Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines
Abstract
1. Introduction
2. Conventional Cytokines Measurements
2.1. Enzyme Linked Immunosorbent Assay (ELISA)
2.2. Radioimmunoassay (RIA)
2.3. Enzyme-Linked Immunospot (ELISPOT)
2.4. mRNA Based Assays
2.5. Immunostaining
2.6. Intra-Cytoplasmic Cytokine Staining (ICC)
2.7. Cytokine Microarrays
3. Biosensor (lab-on-a-Chip) Concept for Cytokines Detection
4. Nanomaterials and Biosensing Application
4.1. Optical Transduction
4.2. Electrochemical Transduction
4.3. Magnetic Transduction
5. Nanomaterials as Biosensor for Inflammatory Cytokines
5.1. Quantum Dots (QDs)
5.2. Noble Metal Nanomaterials (NMNs)
5.3. Metal Oxide Nanomaterials
5.4. Carbon-Based Nanomaterials
5.5. Polymer Nanomaterials
5.6. Bionanomaterials
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinarello, C.A. Historical insights into cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Kelso, A. Cytokines: Principles and prospects. Immunol. Cell Biol. 1998, 76, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. Chapter 27—Cytokines. In Asthma and COPD, 2nd ed.; Barnes, P.J., Ed.; Academic Press: Oxford, UK, 2009; pp. 327–341. [Google Scholar]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Kupsa, T.; Horacek, J.M.; Jebavy, L. The role of cytokines in acute myeloid leukemia: A systematic review. Biomed. Pap. Med. Fac.Univ. Palacky Olomouc Czech Repub. 2012, 156, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, N.; Asthana, D. Cytokine quantitation: Technologies and applications. Front. Biosci. J. Virtual Libr. 2007, 12, 4682–4695. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, A.L.; Singh, S. Advancement in biosensors for inflammatory biomarkers of SARS-CoV-2 during 2019–2020. Biosens. Bioelectron. 2021, 171, 112703. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jiang, C.; Lin, X.; Yang, Y. Point-of-care detection of cytokines in cytokine storm management and beyond: Significance and challenges. VIEW 2021, 2, 20210003. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Electrochemical immunosensors for the detection of cytokine tumor necrosis factor alpha: A review. Talanta 2020, 211, 120758. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. Recent Advances in Electrochemical and Optical Biosensors Designed for Detection of Interleukin 6. Sensors 2020, 20, 646. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Revisiting Electrochemical Biosensing in the 21st Century Society for Inflammatory Cytokines Involved in Autoimmune, Neurodegenerative, Cardiac, Viral and Cancer Diseases. Sensors 2020, 21, 189. [Google Scholar] [CrossRef]
- Tanak, A.S.; Muthukumar, S.; Krishnan, S.; Schully, K.L.; Clark, D.V.; Prasad, S. Multiplexed cytokine detection using electrochemical point-of-care sensing device towards rapid sepsis endotyping. Biosens. Bioelectron. 2021, 171, 112726. [Google Scholar] [CrossRef]
- Chen, P.; Chung, M.T.; McHugh, W.; Nidetz, R.; Li, Y.; Fu, J.; Cornell, T.T.; Shanley, T.P.; Kurabayashi, K. Multiplex Serum Cytokine Immunoassay Using Nanoplasmonic Biosensor Microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef]
- Abreu, C.M.; Soares-Dos-Reis, R.; Melo, P.; Relvas, J.; Guimarães, J.; Sá, M.J.; Cruz, A.P.; Pinto, I.M. Emerging Biosensing Technologies for Neuroinflammatory and Neurodegenerative Disease Diagnostics. Front. Mol. Neurosci. 2018, 11, 164. [Google Scholar] [CrossRef]
- Liu, G.; Qi, M.; Hutchinson, M.; Yang, G.; Goldys, E.M. Recent advances in cytokine detection by immunosensing. Biosens. Bioelectron. 2016, 79, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Dutta, N.; Lillehoj, P.; Estrela, P.; Dutta, G. Electrochemical Biosensors for Cytokine Profiling: Recent Advancements and Possibilities in the Near Future. Biosensors 2021, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Truong, J.; Reeves, W.B.; Hahm, J.-I. Emerging Cytokine Biosensors with Optical Detection Modalities and Nanomaterial-Enabled Signal Enhancement. Sensors 2017, 17, 428. [Google Scholar] [CrossRef]
- Crowther, J.R. Basic Immunology. In ELISA: Theory and Practice; Crowther, J.R., Ed.; Humana Press: Totowa, NJ, USA, 1995; pp. 1–34. [Google Scholar]
- Grange, R.; Thompson, J.; Lambert, D. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef]
- Whiteside, T.L. Cytokine assays. Biotechniques 2002, 10, 12–25. [Google Scholar] [CrossRef]
- Palzer, S.; Bailey, T.; Hartnett, C.; Grant, A.; Tsang, M.; Kalyuzhny, A.E. Simultaneous detection of multiple cytokines in ELISPOT assays. Methods Mol. Biol. 2005, 302, 273–288. [Google Scholar]
- Gazagne, A.; Malkusch, W.; Vingert, B.; Fridman, W.H.; Tartour, E.; Kalyuzhny, A.E. Fluorospot Assay: Methodological Analysis. Methods Mol. Biol. 2005, 302, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Mueller, R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin. Sci. 2005, 109, 365–379. [Google Scholar] [CrossRef]
- Gogos, C.A.; Drosou, E.; Bassaris, H.P.; Skoutelis, A. Pro- versus Anti-inflammatory Cytokine Profile in Patients with Severe Sepsis: A Marker for Prognosis and Future Therapeutic Options. J. Infect. Dis. 2000, 181, 176–180. [Google Scholar] [CrossRef]
- Thorpe, R.; Wadhwa, M.; Bird, C.; Mire-Sluis, A. Detection and measurement of cytokines. Blood Rev. 1992, 6, 133–148. [Google Scholar] [CrossRef]
- Arora, S.K. Analysis of intracellular cytokines using flowcytometry. Methods Cell Sci. 2002, 24, 37–40. [Google Scholar] [CrossRef]
- Picker, L.J.; Singh, M.K.; Zdraveski, Z.; Treer, J.R.; Waldrop, S.L.; Bergstresser, P.R.; Maino, V.C. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 1995, 86, 1408–1419. [Google Scholar] [CrossRef]
- Roberts, W.L. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease. Circulation 2004, 110, e572–e576. [Google Scholar] [CrossRef]
- Napoli, C.; De Nigris, F.; Sica, V. New Advances in Microarrays: Finding the Genes Causally Involved in Disease. Methods Mol. Med. 2005, 108, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Gagna, C.E.; Lambert, W.C. Novel multistranded, alternative, plasmid and helical transitional DNA and RNA microarrays: Implications for therapeutics. Pharmacogenomics 2009, 10, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Shakouri, S.K.; Dolati, S. Biosensors: A novel approach to and recent discovery in detection of cytokines. Cytokine 2020, 136, 155272. [Google Scholar] [CrossRef] [PubMed]
- Baraket, A.; Lee, M.; Zine, N.; Sigaud, M.; Bausells, J.; Errachid, A. A fully integrated electrochemical biosensor platform fabrication process for cytokines detection. Biosens. Bioelectron. 2017, 93, 170–175. [Google Scholar] [CrossRef]
- Bhavsar, K.; Fairchild, A.; Alonas, E.; Bishop, D.K.; La Belle, J.T.; Sweeney, J.; Alford, T.; Joshi, L. A cytokine immunosensor for Multiple Sclerosis detection based upon label-free electrochemical impedance spectroscopy using electroplated printed circuit board electrodes. Biosens. Bioelectron. 2009, 25, 506–509. [Google Scholar] [CrossRef]
- Oh, D.Y.; Na, H.; Song, S.W.; Kim, J.; In, H.; Lee, A.C.; Jeong, Y.; Lee, D.; Jang, J.; Kwon, S. ELIPatch, a thumbnail-size patch with immunospot array for multiplexed protein detection from human skin surface. Biomicrofluidics 2018, 12, 031101. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Wang, Y. Nanostructures and Nanomaterials. In World Scientific Series in Nanoscience and Nanotechnology; World Scientific: Singapore, 2011; Volume 2, 596p. [Google Scholar]
- Batool, R.; Rhouati, A.; Nawaz, M.H.; Hayat, A.; Marty, J.L. A Review of the Construction of Nano-Hybrids for Electrochemical Biosensing of Glucose. Biosensors 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Willner, M.R.; Vikesland, P.J. Nanomaterial enabled sensors for environmental contaminants. J. Nanobiotechnology 2018, 16, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, B.D.; Ali, M.A. Chapter 1—Nanomaterials for Biosensors: Fundamentals and Applications; Malhotra, B.D., Ali, M.A., Eds.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–74. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Das, S.; Mitra, S.; Khurana, S.P.; Debnath, N. Nanomaterials for biomedical applications. Front. Life Sci. 2013, 7, 90–98. [Google Scholar] [CrossRef]
- Sadik, O.A.; Aluoch, A.; Zhou, A. Status of biomolecular recognition using electrochemical techniques. Biosens. Bioelectron. 2009, 24, 2749–2765. [Google Scholar] [CrossRef]
- Kimmel, D.W.; LeBlanc, G.; Meschievitz, M.E.; Cliffel, D.E. Electrochemical Sensors and Biosensors. Anal. Chem. 2012, 84, 685–707. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.W.; Tan, W.; Hong, J.-I. Fluorescent dye-doped silica nanoparticles: New tools for bioapplications. Chem. Commun. 2012, 48, 2270–2282. [Google Scholar] [CrossRef]
- Vikesland, P.J.; Wigginton, K.R. Nanomaterial Enabled Biosensors for Pathogen Monitoring—A Review. Environ. Sci. Technol. 2010, 44, 3656–3669. [Google Scholar] [CrossRef]
- Wei, H.; Abtahi, S.M.H.; Vikesland, P.J. Plasmonic colorimetric and SERS sensors for environmental analysis. Environ. Sci. Nano 2015, 2, 120–135. [Google Scholar] [CrossRef]
- D’Orazio, P. Biosensors in clinical chemistry. Clin. Chim. Acta 2003, 334, 41–69. [Google Scholar] [CrossRef]
- Sanvicens, N.; Pastells, C.; Pascual, N.; Marco, M.-P. Nanoparticle-based biosensors for detection of pathogenic bacteria. TrAC Trends Anal. Chem. 2009, 28, 1243–1252. [Google Scholar] [CrossRef]
- García-Aljaro, C.; Cella, L.N.; Shirale, D.J.; Park, M.; Munoz, P.; Yates, M.V.; Mulchandani, A. Carbon nanotubes-based chemiresistive biosensors for detection of microorganisms. Biosens. Bioelectron. 2010, 26, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Koets, M.; van der Wijk, T.; van Eemeren, J.; van Amerongen, A.; Prins, M. Rapid DNA multi-analyte immunoassay on a magneto-resistance biosensor. Biosens. Bioelectron. 2009, 24, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Perez, J.M.; Simeone, F.J.; Saeki, Y.; Josephson, A.L.; Weissleder, R. Viral-Induced Self-Assembly of Magnetic Nanoparticles Allows the Detection of Viral Particles in Biological Media. J. Am. Chem. Soc. 2003, 125, 10192–10193. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Ma, W.; Guo, J.; Lin, Y.; Wang, C. Uniform Magnetic Core/Shell Microspheres Functionalized with Ni2+–Iminodiacetic Acid for One Step Purification and Immobilization of His-Tagged Enzymes. ACS Appl. Mater. Interfaces 2013, 5, 2626–2633. [Google Scholar] [CrossRef]
- Gomes, S.A.; Vieira, C.S.; Almeida, D.B.; Santos-Mallet, J.R.; Menna-Barreto, R.F.S.; Cesar, C.L.; Feder, D. CdTe and CdSe Quantum Dots Cytotoxicity: A Comparative Study on Microorganisms. Sensors 2011, 11, 11664–11678. [Google Scholar] [CrossRef]
- Şahin, S.; Ünlü, C.; Trabzon, L. Affinity biosensors developed with quantum dots in microfluidic systems. Emergent Mater. 2021, 4, 187–209. [Google Scholar] [CrossRef]
- Frasco, M.F.; Chaniotakis, N. Semiconductor Quantum Dots in Chemical Sensors and Biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef]
- Amelia, M.; Lincheneau, C.; Silvi, S.; Credi, A. Electrochemical properties of CdSe and CdTe quantum dots. Chem. Soc. Rev. 2012, 41, 5728–5743. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, X.; Xie, L.; Shao, M. Facile incorporation of DNA-templated quantum dots for sensitive electrochemical detection of the oral cancer biomarker interleukin-8. Anal. Bioanal. Chem. 2020, 412, 2599–2606. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, K.; Ma, K.; Care, A.; Hutchinson, M.; Goldys, E.M. Graphene quantum dot based “switch-on” nanosensors for intracellular cytokine monitoring. Nanoscale 2017, 9, 4934–4943. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A repertoire of biomedical applications of noble metal nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef] [PubMed]
- Doria, G.; Conde, J.; Veigas, B.; Giestas, L.; Almeida, C.; Assunção, M.; Rosa, J.; Baptista, P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors 2012, 12, 1657–1687. [Google Scholar] [CrossRef]
- Baptista, P.; Pereira, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold nanoparticles for the development of clinical diagnosis methods. Anal. Bioanal. Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef]
- Sperling, R.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. “Plugging into Enzymes”: Nanowiring of Redox Enzymes by a Gold Nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef]
- Li, Y.; Schluesener, H.J.; Xu, S. Gold nanoparticle-based biosensors. Gold Bull. 2010, 43, 29–41. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Chou, T.-H.; Chuang, C.-Y.; Wu, C.-M. Quantification of Interleukin-6 in cell culture medium using surface plasmon resonance biosensors. Cytokine 2010, 51, 107–111. [Google Scholar] [CrossRef]
- Battaglia, T.M.; Masson, J.-F.; Sierks, M.R.; Beaudoin, S.P.; Rogers, J.; Foster, K.N.; Holloway, A.G.A.; Booksh, K.S. Quantification of Cytokines Involved in Wound Healing Using Surface Plasmon Resonance. Anal. Chem. 2005, 77, 7016–7023. [Google Scholar] [CrossRef]
- Stybayeva, G.; Kairova, M.; Ramanculov, E.; Simonian, A.L.; Revzin, A. Detecting interferon-gamma release from human CD4 T-cells using surface plasmon resonance. Colloids Surfaces B Biointerfaces 2010, 80, 251–255. [Google Scholar] [CrossRef]
- Giorgi-Coll, S.; Marín, M.J.; Sule, O.; Hutchinson, P.J.; Carpenter, K.L. Aptamer-modified gold nanoparticles for rapid aggregation-based detection of inflammation: An optical assay for interleukin-6. Microchim. Acta 2019, 187, 1–11. [Google Scholar] [CrossRef]
- Potůčková, L.; Franko, F.; Bambousková, M.; Dráber, P. Rapid and sensitive detection of cytokines using functionalized gold nanoparticle-based immuno-PCR, comparison with immuno-PCR and ELISA. J. Immunol. Methods 2011, 371, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Song, H.W.; Lee, S.; Kim, J.-E.; Kim, Y.H.; Wi, J.-S.; Ok, J.G.; Park, J.S.; Hong, S.; Kwak, M.K.; et al. Gold Nanoparticle-Enhanced and Roll-to-Roll Nanoimprinted LSPR Platform for Detecting Interleukin-10. Front. Chem. 2020, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, K.; Nadort, A.; Hutchinson, M.R.; Goldys, E.M. Sensitive Cytokine Assay Based on Optical Fiber Allowing Localized and Spatially Resolved Detection of Interleukin-6. ACS Sensors 2017, 2, 218–226. [Google Scholar] [CrossRef]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Li, H.; Wang, J.; Gopinath, S.C. Silver nanoparticle in biosensor and bioimaging: Clinical perspectives. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef]
- Caro, C.; Castillo, P.M.; Klippstein, R.; Pozo, D.; Zaderenko, A.P. Silver Nanoparticles: Sensing and Imaging Applications. Silver Nanoparticles 2010. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Khan, R.M.R.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Bergeron, S.; Juncker, D. High-Performance Low-Cost Antibody Microarrays Using Enzyme-Mediated Silver Amplification. J. Proteome Res. 2015, 14, 1872–1879. [Google Scholar] [CrossRef] [PubMed]
- Pugh, V.J.; Szmacinski, H.; Moore, W.E.; Geddes, C.D.; Lakowicz, J.R. Submicrometer spatial resolution of metal-enhanced fluorescence. Appl. Spectrosc. 2003, 57, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Munechika, K.; Ginger, D.S. Dependence of Fluorescence Intensity on the Spectral Overlap between Fluorophores and Plasmon Resonant Single Silver Nanoparticles. Nano Lett. 2007, 7, 690–696. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Chowdhury, M.H.; Lakowicz, J.R. Metal-Enhanced Single-Molecule Fluorescence on Silver Particle Monomer and Dimer: Coupling Effect between Metal Particles. Nano Lett. 2007, 7, 2101–2107. [Google Scholar] [CrossRef]
- Szmacinski, H.; Toshchakov, V.; Piao, W.; Lakowicz, J.R. Imaging of Protein Secretion from a Single Cell Using Plasmonic Substrates. BioNanoScience 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Y.; MacLachlan, A.; Chen, P. Novel Nanoplasmonic-Structure-Based Integrated Microfluidic Biosensors for Label-Free In Situ Immune Functional Analysis: A review of recent progress. IEEE Nanotechnol. Mag. 2020, 14, 46-C3. [Google Scholar] [CrossRef]
- Fan, D.; Xia, L. Development of Human Interleukin-6 electrochemical Immunosensor Based on Pt-Pd Nanocomposite for Evaluation of Intervertebral Disc Degeneration. Int. J. Electrochem. Sci. 2017, 12, 11646–11655. [Google Scholar]
- Prasanna, S.S.; Balaji, K.; Pandey, S.; Rana, S. Chapter 4—Metal Oxide Based Nanomaterials and Their Polymer Nanocomposites. In Nanomaterials and Polymer Nanocomposites; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 123–144. [Google Scholar]
- Fernández-García, M.; Rodriguez, J.A. Metal Oxide Nanoparticles. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- George, J.M.; Antony, A.; Mathew, B. Metal oxide nanoparticles in electrochemical sensing and biosensing: A review. Microchim. Acta 2018, 185, 358. [Google Scholar] [CrossRef] [PubMed]
- Șerban, I.; Enesca, A. Metal Oxides-Based Semiconductors for Biosensors Applications. Front. Chem. 2020, 8, 354. [Google Scholar] [CrossRef]
- Napi, M.L.M.; Sultan, S.M.; Ismail, R.; How, K.W.; Ahmad, M.K. Electrochemical-Based Biosensors on Different Zinc Oxide Nanostructures: A Review. Materials 2019, 12, 2985. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Alabanza, A.; Gonzalez, L.E.; Wang, W.; Reeves, W.B.; Hahm, J.-I. Ultratrace level determination and quantitative analysis of kidney injury biomarkers in patient samples attained by zinc oxide nanorods. Nanoscale 2016, 8, 4613–4622. [Google Scholar] [CrossRef]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef]
- Rahman, S.S.U.; Qureshi, M.T.; Sultana, K.; Rehman, W.; Khan, M.Y.; Asif, M.H.; Farooq, M.; Sultana, N. Single step growth of iron oxide nanoparticles and their use as glucose biosensor. Results Phys. 2017, 7, 4451–4456. [Google Scholar] [CrossRef]
- Chen, C.; Gopinath, S.C.B.; Anbu, P. Longitudinal Zeolite-Iron Oxide Nanocomposite Deposited Capacitance Biosensor for Interleukin-3 in Sepsis Detection. Nanoscale Res. Lett. 2021, 16, 68. [Google Scholar] [CrossRef]
- Liu, G.; Bursill, C.; Cartland, S.; Anwer, A.G.; Parker, L.; Zhang, K.; Feng, S.; He, M.; Inglis, D.; Kavurma, M.M.; et al. A Nanoparticle-Based Affinity Sensor that Identifies and Selects Highly Cytokine-Secreting Cells. iScience 2019, 20, 137–147. [Google Scholar] [CrossRef]
- Shetti, N.P.; Bukkitgar, S.D.; Reddy, K.R.; Reddy, C.V.; Aminabhavi, T.M. Nanostructured titanium oxide hybrids-based electrochemical biosensors for healthcare applications. Colloids Surfaces B Biointerfaces 2019, 178, 385–394. [Google Scholar] [CrossRef]
- Nadzirah, S.; Azizah, N.; Hashim, U.; Gopinath, S.C.B.; Kashif, M. Titanium Dioxide Nanoparticle-Based Interdigitated Electrodes: A Novel Current to Voltage DNA Biosensor Recognizes E. coli O157:H7. PLoS ONE 2015, 10, e0139766. [Google Scholar] [CrossRef]
- Arkusz, K.A.; Nycz, M.A.; Paradowska, E.; Krasicka-Cydzik, E. Electrochemical detection method for interleukin-6 on titania nanotube platforms. Eng. Biomater. 2014, 17, 21–29. [Google Scholar]
- Arkusz, K.; Paradowska, E. Impedimetric Detection of Femtomolar Levels of Interleukin6, Interleukin 8, and Tumor Necrosis Factor Alpha Based on Thermally Modified Nanotubular Titanium Dioxide Arrays. Nanomaterials 2020, 10, 2399. [Google Scholar] [CrossRef]
- Kumar, R. Cerium oxide nanostructures for bio-sensing application. Sci. Lett. 2015, 4, 161. [Google Scholar]
- Li, T.; Si, Z.; Hu, L.; Qi, H.; Yang, M. Prussian Blue-functionalized ceria nanoparticles as label for ultrasensitive detection of tumor necrosis factor-α. Sens. Actuators B Chem. 2012, 171–172, 1060–1065. [Google Scholar] [CrossRef]
- Peng, J.; Guan, J.; Yao, H.; Jin, X. Magnetic colorimetric immunoassay for human interleukin-6 based on the oxidase activity of ceria spheres. Anal. Biochem. 2016, 492, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Liberman, A.; Mendez, N.; Trogler, W.C.; Kummel, A.C. Synthesis and surface functionalization of silica nanoparticles for nanomedicine. Surf. Sci. Rep. 2014, 69, 132–158. [Google Scholar] [CrossRef]
- Lad, A.N.; Agrawal, Y.K. SiO2-based nanobiosensor monitoring toxicological behavior of Mitoxantrone in vitro. Appl. Nanosci. 2014, 4, 523–529. [Google Scholar] [CrossRef][Green Version]
- Gomes, D.G.; Pieretti, J.C.; Rolim, W.R.; Seabra, A.B.; Oliveira, H.C. Advances in nano-based delivery systems of micronutrients for a greener agriculture. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 111–143. [Google Scholar]
- Roy, B.; Krishnan, S.P.; Chandrasekaran, N.; Mukherjee, A. Chapter Five—Toxic effects of engineered nanoparticles (metal/metal oxides) on plants using Allium cepa as a model system. In Comprehensive Analytical Chemistry; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 125–143. [Google Scholar]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Jouyban, A. Nanomaterials based optical biosensing of hepatitis: Recent analytical advancements. TrAC Trends Anal. Chem. 2018, 107, 169–180. [Google Scholar] [CrossRef]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar] [CrossRef]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A novel biosensor based on hafnium oxide: Application for early stage detection of human interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Yuan, L.; Hua, X.; Wu, Y.; Pan, X.; Liu, S. Polymer-Functionalized Silica Nanosphere Labels for Ultrasensitive Detection of Tumor Necrosis Factor-alpha. Anal. Chem. 2011, 83, 6800–6809. [Google Scholar] [CrossRef] [PubMed]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-carbons in biosensor applications: An overview of carbon nanotubes (CNTs) and fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Tung, T.T.; Losic, D. Carbon Nanomaterial Based Biosensors for Non-Invasive Detection of Cancer and Disease Biomarkers for Clinical Diagnosis. Sensors 2017, 17, 1919. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent Advances in Sensing Applications of Graphene Assemblies and Their Composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond graphene: Electrochemical sensors and biosensors for biomarkers detection. Biosens. Bioelectron. 2016, 89, 152–166. [Google Scholar] [CrossRef]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vazquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Shi, J.-J.; He, T.-T.; Jiang, F.; Abdel-Halim, E.; Zhu, J.-J. Ultrasensitive multi-analyte electrochemical immunoassay based on GNR-modified heated screen-printed carbon electrodes and PS@PDA-metal labels for rapid detection of MMP-9 and IL-6. Biosens. Bioelectron. 2014, 55, 51–56. [Google Scholar] [CrossRef]
- Ruecha, N.; Shin, K.; Chailapakul, O.; Rodthongkum, N. Label-free paper-based electrochemical impedance immunosensor for human interferon gamma detection. Sens. Actuators B Chem. 2019, 279, 298–304. [Google Scholar] [CrossRef]
- Farid, S.; Meshik, X.; Choi, M.; Mukherjee, S.; Lan, Y.; Parikh, D.; Poduri, S.; Baterdene, U.; Huang, C.-E.; Wang, Y.Y.; et al. Detection of Interferon gamma using graphene and aptamer based FET-like electrochemical biosensor. Biosens. Bioelectron. 2015, 71, 294–299. [Google Scholar] [CrossRef]
- Mochalin, V.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J. Stochastic sensors. J. Mater. Chem. 2005, 15, 831–840. [Google Scholar] [CrossRef]
- Ilie-Mihai, R.M.; Gheorghe, S.S.; Staden, H.R.S.; Bratei, A. Electroanalysis of Interleukins 1β, 6, and 12 in Biological Samples Using a Needle Stochastic Sensor Based on Nanodiamond Paste. Electroanalysis 2021, 33, 6–10. [Google Scholar] [CrossRef]
- Li, J.; Ebendorff-Heidepriem, H.; Gibson, B.C.; Greentree, A.D.; Hutchinson, M.R.; Jia, P.; Kostecki, R.; Liu, G.; Orth, A.; Ploschner, M.; et al. Perspective: Biomedical sensing and imaging with optical fibers—Innovation through convergence of science disciplines. APL Photon. 2018, 3, 100902. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, G.; Goldys, E.M. Robust immunosensing system based on biotin-streptavidin coupling for spatially localized femtogram mL−1 level detection of interleukin-6. Biosens. Bioelectron. 2018, 102, 80–86. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef]
- Yadav, B.; Kumar, R. Structure, properties and applications of fullerenes. Int. J. Nanotechnol. Appl. 2008, 2, 15–24. [Google Scholar]
- Tonello, S.; Marziano, M.; Abate, G.; Kilic, T.; Memo, M.; Uberti, D.; Carrara, S.; Lopomo, N.F.; Serpelloni, M.; Sardini, E. Enhanced Sensing of Interleukin 8 by Stripping Voltammetry: Carbon Nanotubes versus Fullerene. In EMBEC & NBC 2017; Springer: Singapore, 2018. [Google Scholar]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Khoshroo, A. Label-free electrochemical immunosensor for detection of tumor necrosis factor α based on fullerene-functionalized carbon nanotubes/ionic liquid. J. Electroanal. Chem. 2015, 757, 58–64. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter. 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Wang, G.; Huang, H.; Zhang, G.; Zhang, X.; Fang, B.; Wang, L. Dual Amplification Strategy for the Fabrication of Highly Sensitive Interleukin-6 Amperometric Immunosensor Based on Poly-Dopamine. Langmuir 2011, 27, 1224–1231. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Arellano, L.M.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J. Viologen-functionalized single-walled carbon nanotubes as carrier nanotags for electrochemical immunosensing. Application to TGF-β1 cytokine. Biosens. Bioelectron. 2017, 98, 240–247. [Google Scholar] [CrossRef]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive Electrochemical Immunosensor for Oral Cancer Biomarker IL-6 Using Carbon Nanotube Forest Electrodes and Multilabel Amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Hu, L.-H.; Jiang, L.-P.; Zhu, J.-J. “Proof-of-principle” concept for ultrasensitive detection of cytokines based on the electrically heated carbon paste electrode. Chem. Commun. 2011, 47, 6551–6553. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, S.; Jin, H.; Bao, W.; Huang, S.; Wang, J. An electrochemical impedance sensor for the label-free ultrasensitive detection of interleukin-6 antigen. Sens. Actuators B Chem. 2013, 178, 310–315. [Google Scholar] [CrossRef]
- Khosravi, F.; Loeian, S.M.; Panchapakesan, B. Ultrasensitive Label-Free Sensing of IL-6 Based on PASE Functionalized Carbon Nanotube Micro-Arrays with RNA-Aptamers as Molecular Recognition Elements. Biosensors 2017, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Baiju, J. Polymer Nanocomposite-Based Electrochemical Sensors and Biosensors, Nanorods and Nanocomposites; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S. Recent Development of Morphology Controlled Conducting Polymer Nanomaterial-Based Biosensor. Appl. Sci. 2020, 10, 5889. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile fabrication of polypyrrole nanotubes using reverse microemulsion. Chem. Commun. 2003, 6, 720–721. [Google Scholar] [CrossRef]
- Liao, W.; Randall, B.; Alba, N.; Cui, X.T. Conducting Polymer-based Aptamer Biosensor for in situ Monitoring of Cytokine. MRS Proc. 2011, 1065, 505. [Google Scholar] [CrossRef]
- Nessark, F.; Eissa, M.M.; Baraket, A.; Zine, N.; Nessark, B.; Zouaoui, A.; Bausells, J.; Errachid, A. Capacitance Polypyrrole-based Impedimetric Immunosensor for Interleukin-10 Cytokine Detection. Electroanalysis 2020, 32, 1795–1806. [Google Scholar] [CrossRef]
- Wang, Y.; Mazurek, G.H.; Alocilja, E.C. Measurement of Interferon Gamma Concentration Using an Electrochemical Immunosensor. J. Electrochem. Soc. 2016, 163, B140–B145. [Google Scholar] [CrossRef]
- Liu, P.-Z.; Hu, X.-W.; Mao, C.-J.; Niu, H.-L.; Song, J.-M.; Jin, B.-K.; Zhang, S.-Y. Electrochemiluminescence immunosensor based on graphene oxide nanosheets/polyaniline nanowires/CdSe quantum dots nanocomposites for ultrasensitive determination of human interleukin-6. Electrochim. Acta 2013, 113, 176–180. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The design, fabrication, and applications of flexible biosensing devices. Biosens. Bioelectron. 2019, 124–125, 96–114. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.R.; de Sá, M.H.; Sales, M.G.F. An impedimetric molecularly-imprinted biosensor for Interleukin-1β determination, prepared by in-situ electropolymerization on carbon screen-printed electrodes. Bioelectrochemistry 2019, 130, 107287. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, F.; Zheng, N.; Saive, M.; Fauconnier, M.-L.; Wang, J. Aptamer-Based Biosensor for Detection of Mycotoxins. Front. Chem. 2020, 8, 195. [Google Scholar] [CrossRef] [PubMed]
- Tuleuova, N.; Jones, C.N.; Yan, J.; Ramanculov, E.; Yokobayashi, Y.; Revzin, A. Development of an Aptamer Beacon for Detection of Interferon-Gamma. Anal. Chem. 2010, 82, 1851–1857. [Google Scholar] [CrossRef]
- Tuleuova, N.; Revzin, A. Micropatterning of Aptamer Beacons to Create Cytokine-Sensing Surfaces. Cell. Mol. Bioeng. 2010, 3, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Jagannath, B.; Lin, K.; Pali, M.; Sankhala, D.; Muthukumar, S.; Prasad, S. Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 2021, 6, e10220. [Google Scholar] [CrossRef] [PubMed]
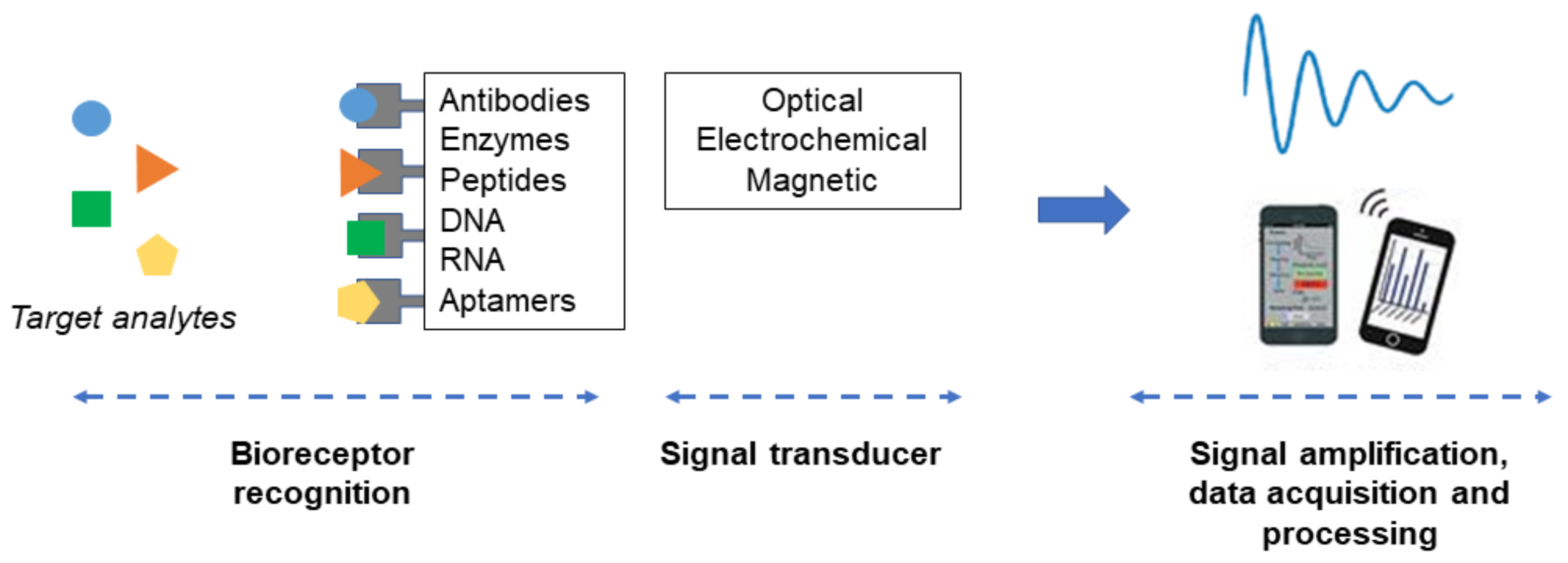
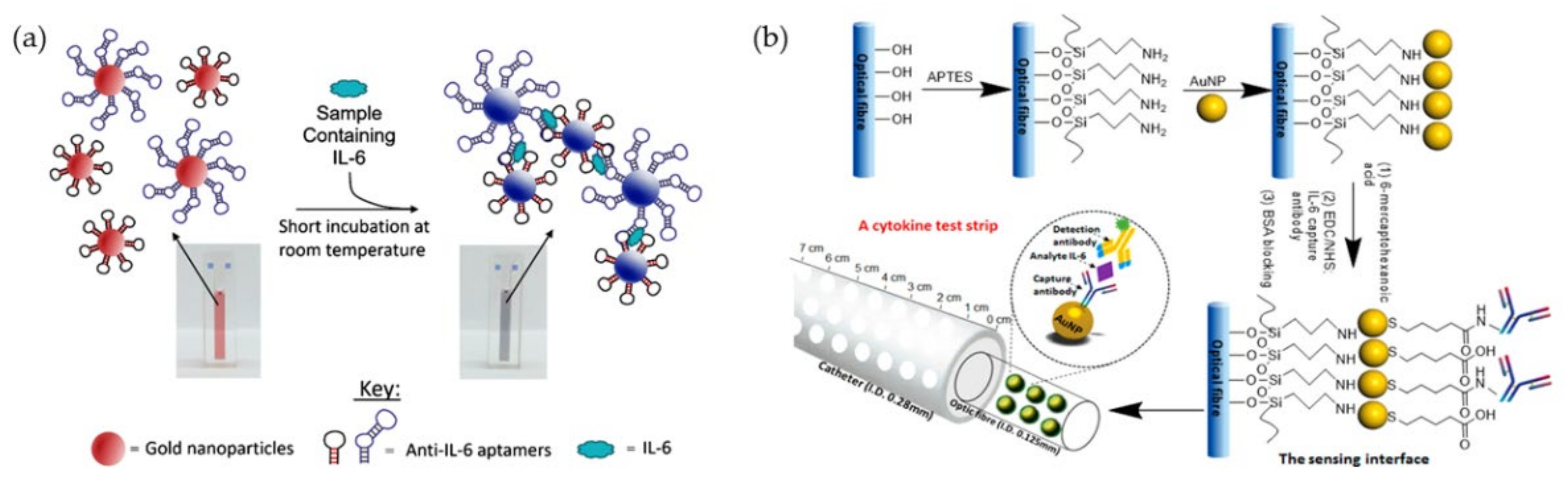
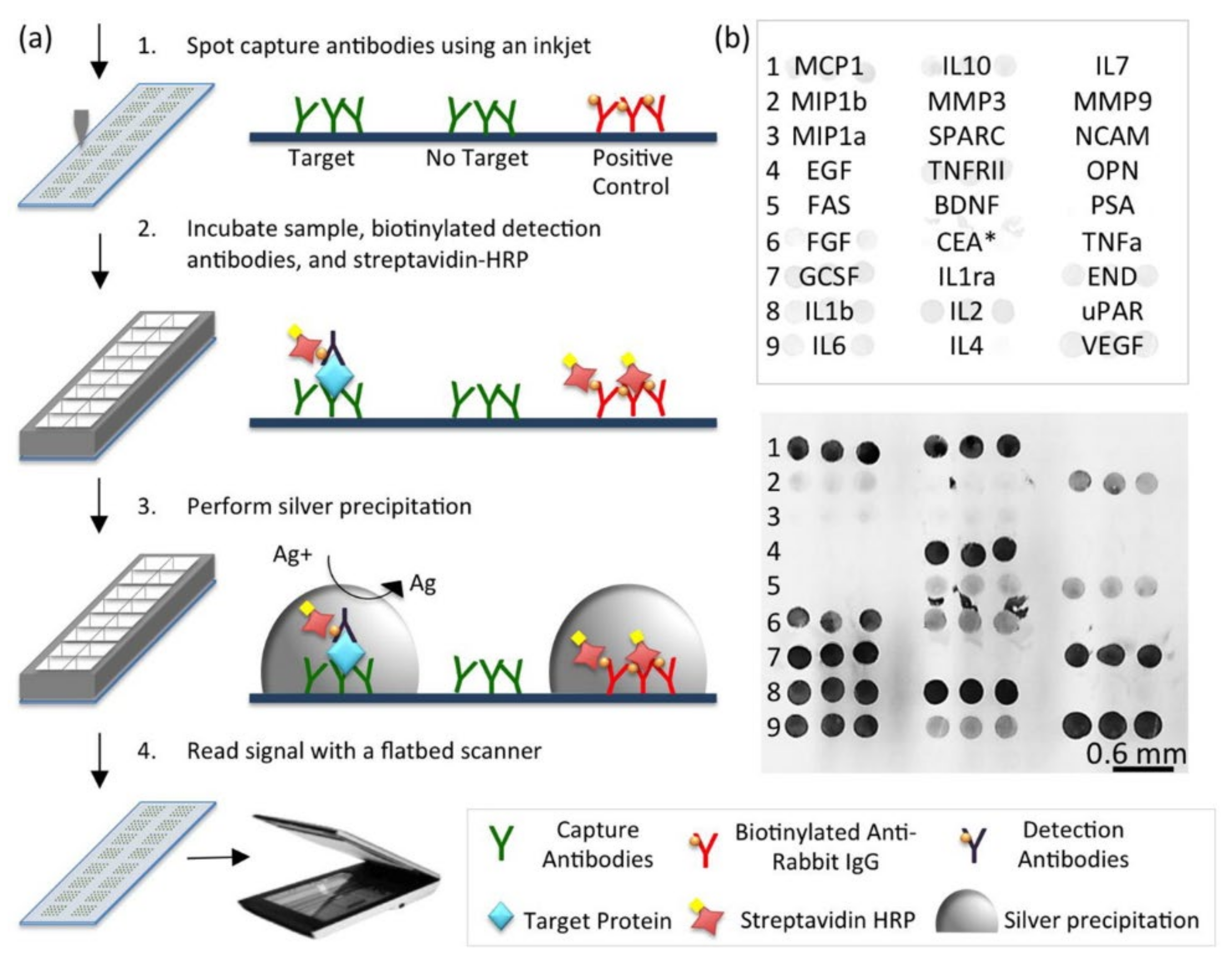

| Cytokine Family | Functions | Example |
|---|---|---|
| Interleukins | -stimulate hematopoiesis | IL-3, IL-7 |
| -regulate pluripotency and inflammation | IL-1, IL-6 | |
| -regulate T cells and B cells | IL-2, IL-4, IL-5, IL-12, and IL-13 | |
| Interferons | -exert antiviral, anti-proliferative effects | INF-α, INF-β |
| -exert antitumor effect | INF-γ | |
| Tumor necrosis factors | -stimulate inflammation, apoptosis, non-specific immune response | TNF-α, TNF-β |
| Chemokines | -regulate migration of granulocytes and lymphocytes, promote angiogenesis and inflammation | CXCL-1, CXCL-8 |
| -regulate migration of monocytes | CCL-3, CCL-5, CCL-7, and CCL-8 | |
| Colony stimulating factors | -stimulate proliferation and maturation of myeloid precursors | G-CSF, GM-CSF |
| Transforming growth factor | -stimulate fibroblast proliferation and extracellular matrix production | TGF-β |
| Method | Advantages | Disadvantages |
|---|---|---|
| ELISA | -well-accepted and standardized protocol -commercial kits available for a wide range of cytokines -high sensitivity and specificity | -laborious assay procedure -high cost |
| RIA | -high sensitivity | -exposure to radiations -time consuming procedure -costly equipment |
| ELISPOT | -detection of cytokines from single cells | -difficult to interpret the result |
| qRT-PCR | -sensitive and well-developed | -require proper handling of samples to avoid RNA degradation -amount of detected RNA may not correlate with protein level |
| Immunostaining | -provide physiologic and pathologic information | -can be invasive to obtain tissue of interest -time consuming procedure -fixation step can denature cytokines -need optimization for each antibody |
| ICC | -multiple cytokines can be detected -fast process compared to other methods | -procedure contains fixation step -costly equipment |
| Microarray | -multiplex | -require extensive validation |
| Allotrope | Sensor Platform/Label | Analyte | Detection Method | Linearity Range | LOD | Ref |
|---|---|---|---|---|---|---|
| Graphene | GNR/HSPCE/PS@PDOP | IL-6 | Electrochemical | 10−5–103 mg/mL | 0.1 pg/mL | [119] |
| PANI/Graphene | IFN-γ | CV | 5–1000 pg/mL | 3.4 pg/mL | [120] | |
| Graphene/PDMS/aptamer | IFN-γ | Electrochemical | NA | 83 pM | [121] | |
| NDs | NDs/PIX | IL-1β, IL-6, IL-12 | Stochastic electrochemical | IL-1β: 4 × 10−9–6.4 × 10−5 µg/mL, IL-6: 4 × 10−9–1 µg/mL, IL-12: 5.1 × 10−7–8 × 10−3 µg/mL | IL-1β: 4 × 10−9 µg/mL IL-6: 4 × 10−9 µg/mL IL-12: 5.1 × 10−7 µg/mL | [124] |
| NDs optic fiber/magnetic NPs | IL-6 | Fluorescence | 0.4–400 pg/mL | 0.1 pg/mL | [126] | |
| Fullerenes | SPES/Fullerenes | IL-8 | ASV | NA | 0.61 g/mL | [129] |
| Fullerenes/CNTs/Ionic liquid | TNF-α | Electrochemical | 5–75 pg/mL | 2 pg/mL | [130] | |
| CNTs | AuNPs/PDOP/CNTs | IL-6 | Amperometry | 4–800 pg/mL | 1 pg/mL | [133] |
| HOOC-Phe-SWCNTs/Viologen/HRP | TGF-β | Electrochemical | 2.5–1000 pg/mL | 0.95 pg/mL | [134] | |
| CNTs/Iron oxide/HRP | IL-6 | Amperometry | NA | 0.5 pg/mL | [135] | |
| Cu catalyzed IgG/azide-MWCNTs | IL-1β | DPV | 10–1200 pg/mL | 5.2 pg/mL | [136] | |
| AuNPs/SWCNTs/SiO2 | IL-6 | EIS | 0.01–100 fg/mL | 0.01 fg/mL | [138] | |
| SWCNTs/aptamer-PASE complex | IL-6 | Conductometry | NA | 10 pg/mL | [139] |
| Nanomaterials | Advantages | Disadvantages |
|---|---|---|
| QDs | High photostability Long lifetime | Toxicity in in vivo system Interact with protein in biological fluid |
| NMNs | Can be used in both biorecognition and signal amplification purposes Several transduction methods can be applied | Difficult to control morphology during synthesis Cost-ineffective for large scale production |
| Metal oxide | Charge and size controllable Fast response and recovery time Several transduction methods can be applied | Questionable toxicity for some of them Often need to be used with other nanomaterials as a hybrid nanocomposite for better sensitivity |
| Carbon-based | High degree of selectivity when functionalized Several transduction methods can be applied | Toxicity in in vivo system |
| Nanopolymer | Flexibility Wide range of polymers | Difficult to functionalize due to complex structure |
| Bionanomaterials | Superior specificity | Need other materials as transducer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lohcharoenkal, W.; Abbas, Z.; Rojanasakul, Y. Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors 2021, 11, 364. https://doi.org/10.3390/bios11100364
Lohcharoenkal W, Abbas Z, Rojanasakul Y. Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors. 2021; 11(10):364. https://doi.org/10.3390/bios11100364
Chicago/Turabian StyleLohcharoenkal, Warangkana, Zareen Abbas, and Yon Rojanasakul. 2021. "Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines" Biosensors 11, no. 10: 364. https://doi.org/10.3390/bios11100364
APA StyleLohcharoenkal, W., Abbas, Z., & Rojanasakul, Y. (2021). Advances in Nanotechnology-Based Biosensing of Immunoregulatory Cytokines. Biosensors, 11(10), 364. https://doi.org/10.3390/bios11100364





