Fibre-Optic Surface Plasmon Resonance Biosensor for Monoclonal Antibody Titer Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabricating the Gold Sensing Surface
2.2. Fabricating and Assembling the Flow Cell
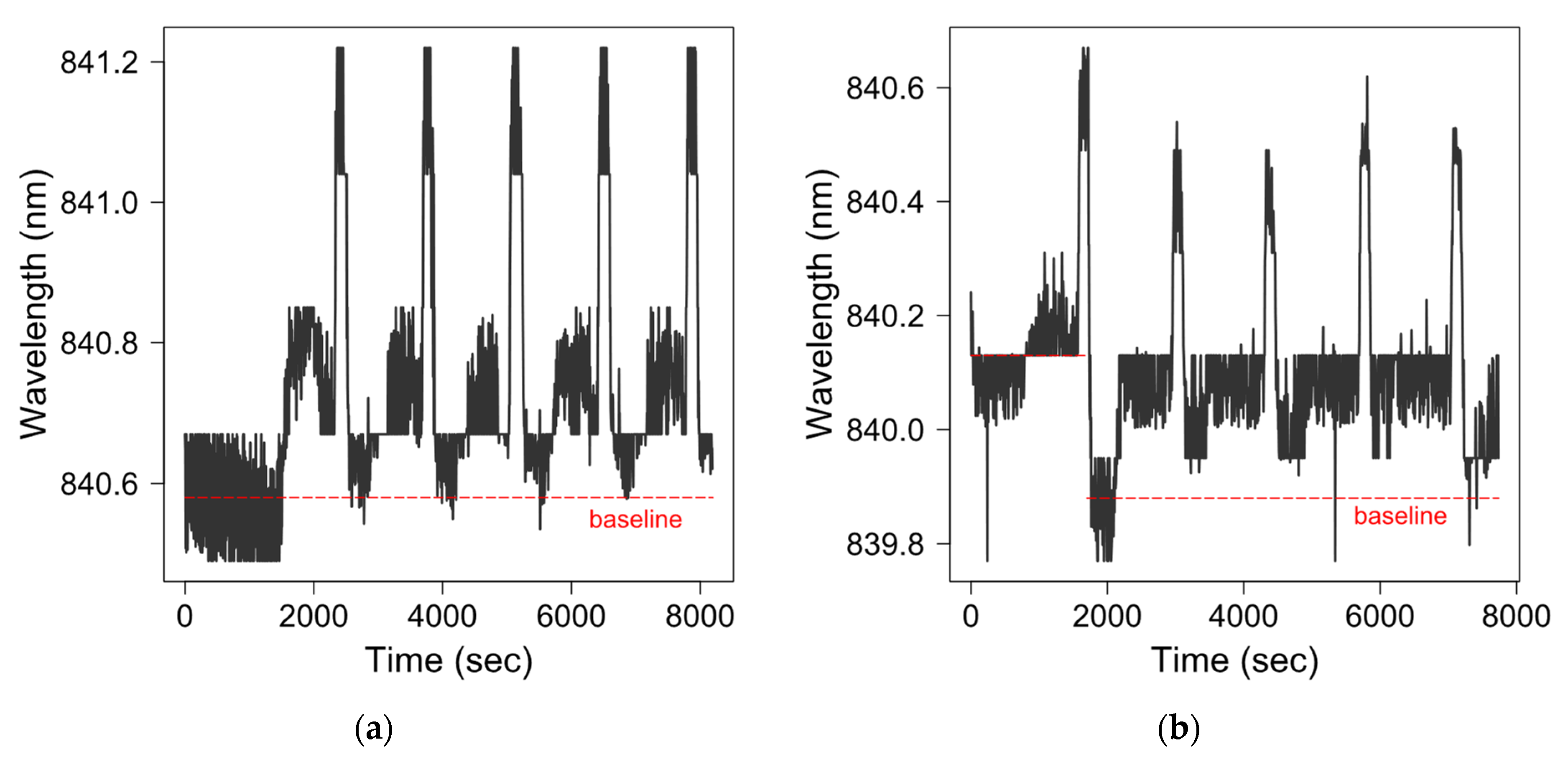
2.3. Data Interpretation
2.4. Sensitivity Test
2.5. Protein A Immobilisation Using Spacer Arm and Aero-Length Crosslinker
2.6. Monoclonal Antibody Detection
2.7. Protein A Regeneration
3. Results
3.1. Fabrication of Fibre-Optic EOT Biosensor-Incorporated Mab Detection Device
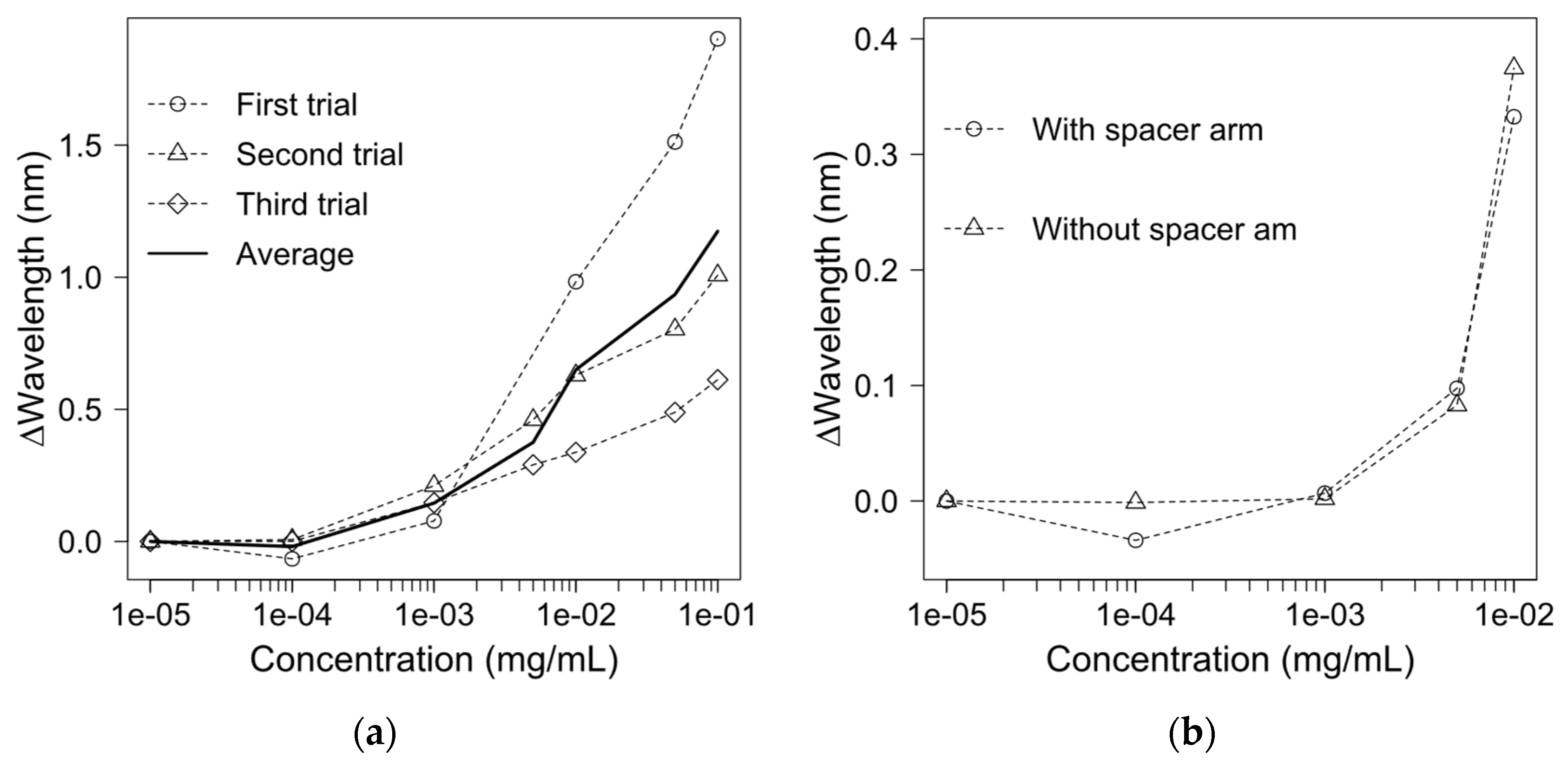
3.2. Sensitivity of the Fibre-Optic EOT Biosensor
3.3. Monoclonal Antibody Detection
3.4. Monoclonal Antibody Detection Using a Zero-Length Crosslinker
3.5. Protein A Regeneration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gody, J.; Khouri, J.; Durve, A.; Zimmermann, E.; Furuya, K. Control of Protein A Column Loading During Continuous Antibody Production: A Technology Overview of Real-Time Titer Measurement Methods. Bioprocess Int. 2019, 17. Available online: https://bioprocessintl.com/analytical/pat/control-of-protein-a-column-loading-during-continuous-antibody-production-real-time-titer-measurement-methods/ (accessed on 5 October 2021).
- Jenzsch, M.; Bell, C.; Buziol, S.; Kepert, F.; Wegele, H.; Hakemeyer, C. Trends in Process Analytical Technology: Present State in Bioprocessing. Adv. Biochem. Eng. Biotechnol. 2018, 165, 211–252. [Google Scholar] [CrossRef] [PubMed]
- Jackman, J.A.; Rahim Ferhan, A.; Cho, N.-J. Nanoplasmonic sensors for biointerfacial science. Chem. Soc. Rev. 2017, 46, 3615–3660. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Lezec, H.J.; Ghaemi, H.F.; Thio, T.; Wolff, P.A. Extraordinary optical transmission through sub-wavelength hole arrays. Nature 1998, 391, 667. [Google Scholar] [CrossRef]
- Zhao, E.; Jia, P.; Ebendorff-Heidepriem, H.; Li, H.; Huang, P.; Liu, D.; Li, H.; Yang, X.; Liu, L.; Guan, C. Localized surface plasmon resonance sensing structure based on gold nanohole array on beveled fiber edge. Nanotechnology 2017, 28, 435504. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Ruan, Y.; Ly, T.-T.; Jia, P.; Sun, Q.; Feng, Q.; Yang, D.; Ebendorff-Heidepriem, H. MoS2-enhanced epoxy-based plasmonic fiber-optic sensor for selective and sensitive detection of methanol. Sens. Actuators B Chem. 2020, 305, 127513. [Google Scholar] [CrossRef]
- Aliberti, A.; Ricciardi, A.; Giaquinto, M.; Micco, A.; Bobeico, E.; La Ferrara, V.; Ruvo, M.; Cutolo, A.; Cusano, A. Microgel assisted Lab-on-Fiber Optrode. Sci. Rep. 2017, 7, 14459. [Google Scholar] [CrossRef]
- Briscoe, J.L.; Cho, S.Y.; Brener, I. Part-Per-Trillion Level Detection of Microcystin-LR Using a Periodic Nanostructure. IEEE Sens. J. 2015, 15, 1366–1371. [Google Scholar] [CrossRef]
- Dhawan, A.; Muth, J.F. Engineering surface plasmon based fiber-optic sensors. Mater. Sci. Eng. B 2008, 149, 237–241. [Google Scholar] [CrossRef]
- Escobedo, C.; Vincent, S.; I K Choudhury, A.; Campbell, J.; Brolo, A.; Sinton, D.; Gordon, R. Integrated nanohole array surface plasmon resonance sensing device using a dual-wavelength source. J. Micromech. Microeng. 2011, 21, 115001. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, C.; Brolo, A.G.; Gordon, R.; Sinton, D. Optofluidic Concentration: Plasmonic Nanostructure as Concentrator and Sensor. Nano Lett. 2012, 12, 1592–1596. [Google Scholar] [CrossRef]
- Ferreira, J.; Santos, M.J.L.; Rahman, M.M.; Brolo, A.G.; Gordon, R.; Sinton, D.; Girotto, E.M. Attomolar Protein Detection Using in-Hole Surface Plasmon Resonance. J. Am. Chem. Soc. 2009, 131, 436–437. [Google Scholar] [CrossRef]
- Im, H.; Lesuffleur, A.; Lindquist, N.C.; Oh, S.-H. Plasmonic Nanoholes in a Multi-Channel Microarray Format for Parallel Kinetic Assays and Differential Sensing. Anal. Chem. 2009, 81, 2854–2859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, H.; Lindquist, N.C.; Lesuffleur, A.; Oh, S.-H. Atomic Layer Deposition of Dielectric Overlayers for Enhancing the Optical Properties and Chemical Stability of Plasmonic Nanoholes. ACS Nano 2010, 4, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Cheng, B.; Yang, Q.; Huang, J.; Wang, H.; Ma, Y.; Shi, H.; Xiao, H. Reflection based extraordinary optical transmission fiber optic probe for refractive index sensing. Sens. Actuators B Chem. 2014, 193, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, L.; Zhou, J.; Wu, H. Well-designed metal nanostructured arrays for label-free plasmonic biosensing. J. Mater. Chem. C 2015, 3, 6479–6492. [Google Scholar] [CrossRef]
- Monteiro, J.P.; de Oliveira, J.H.; Radovanovic, E.; Brolo, A.G.; Girotto, E.M. Microfluidic Plasmonic Biosensor for Breast Cancer Antigen Detection. Plasmonics 2016, 11, 45–51. [Google Scholar] [CrossRef]
- Thio, T.; Lezec, H.J.; Ebbesen, T.W. Strongly enhanced optical transmission through subwavelength holes in metal films. Phys. B Condens. Matter 2000, 279, 90–93. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Liu, H.; Bae, H.; Olson, D.A.; Gupta, A.K.; Yu, M. On-fiber plasmonic interferometer for multi-parameter sensing. Opt. Express 2015, 23, 10732–10740. [Google Scholar] [CrossRef]
- Couture, M.; Live, L.S.; Dhawan, A.; Masson, J.-F. EOT or Kretschmann configuration? Comparative study of the plasmonic modes in gold nanohole arrays. Analyst 2012, 137, 4162–4170. [Google Scholar] [CrossRef]
- Junesch, J.; Emilsson, G.; Xiong, K.; Kumar, S.; Sannomiya, T.; Pace, H.; Voros, J.; Oh, S.-H.; Bally, M.; Dahlin, A.B. Location-specific nanoplasmonic sensing of biomolecular binding to lipid membranes with negative curvature. Nanoscale 2015, 7, 15080–15085. [Google Scholar] [CrossRef] [Green Version]
- Xiong, K.; Emilsson, G.; Dahlin, A.B. Biosensing using plasmonic nanohole arrays with small, homogenous and tunable aperture diameters. Analyst 2016, 141, 3803–3810. [Google Scholar] [CrossRef]
- Dahlin, A.B.; Mapar, M.; Xiong, K.; Mazzotta, F.; Höök, F.; Sannomiya, T. Plasmonic Nanopores in Metal-Insulator-Metal Films. Adv. Opt. Mater. 2014, 2, 556–564. [Google Scholar] [CrossRef]
- Couture, M.; Ray, K.K.; Poirier-Richard, H.-P.; Crofton, A.; Masson, J.-F. 96-Well Plasmonic Sensing with Nanohole Arrays. ACS Sens. 2016, 1, 287–294. [Google Scholar] [CrossRef]
- Cetin, A.E.; Etezadi, D.; Galarreta, B.C.; Busson, M.P.; Eksioglu, Y.; Altug, H. Plasmonic Nanohole Arrays on a Robust Hybrid Substrate for Highly Sensitive Label-Free Biosensing. ACS Photonics 2015, 2, 1167–1174. [Google Scholar] [CrossRef]
- Ding, T.; Hong, M.; Richards, A.M.; Wong, T.I.; Zhou, X.; Drum, C.L. Quantification of a Cardiac Biomarker in Human Serum Using Extraordinary Optical Transmission (EOT). PLoS ONE 2015, 10, e0120974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, J.S.; Lim, S.Y.; Perera, A.P.; Zhang, Y.; Park, M.K. Plasmonic nanohole arrays for monitoring growth of bacteria and antibiotic susceptibility test. Sens. Actuators B Chem. 2013, 182, 576–583. [Google Scholar] [CrossRef]
- Im, H.; Lee, S.H.; Wittenberg, N.J.; Johnson, T.W.; Lindquist, N.C.; Nagpal, P.; Norris, D.J.; Oh, S.-H. Template-Stripped Smooth Ag Nanohole Arrays with Silica Shells for Surface Plasmon Resonance Biosensing. ACS Nano 2011, 5, 6244–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagpal, P.; Lindquist, N.C.; Oh, S.-H.; Norris, D.J. Ultrasmooth Patterned Metals for Plasmonics and Metamaterials. Science 2009, 325, 594. [Google Scholar] [CrossRef]
- Im, H.; Sutherland, J.N.; Maynard, J.A.; Oh, S.-H. Nanohole-based SPR Instruments with Improved Spectral Resolution Quantify a Broad Range of Antibody-Ligand Binding Kinetics. Anal. Chem. 2012, 84, 1941–1947. [Google Scholar] [CrossRef] [Green Version]
- Jackman, J.A.; Linardy, E.; Yoo, D.; Seo, J.; Ng, W.B.; Klemme, D.J.; Wittenberg, N.J.; Oh, S.-H.; Cho, N.-J. Plasmonic Nanohole Sensor for Capturing Single Virus-Like Particles toward Virucidal Drug Evaluation. Small 2016, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Jiang, H.; Sabarinathan, J.; Yang, J. Plasmonic nanohole array sensors fabricated by template transfer with improved optical performance. Nanotechnology 2013, 24, 195501. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Yang, J. Integration of large-area metallic nanohole arrays with multimode optical fibers for surface plasmon resonance sensing. Appl. Phys. Lett. 2013, 102, 243107. [Google Scholar] [CrossRef]
- Jia, P.; Yang, Z.; Yang, J.; Ebendorff-Heidepriem, H. Quasiperiodic Nanohole Arrays on Optical Fibers as Plasmonic Sensors: Fabrication and Sensitivity Determination. ACS Sens. 2016, 1, 1078–1083. [Google Scholar] [CrossRef]
- Lee, S.H.; Lindquist, N.C.; Wittenberg, N.J.; Jordan, L.R.; Oh, S.-H. Real-time Full-spectral Imaging and Affinity Measurements from 50 Microfluidic Channels using Nanohole Surface Plasmon Resonance. Lab Chip 2012, 12, 3882–3890. [Google Scholar] [CrossRef]
- Yang, J.-C.; Ji, J.; Hogle, J.M.; Larson, D.N. Metallic Nanohole Arrays on Fluoropolymer Substrates as Small Label-Free Real-Time Bioprobes. Nano Lett. 2008, 8, 2718–2724. [Google Scholar] [CrossRef] [Green Version]
- Hegner, M.; Wagner, P.; Semenza, G. Ultralarge atomically flat template-stripped Au surfaces for scanning probe microscopy. Surf. Sci. 1993, 291, 39–46. [Google Scholar] [CrossRef]
- Jia, P.; Yang, J. A plasmonic optical fiber patterned by template transfer as a high-performance flexible nanoprobe for real-time biosensing. Nanoscale 2014, 6. [Google Scholar] [CrossRef]
- GE Healthcare Affinity Chromatography—Vol. 1 Antibodies. In Handbook of Affinity Chromatography; Routledge: New York, NY, USA, 2016; Volume 1, p. 53.
- Zhang, B.; Yu, J.; Liu, C.; Wang, J.; Han, H.; Zhang, P.; Shi, D. Improving detection sensitivity by oriented bioconjugation of antibodies to quantum dots with a flexible spacer arm for immunoassay. RSC Adv. 2016, 6, 50119–50127. [Google Scholar] [CrossRef]
- Yang, L.; Biswas, M.E.; Chen, P. Study of binding between protein A and immunoglobulin G using a surface tension probe. Biophys. J. 2003, 84, 509–522. [Google Scholar] [CrossRef] [Green Version]
- Esmonde-White, K.A.; Cuellar, M.; Uerpmann, C.; Lenain, B.; Lewis, I.R. Raman spectroscopy as a process analytical technology for pharmaceutical manufacturing and bioprocessing. Anal. Bioanal. Chem. 2017, 409, 637–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-T.; Lee, Y.-C.; Lai, Y.-H.; Lim, J.-C.; Huang, N.-T.; Lin, C.-T.; Huang, J.-J. Review of Integrated Optical Biosensors for Point-Of-Care Applications. Biosensors 2020, 10, 209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.; Choi, J.; Jia, Z.; Park, S.; Gartia, M.R. Nanohole array plasmonic biosensors: Emerging point-of-care applications. Biosens. Bioelectron. 2019, 130, 185–203. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, T.T.; Ruan, Y.; Du, B.; Jia, P.; Zhang, H. Fibre-Optic Surface Plasmon Resonance Biosensor for Monoclonal Antibody Titer Quantification. Biosensors 2021, 11, 383. https://doi.org/10.3390/bios11100383
Ly TT, Ruan Y, Du B, Jia P, Zhang H. Fibre-Optic Surface Plasmon Resonance Biosensor for Monoclonal Antibody Titer Quantification. Biosensors. 2021; 11(10):383. https://doi.org/10.3390/bios11100383
Chicago/Turabian StyleLy, Thai Thao, Yinlan Ruan, Bobo Du, Peipei Jia, and Hu Zhang. 2021. "Fibre-Optic Surface Plasmon Resonance Biosensor for Monoclonal Antibody Titer Quantification" Biosensors 11, no. 10: 383. https://doi.org/10.3390/bios11100383
APA StyleLy, T. T., Ruan, Y., Du, B., Jia, P., & Zhang, H. (2021). Fibre-Optic Surface Plasmon Resonance Biosensor for Monoclonal Antibody Titer Quantification. Biosensors, 11(10), 383. https://doi.org/10.3390/bios11100383






