Applications of Microfluidics in Liquid Crystal-Based Biosensors
Abstract
:1. Introduction
2. Microfluidics in LC Planar Sensing Platforms
2.1. LC-Solid Interface in Microfluidic Channels for Biosensing Applications
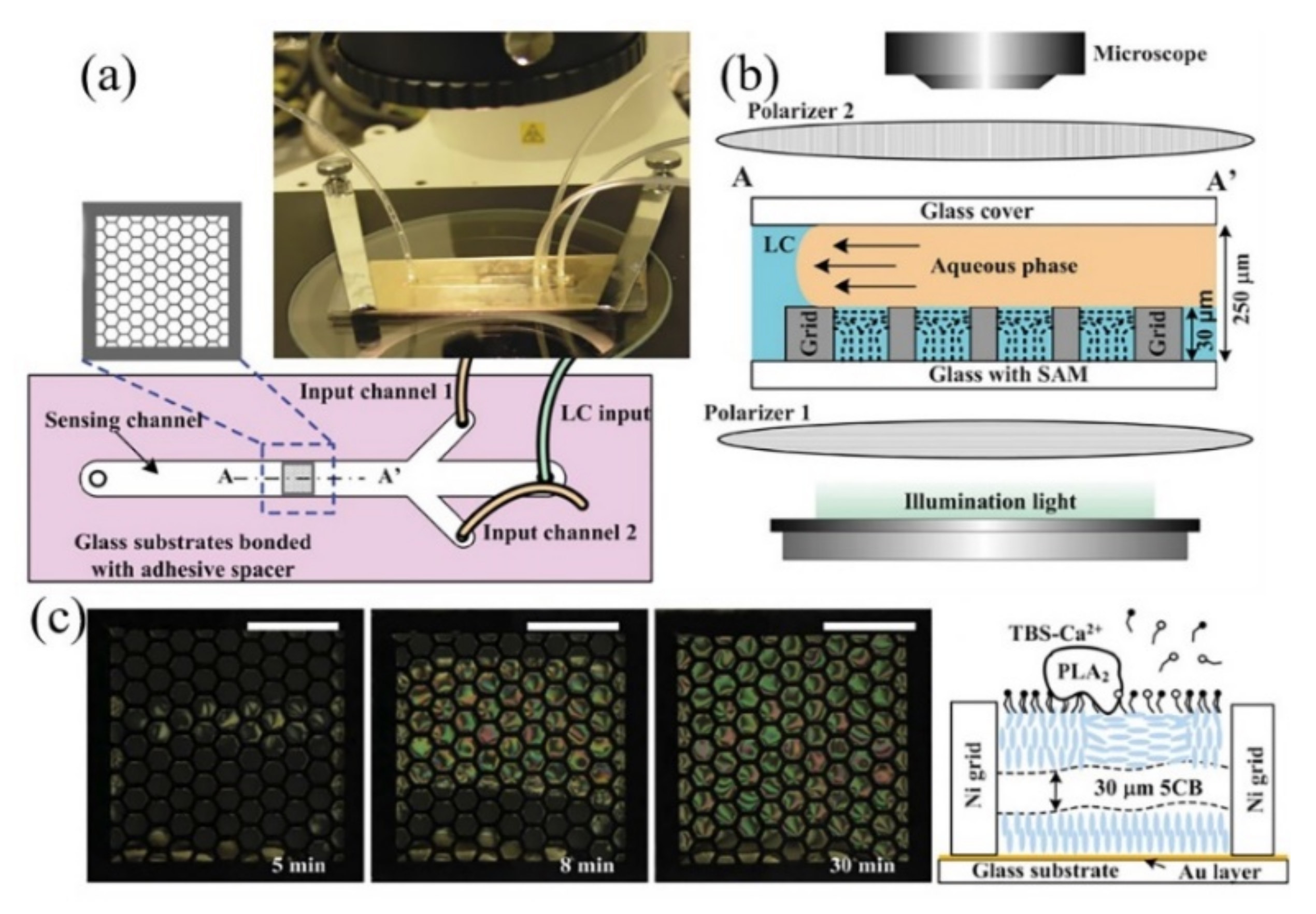
2.1.1. Microfluidic Channel Integrated with LC-Solid Interface
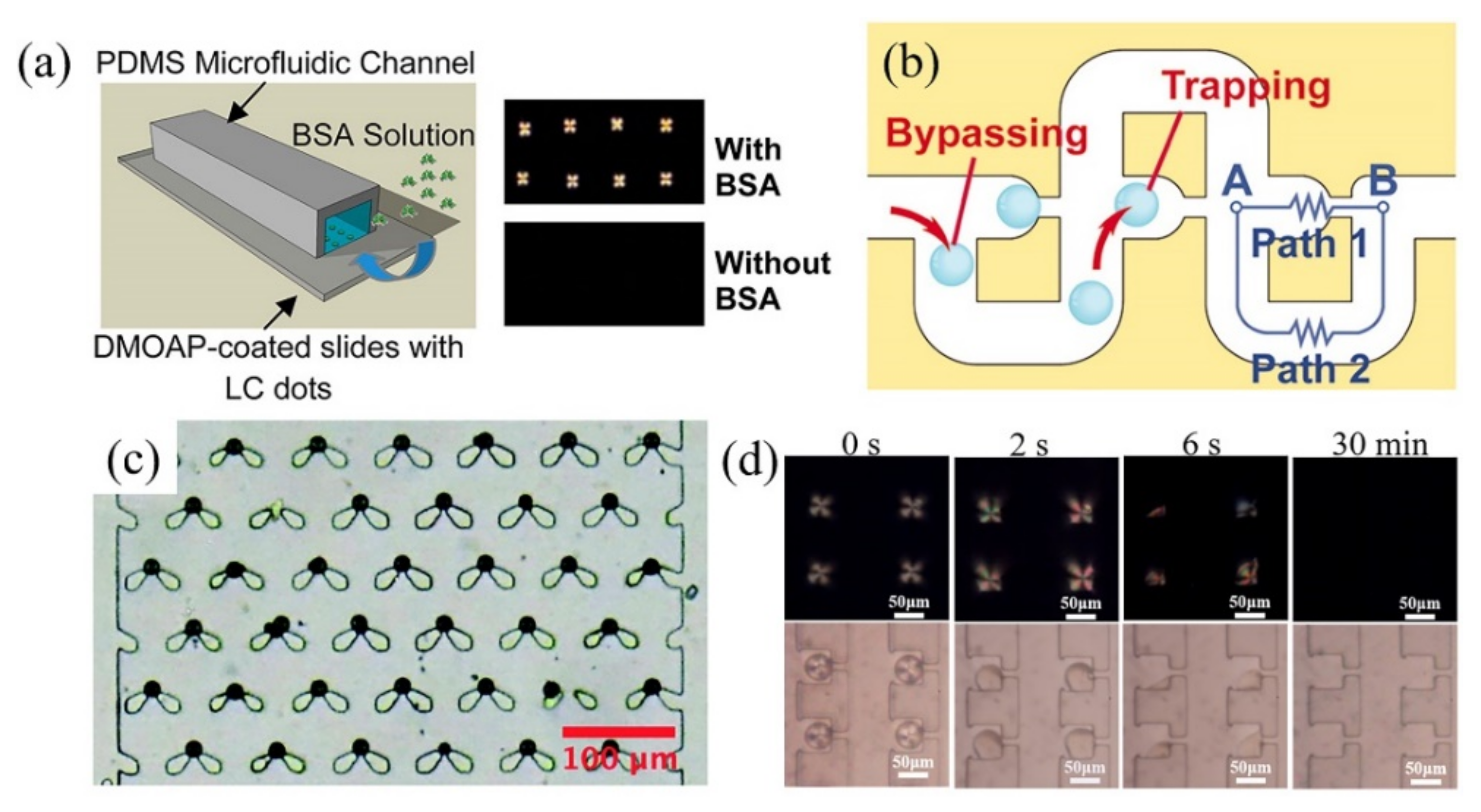
2.1.2. LC-Solid Interface-Based Microfluidic Device for Biosensing Applications
2.2. LC-Aqueous Interface in Microfluidic Channels for Biosensing Applications
2.2.1. Fabrication of LC-Aqueous Interface within Microfluidic Channels
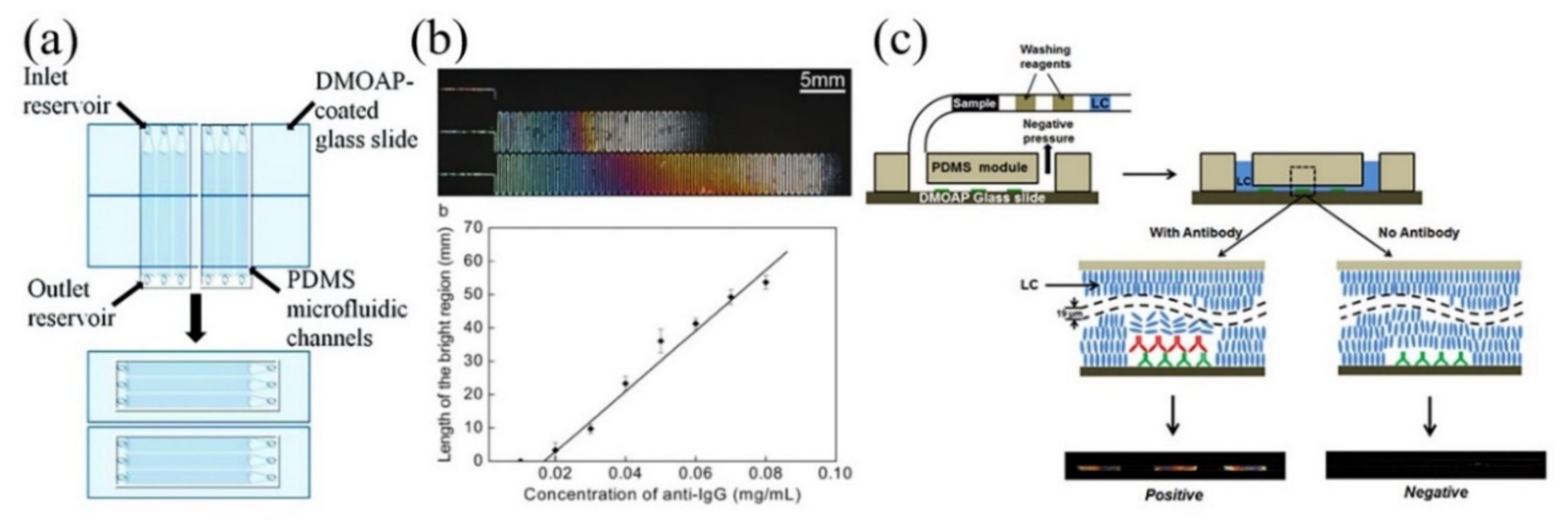
2.2.2. LC-Aqueous Interface-Based Microfluidic Device for Biosensing Applications
3. Microfluidics in LC Droplet-Based Biosensors
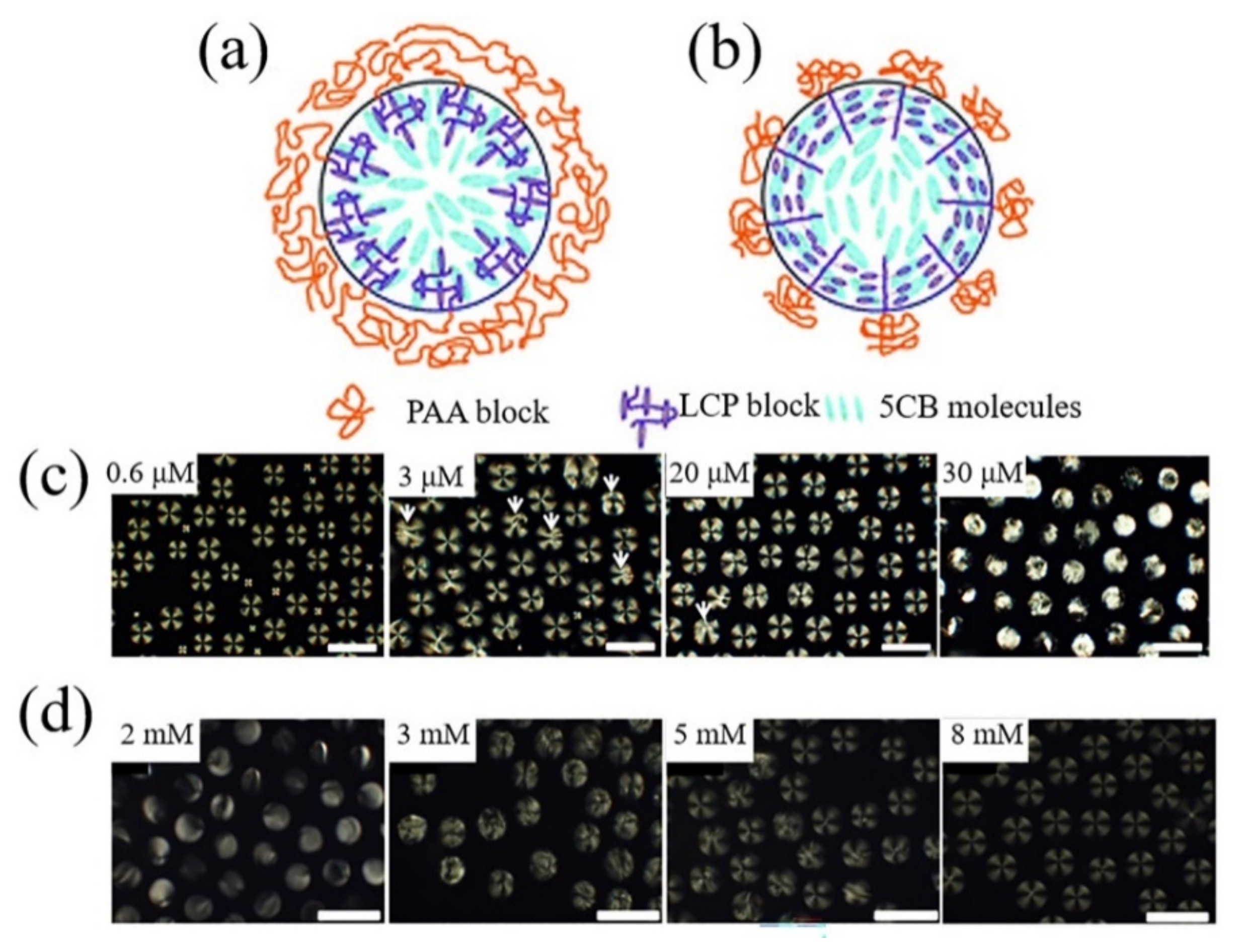
3.1. Microfluidic Fabrication of LC Droplets for Biosensing Applications
3.1.1. Fabrication Methods for Monodisperse LC Droplets
3.1.2. Biosensing Applications with Monodisperse LC Droplets
3.2. Confinement of LC Droplet within Microfluidic Channel for Real-Time Detection
3.2.1. LC Droplet-Based Static Microarrays
3.2.2. LC Droplet-Based Dynamic Microarrays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.; Gou, F.; Chen, R.; Yin, K.; Zhan, T.; Wu, S.T. Liquid crystal beam steering devices: Principles, recent advances, and future developments. Crystals 2019, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Bisoyi, H.K.; Li, Q. Light-driven liquid crystalline materials: From photo-induced phase transitions and property modulations to applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Bremer, M.; Kirsch, P.; Klasen-Memmer, M.; Tarumi, K. The TV in your pocket: Development of liquid-crystal materials for the new millennium. Angew. Chem. Int. Ed. 2013, 52, 8880–8896. [Google Scholar] [CrossRef] [PubMed]
- Woltman, S.J.; Jay, G.D.; Crawford, G.P. Liquid-crystal materials find a new order in biomedical applications. Nat. Mater. 2007, 6, 929–938. [Google Scholar] [CrossRef]
- Prakash, J.; Parveen, A.; Mishra, Y.K.; Kaushik, A. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 168, 112562. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Noh, J.; Nayani, K.; Abbott, N.L. Soft matter from liquid crystals. Soft Matter 2019, 15, 6913–6929. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Herminghaus, S.; Bahr, C. Liquid crystal microfluidics: Surface, elastic and viscous interactions at microscales. Liq. Cryst. Rev. 2014, 2, 73–110. [Google Scholar] [CrossRef]
- Lockwood, N.A.; Gupta, J.K.; Abbott, N.L. Self-assembly of amphiphiles, polymers and proteins at interfaces between thermotropic liquid crystals and aqueous phases. Surf. Sci. Rep. 2008, 63, 255–293. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Abnous, K.; Taghdisi, S.M.; Verdian, A. Liquid crystal-based biosensors as lab-on-chip tools: Promising for future on-site detection test kits. TrAC Trends Anal. Chem. 2021, 142, 116325. [Google Scholar] [CrossRef]
- Zhou, L.; Hu, Q.; Kang, Q.; Yu, L. Construction of liquid crystal droplet-based sensing platform for sensitive detection of organophosphate pesticide. Talanta 2018, 190, 375–381. [Google Scholar] [CrossRef]
- Gold, J.; Szilvasi, T.; Abbott, N.L.; Mavrikakis, M. Binding of organophosphorus nerve agents and their simulants to metal salts. ACS Appl. Mater. Interfaces 2020, 12, 30941–30953. [Google Scholar] [CrossRef]
- Yu, H.; Szilvási, T.; Rai, P.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Computational chemistry-guided design of selective chemoresponsive liquid crystals using pyridine and pyrimidine functional groups. Adv. Funct. Mater. 2018, 28, 1703581. [Google Scholar] [CrossRef]
- Jiang, S.; Noh, J.; Park, C.; Smith, A.D.; Abbott, N.L.; Zavala, V.M. Using machine learning and liquid crystal droplets to identify and quantify endotoxins from different bacterial species. Analyst 2021, 146, 1224–1233. [Google Scholar] [CrossRef]
- Lin, I.H.; Miller, D.S.; Bertics, P.J.; Murphy, C.J.; de Pablo, J.J.; Abbott, N.L. Endotoxin-induced structural transformations in liquid crystalline droplets. Science 2011, 332, 1297–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, Z.; Jang, C.H. Simple and label-free liquid crystal-based optical sensor for highly sensitive and selective endotoxin detection by aptamer binding and separation. ChemistrySelect 2019, 4, 1416–1422. [Google Scholar] [CrossRef]
- Manna, U.; Zayas-Gonzalez, Y.M.; Carlton, R.J.; Caruso, F.; Abbott, N.L.; Lynn, D.M. Liquid crystal chemical sensors that cells can wear. Angew. Chem. Int. Ed. 2013, 52, 14011–14015. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Gupta, K.C.; Borah, J.S.; Park, S.Y.; Kim, Y.K.; Lee, J.H.; Kang, I.K. Folate ligand anchored liquid crystal microdroplets emulsion for in vitro detection of KB cancer cells. Langmuir 2014, 30, 10668–10677. [Google Scholar] [CrossRef] [PubMed]
- Han, G.R.; Jang, C.H. Detection of heavy-metal ions using liquid crystal droplet patterns modulated by interaction between negatively charged carboxylate and heavy-metal cations. Talanta 2014, 128, 44–50. [Google Scholar] [CrossRef]
- Yang, S.; Wu, C.; Tan, H.; Wu, Y.; Liao, S.; Wu, Z.; Shen, G.; Yu, R. Label-free liquid crystal biosensor based on specific oligonucleotide probes for heavy metal ions. Anal. Chem. 2013, 85, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Li, Y.; Li, H.; Yang, J. Detection of heavy metal ions using whispering gallery mode lasing in functionalized liquid crystal microdroplets. Biomed. Opt. Express 2019, 10, 6073–6083. [Google Scholar] [CrossRef]
- Alino, V.J.; Pang, J.; Yang, K.L. Liquid crystal droplets as a hosting and sensing platform for developing immunoassays. Langmuir 2011, 27, 11784–11789. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Gupta, K.C.; Park, S.Y.; Kang, I.K. Anti-IgG-anchored liquid crystal microdroplets for label free detection of IgG. J. Mater. Chem. B 2016, 4, 704–715. [Google Scholar] [CrossRef]
- Qi, L.; Liu, S.; Jiang, Y.; Lin, J.M.; Yu, L.; Hu, Q. Simultaneous detection of multiple tumor markers in blood by functional liquid crystal sensors assisted with target-induced dissociation of aptamer. Anal. Chem. 2020, 92, 3867–3873. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Liao, S.; Yu, R.; Wu, Z. Highly-sensitive liquid crystal biosensor based on DNA dendrimers-mediated optical reorientation. Biosens. Bioelectron. 2014, 62, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.L.; Tan, W.L.; Yang, K.L. Detection of DNA targets hybridized to solid surfaces using optical images of liquid crystals. ACS Appl. Mat. Interfaces 2011, 3, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Tan, H.; Wu, C.; Wu, Z.; Shen, G.; Yu, R. Gold nanoparticle based signal enhancement liquid crystal biosensors for DNA hybridization assays. Chem. Commun. 2012, 48, 2861–2863. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Khan, M.; Park, S.Y. Glucose sensor using liquid-crystal droplets made by microfluidics. ACS Appl. Mat. Interfaces 2013, 5, 13135–13139. [Google Scholar] [CrossRef]
- Qi, L.; Hu, Q.; Kang, Q.; Yu, L. Fabrication of liquid-crystal-based optical sensing platform for detection of hydrogen peroxide and blood glucose. Anal. Chem. 2018, 90, 11607–11613. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Park, S.Y. Label- and enzyme-free detection of glucose by boronic acid-coupled poly(styrene-b-acrylic acid) at liquid crystal/aqueous interfaces. Anal. Chim. Acta 2018, 1032, 122–129. [Google Scholar] [CrossRef]
- Bera, T.; Fang, J. Optical detection of lithocholic acid with liquid crystal emulsions. Langmuir 2013, 29, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Lu, X.; Constant, C.; Dogariu, A.; Fang, J. Design of beta-CD-surfactant complex-coated liquid crystal droplets for the detection of cholic acid via competitive host-guest recognition. Chem. Commun. 2015, 51, 8912–8915. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, D.; Chen, R.; Wang, F.; Sun, X.; Dai, H. Optical biosensor based on liquid crystal droplets for detection of cholic acid. Opt. Commun. 2016, 381, 286–291. [Google Scholar] [CrossRef]
- Ma, H.; Kang, Q.; Wang, T.; Xiao, J.; Yu, L. Liquid crystals-based sensor for the detection of lithocholic acid coupled with competitive host-guest inclusion. Colloids Surf. B 2019, 173, 178–184. [Google Scholar] [CrossRef]
- Bukusoglu, E.; Bedolla Pantoja, M.; Mushenheim, P.C.; Wang, X.; Abbott, N.L. Design of responsive and active (soft) materials using liquid crystals. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 163–196. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Ratchford, D.; Casalini, R.; Thum, M.D.; Wynne, J.H.; Lundin, J.G. Surfactant modulated phase transitions of liquid crystals confined in electrospun coaxial fibers. Langmuir 2020, 36, 7916–7924. [Google Scholar] [CrossRef]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef]
- Shin, M.J.; Yoon, D.K. Role of stimuli on liquid crystalline defects: From defect engineering to switchable functional materials. Materials 2020, 13, 5466. [Google Scholar] [CrossRef] [PubMed]
- Karausta, A.; Kocaman, C.; Bukusoglu, E. Controlling the shapes and internal complexity of the polymeric particles using liquid crystal-templates confined into microwells. J. Mol. Liq. 2021, 324, 114710. [Google Scholar] [CrossRef]
- Nguyen, D.K.; Jang, C.H. An acetylcholinesterase-based biosensor for the detection of pesticides using liquid crystals confined in microcapillaries. Colloids Surf. B Biointerfaces 2021, 200, 111587. [Google Scholar] [CrossRef]
- Carlton, R.J.; Gupta, J.K.; Swift, C.L.; Abbott, N.L. Influence of simple electrolytes on the orientational ordering of thermotropic liquid crystals at aqueous interfaces. Langmuir 2012, 28, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Zhao, Q.; Cui, H.; Wang, Y.; Du, X. Microfluidic platforms toward rational material fabrication for biomedical applications. Small 2019, 16, 1903798. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, S.; Jiang, D.; Zhu, L.; Yang, J.; Xiang, N. Channel innovations for inertial microfluidics. Lab Chip 2020, 20, 3485–3502. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Li, L.; Han, Z.; Wang, Y.; Tang, B. Recent advance in microfluidic technologies for circulating tumor cells: From enrichment, single cell analysis to liquid biopsy for clinical applications. Lab Chip 2020, 20, 3854–3875. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in microfluidic materials, functions, integration, and applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [Green Version]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef] [PubMed]
- Sassa, F.; Biswas, G.C.; Suzuki, H. Microfabricated electrochemical sensing devices. Lab Chip 2020, 20, 1358–1389. [Google Scholar] [CrossRef]
- Al Mughairy, B.; Al-Lawati, H.A.J. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. TrAC Trends Anal. Chem. 2020, 124, 115802. [Google Scholar] [CrossRef]
- Mei, H.; Pan, J.; Zhang, Z.; Zhang, L.; Tong, L. Coiled optical nanofiber for optofluidic absorbance detection. ACS Sens. 2019, 4, 2267–2271. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, D.; Lin, I.H.; Abbott, N.L.; Jiang, H. Microfluidic sensing devices employing in situ-formed liquid crystal thin film for detection of biochemical interactions. Lab Chip 2012, 12, 3746–3753. [Google Scholar] [CrossRef] [Green Version]
- Khan, W.; Choi, J.H.; Kim, G.M.; Park, S.Y. Microfluidic formation of pH responsive 5CB droplets decorated with PAA-b-LCP. Lab Chip 2011, 11, 3493–3498. [Google Scholar] [CrossRef]
- Myung, D.B.; Park, S.Y. Optical properties and applications of photonic shells. ACS Appl. Mat. Interfaces 2019, 11, 20350–20359. [Google Scholar] [CrossRef]
- Aliño, V.J.; Sim, P.H.; Choy, W.T.; Fraser, A.; Yang, K.L. Detecting proteins in microfluidic channels decorated with liquid crystal sensing dots. Langmuir 2012, 28, 17571–17577. [Google Scholar] [CrossRef]
- Han, X.; Han, D.; Zeng, J.; Deng, J.; Hu, N.; Yang, J. Fabrication and performance of monodisperse liquid crystal droplet-based microchips for the on-chip detection of bile acids. Microchem. J. 2020, 157, 105057. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Liang, W.; Richardson, D.; Lu, Q.; Fang, J. Surface modified liquid crystal droplets as an optical probe for the detection of bile acids in microfluidic channels. Colloids Surf. A 2018, 542, 52–58. [Google Scholar] [CrossRef]
- Bao, P.; Paterson, D.A.; Harrison, P.L.; Miller, K.; Peyman, S.; Jones, J.C.; Sandoe, J.; Evans, S.D.; Bushby, R.J.; Gleeson, H.F. Lipid coated liquid crystal droplets for the on-chip detection of antimicrobial peptides. Lab Chip 2019, 19, 1082–1089. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Su, X.; Yang, D.; Luan, C. Label-free liquid crystal biosensor for cecropin B detection. Talanta 2018, 186, 60–64. [Google Scholar] [CrossRef]
- Xue, C.Y.; Yang, K.L. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Xiong, X.; Deng, S. Gold nanoparticle-based signal enhancement of an aptasensor for ractopamine using liquid crystal based optical imaging. Microchim. Acta 2019, 186, 697. [Google Scholar] [CrossRef] [PubMed]
- Kim Hong, P.T.; Jang, C.H. Sensitive and label-free liquid crystal-based optical sensor for the detection of malathion. Anal. Biochem. 2020, 593, 113589. [Google Scholar] [CrossRef]
- Brake, J.M.; Daschner, M.K.; Luk, Y.Y.; Abbott, N.L. Biomolecular interactions at phospholipid-decorated surfaces of liquid crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Deng, J.; Fang, J.; Wu, S.T. Lag-burst kinetics of surfactant displacement from the liquid crystal/aqueous interface by bile acids. Colloids Surf. A 2015, 471, 148–152. [Google Scholar] [CrossRef]
- Gupta, J.K.; Meli, M.V.; Teren, S.; Abbott, N.L. Elastic energy-driven phase separation of phospholipid monolayers at the nematic liquid-crystal–aqueous interface. Phys. Rev. Lett. 2008, 100, 048301. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Carlton, R.J.; Mushenheim, P.C.; Abbott, N.L. Introduction to optical methods for characterizing liquid crystals at interfaces. Langmuir 2013, 29, 3154–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.; Lin, I.H.; Abbott, N.L.; Jiang, H. Autonomous microfluidic sensing device employing liquid crystal for detection of biological interactions. In Proceedings of the Transducers 2009—15th International Conference on Solid-State Sensors, Actuators and Microsystems, Denver, CO, USA, 21–25 June 2009; pp. 116–119. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, K.L. Microfluidic immunoassay with plug-in liquid crystal for optical detection of antibody. Anal. Chim. Acta 2015, 853, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.Y.; Khan, S.A.; Yang, K.L. Exploring optical properties of liquid crystals for developing label-free and high-throughput microfluidic immunoassays. Adv. Mater. 2009, 21, 198–202. [Google Scholar] [CrossRef]
- Zhang, W.; Ang, W.T.; Xue, C.Y.; Yang, K.L. Minimizing nonspecific protein adsorption in liquid crystal immunoassays by using surfactants. ACS Appl. Mat. Interfaces 2011, 3, 3496–3500. [Google Scholar] [CrossRef]
- Wang, I.T.; Lee, Y.H.; Chuang, E.Y.; Hsiao, Y.C. Sensitive, color-indicating and labeling-free multi-detection cholesteric liquid crystal biosensing chips for detecting albumin. Polymers 2021, 13, 1463. [Google Scholar] [CrossRef]
- Fan, Y.J.; Chen, F.L.; Liou, J.C.; Huang, Y.W.; Chen, C.H.; Hong, Z.Y.; Lin, J.D.; Hsiao, Y.C. Label-free multi-microfluidic immunoassays with liquid crystals on polydimethylsiloxane biosensing chips. Polymers 2020, 12, 395. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Q.; Tang, R.Z.; Chen, Y.; Yin, S.; Huang, Y.; Wong, T.N. Investigation of shear-driven and pressure-driven liquid crystal flow at microscale: A quantitative approach for the flow measurement. Micromachines 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Zou, Y.; Lin, Y.; Lindquist, R. Microfluidic biosensor using liquid crystal. In Proceedings of the IEEE SoutheastCon, Nashville, TN, USA, 17–20 March 2011; pp. 42–44. [Google Scholar] [CrossRef]
- Chang, J.J.; Huang, J.W.; Lin, C.F.; Liu, S.W.; Chen, C.H. Enhancing the signal contrast ratio and stability of liquid crystal-based sensors by using fine grids made by photolithography of photoresists. Analyst 2021, 146, 3834–3840. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, D.; Yang, K.L. Flow-driven disclination lines of nematic liquid crystals inside a rectangular microchannel. Soft Matter 2019, 15, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.; Tsuei, M.; Abbott, N.L. Using liquid crystals for in situ optical mapping of interfacial mobility and surfactant concentrations at flowing aqueous–oil interfaces. Langmuir 2021, 37, 5810–5822. [Google Scholar] [CrossRef]
- Lin, C.T.; Hsu, W.T.; Hwang, S.J. Real-time liquid crystal-based creatinine sensor using a micro-patterned flexible substrate. Liq. Cryst. 2021, 1–11. [Google Scholar] [CrossRef]
- Brake, J.M.; Daschner, M.K.; Abbott, N.L. Formation and characterization of phospholipid monolayers spontaneously assembled at interfaces between aqueous phases and thermotropic liquid crystals. Langmuir 2005, 21, 2218–2228. [Google Scholar] [CrossRef]
- Miller, D.S.; Abbott, N.L. Influence of droplet size, pH and ionic strength on endotoxin-triggered ordering transitions in liquid crystalline droplets. Soft Matter 2013, 9, 374–382. [Google Scholar] [CrossRef]
- Gupta, J.K.; Sivakumar, S.; Caruso, F.; Abbott, N.L. Size-dependent ordering of liquid crystals observed in polymeric capsules with micrometer and smaller diameters. Angew. Chem. Int. Ed. 2009, 48, 1652–1655. [Google Scholar] [CrossRef]
- Wang, X.; Bukusoglu, E.; Abbott, N.L. A practical guide to the preparation of liquid crystal-templated microparticles. Chem. Mater. 2017, 29, 53–61. [Google Scholar] [CrossRef]
- Sattari, A.; Hanafizadeh, P.; Hoorfar, M. Multiphase flow in microfluidics: From droplets and bubbles to the encapsulated structures. Adv. Colloid Interface Sci. 2020, 282, 102208. [Google Scholar] [CrossRef]
- Suea-Ngam, A.; Howes, P.; Srisa-Art, M.; deMello, A. Droplet microfluidics: From proof-of-concept to real-world utility? Chem. Commun. 2019, 55, 9895–9903. [Google Scholar] [CrossRef] [Green Version]
- Priest, C.; Quinn, A.; Postma, A.; Zelikin, A.N.; Ralston, J.; Caruso, F. Microfluidic polymer multilayer adsorption on liquid crystal droplets for microcapsule synthesis. Lab Chip 2008, 8, 2182–2187. [Google Scholar] [CrossRef] [PubMed]
- Doufene, K.; Tourne-Peteilh, C.; Etienne, P.; Aubert-Pouessel, A. Microfluidic systems for droplets generation in aqueous-continuous phases: A focus review. Langmuir 2019, 35, 12597–12612. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Ge, X.; Xu, B.; Zhang, W.; Qu, L.; Choi, C.H.; Xu, J.; Zhang, A.; Lee, H.; et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018, 47, 5646–5683. [Google Scholar] [CrossRef] [PubMed]
- Lashkaripour, A.; Rodriguez, C.; Ortiz, L.; Densmore, D. Performance tuning of microfluidic flow-focusing droplet generators. Lab Chip 2019, 19, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Lee, H.; Weitz, D.A. Rapid patterning of PDMS microfluidic device wettability using syringe-vacuum-induced segmented flow in nonplanar geometry. ACS Appl. Mat. Interfaces 2018, 10, 3170–3174. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Khan, M.; Park, S.Y. pH-responsive liquid crystal double emulsion droplets prepared using microfluidics. RSC Adv. 2016, 6, 55976–55983. [Google Scholar] [CrossRef]
- Durey, G.; Ishii, Y.; Lopez-Leon, T. Temperature-driven anchoring transitions at liquid crystal/water interfaces. Langmuir 2020, 36, 9368–9376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, S.K.; Won, J.C.; Kim, Y.H.; Kim, S.H. Reconfigurable photonic capsules containing cholesteric liquid crystals with planar alignment. Angew. Chem. Int. Ed. 2015, 54, 15266–15270. [Google Scholar] [CrossRef]
- Lee, S.S.; Seo, H.J.; Kim, Y.H.; Kim, S.H. Structural color palettes of core–shell photonic ink capsules containing cholesteric liquid crystals. Adv. Mater. 2017, 29, 1606894. [Google Scholar] [CrossRef]
- Takenaka, Y.; Škarabot, M.; Muševič, I. Nematic liquid-crystal necklace structure made by microfluidics system. Langmuir 2020, 36, 3234–3241. [Google Scholar] [CrossRef]
- Chen, H.Q.; Wang, X.Y.; Bisoyi, H.K.; Chen, L.J.; Li, Q. Liquid crystals in curved confined geometries: Microfluidics bring new capabilities for photonic applications and beyond. Langmuir 2021, 37, 3789–3807. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Park, S.Y. General liquid-crystal droplets produced by microfluidics for urea detection. Sens. Actuators B Chem. 2014, 202, 516–522. [Google Scholar] [CrossRef]
- Gollapelli, B.; Tatipamula, A.K.; Dewanjee, S.; Pathinti, R.S.; Vallamkondu, J. Detection of bile acids using optical biosensors based on cholesteric liquid crystal droplets. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Khan, M.; Park, S.Y. Specific detection of avidin–biotin binding using liquid crystal droplets. Colloids Surf. B 2015, 127, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Aliño, V.J.; Tay, K.X.; Khan, S.A.; Yang, K.L. Inkjet printing and release of monodisperse liquid crystal droplets from solid surfaces. Langmuir 2012, 28, 14540–14546. [Google Scholar] [CrossRef]
- Huebner, A.; Bratton, D.; Whyte, G.; Yang, M.; Demello, A.J.; Abell, C.; Hollfelder, F. Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 2009, 9, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.H.; Takeuchi, S. A trap-and-release integrated microfluidic system for dynamic microarray applications. Proc. Natl. Acad. Sci. USA 2007, 104, 1146–1151. [Google Scholar] [CrossRef] [Green Version]
- Karimi, A.; Yazdi, S.; Ardekani, A.M. Hydrodynamic mechanisms of cell and particle trapping in microfluidics. Biomicrofluidics 2013, 7, 21501. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Nieves, A.; Link, D.R.; Márquez, M.; Weitz, D.A. Topological changes in bipolar nematic droplets under flow. Phys. Rev. Lett. 2007, 98, 087801. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.L.; Luh, H.T.; Hsiao, Y.C. Label-free, color-indicating, polarizer-free dye-doped liquid crystal microfluidic polydimethylsiloxane biosensing chips for detecting albumin. Polymers 2021, 13, 2587. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.D.; Khan, M.; Park, S.Y. Fabrication of temperature- and pH-sensitive liquid-crystal droplets with PNIPAM-b-LCP and SDS coatings by microfluidics. J. Mater. Chem. B 2014, 2, 4922–4928. [Google Scholar] [CrossRef]
- Yang, L.; Khan, M.; Park, S.Y. Liquid crystal droplets functionalized with charged surfactant and polyelectrolyte for non-specific protein detection. RSC Adv. 2015, 5, 97264–97271. [Google Scholar] [CrossRef]
- Lee, H.G.; Munir, S.; Park, S.Y. Cholesteric liquid crystal droplets for biosensors. ACS Appl. Mat. Interfaces 2016, 8, 26407–26417. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.K.; Jang, C.H. Label-free liquid crystal-based biosensor for detection of dopamine using DNA aptamer as a recognition probe. Anal. Biochem. 2020, 605, 113807. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, X.; Liu, F.; Li, H.; Zhang, C.X.; Yang, Z. Simple, rapid and sensitive detection of Parkinson’s disease related alpha-synuclein using a DNA aptamer assisted liquid crystal biosensor. Soft Matter 2021, 17, 4842–4847. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441–5444. [Google Scholar] [CrossRef]
- Smith, A.D.; Abbott, N.; Zavala, V.M. Convolutional network analysis of optical micrographs for liquid crystal sensors. J. Phys. Chem. C 2020, 124, 15152–15161. [Google Scholar] [CrossRef]
- Cao, Y.; Yu, H.; Abbott, N.L.; Zavala, V.M. Machine learning algorithms for liquid crystal-based sensors. ACS Sens. 2018, 3, 2237–2245. [Google Scholar] [CrossRef] [PubMed]
- Nayani, K.; Yang, Y.; Yu, H.; Jani, P.; Mavrikakis, M.; Abbott, N. Areas of opportunity related to design of chemical and biological sensors based on liquid crystals. Liq. Cry. Today 2020, 29, 24–35. [Google Scholar] [CrossRef]




| Sensing Structure | Type of LC | Surface Modification of LC | Target | Linear Range | Detection Limit | Ref. |
|---|---|---|---|---|---|---|
| LC-solid interface | 5CB | IgG | Anti-IgG | 0.02–0.08 mg/mL | 0.02 mg/mL | [68] |
| 5CB | Biotin-BSA | Cy3 antibiotin | Not reported | 5 μg/mL | [69] | |
| 5CB | Rabbit IgG | Anti-rabbit IgG | Not reported | 1 μg/mL | [67] | |
| E44 | BSA | Anti-BSA | Not reported | 1 μg/mL | [71] | |
| E7 doped with dichroic dye | BSA | Anti-BSA | Not reported | 1 μg/mL | [103] | |
| LC-aqueous interface | 5CB | L-DLPC | Phospholipase | Not reported | 100 nM | [51] |
| 5CB doped with HBA | None | Creatinine | 50–250 μM | 50 μM | [77] | |
| Mobile LC droplets | 5CB | PAA-b-LCP-glucose oxidase | Glucose | Not reported | 0.03 mM | [27] |
| 5CB | PNIPAM-b-LCP/SDS | BSA Lysozyme Hemoglobin Chymotrypsinogen | Not reported | 0.95 μM 1.1 μM 0.12 μM 0.07 μM | [104] | |
| 5CB | SDS/QP4VP | BSA Hemoglobin | Not reported | 0.005 wt% 0.03 wt% | [105] | |
| 5CB | PAA-b-LCP-urease | Urea | Not reported | 3 mM | [95] | |
| 5CB | PAA-b-LCP-avidin | Avidin | Not reported | 0.5 μg/mL | [97] | |
| MLC-2132 doped with CB15 | PAA-b-LCP-glucose oxidase | Glucose | Not reported | 0.5 μM | [106] | |
| PAA-b-LCP-cholesterol oxidase | Cholesterol | Not reported | 2.5 μM | |||
| E7 doped with R5011 | PVA/SDS | Cholic acid Deoxycholic acid | Not reported | 1 μM 0.5 μM | [96] | |
| LC droplet microarray | 5CB | CTAB | BSA | 4–40 μg/mL | 4 μg/mL | [54] |
| E7 | Lipid mixture | Smp43 | Not reported | 4.8 μM at 2 h 3.6 μM at 4 h | [57] | |
| 5CB | PVA/SDS | Cholic acid | 10–30 μM | 10 μM | [55] | |
| Deoxycholic acid | 1–10 μM | 1 μM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Han, D.; Yang, J. Applications of Microfluidics in Liquid Crystal-Based Biosensors. Biosensors 2021, 11, 385. https://doi.org/10.3390/bios11100385
Deng J, Han D, Yang J. Applications of Microfluidics in Liquid Crystal-Based Biosensors. Biosensors. 2021; 11(10):385. https://doi.org/10.3390/bios11100385
Chicago/Turabian StyleDeng, Jinan, Dandan Han, and Jun Yang. 2021. "Applications of Microfluidics in Liquid Crystal-Based Biosensors" Biosensors 11, no. 10: 385. https://doi.org/10.3390/bios11100385
APA StyleDeng, J., Han, D., & Yang, J. (2021). Applications of Microfluidics in Liquid Crystal-Based Biosensors. Biosensors, 11(10), 385. https://doi.org/10.3390/bios11100385






