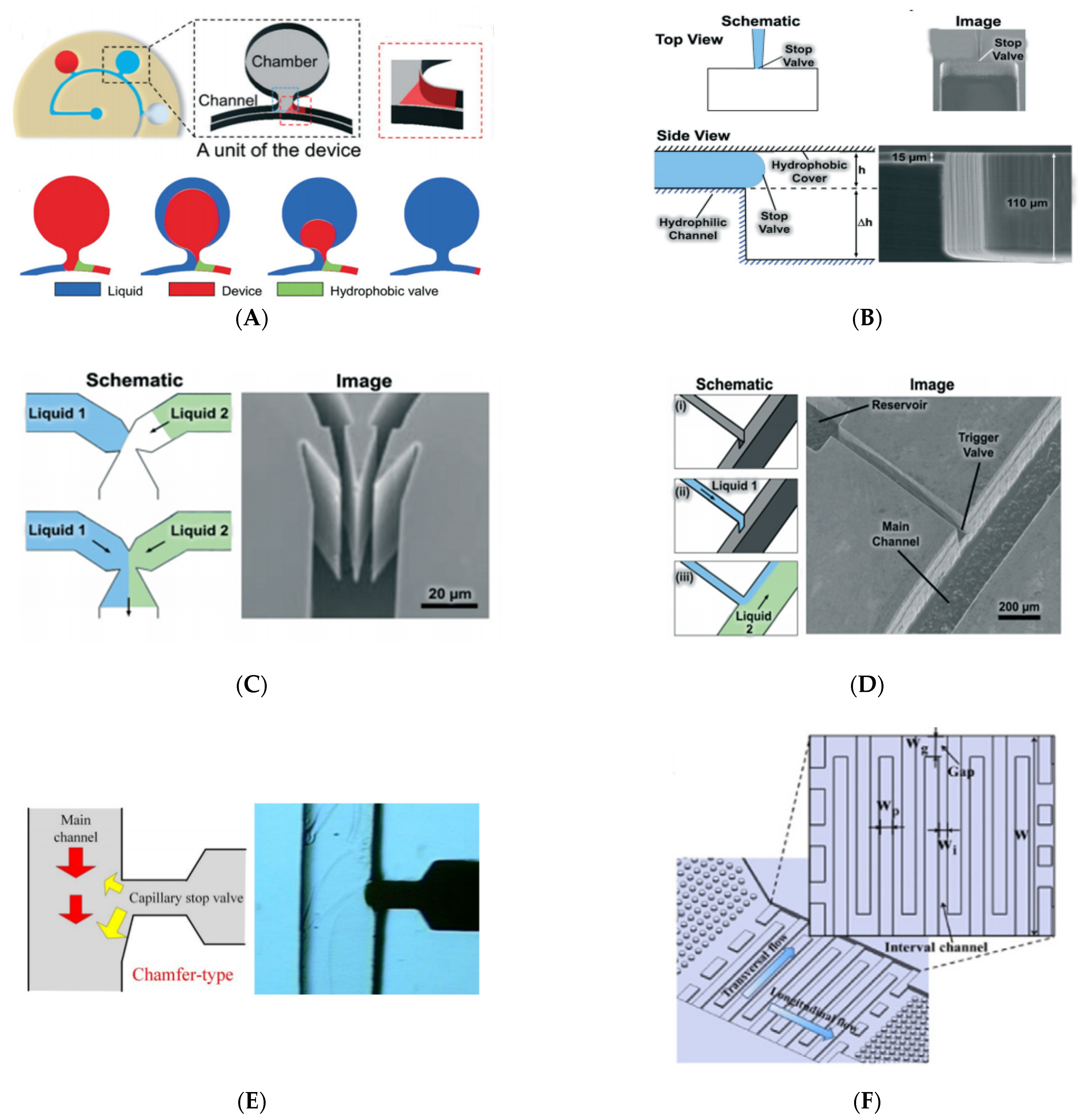
2.4.1. Stop Valves
2.4.2. Trigger Valves
2.4.3. Delay Valves
3. Applications and Impacts of CPCV
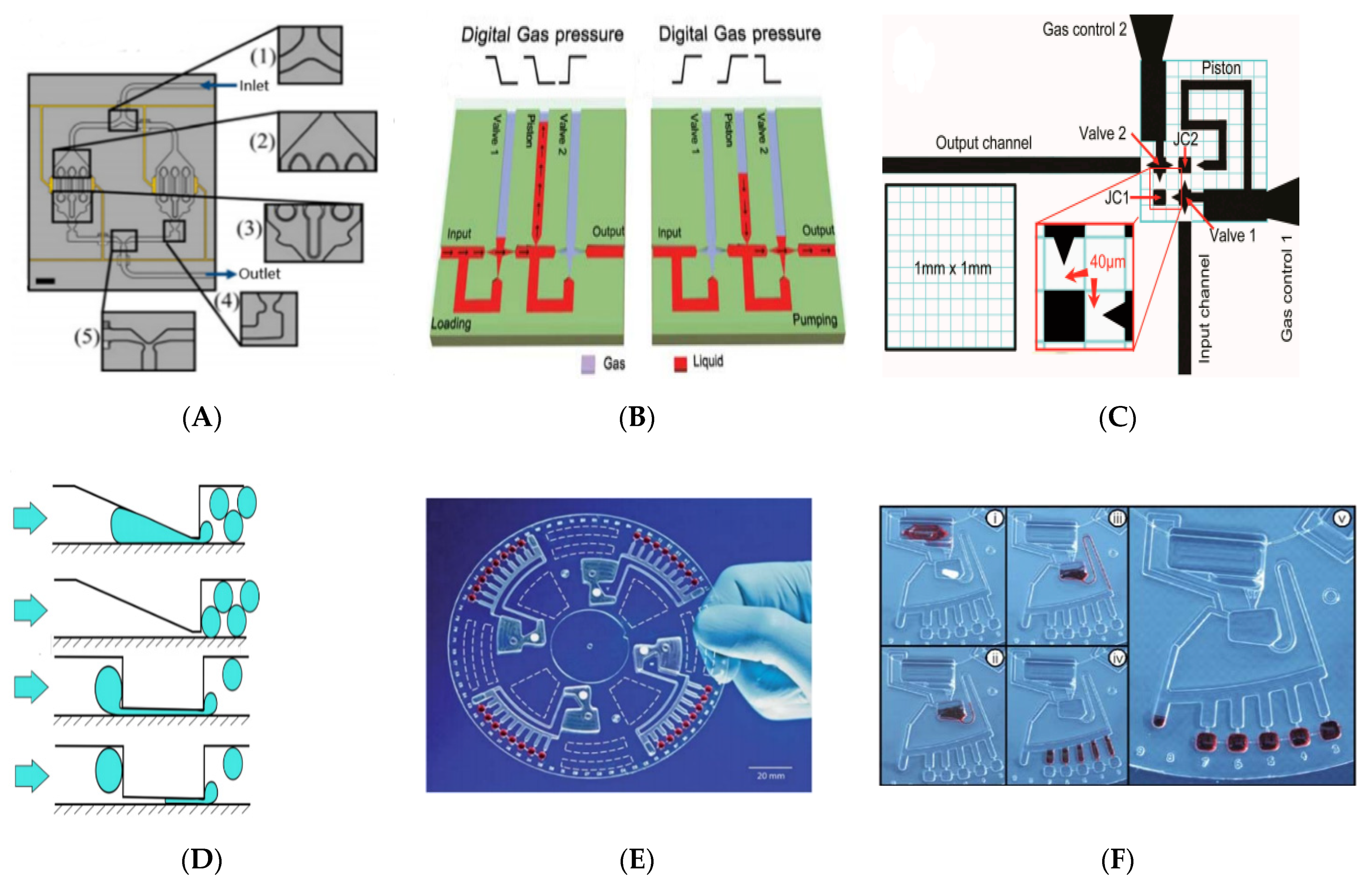
3.1. Simplifying Automation of Microfluidic Networks
3.1.1. Microfluidic Networks
3.1.2. Micropump
3.1.3. Droplet Generation
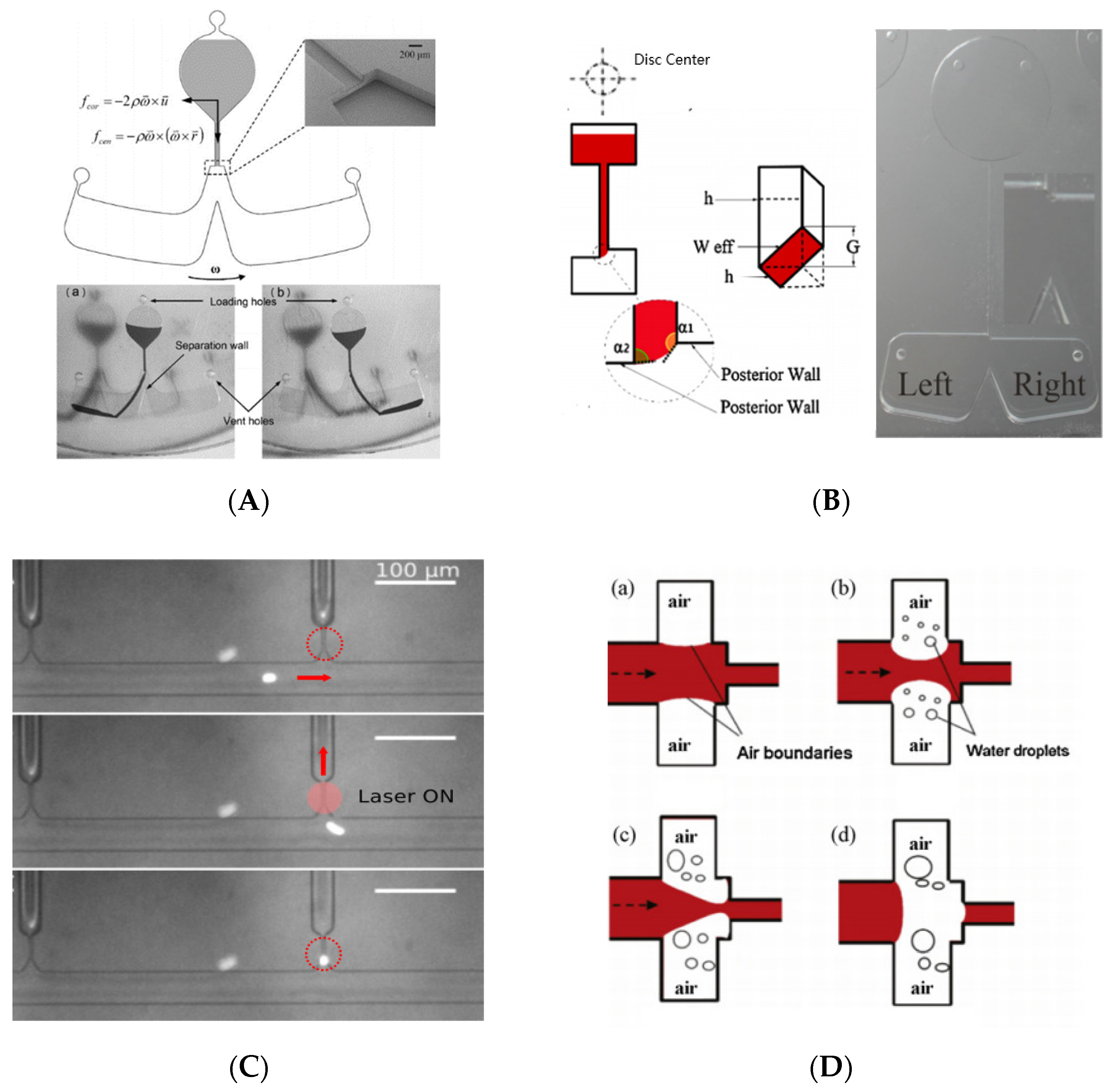
3.1.4. One Example: Lab on a Disk
3.2. Various Valve Actuation Mode
3.2.1. External Forces
3.2.2. By Changing Surface Tension
3.3. Make Broader Use of Microfluidics by Open Channel
3.4. Sampling and Delivery with Controlled Manner
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thorsen, T.; Maerkl, S.J.; Quake, S.R. Microfluidic large-scale integration. Science 2002, 298, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Skelley, A.M.; Anwer, A.G.; Liu, G.; Inglis, D.W. Maximizing particle concentration in deterministic lateral displacement arrays. Biomicrofluidics 2017, 11, 024121. [Google Scholar] [CrossRef] [PubMed]
- Inglis, D.; Vernekar, R.; Krüger, T.; Feng, S. The fluidic resistance of an array of obstacles and a method for improving boundaries in deterministic lateral displacement arrays. Microfluid. Nanofluid. 2020, 24, 18. [Google Scholar] [CrossRef]
- Liu, G.; Cao, C.; Ni, S.; Feng, S.; Wei, H. On-chip structure-switching aptamer-modified magnetic nanobeads for the continuous monitoring of interferon-gamma ex vivo. Microsyst. Nanoeng. 2019, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Kanitthamniyom, P.; Zhou, A.; Feng, S.; Liu, A.; Vasoo, S.; Zhang, Y. A 3D-printed modular magnetic digital microfluidic architecture for on-demand bioanalysis. Microsyst. Nanoeng. 2020, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Ghodbane, M.; Stucky, E.C.; Maguire, T.J.; Schloss, R.S.; Shreiber, D.I.; Zahn, J.D.; Yarmush, M.L. Development and validation of a microfluidic immunoassay capable of multiplexing parallel samples in microliter volumes. Lab Chip 2015, 15, 3211–3221. [Google Scholar] [CrossRef]
- Han, S.W.; Jang, E.; Koh, W.-G. Microfluidic-based multiplex immunoassay system integrated with an array of QD-encoded microbeads. Sens. Actuators B-Chem. 2015, 209, 242–251. [Google Scholar] [CrossRef]
- Soares, R.R.G.; Ramadas, D.; Chu, V.; Aires-Barros, M.R.; Conde, J.P.; Viana, A.S.; Cascalheira, A.C. An ultrarapid and regenerable microfluidic immunoassay coupled with integrated photosensors for point-of-use detection of ochratoxin A. Sens. Actuators B-Chem. 2016, 235, 554–562. [Google Scholar] [CrossRef]
- Amasia, M.; Cozzens, M.; Madou, M.J. Centrifugal microfluidic platform for rapid PCR amplification using integrated thermoelectric heating and ice-valving. Sens. Actuators B-Chem. 2012, 161, 1191–1197. [Google Scholar] [CrossRef]
- Kieu The Loan, T.; Wu, W.; Lee, N.Y. Planar poly(dimethylsiloxane) (PDMS)-glass hybrid microdevice for a flow-through polymerase chain reaction (PCR) employing a single heater assisted by an intermediate metal alloy layer for temperature gradient formation. Sens. Actuators B-Chem. 2014, 190, 177–184. [Google Scholar] [CrossRef]
- Tachibana, H.; Saito, M.; Tsuji, K.; Yamanaka, K.; Le Quynh, H.; Tamiya, E. Self-propelled continuous-flow PCR in capillary-driven microfluidic device: Microfluidic behavior and DNA amplification. Sens. Actuators B-Chem. 2015, 206, 303–310. [Google Scholar] [CrossRef]
- Idota, N.; Kikuchi, A.; Kobayashi, J.; Sakai, K.; Okano, T. Microfluidic valves comprising nanolayered thermoresponsive polymer-grafted capillaries. Adv. Mater. 2005, 17, 2723–2727. [Google Scholar] [CrossRef]
- Olanrewaju, A.; Beaugrand, M.; Yafia, M.; Juncker, D. Capillary microfluidics in microchannels: From microfluidic networks to capillaric circuits. Lab Chip 2018, 18, 2323–2347. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Lin, Y.-T.; Liao, C.-C. Chamfer-Type Capillary Stop Valve and Its Microfluidic Application to Blood Typing Tests. SLAS Technol. 2019, 24, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Faqheri, W.; Ibrahim, F.; Thio, T.H.G.; Aeinehvand, M.M.; Arof, H.; Madou, M. Development of novel passive check valves for the microfluidic CD platform. Sens. Actuators A-Phys. 2015, 222, 245–254. [Google Scholar] [CrossRef]
- Kim, D.; Beebe, D.J. A bi-polymer micro one-way valve. Sens. Actuators A-Phys. 2007, 136, 426–433. [Google Scholar] [CrossRef]
- Li, J.; Liang, C.; Zhang, B.; Liu, C. A comblike time-valve used in capillary-driven microfluidic devices. Microelectron. Eng. 2017, 173, 48–53. [Google Scholar] [CrossRef]
- Zhang, L.; Jones, B.; Majeed, B.; Nishiyama, Y.; Okumura, Y.; Stakenborg, T. Study on stair-step liquid triggered capillary valve for microfluidic systems. J. Micromech. Microeng. 2018, 28, 065005. [Google Scholar] [CrossRef]
- Siegrist, J.; Gorkin, R.; Clime, L.; Roy, E.; Peytavi, R.; Kido, H.; Bergeron, M.; Veres, T.; Madou, M. Serial siphon valving for centrifugal microfluidic platforms. Microfluid. Nanofluid. 2010, 9, 55–63. [Google Scholar] [CrossRef]
- Desai, A.V.; Tice, J.D.; Apblett, C.A.; Kenis, P.J.A. Design considerations for electrostatic microvalves with applications in poly(dimethylsiloxane)-based microfluidics. Lab Chip 2012, 12, 1078–1088. [Google Scholar] [CrossRef]
- Li, H.Q.; Roberts, D.C.; Steyn, J.L.; Turner, K.T.; Yaglioglu, O.; Hagood, N.W.; Spearing, S.M.; Schmidt, M.A. Fabrication of a high frequency piezoelectric microvalve. Sens. Actuators A-Phys. 2004, 111, 51–56. [Google Scholar] [CrossRef]
- Tice, J.D.; Desai, A.V.; Bassett, T.A.; Apblett, C.A.; Kenis, P.J.A. Control of pressure-driven components in integrated microfluidic devices using an on-chip electrostatic microvalve. RSC Adv. 2014, 4, 51593–51602. [Google Scholar] [CrossRef]
- Hartshorne, H.; Backhouse, C.J.; Lee, W.E. Ferrofluid-based microchip pump and valve. Sens. Actuators B-Chem. 2004, 99, 592–600. [Google Scholar] [CrossRef]
- Luharuka, R.; LeBlanc, S.; Bintoro, J.S.; Berthelot, Y.H.; Hesketh, P.J. Simulated and experimental dynamic response characterization of an electromagnetic microvalve. Sens. Actuators A-Phys. 2008, 143, 399–408. [Google Scholar] [CrossRef]
- Rich, C.A.; Wise, K.D. A high-flow thermopneumatic microvalve with improved efficiency and integrated state sensing. J. Microelectromechan. Syst. 2003, 12, 201–208. [Google Scholar] [CrossRef]
- Huang, C.; Tsou, C. The implementation of a thermal bubble actuated microfluidic chip with microvalve, micropump and micromixer. Sens. Actuators A-Phys. 2014, 210, 147–156. [Google Scholar] [CrossRef]
- Liu, R.H.; Bonanno, J.; Yang, J.N.; Lenigk, R.; Grodzinski, P. Single-use, thermally actuated paraffin valves for microfluidic applications. Sens. Actuators B-Chem. 2004, 98, 328–336. [Google Scholar] [CrossRef]
- Zahra, A.; Scipinotti, R.; Caputo, D.; Nascetti, A.; de Cesare, G. Design and fabrication of microfluidics system integrated with temperature actuated microvalve. Sens. Actuators A-Phys. 2015, 236, 206–213. [Google Scholar] [CrossRef]
- Casals-Terre, J.; Duch, M.; Plaza, J.A.; Esteve, J.; Perez-Castillejos, R.; Valles, E.; Gomez, E. Design, fabrication and characterization of an externally actuated ON/OFF microvalve. Sens. Actuators A-Phys. 2008, 147, 600–606. [Google Scholar] [CrossRef]
- Kong, M.C.R.; Salin, E.D. Pneumatic Flow Switching on Centrifugal Microfluidic Platforms in Motion. Anal. Chem. 2011, 83, 1148–1151. [Google Scholar] [CrossRef]
- Al-Faqheri, W.; Ibrahim, F.; Thio, T.H.G.; Moebius, J.; Joseph, K.; Arof, H.; Madou, M. Vacuum/Compression Valving (VCV) Using Parrafin-Wax on a Centrifugal Microfluidic CD Platform. PLoS ONE 2013, 8, e58523. [Google Scholar] [CrossRef]
- Abi-Samra, K.; Hanson, R.; Madou, M.; Gorkin, R.A., III. Infrared controlled waxes for liquid handling and storage on a CD-microfluidic platform. Lab Chip 2011, 11, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, S.; Szilagyi, A.; Sumaru, K.; Hattori, K.; Takagi, T.; Filipcsei, G.; Zrinyi, M.; Kanamori, T. On-demand microfluidic control by micropatterned light irradiation of a photoresponsive hydrogel sheet. Lab Chip 2009, 9, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Ducree, J.; Haeberle, S.; Lutz, S.; Pausch, S.; von Stetten, F.; Zengerle, R. The centrifugal microfluidic bio-disk platform. J. Micromech. Microeng. 2007, 17, S103–S115. [Google Scholar] [CrossRef]
- Cho, H.; Kim, H.-Y.; Kang, J.Y.; Kim, T.S. How the capillary burst microvalve works. J. Colloid Interface Sci. 2007, 306, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Thio, T.; Nozari, A.A.; Soin, N.; Kahar, M.K.B.A.; Dawal, S.Z.M.; Samra, K.A.; Madou, M.; Ibrahim, F. Hybrid Capillary-Flap Valve for Vapor Control in Point-of-Care Microfluidic CD. In Proceedings of the 5th Kuala Lumpur International Conference on Biomedical Engineering 2011, Kuala Lumpur, Malaysia, 20–23 June 2011; Volume 35, pp. 578–581. [Google Scholar]
- Brask, A.; Snakenborg, D.; Kutter, J.P.; Bruus, H. AC electroosmotic pump with bubble-free palladium electrodes and rectifying polymer membrane valves. Lab Chip 2006, 6, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Truong, T.Q.; Wong, K.K.; Ho, S.S.; Low, C.L.N. Micro check valves for integration into polymeric microfluidic devices. J. Micromech. Microeng. 2004, 14, 69–75. [Google Scholar] [CrossRef]
- Ni, J.; Huang, F.; Wang, B.; Li, B.; Lin, Q. A planar PDMS micropump using in-contact minimized-leakage check valves. J. Micromech. Microeng. 2010, 20, 095033. [Google Scholar] [CrossRef]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef]
- Suk, J.W.; Cho, J.-H. Capillary flow control using hydrophobic patterns. J. Micromech. Microeng. 2007, 17, N11–N15. [Google Scholar] [CrossRef]
- Andersson, H.; van der Wijngaart, W.; Griss, P.; Niklaus, F.; Stemme, G. Hydrophobic valves of plasma deposited octafluorocyclobutane in DRIE channels. Sens. Actuators B-Chem. 2001, 75, 136–141. [Google Scholar] [CrossRef]
- Lu, C.; Xie, Y.; Yang, Y.; Cheng, M.M.C.; Koh, C.-G.; Bai, Y.; Lee, L.J. New valve and bonding designs for microfluidic biochips containing proteins. Anal. Chem. 2007, 79, 994–1001. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Huang, J.; Liu, Y.; Peng, J.; Chen, S.; Song, K.; Ouyang, X.; Cheng, H.; Wang, X. Skin-interfaced microfluidic devices with one-opening chambers and hydrophobic valves for sweat collection and analysis. Lab Chip 2020, 20, 2635–2645. [Google Scholar] [CrossRef]
- Gliere, A.; Delattre, C. Modeling and fabrication of capillary stop valves for planar microfluidic systems. Sens. Actuators A-Phys. 2006, 130, 601–608. [Google Scholar] [CrossRef]
- Man, P.F.; Mastrangelo, C.H.; Burns, M.A.; Burke, D.T. Microfabricated capillarity-driven stop valve and sample injector. In Proceedings of the Proceedings MEMS 98. IEEE. Eleventh Annual International Workshop on Micro Electro Mechanical Systems, Heideberg, Germany, 25–29 January 1998; pp. 45–50. [Google Scholar] [CrossRef]
- Kong, L.X.; Perebikovsky, A.; Moebius, J.; Kulinsky, L.; Madou, M. Lab-on-a-CD: A Fully Integrated Molecular Diagnostic System. J. Lab. Autom. 2016, 21, 323–355. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Liu, Y.; Lau, W.M.; Yang, J. Modeling of flow burst, flow timing in Lab-on-a-CD systems and its application in digital chemical analysis. Chem. Eng. Technol. 2008, 31, 1328–1335. [Google Scholar] [CrossRef]
- Chen, J.M.; Huang, P.-C.; Lin, M.-G. Analysis and experiment of capillary valves for microfluidics on a rotating disk. Microfluid. Nanofluid. 2008, 4, 427–437. [Google Scholar] [CrossRef]
- Zhang, H.; Hong Hanh, T.; Chung, B.H.; Lee, N.Y. Solid-phase based on-chip DNA purification through a valve-free stepwise injection of multiple reagents employing centrifugal force combined with a hydrophobic capillary barrier pressure. Analyst 2013, 138, 1750–1757. [Google Scholar] [CrossRef]
- Melin, J.; Roxhed, N.; Gimenez, G.; Griss, P.; van der Wijngaart, W.; Stemme, G. A liquid-triggered liquid microvalve for on-chip flow control. Sens. Actuators B-Chem. 2004, 100, 463–468. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 9780470541944. [Google Scholar] [CrossRef]
- Kumar, K.; Knie, C.; Bléger, D.; Peletier, M.A.; Friedrich, H.; Hecht, S.; Broer, D.J.; Debije, M.G.; Schenning, A.P.H.J. A chaotic self-oscillating sunlight-driven polymer actuator. Nat. Commun. 2016, 7, 11975. [Google Scholar] [CrossRef]
- Amiri, M.; Dadkhah, A.A. On reduction in the surface tension of water due to magnetic treatment. Colloids Surf. A Physicochem. Eng. Asp. 2006, 278, 252–255. [Google Scholar] [CrossRef]
- Chen, J.M.; Chen, C.-Y.; Liu, C.-H. Pressure barrier in an axisymmetric capillary microchannel with sudden expansion. Jpn. J. Appl. Phys. 2008, 47, 1683–1689. [Google Scholar] [CrossRef]
- Grundner, M.; Jacob, H. Investigations on hydrophilic and hydrophobic silicon (100) wafer surfaces by X-ray photoelectron and high-resolution electron-energy loss-spectroscopy. Appl. Phys. A-Mater. 1986, 39, 73–82. [Google Scholar] [CrossRef]
- Zhao, B.; Moore, J.S.; Beebe, D.J. Surface-directed liquid flow inside microchannels. Science 2001, 291, 1023–1026. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- Tihan, T.G.; Ionita, M.D.; Popescu, R.G.; Iordachescu, D. Effect of hydrophilic-hydrophobic balance on biocompatibility of poly(methyl methacrylate) (PMMA)-hydroxyapatite (HA) composites. Mater. Chem. Phys. 2009, 118, 265–269. [Google Scholar] [CrossRef]
- Chi, F.; Liu, D.; Wu, H.; Lei, J. Mechanically robust and self-cleaning antireflection coatings from nanoscale binding of hydrophobic silica nanoparticles. Sol. Energy Mater. Sol. Cells 2019, 200, 109939. [Google Scholar] [CrossRef]
- Larsen, S.T.; Andersen, N.K.; Sogaard, E.; Taboryski, R. Structure Irregularity Impedes Drop Roll-Off at Superhydrophobic Surfaces. Langmuir 2014, 30, 5041–5045. [Google Scholar] [CrossRef]
- Zhong, J.; Chinn, J.; Roberts, C.B.; Ashurst, W.R. Vapor-Phase Deposited Chlorosilane-Based Self-Assembled Monolayers on Various Substrates for Thermal Stability Analysis. Ind. Eng. Chem. Res. 2017, 56, 5239–5252. [Google Scholar] [CrossRef]
- Li, D.W.; Wang, H.Y.; Liu, Y.; Wei, D.S.; Zhao, Z.X. Large-scale fabrication of durable and robust super-hydrophobic spray coatings with excellent repairable and anti-corrosion performance. Chem. Eng. J. 2019, 367, 169–179. [Google Scholar] [CrossRef]
- Chaudhary, S.; Kamra, T.; Uddin, K.M.A.; Snezhkova, O.; Jayawardena, H.S.N.; Yan, M.; Montelius, L.; Schnadt, J.; Ye, L. Controlled short-linkage assembly of functional nano-objects. Appl. Surf. Sci. 2014, 300, 22–28. [Google Scholar] [CrossRef]
- Rezaei, S.; Manoucheri, I.; Moradian, R.; Pourabbas, B. One-step chemical vapor deposition and modification of silica nanoparticles at the lowest possible temperature and superhydrophobic surface fabrication. Chem. Eng. J. 2014, 252, 11–16. [Google Scholar] [CrossRef]
- Cordeiro, A.L.; Zschoche, S.; Janke, A.; Nitschke, M.; Werner, C. Functionalization of Poly(dimethylsiloxane) Surfaces with Maleic Anhydride Copolymer Films. Langmuir 2009, 25, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.; Ho, C.-M. Surface molecular property modifications for poly(dimethylsiloxane) (PDMS) based microfluidic devices. Microfluid. Nanofluid. 2009, 7, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Vickers, J.A.; Caulum, M.M.; Henry, C.S. Generation of hydrophilic poly(dimethylsiloxane) for high-performance microchip electrophoresis. Anal. Chem. 2006, 78, 7446–7452. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.L.; McDonald, A.; Gourley, P.L.; Sasaki, D.Y. Poly(dimethylsiloxane) thin films as biocompatible coatings for microfluidic devices: Cell culture and flow studies with glial cells. J. Biomed. Mater. Res. Part A 2005, 72A, 10–18. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Abate, A.R.; Lee, D.; Do, T.; Holtze, C.; Weitz, D.A. Glass coating for PDMS microfluidic channels by sol–gel methods. Lab Chip 2008, 8, 516–518. [Google Scholar] [CrossRef]
- Khamova, T.V.; Shilova, O.A.; Krasil’nikova, L.N.; Ladilina, E.Y.; Lyubova, T.S.; Baten’kin, M.A.; Kruchinina, I.Y. Sol–gel synthesis and study of the hydrophobicity of coatings prepared using modified aerosils. Glass Phys. Chem. 2016, 42, 194–201. [Google Scholar] [CrossRef]
- Guo, W.; Hansson, J.; van der Wijngaart, W. Synthetic Paper Separates Plasma from Whole Blood with Low Protein Loss. Anal. Chem. 2020, 92, 6194–6199. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lopez-Martinez, M.J.; Baraket, A.; Zine, N.; Esteve, J.; Plaza, J.A.; Jaffrezic-Renault, N.; Errachid, A. Polymer micromixers bonded to thermoplastic films combining soft-lithography with plasma and aptes treatment processes. J. Polym. Sci. Part A-Polym. Chem. 2013, 51, 59–70. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, J.Y.; Seong, S.; Cha, S.H.; Lee, S.H.; Kim, J.J.; Park, T.H. Fabrication and characterization of a PDMS-glass hybrid continuous-flow PCR chip. Biochem. Eng. J. 2006, 29, 91–97. [Google Scholar] [CrossRef]
- Hitzbleck, M.; Delamarche, E. Advanced Capillary Soft Valves for Flow Control in Self-Driven Microfluidics. Micromachines 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Zhou, S.; Li, M.; Tang, Q.; Song, Z.; Tong, Y.; Liu, Y. Deposition of Pentacene Thin Film on Polydimethylsiloxane Elastic Dielectric Layer for Flexible Thin-Film Transistors. IEEE Electron Device Lett. 2017, 38, 1031–1034. [Google Scholar] [CrossRef]
- Feng, S.; Liu, G.; Jiang, L.; Zhu, Y.; Goldys, E.; Inglis, D. A Microfluidic Needle For Sampling And Delivery Of Chemical Signals By Segmented Flows. Appl. Phys. Lett. 2017, 111, 183702. [Google Scholar] [CrossRef]
- Shirtcliffe, N.; McHale, G.; Newton, M.; Perry, C. Intrinsically superhydrophobic organosilica sol–gel foams. Langmuir 2003, 19, 5626–5631. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol–gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Basu, B.J.; Hariprakash, V.; Aruna, S.; Lakshmi, R.; Manasa, J.; Shruthi, B. Effect of microstructure and surface roughness on the wettability of superhydrophobic sol–gel nanocomposite coatings. J. Sol-Gel Sci. Technol. 2010, 56, 278–286. [Google Scholar] [CrossRef]
- Ogihara, H.; Katayama, T.; Saji, T. One-step electrophoretic deposition for the preparation of superhydrophobic silica particle/trimethylsiloxysilicate composite coatings. J. Colloid Interface Sci. 2011, 362, 560–566. [Google Scholar] [CrossRef]
- Mahadik, S.A.; Mahadik, D.; Kavale, M.; Parale, V.; Wagh, P.; Barshilia, H.C.; Gupta, S.C.; Hegde, N.; Rao, A.V. Thermally stable and transparent superhydrophobic sol–gel coatings by spray method. J. Sol-Gel Sci. Technol. 2012, 63, 580–586. [Google Scholar] [CrossRef]
- Graham, P.; Stone, M.; Thorpe, A.; Nevell, T.G.; Tsibouklis, J. Fluoropolymers with very low surface energy characteristics. J. Fluor. Chem. 2000, 104, 29–36. [Google Scholar] [CrossRef]
- Thorpe, A.A.; Peters, V.; Smith, J.R.; Nevell, T.G.; Tsibouklis, J. Poly(methylpropenoxyfluoroalkylsiloxane)s: A class of fluoropolymers capable of inhibiting bacterial adhesion onto surfaces. J. Fluor. Chem. 2000, 104, 37–45. [Google Scholar] [CrossRef]
- Yarosh, A.A.; Krukovsky, S.P.; Pryakhina, T.A.; Kotov, V.M.; Zavin, B.G.; Sakharov, A.M. Synthesis of water- and oil-repellent organofluorosilicon compounds. Mendeleev Commun. 2006, 16, 190–192. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Zhang, Q.; He, M.; Chen, J.; Wang, J.; Song, Y.; Jiang, L. Anti-icing surfaces based on enhanced self-propelled jumping of condensed water microdroplets. Chem. Commun. 2013, 49, 4516–4518. [Google Scholar] [CrossRef]
- Cho, S.J.; An, T.; Kim, J.Y.; Sung, J.; Lim, G. Superhydrophobic nanostructured silicon surfaces with controllable broadband reflectance. Chem. Commun. 2011, 47, 6108–6110. [Google Scholar] [CrossRef]
- Ali, U.; Abd Karim, K.J.B.; Buang, N.A. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface modification of Sylgard-184 poly(dimethyl siloxane) networks by ultraviolet and ultraviolet/ozone treatment. J. Colloid Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef]
- Berdichevsky, Y.; Khandurina, J.; Guttman, A.; Lo, Y.H. UV/ozone modification of poly(dimethylsiloxane) microfluidic channels. Sens. Actuators B-Chem. 2004, 97, 402–408. [Google Scholar] [CrossRef]
- Chen, H.-Y.; McClelland, A.A.; Chen, Z.; Lahann, J. Solventless adhesive bonding using reactive polymer coatings. Anal. Chem. 2008, 80, 4119–4124. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lahann, J. Fabrication of discontinuous surface patterns within microfluidic channels using photodefinable vapor-based polymer coatings. Anal. Chem. 2005, 77, 6909–6914. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.H.; Timm, A.C.; Timm, C.M.; Bible, A.N.; Morrell-Falvey, J.L.; Pelletier, D.A.; Simpson, M.L.; Doktycz, M.J.; Retterer, S.T. Stochastic Assembly of Bacteria in Microwell Arrays Reveals the Importance of Confinement in Community Development. PLoS ONE 2016, 11, e0155080. [Google Scholar] [CrossRef]
- Zandi Shafagh, R.; Decrop, D.; Ven, K.; Vanderbeke, A.; Hanusa, R.; Breukers, J.; Pardon, G.; Haraldsson, T.; Lammertyn, J.; van der Wijngaart, W. Reaction injection molding of hydrophilic-in-hydrophobic femtolitre-well arrays. Microsyst. Nanoeng. 2019, 5, 25. [Google Scholar] [CrossRef]
- Feng, S.; Nguyen, M.N.; Inglis, D.W. Microfluidic Droplet Extraction by Hydrophilic Membrane. Micromachines 2017, 8, 331. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Abraham, E.; Desmulliez, M.P.Y. Rapid laser prototyping of valves for microfluidic autonomous systems. J. Micromech. Microeng. 2013, 23, 035034. [Google Scholar] [CrossRef]
- Weigl, B.; Klein, G. Surface Tension Valves for Microfluidic Applications. International Patent WO0190614, 13 June 2002. [Google Scholar]
- Wang, M.Y.; Wan, Z.; Surangalikar, H.; Wu, G.; Ata, E. Micromachined Electrowetting Microfluidic Valve. U.S. Patent US20080257438, 23 October 2008. [Google Scholar]
- Field, L.A.; Schiaffino, S.; Barth, P.W.; Hoen, S.T.; Kawamura, N.A.; Donald, D.K.; Robertson, C.R.; Servaites, J.D. Bubble Valve and Bubble Valve-Based Pressure Regulator. U.S. Patent US6062681, 16 May 2000. [Google Scholar]
- Krulevitch, P.A.; Benett, W.J.; Rose, K.A.; Hamilton, J.; Maghribi, M. Low Power Integrated Pumping and Valving Arrays for Microfluidic Systems. International Patent WO03027508, 3 April 2003. [Google Scholar]
- Larsson, O.; Tiensuu, A.-L. Hydrophobic Barriers. U.S. Patent US2007059216, 15 March 2007. [Google Scholar]
- Lee, L.; Lu, C.; Juang, Y.-J. Valve for Microfluidic Chips. U.S. Patent US2007113908, 24 May 2007. [Google Scholar]
- Kozicki, M.N. Programmable Surface Control Devices and Method of Making Same. European Patent EP1440485, 21 June 2006. [Google Scholar]
- Shartle, R.; Besemer, D.; Gorin, M. Capillary Stop-Flow Junction Having Improved Stability against Accidental Fluid Flow. U.S. Patent US5230866, 27 July 1993. [Google Scholar]
- Banerjee, D.; Faulstich, K.; Lau, A.; Ulmanella, U.; Xie, J. Device Including a Dissolvable Structure for Flow Control. U.S. Patent US2006093528, 4 May 2006. [Google Scholar]
- Chung, K.H.; Ko, J.S.; Yoon, H.C.; Yang, H.S.; Pyo, H.B.; Kim, S.J.; Kim, Y.T. Device for Controlling Fluid Using Surface Tension. U.S. Patent US7445754, 4 November 2008. [Google Scholar]
- Gerhardt, G.C.; Bouvier, E.S.; Dourdeville, T. Fluid Flow Control Freeze/Thaw Valve for Narrow Bore Capillaries or Microfluidic Devices. European Patent EP1446601, 26 March 2008. [Google Scholar]
- Zimmermann, M.; Hunziker, P.; Delamarche, E. Valves for autonomous capillary systems. Microfluid. Nanofluid. 2008, 5, 395–402. [Google Scholar] [CrossRef]
- Takei, G.; Nonogi, M.; Hibara, A.; Kitamori, T.; Kim, H.-B. Tuning microchannel wettability and fabrication of multiple-step Laplace valves. Lab Chip 2007, 7, 596–602. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Superhydrophobic, passive microvalves with controllable opening threshold: Exploiting plasma nanotextured microfluidics for a programmable flow switchboard. Microfluid. Nanofluid. 2014, 17, 489–498. [Google Scholar] [CrossRef]
- Olanrewaju, A.O.; Robillard, A.; Dagher, M.; Juncker, D. Autonomous microfluidic capillaric circuits replicated from 3D-printed molds. Lab Chip 2016, 16, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Safavieh, R.; Juncker, D. Capillarics: Pre-programmed, self-powered microfluidic circuits built from capillary elements. Lab Chip 2013, 13, 4180–4189. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, S.; Zhang, Y.; Yang, H. Study on Functionality and Surface Modification of a Stair-Step Liquid-Triggered Valve for On-Chip Flow Control. Micromachines 2020, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Olanrewaju, A.O.; Ng, A.; DeCorwin-Martin, P.; Robillard, A.; Juncker, D. Microfluidic Capillaric Circuit for Rapid and Facile Bacteria Detection. Anal. Chem. 2017, 89, 6846–6853. [Google Scholar] [CrossRef]
- Hagmeyer, B.; Zechnall, F.; Stelzle, M. Towards plug and play filling of microfluidic devices by utilizing networks of capillary stop valves. Biomicrofluidics 2014, 8, 056501. [Google Scholar] [CrossRef]
- Peng, X.Y. A micro surface tension pump (MISPU) in a glass microchip. Lab Chip 2011, 11, 132–138. [Google Scholar] [CrossRef]
- Peng, X.Y. A One-Square-Millimeter Compact Hollow Structure for Microfluidic Pumping on an All-Glass Chip. Micromachines 2016, 7, 63. [Google Scholar] [CrossRef]
- Postek, W.; Kaminski, T.S.; Garstecki, P. A passive microfluidic system based on step emulsification allows the generation of libraries of nanoliter-sized droplets from microliter droplets of varying and known concentrations of a sample. Lab Chip 2017, 17, 1323–1331. [Google Scholar] [CrossRef]
- Focke, M.; Stumpf, F.; Faltin, B.; Reith, P.; Bamarni, D.; Wadle, S.; Mueller, C.; Reinecke, H.; Schrenzel, J.; Francois, P.; et al. Microstructuring of polymer films for sensitive genotyping by real-time PCR on a centrifugal microfluidic platform. Lab Chip 2010, 10, 2519–2526. [Google Scholar] [CrossRef]
- Lutz, S.; Weber, P.; Focke, M.; Faltin, B.; Hoffmann, J.; Mueller, C.; Mark, D.; Roth, G.; Munday, P.; Armes, N.; et al. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA). Lab Chip 2010, 10, 887–893. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Mittal, N.; Cohen, C.; Bibette, J.; Bremond, N. Dynamics of step-emulsification: From a single to a collection of emulsion droplet generators. Phys. Fluids 2014, 26, 082109. [Google Scholar] [CrossRef]
- Amstad, E.; Chemama, M.; Eggersdorfer, M.; Arriaga, L.; Brenner, M.; Weitz, D. Robust scalable high throughput production of monodisperse drops. Lab Chip 2016, 16, 4163–4172. [Google Scholar] [CrossRef] [PubMed]
- Dangla, R.; Kayi, S.C.; Baroud, C.N. Droplet microfluidics driven by gradients of confinement. Proc. Natl. Acad. Sci. USA 2013, 110, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Burger, R.; Kirby, D.; Glynn, M.; Nwankire, C.; O’Sullivan, M.; Siegrist, J.; Kinahan, D.; Aguirre, G.; Kijanka, G.; Gorkin, R.A.; et al. Centrifugal microfluidics for cell analysis. Curr. Opin. Chem. Biol. 2012, 16, 409–414. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; von Stetten, F.; Zengerle, R. Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 2010, 39, 1153–1182. [Google Scholar] [CrossRef]
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; von Stetten, F. Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid. Nanofluid. 2011, 10, 1279–1288. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Segal, E. Lab-on-a-Chip Devices for Point-of-Care Medical Diagnostics. Adv. Biochem. Eng./Biotechnol. 2020, 1–19. [Google Scholar] [CrossRef]
- Kim, J.; Kido, H.; Rangel, R.H.; Madou, M.J. Passive flow switching valves on a centrifugal microfluidic platform. Sens. Actuators B-Chem. 2008, 128, 613–621. [Google Scholar] [CrossRef]
- Kazemzadeh, A.; Ganesan, P.; Ibrahim, F.; Aeinehvand, M.M.; Kulinsky, L.; Madou, M.J. Gating valve on spinning microfluidic platforms: A flow switch/control concept. Sens. Actuators B-Chem. 2014, 204, 149–158. [Google Scholar] [CrossRef]
- Eriksen, J.; Bilenberg, B.; Kristensen, A.; Marie, R. Optothermally actuated capillary burst valve. Rev. Sci. Instrum. 2017, 88, 045101. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wu, L.L.; Zhang, Y.; Xue, H.; Li, G.-P.; Bachman, M. A vapor based microfluidic flow regulator. Sens. Actuators B-Chem. 2009, 142, 355–361. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shih, C.-H.; Chen, W.-H.; Lin, M.-H. Design and Analysis of a Robust Sequential Flow Control Using Burst Valves on the Centrifugal Platform. J. Nanosci. Nanotechnol. 2016, 16, 12602–12608. [Google Scholar] [CrossRef]
- Madou, M.J.; Lee, L.J.; Daunert, S.; Lai, S.; Shih, C.-H. Design and Fabrication of CD-like Microfluidic Platforms for Diagnostics: Microfluidic Functions. Biomed. Microdevices 2001, 3, 245–254. [Google Scholar] [CrossRef]
- Zoval, J.V.; Madou, M.J. Centrifuge-based fluidic platforms. Proc. IEEE 2004, 92, 140–153. [Google Scholar] [CrossRef]
- Leu, T.S.; Chang, P.Y. Pressure barrier of capillary stop valves in micro sample separators. Sens. Actuators A-Phys. 2004, 115, 508–515. [Google Scholar] [CrossRef]
- Londe, G.; Chunder, A.; Wesser, A.; Zhai, L.; Cho, H.J. Microfluidic valves based on superhydrophobic nanostructures and switchable thermosensitive surface for lab-on-a-chip (LOC) systems. Sens. Actuators B-Chem. 2008, 132, 431–438. [Google Scholar] [CrossRef]
- Li, L.; Westerbeek, E.Y.; Vollenbroek, J.C.; de Beer, S.; Shui, L.; Odijk, M.; Eijkel, J.C.T. Autonomous capillary microfluidic devices with constant flow rate and temperature-controlled valving. Soft Matter. 2021. [Google Scholar] [CrossRef]
- Bouaidat, S.; Hansen, O.; Bruus, H.; Berendsen, C.; Bau-Madsen, N.K.; Thomsen, P.; Wolff, A.; Jonsmann, J. Surface-directed capillary system; theory, experiments and applications. Lab Chip 2005, 5, 827–836. [Google Scholar] [CrossRef]
- Lam, P.; Wynne, K.J.; Wnek, G.E. Surface-tension-confined microfluidics. Langmuir 2002, 18, 948–951. [Google Scholar] [CrossRef]
- You, I.; Yun, N.; Lee, H. Surface-Tension-Confined Microfluidics and Their Applications. ChemPhysChem 2013, 14, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Niu, C.; Zhang, C.; Wang, Z. Steady flow of pressure-driven water-in-oil droplets in closed-open-closed microchannels. AIP Adv. 2019, 9, 125040. [Google Scholar] [CrossRef]
- Papadimitriou, V.A.; Segerink, L.I.; van den Berg, A.; Eijkel, J.C.T. 3D capillary stop valves for versatile patterning inside microfluidic chips. Anal. Chim. Acta 2018, 1000, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-H.; Xu, W.; Allbritton, N.L. Use of a virtual wall valve in polydimethylsiloxane microfluidic devices for bioanalytical applications. Biomicrofluidics 2011, 5, 024105. [Google Scholar] [CrossRef]
- Choi, J.; Kang, D.; Han, S.; Kim, S.B.; Rogers, J.A. Thin, Soft, Skin-Mounted Microfluidic Networks with Capillary Bursting Valves for Chrono-Sampling of Sweat. Adv. Healthc. Mater. 2017, 6, 1601355. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Li, J.; Tesler, A.B.; Yao, Y.; Wang, M.; Min, L.; Sheng, Z.; Aizenberg, J. Dynamic air/liquid pockets for guiding microscale flow. Nat. Commun. 2018, 9, 733. [Google Scholar] [CrossRef]
- Feng, S.; Clement, S.; Zhu, Y.; Goldys, E.M.; Inglis, D.W. Microfabricated needle for hydrogen peroxide detection. RSC Adv. 2019, 9, 18176–18181. [Google Scholar] [CrossRef]
- Feng, S.; Shirani, E.; Inglis, D.W. Droplets for Sampling and Transport of Chemical Signals in Biosensing: A Review. Biosensors 2019, 9, 80. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, X.; Ma, C.; Yan, S.; Inglis, D.; Feng, S. A Review of Capillary Pressure Control Valves in Microfluidics. Biosensors 2021, 11, 405. https://doi.org/10.3390/bios11100405
Wang S, Zhang X, Ma C, Yan S, Inglis D, Feng S. A Review of Capillary Pressure Control Valves in Microfluidics. Biosensors. 2021; 11(10):405. https://doi.org/10.3390/bios11100405
Chicago/Turabian StyleWang, Shaoxi, Xiafeng Zhang, Cong Ma, Sheng Yan, David Inglis, and Shilun Feng. 2021. "A Review of Capillary Pressure Control Valves in Microfluidics" Biosensors 11, no. 10: 405. https://doi.org/10.3390/bios11100405
APA StyleWang, S., Zhang, X., Ma, C., Yan, S., Inglis, D., & Feng, S. (2021). A Review of Capillary Pressure Control Valves in Microfluidics. Biosensors, 11(10), 405. https://doi.org/10.3390/bios11100405








