Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers
Abstract
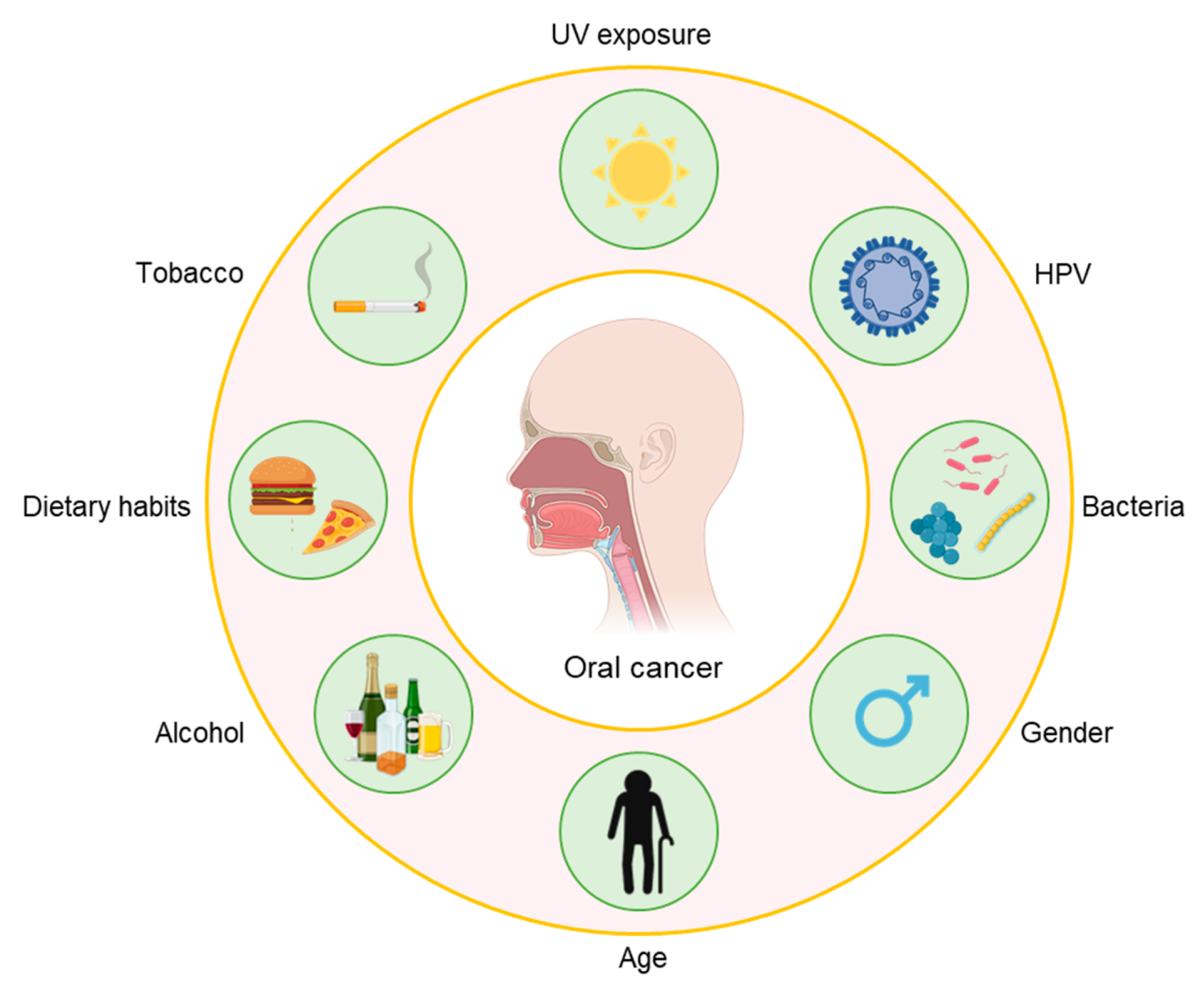
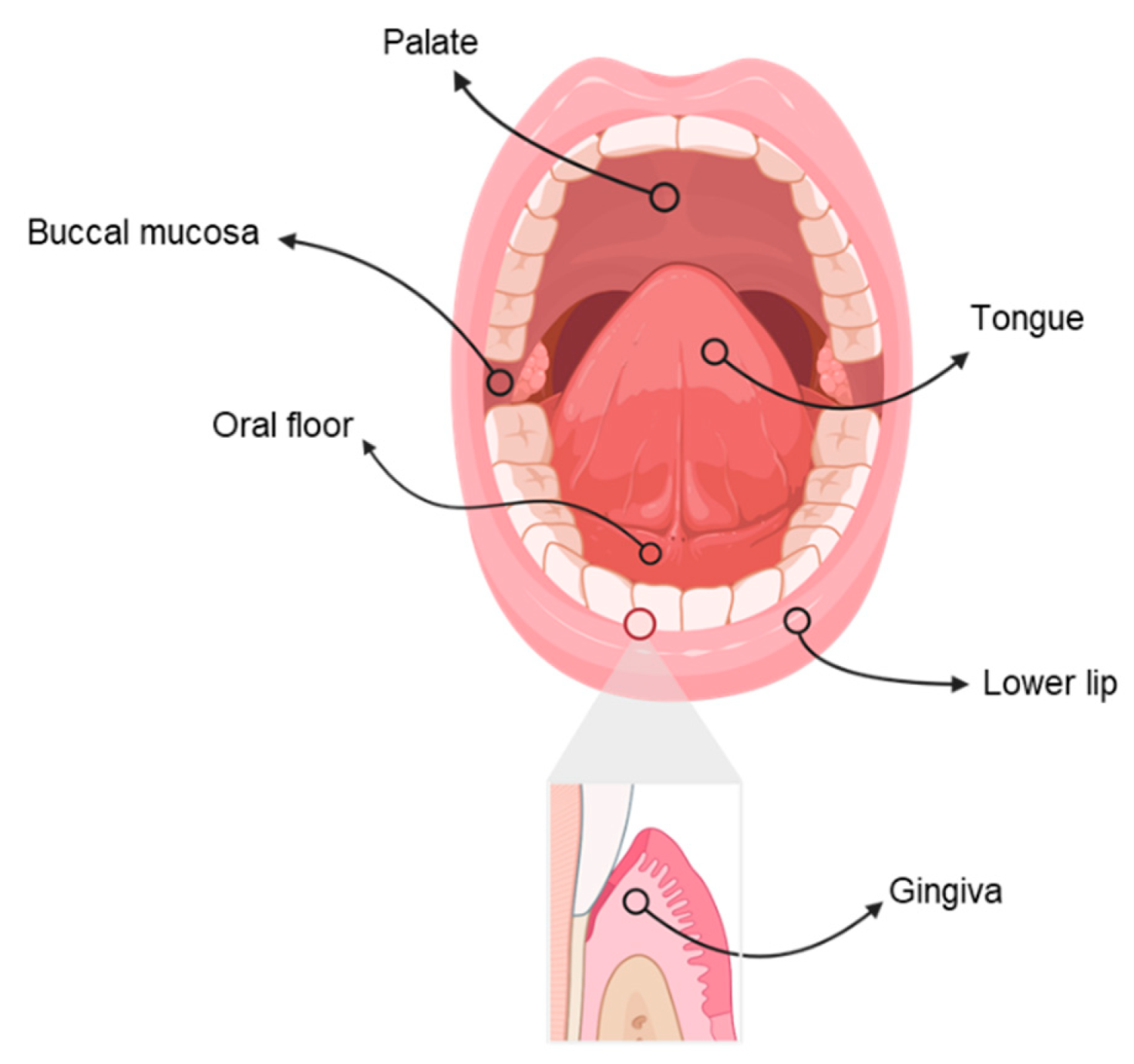
:1. Introduction
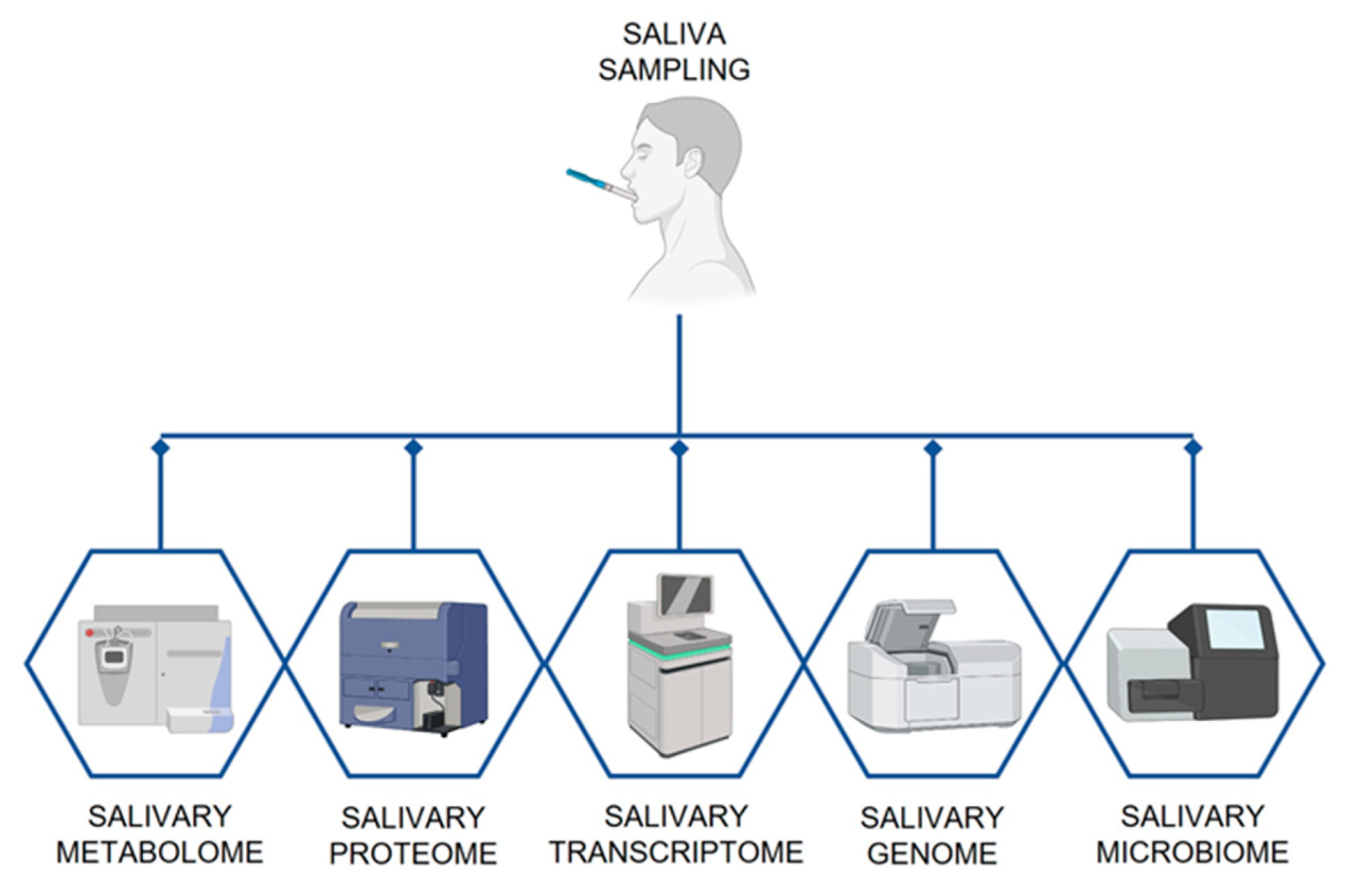
2. Saliva as Diagnostic and Monitoring Tool
3. Salivary Biomarkers of Oral Cancer
3.1. Micro RNA (miRNA)
3.2. Cell Free DNA (cfDNA)
3.3. miRNA vs. cfDNA
3.4. Messenger RNA (mRNA)
3.5. Protein Biomarkers
3.6. Metabolic Biomarkers
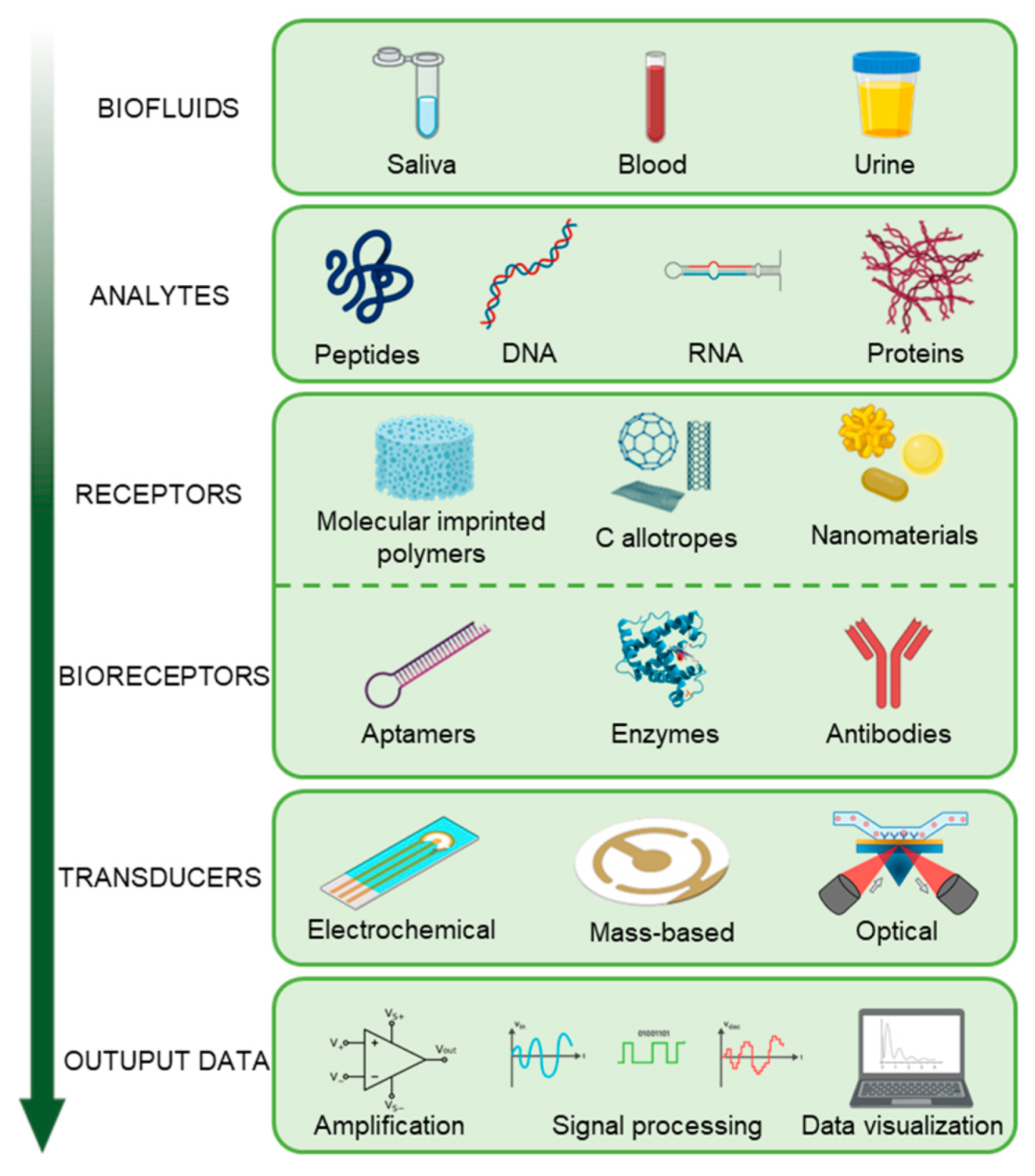
4. Biosensors and Bioelectronic Platforms for the Detection of Oral Cancer Biomarkers in Saliva
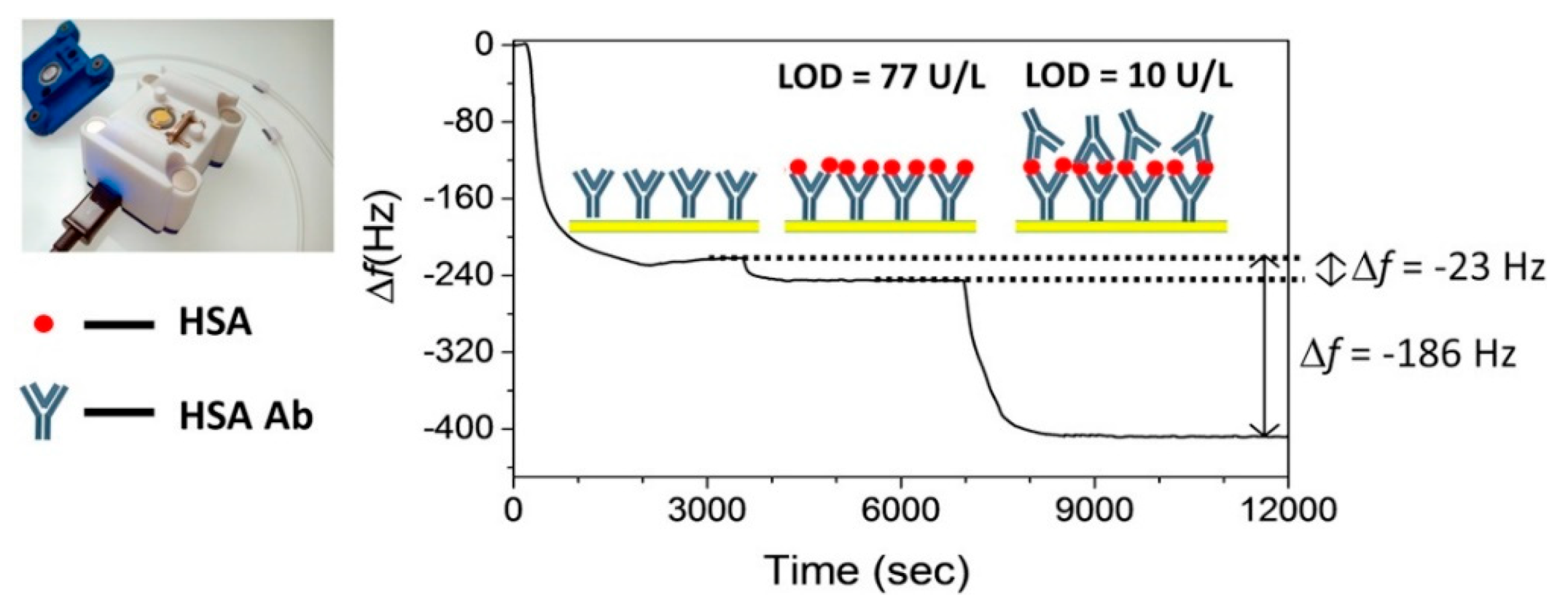
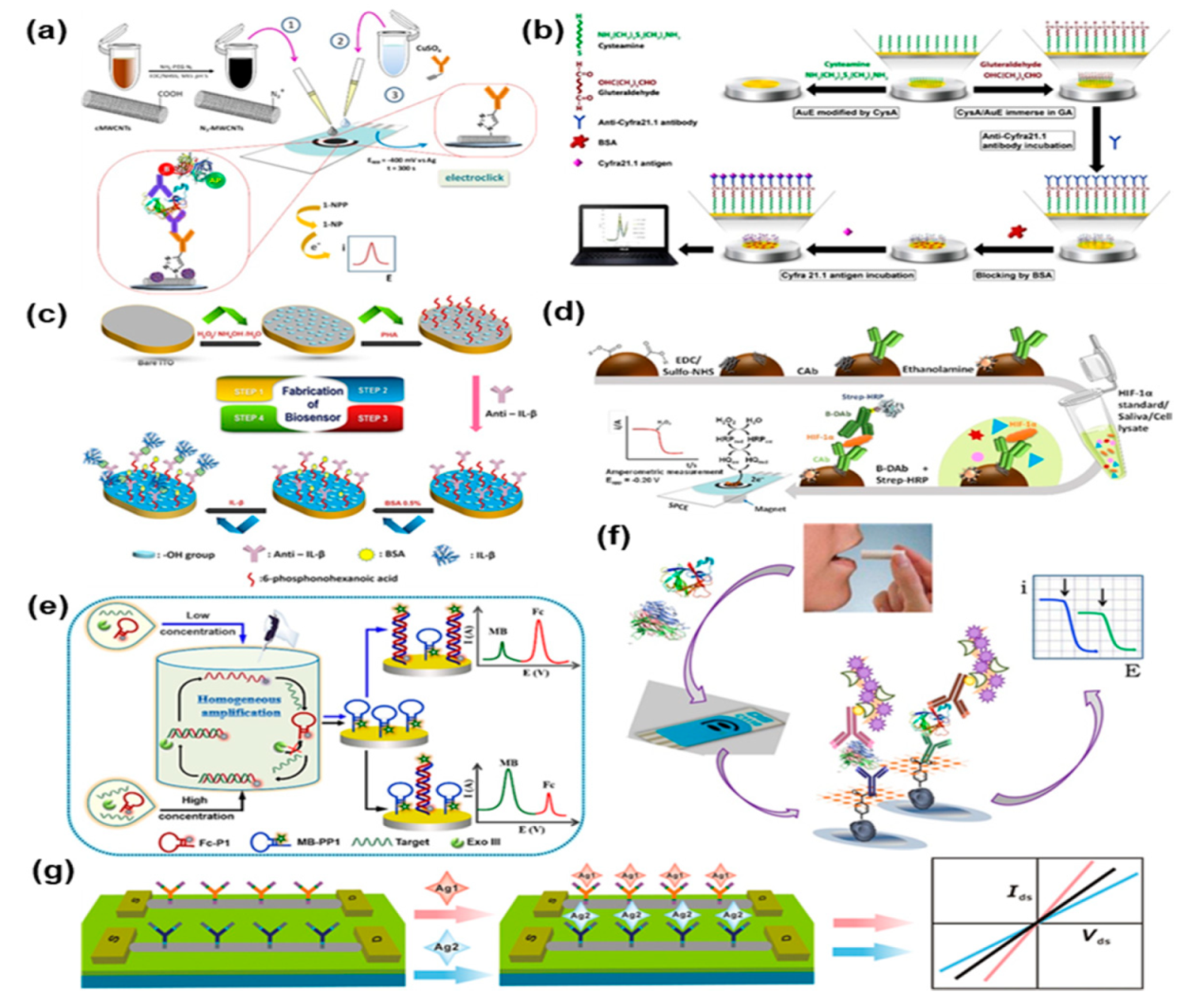
4.1. Electrochemical Biosensors
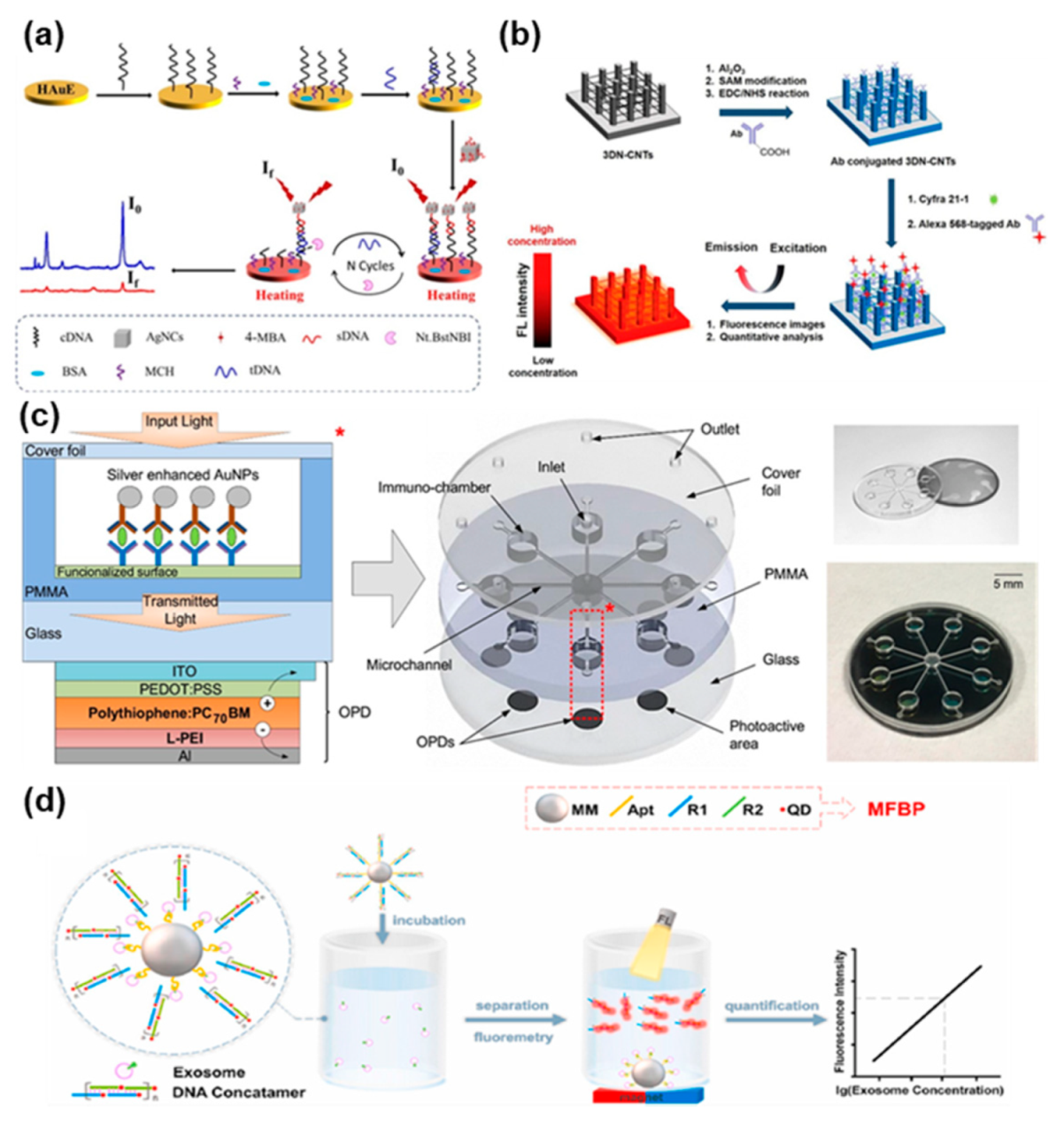
4.2. Optical Biosensors
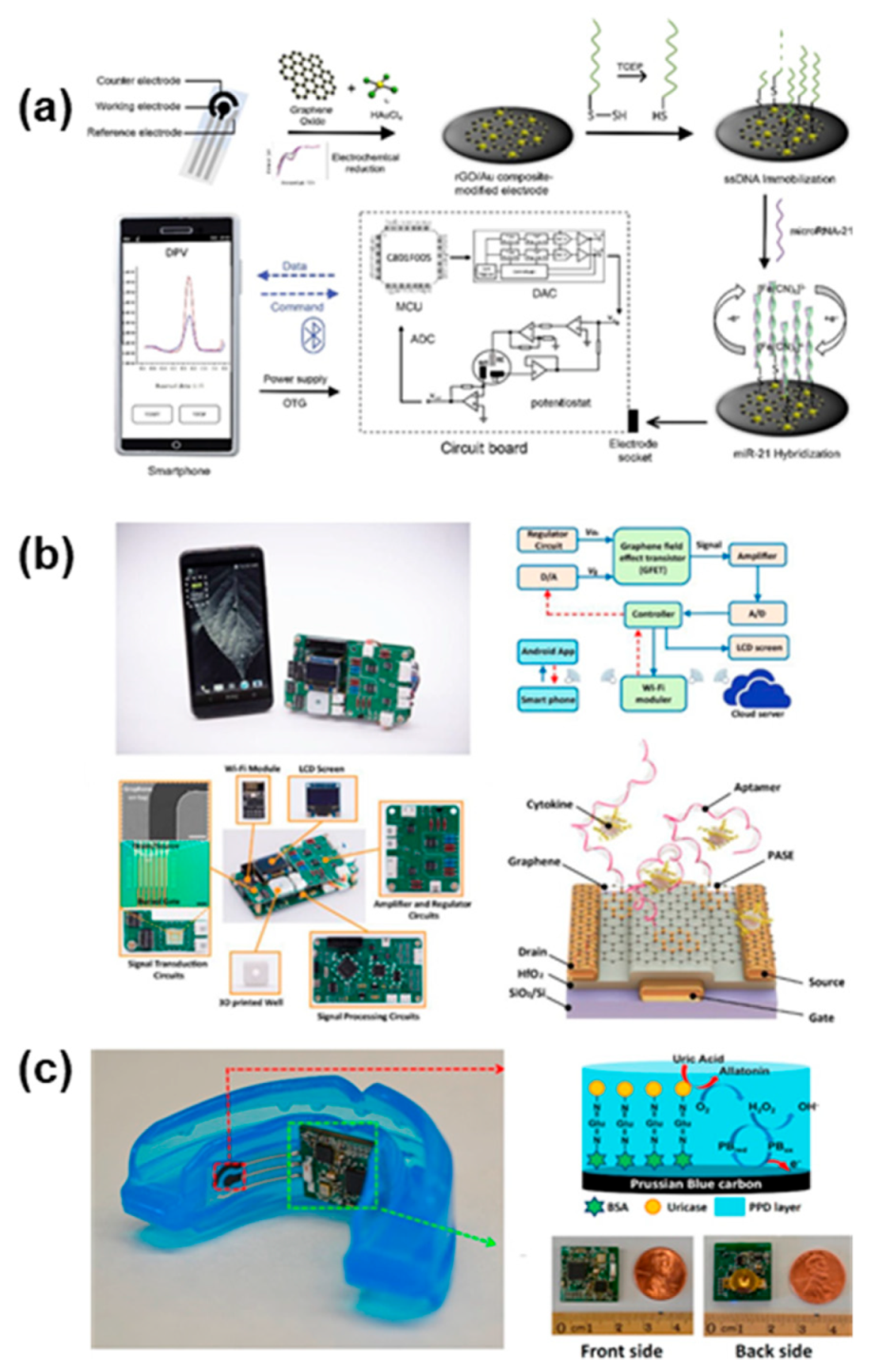
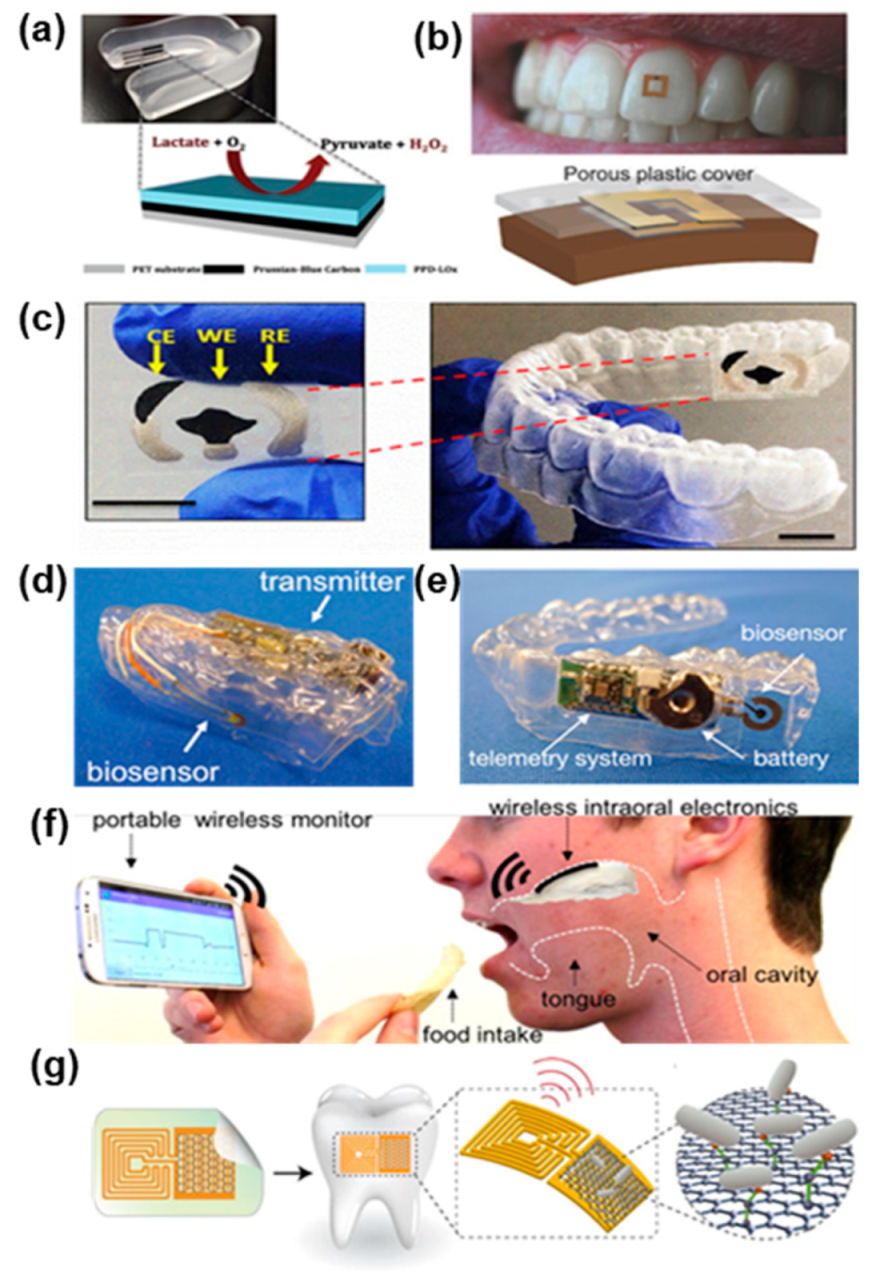
4.3. Integrated Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 14 June 2021).
- Seethala, R.R. Update from the 4th edition of the World Health Organization classification of head and neck tumours: Preface. Head Neck Pathol. 2017, 11, 1–2. [Google Scholar] [CrossRef]
- Petti, S.; Masood, M.; Scully, C. The magnitude of tobacco smoking-betel quid chewing-alcohol drinking interaction effect on oral cancer in South-East Asia. A meta-analysis of observational studies. PLoS ONE 2013, 8, e78999. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Rivera, C. Essentials of oral cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11884–11894. [Google Scholar]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Hu, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L. Role of oral bacteria in the development of oral squamous cell carcinoma. Cancers 2020, 12, 2797. [Google Scholar] [CrossRef]
- Panarese, I.; Aquino, G.; Ronchi, A.; Longo, F.; Montella, M.; Cozzolino, I.; Roccuzzo, G.; Colella, G.; Caraglia, M.; Franco, R. Oral and oropharyngeal squamous cell carcinoma: Prognostic and predictive parameters in the etiopathogenetic route. Expert Rev. Anticancer Ther. 2019, 19, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Sasahira, T.; Kirita, T. Hallmarks of cancer-related newly prognostic factors of oral squamous cell carcinoma. Int. J. Mol. Sci. 2018, 19, 2413. [Google Scholar] [CrossRef] [Green Version]
- Bagan, J.; Sarrion, G.; Jimenez, Y. Oral cancer: Clinical features. Oral Oncol. 2010, 46, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Neville, B.; Damm, D.; Allen, C.; Bouquot, J. Oral and Maxillofacial Pathology, 3rd ed.; Saunders: St. Louis, MO, USA, 2008; p. 984. [Google Scholar]
- Yellowitz, J.A.; Horowitz, A.M.; Drury, T.F.; Goodman, H.S. Survey of U.S. dentists’ knowledge and opinions about oral pharyngeal cancer. J. Am. Dent. Assoc. 2000, 131, 653–661. [Google Scholar] [CrossRef]
- Neville, B.W.; Day, T.A. Oral cancer and precancerous lesions. CA Cancer J. Clin. 2002, 52, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Wiesenfeld, D. Oral cancer. Aust. Dent. J. 2018, 63, S91–S99. [Google Scholar] [CrossRef] [PubMed]
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral cancer and precancer: A narrative review on the relevance of early diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Chakraborty, D.; Natarajan, C.; Mukherjee, A. Advances in oral cancer detection. Adv. Clin. Chem. 2019, 91, 181–200. [Google Scholar] [PubMed]
- Khurshid, Z.; Zafar, M.S.; Khan, R.S.; Najeeb, S.; Slowey, P.D.; Rehman, I.U. Role of salivary biomarkers in oral cancer detection. Adv. Clin. Chem. 2018, 86, 23–70. [Google Scholar]
- Sarrión Pérez, M.G.; Bagán, J.V.; Jiménez, Y.; Margaix, M.; Marzal, C. Utility of imaging techniques in the diagnosis of oral cancer. J. Craniomaxillofac. Surg. 2015, 43, 1880–1894. [Google Scholar] [CrossRef]
- Kaur, J.; Jacobs, R.; Huang, Y.; Salvo, N.; Politis, C. Salivary biomarkers for oral cancer and pre-cancer screening: A review. Clin. Oral Investig. 2018, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzi, M.; Darijani, M.; Momeni, F.; Moradi, P.; Ebrahimnejad, H.; Masoudifar, A.; Mirzaei, H. Molecular imaging and oral cancer diagnosis and therapy. J. Cell. Biochem. 2017, 118, 3055–3060. [Google Scholar] [CrossRef]
- Santosh, A.B.; Jones, T.; Harvey, J. Areview on oral cancer biomarkers: Understanding the past and learning from the present. J. Cancer Res. Ther. 2016, 12, 486–492. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Rees, T.; Wright, J. A review of research on salivary biomarkers for oral cancer detection. Clin. Transl. Med. 2014, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Fridman, E.; Na’ara, S.; Agarwal, J.; Amit, M.; Bachar, G.; Villaret, A.B.; Brandao, J.; Cernea, C.R.; Chaturvedi, P.; Clark, J.; et al. The role of adjuvant treatment in early-stage oral cavity squamous cell carcinoma: An international collaborative study. Cancer 2018, 124, 2948–2955. [Google Scholar] [CrossRef]
- Ganly, I.; Goldstein, D.; Carlson, D.L.; Patel, S.G.; O’Sullivan, B.; Lee, N.; Gullane, P.; Shah, J.P. Long-term regional control and survival in patients with “low-risk”, early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: The importance of tumor thickness. Cancer 2013, 119, 1168–1176. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. Areview of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Holmberg, K.V.; Hoffman, M.P. Anatomy, biogenesis and regeneration of salivary glands. Monogr. Oral Sci. 2014, 24, 1–13. [Google Scholar]
- Pereira, J.A.; Porto-Figueira, P.; Taware, R.; Sukul, P.; Rapole, S.; Câmara, J.S. Unravelling the potential of salivary volatile metabolites in oral diseases. A Review. Molecules 2020, 25, 3098. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.; Bernardini, S. AD biomarker discovery in CSF and in alternative matrices. Clin. Biochem. 2019, 72, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Malon, R.S.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. BioMed Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef]
- Schepici, G.; Silvestro, S.; Trubiani, O.; Bramanti, P.; Mazzon, E. Salivary biomarkers: Future approaches for early diagnosis of neurodegenerative diseases. Brain Sci. 2020, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Kist, T.B.L. Areview of biomarkers of Alzheimer’s disease in noninvasive samples. Biomark. Med. 2018, 12, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Kaczor-Urbanowicz, K.E.; Martin Carreras-Presas, C.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wren, M.E.; Shirtcliff, E.A.; Drury, S.S. Not all biofluids are created equal: Chewing over salivary diagnostics and the epigenome. Clin. Ther. 2015, 37, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, N.J.; Li, Y.; Yu, T.; Brinkman, B.M.; Wong, D.T. Characterization of RNA in saliva. Clin. Chem. 2006, 52, 988–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeda, I.; Stretch, C.; Barnaby, P.; Bhatnager, K.; Rankin, K.; Fu, H.; Weljie, A.; Jha, N.; Slupsky, C. Understanding the human salivary metabolome. NMR Biomed. 2009, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Amado, F.M.; Ferreira, R.P.; Vitorino, R. One decade of salivary proteomics: Current approaches and outstanding challenges. Clin. Biochem. 2013, 46, 506–517. [Google Scholar] [CrossRef]
- Spielmann, N.; Wong, D.T. Saliva: Diagnostics and therapeutic perspectives. Oral Dis. 2011, 17, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Sapienza, C.; Lee, J.; Powell, J.; Erinle, O.; Yafai, F.; Reichert, J.; Siraj, E.S.; Madaio, M. DNA methylation profiling identifies epigenetic differences between diabetes patients with ESRD and diabetes patients without nephropathy. Epigenetics 2011, 6, 20–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, M.; Lacey, M. DNA hypomethylation and hemimethylation in cancer. Adv. Exp. Med. Biol. 2013, 754, 31–56. [Google Scholar]
- Langie, S.A.; Szarc Vel Szic, K.; Declerck, K.; Traen, S.; Koppen, G.; van Camp, G.; Schoeters, G.; Vanden Berghe, W.; de Boever, P. Whole-genome saliva and blood DNA methylation profiling in individuals with a respiratory allergy. PLoS ONE 2016, 11, e0151109. [Google Scholar] [CrossRef]
- Perkins, D.O.; Jeffries, C.D.; Jarskog, L.F.; Thomson, J.M.; Woods, K.; Newman, M.A.; Parker, J.S.; Jin, J.; Hammond, S.M. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007, 8, R27. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.H.; Parker, E.K.; Williamson, V.; McMichael, G.O.; Fanous, A.H.; Vladimirov, V.I. Experimental validation of candidate schizophrenia gene ZNF804A as target for hsa-miR-137. Schizophr. Res. 2012, 141, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hicks, S.D.; Middleton, F.A. A comparative review of microRNA expression patterns in autism spectrum disorder. Front. Psychiatry 2016, 7, 176. [Google Scholar] [CrossRef] [Green Version]
- Sembler-Møller, M.L.; Belstrøm, D.; Locht, H.; Pedersen, A.M.L. Distinct microRNA expression profiles in saliva and salivary gland tissue differentiate patients with primary Sjögren’s syndrome from non-Sjögren’s sicca patients. J. Oral Pathol. Med. 2020, 49, 1044–1052. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Q.; Deng, M.; Miao, J.; Guo, Y.; Gao, W.; Cui, Q. An analysis of human microRNA and disease associations. PLoS ONE 2008, 3, e3420. [Google Scholar] [CrossRef] [Green Version]
- Spielmann, N.; Ilsley, D.; Gu, J.; Lea, K.; Brockman, J.; Heater, S.; Setterquist, R.; Wong, D.T. The human salivary RNA transcriptome revealed by massively parallel sequencing. Clin. Chem. 2012, 58, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Tomei, S.; Manjunath, H.S.; Murugesan, S.; Al Khodor, S. The Salivary miRNome: A promising biomarker of disease. MicroRNA 2021, 10, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Surdacka, A. Salivary biomarkers for diagnosis of inflammatory bowel diseases: A systematic review. Int. J. Mol. Sci. 2020, 21, 7477. [Google Scholar] [CrossRef]
- Hu, S.; Li, Y.; Wang, J.; Xie, Y.; Tjon, K.; Wolinsky, L.; Loo, R.R.; Loo, J.A.; Wong, D.T. Human saliva proteome and transcriptome. J. Dent. Res. 2006, 85, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhou, X.; St John, M.A.; Wong, D.T. RNA profiling of cell-free saliva using microarray technology. J. Dent. Res. 2004, 83, 199–203. [Google Scholar] [CrossRef]
- Park, N.J.; Zhou, X.; Yu, T.; Brinkman, B.M.; Zimmermann, B.G.; Palanisamy, V.; Wong, D.T. Characterization of salivary RNA by cDNA library analysis. Arch. Oral Biol. 2007, 52, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Pisano, E.; Cabras, T.; Montaldo, C.; Piras, V.; Inzitari, R.; Olmi, C.; Castagnola, M.; Messana, I. Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral Sci. 2005, 113, 462–468. [Google Scholar] [CrossRef]
- Inzitari, R.; Cabras, T.; Pisano, E.; Fanali, C.; Manconi, B.; Scarano, E.; Fiorita, A.; Paludetti, G.; Manni, A.; Nemolato, S.; et al. HPLC-ESI-MS analysis of oral human fluids reveals that gingival crevicular fluid is the main source of oral thymosins beta(4) and beta(10). J. Sep. Sci. 2009, 32, 57–63. [Google Scholar] [CrossRef]
- Denny, P.; Hagen, F.K.; Hardt, M.; Liao, L.; Yan, W.; Arellanno, M.; Bassilian, S.; Bedi, G.S.; Boontheung, P.; Cociorva, D.; et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J. Proteome Res. 2008, 7, 1994–2006. [Google Scholar] [CrossRef] [Green Version]
- Al Kawas, S.; Rahim, Z.H.; Ferguson, D.B. Potential uses of human salivary protein and peptide analysis in the diagnosis of disease. Arch. Oral Biol. 2012, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Gardner, A.; Carpenter, G.; So, P.W. Salivary metabolomics: From diagnostic biomarker discovery to investigating biological function. Metabolites 2020, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Avila, M.; Ojcius, D.M.; Yilmaz, O. The oral microbiota: Living with a permanent guest. DNA Cell Biol. 2009, 28, 405–411. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Sodnom-Ish, B.; Choi, S.W.; Jung, H.-I.; Cho, J.; Hwang, I.; Kim, S.M. Salivary biomarkers in oral squamous cell carcinoma. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 301–312. [Google Scholar] [CrossRef]
- Hu, S.; Loo, J.A.; Wong, D.T. Human saliva proteome analysis and disease biomarker discovery. Expert Rev. Proteom. 2007, 4, 531–538. [Google Scholar] [CrossRef]
- Mishra, A.; Verma, M. Cancer biomarkers: Are we ready for the prime time? Cancers 2010, 2, 190–208. [Google Scholar] [CrossRef]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary proteomics for oral cancer biomarker discovery. Clin. Cancer Res. 2008, 14, 6246–6252. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; de Giovanni, E.; Bagnasco, F.; Delucchi, F.; Pera, F.; Baldi, D.; Pesce, P. Salivary micro-RNA and oral squamous cell carcinoma: A systematic review. J. Pers. Med. 2021, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.; Seers, C.; Koo, K.; Cheng, L.; Vella, L.J.; Hill, A.F.; Reynolds, E.; Nastri, A.; Cirillo, N.; McCullough, M. Non-invasive screening of a microRNA-based dysregulation signature in oral cancer and oral potentially malignant disorders. Oral Oncol. 2019, 96, 113–120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ping, F.; Fan, Z.; Zhang, C.; Deng, M.; Cheng, B.; Xia, J. Salivary exosomal miR-24-3p serves as a potential detective biomarker for oral squamous cell carcinoma screening. Biomed. Pharmacother. 2020, 121, 109553. [Google Scholar] [CrossRef]
- Shukla, D.; Kale, A.D.; Hallikerimath, S.; Yerramalla, V.; Subbiah, V. Can quantifying free-circulating DNA be a diagnostic and prognostic marker in oral epithelial dysplasia and oral squamous cell carcinoma? J. Oral Maxillofac. Surg. 2013, 71, 414–418. [Google Scholar] [CrossRef]
- Schröck, A.; Leisse, A.; de Vos, L.; Gevensleben, H.; Dröge, F.; Franzen, A.; Wachendörfer, M.; Schröck, F.; Ellinger, J.; Teschke, M.; et al. Free-circulating methylated DNA in blood for diagnosis, staging, prognosis, and monitoring of head and neck squamous cell carcinoma patients: An observational prospective cohort study. Clin. Chem. 2017, 63, 1288–1296. [Google Scholar] [CrossRef]
- Li, Y.; St John, M.A.; Zhou, X.; Kim, Y.; Sinha, U.; Jordan, R.C.; Eisele, D.; Abemayor, E.; Elashoff, D.; Park, N.H.; et al. Salivary transcriptome diagnostics for oral cancer detection. Clin. Cancer Res. 2004, 10, 8442–8450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katakura, A.; Kamiyama, I.; Takano, N.; Shibahara, T.; Muramatsu, T.; Ishihara, K.; Takagi, R.; Shouno, T. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull. Tokyo Dent. Coll. 2007, 48, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.A.; Taylor, J.M.; Terrell, J.E.; Islam, M.; Li, Y.; Fowler, K.E.; Wolf, G.T.; Teknos, T.N. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer 2008, 113, 750–757. [Google Scholar] [CrossRef]
- Zhong, L.P.; Chen, G.F.; Xu, Z.F.; Zhang, X.; Ping, F.Y.; Zhao, S.F. Detection of telomerase activity in saliva from oral squamous cell carcinoma patients. Int. J. Oral Maxillofac. Surg. 2005, 34, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Almadori, G.; Bussu, F.; Galli, J.; Limongelli, A.; Persichilli, S.; Zappacosta, B.; Minucci, A.; Paludetti, G.; Giardina, B. Salivary glutathione and uric acid levels in patients with head and neck squamous cell carcinoma. Head Neck 2007, 29, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.J.; Lin, C.D.; Lai, C.H.; Chen, C.H.; Kao, J.Y.; Chen, S.Y.; Tsai, M.H.; Huang, S.H.; Lin, C.W. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal. Chim. Acta 2010, 681, 41–48. [Google Scholar] [CrossRef]
- Reddy, I.; Sherlin, H.J.; Ramani, P.; Premkumar, P.; Natesan, A.; Chandrasekar, T. Amino acid profile of saliva from patients with oral squamous cell carcinoma using high performance liquid chromatography. J. Oral Sci. 2012, 54, 279–283. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef] [Green Version]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th Edition oral cavity squamous cell carcinoma staging—Is it an improvement on the AJCC 7th Edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef]
- Acker-man, L.V.; del Regato, J.A.; Stokes, T.L. Cancer diagnosis, treatment and prognosis. Ann. Surg. 1955, 142, 1001. [Google Scholar] [CrossRef]
- Soper, S.A.; Brown, K.; Ellington, A.; Frazier, B.; Garcia-Manero, G.; Gau, V.; Gutman, S.I.; Hayes, D.F.; Korte, B.; Landers, J.L.; et al. Point-of-care biosensor systems for cancer diagnostics/prognostics. Biosens. Bioelectron. 2006, 21, 1932–1942. [Google Scholar] [CrossRef]
- Chen, X.; Gole, J.; Gore, A.; He, Q.; Lu, M.; Min, J.; Yuan, Z.; Yang, X.; Jiang, Y.; Zhang, T.; et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat. Commun. 2020, 11, 3475. [Google Scholar] [CrossRef]
- Goldoni, R.; Farronato, M.; Connelly, S.T.; Tartaglia, G.M.; Yeo, W.-H. Recent advances in graphene-based nanobiosensors for salivary biomarker detection. Biosens. Bioelectron. 2021, 171, 112723. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors for cancer markers diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Tothill, I. Biomarkers and biosensors for the early diagnosis of lung cancer. Sens. Actuators B Chem. 2013, 188, 988–998. [Google Scholar] [CrossRef]
- Jayanthi, V.S.A.; Das, A.B.; Saxena, U. Recent advances in biosensor development for the detection of cancer biomarkers. Biosens. Bioelectron. 2017, 91, 15–23. [Google Scholar] [CrossRef]
- Diamandis, E.P. Analysis of serum proteomic patterns for early cancer diagnosis: Drawing attention to potential problems. J. Natl. Cancer Inst. 2004, 96, 353–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, M.P.; Korc, M.; Malfertheiner, P.; Röcken, C. Advances, challenges, and limitations in serum-proteome-based cancer diagnosis. J. Proteome Res. 2006, 5, 19–25. [Google Scholar] [CrossRef]
- Lee, J.; Garon, E.; Wong, D. Salivary diagnostics. Orthod. Craniofac. Res. 2009, 12, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Denny, P.; Ho, C.-M.; Montemagno, C.; Shi, W.; Qi, F.; Wu, B.; Wolinsky, L.; Wong, D. The oral fluid MEMS/NEMS chip (OFMNC): Diagnostic & translational applications. Adv. Dent. Res. 2005, 18, 3–5. [Google Scholar]
- Tan, W.; Sabet, L.; Li, Y.; Yu, T.; Klokkevold, P.R.; Wong, D.T.; Ho, C.-M. Optical protein sensor for detecting cancer markers in saliva. Biosens. Bioelectron. 2008, 24, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Wong, D. Salivary diagnostics. Oper. Dent. 2012, 37, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, D.T. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J. Am. Dent. Assoc. 2006, 137, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Saadat, D.; Kwon, O.; Lee, Y.; Choi, W.-S.; Kim, J.-H.; Yeo, W.-H. Recent advances in salivary cancer diagnostics enabled by biosensors and bioelectronics. Biosens. Bioelectron. 2016, 81, 181–197. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Philos. Trans. R. Soc. A 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Carpenter, G.H. The secretion, components, and properties of saliva. Annu. Rev. Food Sci. Technol. 2013, 4, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, S.-H.; Du, X.-Y.; Sun, J.-J. Plasmonic Ag nanocube enhanced SERS biosensor for sensitive detection of oral cancer DNA based on nicking endonuclease signal amplification and heated electrode. Sens. Actuators B Chem. 2021, 338, 129854. [Google Scholar] [CrossRef]
- Hao, Z.; Pan, Y.; Shao, W.; Lin, Q.; Zhao, X. Graphene-based fully integrated portable nanosensing system for on-line detection of cytokine biomarkers in saliva. Biosens. Bioelectron. 2019, 134, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.-N.; Wang, L.-L.; Wang, H.-F.; Jia, L.-P.; Zhang, W.; Shang, L.; Xue, Q.-W.; Jia, W.-L.; Liu, Q.-Y.; Wang, H.-S. Highly sensitive ratiometric electrochemical DNA biosensor based on homogeneous exonuclease III-assisted target recycling amplification and one-step triggered dual-signal output. Sens. Actuators B Chem. 2018, 269, 173–179. [Google Scholar] [CrossRef]
- Barhoumi, L.; Baraket, A.; Bellagambi, F.G.; Karanasiou, G.S.; Ali, M.B.; Fotiadis, D.I.; Bausells, J.; Zine, N.; Sigaud, M.; Errachid, A. Anovel chronoamperometric immunosensor for rapid detection of TNF-α in human saliva. Sens. Actuators B Chem. 2018, 266, 477–484. [Google Scholar] [CrossRef]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Tiwari, S.; Srivastava, S.; Srivastava, M.; Yadav, B.K.; Kumar, S.; Tran, T.T.; Dewan, A.K.; Mulchandani, A.; et al. Biofunctionalized nanostructured zirconia for biomedical application: A smart approach for oral cancer detection. Adv. Sci. 2015, 2, 1500048. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, R.; Xu, L.; Ning, Y.; Xie, S.; Zhang, G.-J. Silicon nanowire biosensor for highly sensitive and multiplexed detection of oral squamous cell carcinoma biomarkers in saliva. Anal. Sci. 2015, 31, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Aydın, E.B.; Sezgintürk, M.K. Ultrasensitive detection of interleukin 1α using 3-phosphonopropionic acid modified FTO surface as an effective platform for disposable biosensor fabrication. Bioelectrochemistry 2021, 138, 107698. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gupta, N.; Malhotra, B.D. Ultrasensitive biosensing platform based on yttria doped zirconia-reduced graphene oxide nanocomposite for detection of salivary oral cancer biomarker. Bioelectrochemistry 2021, 140, 107799. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Z.; Xie, Q.; Xiao, B.; Zhou, G.; Chen, G.; Bian, Z. One-step quantification of salivary exosomes based on combined aptamer recognition and quantum dot signal amplification. Biosens. Bioelectron. 2021, 171, 112733. [Google Scholar] [CrossRef]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef]
- Jafari, M.; Hasanzadeh, M. Non-invasive bioassay of cytokeratin Fragment 21.1 (Cyfra 21.1) protein in human saliva samples using immunoreaction method: An efficient platform for early-stage diagnosis of oral cancer based on biomedicine. Biomed. Pharmacother. 2020, 131, 110671. [Google Scholar] [CrossRef]
- Shin Low, S.; Pan, Y.; Ji, D.; Li, Y.; Lu, Y.; He, Y.; Chen, Q.; Liu, Q. Smartphone-based portable electrochemical biosensing system for detection of circulating microRNA-21 in saliva as a proof-of-concept. Sens. Actuators B Chem. 2020, 308, 127718. [Google Scholar] [CrossRef]
- Mo, F.; Chen, M.; Meng, H.; Wu, J.; Fu, Y. ADNA rolling motor for photoelectrochemical biosensing of oral cancer overexpressed 1. Sens. Actuators B Chem. 2020, 309, 127824. [Google Scholar] [CrossRef]
- Muñoz-San Martín, C.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Campuzano, S.; Pingarrón, J.M. Magnetic beads-based electrochemical immunosensing of HIF-1α, a biomarker of tumoral hypoxia. Sens. Actuators B Chem. 2020, 307, 127623. [Google Scholar] [CrossRef]
- Pachauri, N.; Lakshmi, G.B.V.S.; Sri, S.; Gupta, P.K.; Solanki, P.R. Silver molybdate nanoparticles based immunosensor for the non-invasive detection of Interleukin-8 biomarker. Mater. Sci. Eng. C 2020, 113, 110911. [Google Scholar] [CrossRef]
- Han, S.; Locke, A.; Oaks, L.; Cheng, Y.-S.L.; Coté, G. Nanoparticle-based assay for detection of S100P mRNA using surface-enhanced Raman spectroscopy. J. Biomed. Opt. 2019, 24, 055001. [Google Scholar]
- Verma, S.; Singh, S.P. Non-invasive oral cancer detection from saliva using zinc oxide—Reduced graphene oxide nanocomposite based bioelectrode. MRS Commun. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Aydın, E.B.; Sezgintürk, M.K. Adisposable and ultrasensitive ITO based biosensor modified by 6-phosphonohexanoic acid for electrochemical sensing of IL-1β in human serum and saliva. Anal. Chim. Acta 2018, 1039, 41–50. [Google Scholar] [CrossRef]
- Kumar, S.; Ashish; Kumar, S.; Augustine, S.; Yadav, S.; Yadav, B.K.; Chauhan, R.P.; Dewan, A.K.; Malhotra, B.D. Effect of Brownian motion on reduced agglomeration of nanostructured metal oxide towards development of efficient cancer biosensor. Biosens. Bioelectron. 2018, 102, 247–255. [Google Scholar] [CrossRef]
- Ding, S.; Das, S.R.; Brownlee, B.J.; Parate, K.; Davis, T.M.; Stromberg, L.R.; Chan, E.K.L.; Katz, J.; Iverson, B.D.; Claussen, J.C. CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening. Biosens. Bioelectron. 2018, 117, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Aydın, M.; Aydın, E.B.; Sezgintürk, M.K. Ahighly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva. Biosens. Bioelectron. 2018, 117, 720–728. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Highly sensitive electrochemical immunosensor based on polythiophene polymer with densely populated carboxyl groups as immobilization matrix for detection of interleukin 1β in human serum and saliva. Sens. Actuators B Chem. 2018, 270, 18–27. [Google Scholar] [CrossRef]
- Song, C.K.; Oh, E.; Kang, M.S.; Shin, B.S.; Han, S.Y.; Jung, M.; Lee, E.S.; Yoon, S.-Y.; Sung, M.M.; Ng, W.B.; et al. Fluorescence-based immunosensor using three-dimensional CNT network structure for sensitive and reproducible detection of oral squamous cell carcinoma biomarker. Anal. Chim. Acta 2018, 1027, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Sezgintürk, M.K. An impedimetric immunosensor for highly sensitive detection of IL-8 in human serum and saliva samples: A new surface modification method by 6-phosphonohexanoic acid for biosensing applications. Anal. Biochem. 2018, 554, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, N.; Hosseinkhani, S.; Ranjbar, B. Afacile and rapid aptasensor based on split peroxidase DNAzyme for visual detection of carcinoembryonic antigen in saliva. Sens. Actuators B Chem. 2017, 253, 794–803. [Google Scholar] [CrossRef]
- Dong, T.; Pires, N.M.M. Immunodetection of salivary biomarkers by an optical microfluidic biosensor with polyethylenimine-modified polythiophene-C70 organic photodetectors. Biosens. Bioelectron. 2017, 94, 321–327. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-rGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef] [PubMed]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Ahighly sensitive immunosensor based on ITO thin films covered by a new semi-conductive conjugated polymer for the determination of TNFα in human saliva and serum samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Salvo, C.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Electrochemical immunosensor for simultaneous determination of interleukin-1 beta and tumor necrosis factor alpha in serum and saliva using dual screen printed electrodes modified with functionalized double–walled carbon nanotubes. Anal. Chim. Acta 2017, 959, 66–73. [Google Scholar] [CrossRef]
- Della Ventura, B.; Sakač, N.; Funari, R.; Velotta, R. Flexible immunosensor for the detection of salivary α-amylase in body fluids. Talanta 2017, 174, 52–58. [Google Scholar] [CrossRef]
- Choudhary, M.; Yadav, P.; Singh, A.; Kaur, S.; Ramirez-Vick, J.; Chandra, P.; Arora, K.; Singh, S.P. CD 59 targeted ultrasensitive electrochemical immunosensor for fast and noninvasive diagnosis of oral cancer. Electroanalysis 2016, 28, 2565–2574. [Google Scholar] [CrossRef]
- Majidi, M.R.; Omidi, Y.; Karami, P.; Johari-Ahar, M. Reusable potentiometric screen-printed sensor and label-free aptasensor with pseudo-reference electrode for determination of tryptophan in the presence of tyrosine. Talanta 2016, 150, 425–433. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Abiocompatible serine functionalized nanostructured zirconia based biosensing platform for non-invasive oral cancer detection. RSC Adv. 2016, 6, 77037–77046. [Google Scholar] [CrossRef]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Gamella, M.; Pingarrón, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 mRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 543–548. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly sensitive protein functionalized nanostructured hafnium oxide based biosensing platform for non-invasive oral cancer detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef]
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical magnetoimmunosensor for the ultrasensitive determination of interleukin-6 in saliva and urine using poly-HRP streptavidin conjugates as labels for signal amplification. Anal. Bioanal. Chem. 2014, 406, 6363–6371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fan, Y.; Chen, J.; Guo, Y.; Wu, W.; He, Y.; Xu, L.; Fu, F. Amicrofluidic chip-based fluorescent biosensor for the sensitive and specific detection of label-free single-base mismatch via magnetic beads-based “sandwich” hybridization strategy. Electrophoresis 2013, 34, 2177–2184. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Guo, Y.; Wu, X.; Yang, W.; Xu, L.; Chen, J.; Fu, F. Anovel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens. Bioelectron. 2013, 45, 108–113. [Google Scholar] [CrossRef]
- Aydın, M. Asensitive and selective approach for detection of IL 1α cancer biomarker using disposable ITO electrode modified with epoxy-substituted polythiophene polymer. Biosens. Bioelectron. 2019, 144, 111675. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-J.; Zhang, X.-Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018, 16, 52. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Biotechnol. 2014, 32, 363–371. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Wang, J. Wearable electrochemical sensors and biosensors: A review. Electroanalysis 2013, 25, 29–46. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Jia, W.; Wang, J. Tattoo-based wearable electrochemical devices: A review. Electroanalysis 2015, 27, 562–572. [Google Scholar] [CrossRef]
- Tu, J.; Torrente-Rodríguez, R.M.; Wang, M.; Gao, W. The era of digital health: A review of portable and wearable affinity biosensors. Adv. Funct. Mater. 2020, 30, 1906713. [Google Scholar] [CrossRef]
- Radhika, T.; Jeddy, N.; Nithya, S.; Muthumeenakshi, R.M. Salivary biomarkers in oral squamous cell carcinoma—An insight. J. Oral Biol. Craniofac. Res. 2016, 6, S51–S54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Huang, S.; Wang, L.; Yuan, X.; Dong, Q.; Zhang, D.; Wang, X. Clinical and prognostic significance of HIF-1α overexpression in oral squamous cell carcinoma: A meta-analysis. World J. Surg. Oncol. 2017, 15, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of integrated optical biosensors for point-of-care applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Bounik, R.; Gusmaroli, M.; Misun, P.M.; Viswam, V.; Hierlemann, A.; Modena, M.M. Integration of discrete sensors and microelectrode arrays into open microfluidic hanging-drop networks. In Proceedings of the 32nd International Conference on Micro Electro Mechanical Systems (MEMS), Seoul, Korea, 27–31 January 2019; pp. 441–444. [Google Scholar]
- Wen, S.; Su, Y.; Dai, C.; Jia, J.; Fan, G.-C.; Jiang, L.-P.; Song, R.-B.; Zhu, J.-J. Plasmon coupling-enhanced raman sensing platform integrated with exonuclease-assisted target recycling amplification for ultrasensitive and selective detection of microRNA-21. Anal. Chem. 2019, 91, 12298–12306. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Valdés-Ramírez, G.; Bandodkar, A.J.; Jia, W.; Martinez, A.G.; Ramírez, J.; Mercier, P.; Wang, J. Non-invasive mouthguard biosensor for continuous salivary monitoring of metabolites. Analyst 2014, 139, 1632–1636. [Google Scholar] [CrossRef]
- Tseng, P.; Napier, B.; Garbarini, L.; Kaplan, D.L.; Omenetto, F.G. Functional, RF-trilayer sensors for tooth-mounted, wireless monitoring of the oral cavity and food consumption. Adv. Mater. 2018, 30, 1703257. [Google Scholar] [CrossRef] [PubMed]
- Ciui, B.; Tertis, M.; Feurdean, C.N.; Ilea, A.; Sandulescu, R.; Wang, J.; Cristea, C. Cavitas electrochemical sensor toward detection of N-epsilon (carboxymethyl)lysine in oral cavity. Sens. Actuators B Chem. 2019, 281, 399–407. [Google Scholar] [CrossRef]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.; Takeuchi, S.; Sekita, T.; Akiyoshi, K.; Minakuchi, S.; et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft material-enabled, flexible hybrid electronics for medicine, healthcare, and human-machine interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.R.; Kim, H.S.; Qazi, R.; Kwon, Y.T.; Jeong, J.W.; Yeo, W.H. Advanced soft materials, sensor integrations, and applications of wearable flexible hybrid electronics in healthcare, energy, and environment. Adv. Mater. 2020, 32, 1901924. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Howe, C.; Mishra, S.; Lee, D.S.; Mahmood, M.; Piper, M.; Kim, Y.; Tieu, K.; Byun, H.-S.; Coffey, J.P.; et al. Wireless, intraoral hybrid electronics for real-time quantification of sodium intake toward hypertension management. Proc. Natl. Acad. Sci. USA 2018, 115, 5377–5382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mannoor, M.S.; Tao, H.; Clayton, J.D.; Sengupta, A.; Kaplan, D.L.; Naik, R.R.; Verma, N.; Omenetto, F.G.; McAlpine, M.C. Graphene-based wireless bacteria detection on tooth enamel. Nat. Commun. 2012, 3, 763. [Google Scholar] [CrossRef]
- Sukul, P.; Richter, A.; Schubert, J.K.; Miekisch, W. Deficiency and absence of endogenous isoprene in adults, disqualified its putative origin. Heliyon 2021, 7, e05922. [Google Scholar] [CrossRef]
| Analyte | Detection | LOD | R/I 1 Time | Sample Type | Validation | Ref. |
|---|---|---|---|---|---|---|
| ORAOV-1 | SERS 2 | 3.1 fM | 60 min | Artificial and real saliva | N/A | [100] |
| IL-1α | EIS 3 | 6 fg/mL | 60 min | Saliva and serum | N/A | [108] |
| CYFRA 21-1 | DPV 3 | 7.2 pg/mL | 15 min | Saliva | N/A | [109] |
| CD63 | Fluorescence 2 | 500 particles/μL | 30 min | Saliva | NanoFCM | [110] |
| IL-1β | DPV 3 | 5.2 pg/mL | 150 min | Saliva | ELISA | [111] |
| CYFRA 21-1 | SWV 3 | 2.5 ng/mL | N/A | Saliva | ELISA | [112] |
| miRNA-21 | DPV 3 | 1 pM | 60 min | Saliva | N/A | [113] |
| ORAOV-1 | PEC 4 | 33 fM | 40 min | Saliva | N/A | [114] |
| HIF-1α | Amperometry 3 | 76 pg/mL | 105 min | Saliva | ELISA | [115] |
| IL-8 | DPV 3 | 90 pg/mL | 10 min | Saliva | N/A | [116] |
| S100p mRNA | SERS 2 | 1.1 nM | 53 min | Saliva | N/A | [117] |
| IL-8 | DPV 3 | 51.53 pg/mL | 10 min | Saliva | N/A | [118] |
| IL-6 | Voltammetry 3 | 12 pM | 400 s | Artificial and real saliva | N/A | [101] |
| IL-1β | EIS 3 | 7.5 fg/mL | 45 min | Saliva and serum | N/A | [119] |
| ORAOV-1 | ACV 3 | 12.8 fM | 120 min | Artificial saliva | N/A | [102] |
| CYFRA 21-1 | DPV 3 | 0.16 ng/mL | 15 min | Saliva | ELISA | [120] |
| CIP2A | EIS 3 | 0.24 pg/mL | 35 min | Saliva | ELISA | [121] |
| IL-8 | EIS 3 | 3.3 fg/mL | 45 min | Saliva and serum | ELISA | [122] |
| IL-1β | EIS 3 | 3 fg/mL | 45 min | Saliva and serum | N/A | [123] |
| CYFRA 21-1 | Fluorescence 2 | 0.5 ng/mL | N/A | Saliva | ECL | [124] |
| IL-8 | SFI 3 | 6 fg/mL | 45 min | Saliva and serum | ELISA | [125] |
| TNF-α | Chronoamperometry 3 | 0.001 ng/mL | N/A | Artificial and real saliva | ELISA | [103] |
| CEA | Colorimetry 2 | 1 ng/mL | 1 min | Saliva | N/A | [126] |
| IL-8 | Absorbance 2 | 90 pg/mL | 30 min | Saliva | ELISA | [127] |
| IL-1β | Absorbance 2 | 80 pg/mL | 30 min | Saliva | ELISA | [127] |
| MMP-8 | Absorbance 2 | 120 pg/mL | 30 min | Saliva | ELISA | [127] |
| IL-8 | DPV 3 | 72.73 pg/mL | 9 min | Saliva | N/A | [128] |
| TNF-α | EIS 3 | 3.7 fg/mL | 45 min | Saliva and serum | ELISA | [129] |
| CYFRA 21-1 | DPV 3 | 0.001 ng/mL | 5 min | Artificial saliva | ELISA | [104] |
| IL-1β | Amperometry 3 | 0.38 pg/mL | 150 min | Saliva and serum | ELISA | [130] |
| TNF-α | Amperometry 3 | 0.85 pg/mL | 150 min | Saliva and serum | ELISA | [130] |
| α-amylase | QCM 5 | 1 μg/mL | N/A | Saliva, serum and urine | Phadebas test | [131] |
| CD59 | EIS 3 | 0.84 fg/mL | 10 min | Saliva | N/A | [132] |
| Tryptophan | CC-PSA 3 | 4.9 pM | 5 min | Saliva | N/A | [133] |
| CYFRA 21-1 | DPV 3 | 0.01 ng/mL | 6 min | Saliva | ELISA | [134] |
| IL-8 | Amperometry 3 | 72.4 pg/mL | 5 h | Saliva | ELISA | [135] |
| IL-8 mRNA | Amperometry 3 | 0.21 nM | 5 h | Saliva | ELISA | [135] |
| CYFRA 21-1 | CV 3 | 0.21 ng/mL | 15 min | Saliva | ELISA | [136] |
| CYFRA 21-1 | DPV 3 | 0.122 ng/mL | 16 min | Saliva | ELISA | [137] |
| CYFRA 21-1 | CV 3 | 0.08 ng/mL | 20 min | Artificial and real saliva | ELISA | [105] |
| Uric Acid | Chronoamperometry 3 | 600 Um | 1 min | Artificial and real saliva | N/A | [106] |
| ORAOV-1 | DPV 3 | 0.35 pM | 60 min | Saliva | N/A | [94] |
| IL-8 + TNF-α | FET 3 | 100 fg/mL | N/A | Artificial saliva | ELISA | [107] |
| IL-6 | DPV 3 | 0.39 pg/mL | 3 h | Saliva and urine | ELISA | [138] |
| DNA sequence | Fluorescence 2 | 56 pM | 15 min | Saliva and serum | N/A | [139] |
| hsa-miR-200a | Amperometry 3 | 0.22 aM | 100 s | Artificial saliva | N/A | [140] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldoni, R.; Scolaro, A.; Boccalari, E.; Dolci, C.; Scarano, A.; Inchingolo, F.; Ravazzani, P.; Muti, P.; Tartaglia, G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors 2021, 11, 396. https://doi.org/10.3390/bios11100396
Goldoni R, Scolaro A, Boccalari E, Dolci C, Scarano A, Inchingolo F, Ravazzani P, Muti P, Tartaglia G. Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors. 2021; 11(10):396. https://doi.org/10.3390/bios11100396
Chicago/Turabian StyleGoldoni, Riccardo, Alessandra Scolaro, Elisa Boccalari, Carolina Dolci, Antonio Scarano, Francesco Inchingolo, Paolo Ravazzani, Paola Muti, and Gianluca Tartaglia. 2021. "Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers" Biosensors 11, no. 10: 396. https://doi.org/10.3390/bios11100396
APA StyleGoldoni, R., Scolaro, A., Boccalari, E., Dolci, C., Scarano, A., Inchingolo, F., Ravazzani, P., Muti, P., & Tartaglia, G. (2021). Malignancies and Biosensors: A Focus on Oral Cancer Detection through Salivary Biomarkers. Biosensors, 11(10), 396. https://doi.org/10.3390/bios11100396









