Solid State Sensors for Hydrogen Peroxide Detection
Abstract
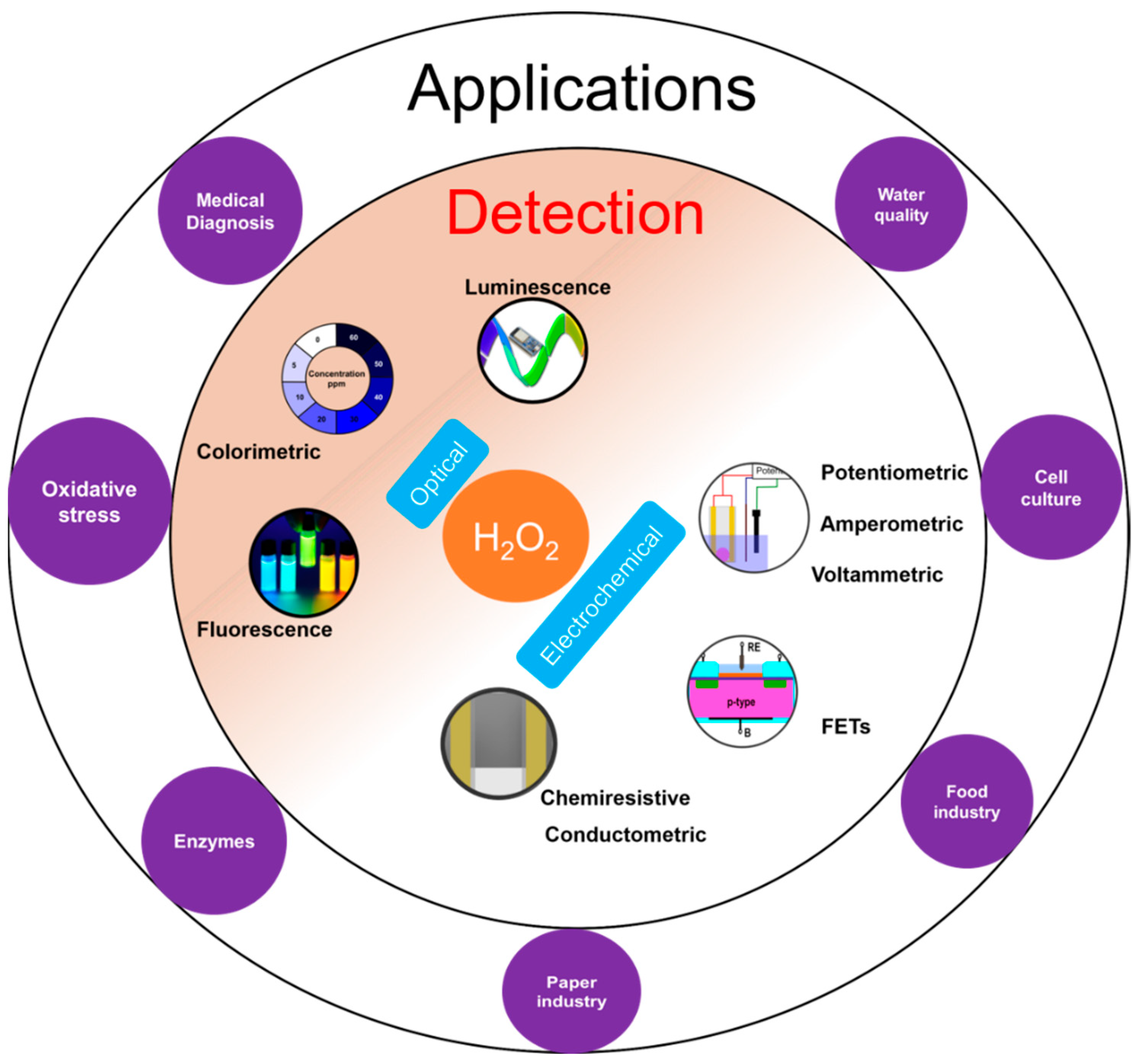
1. Introduction
2. Sensing Mechanism
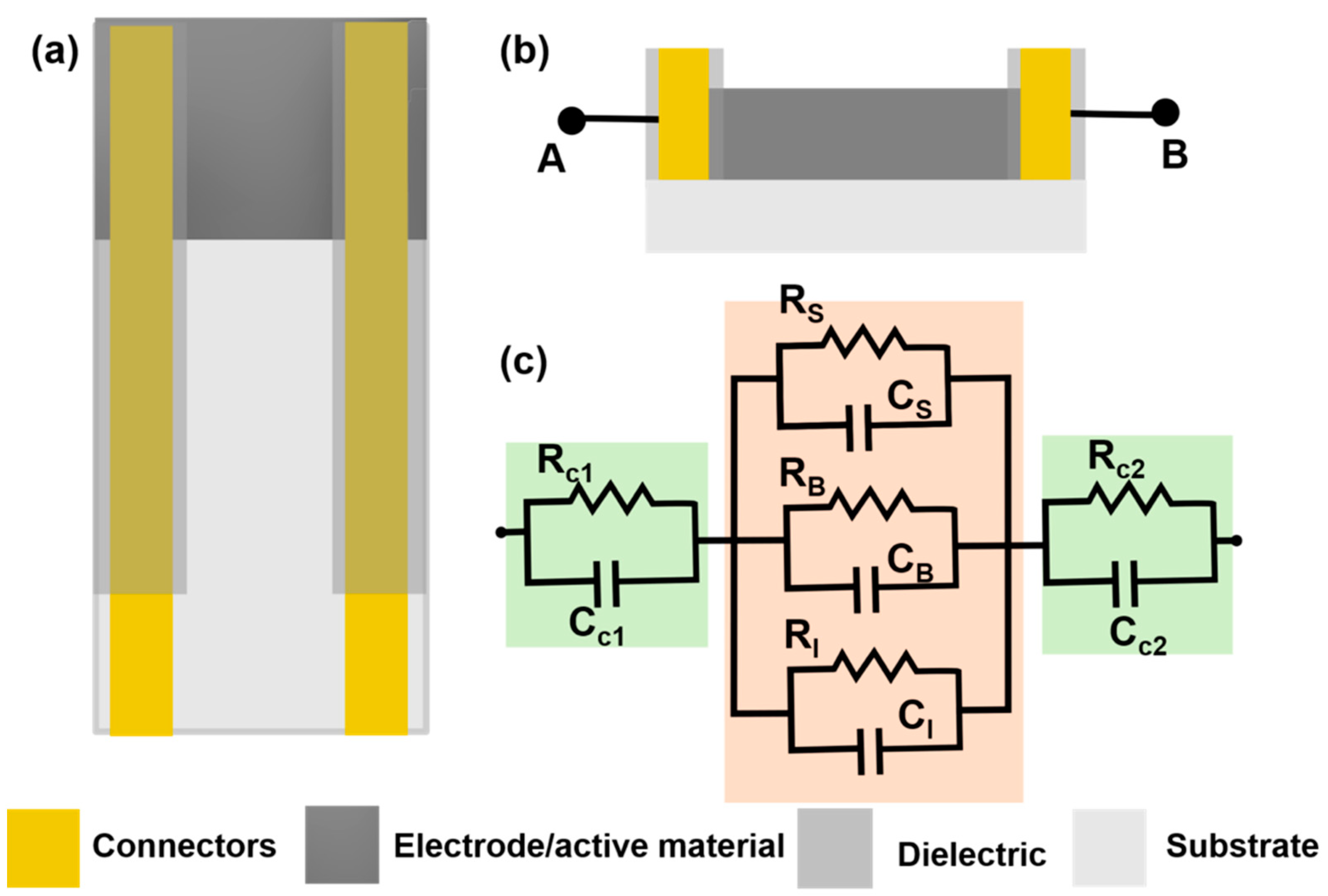
2.1. Chemiresistive Sensors
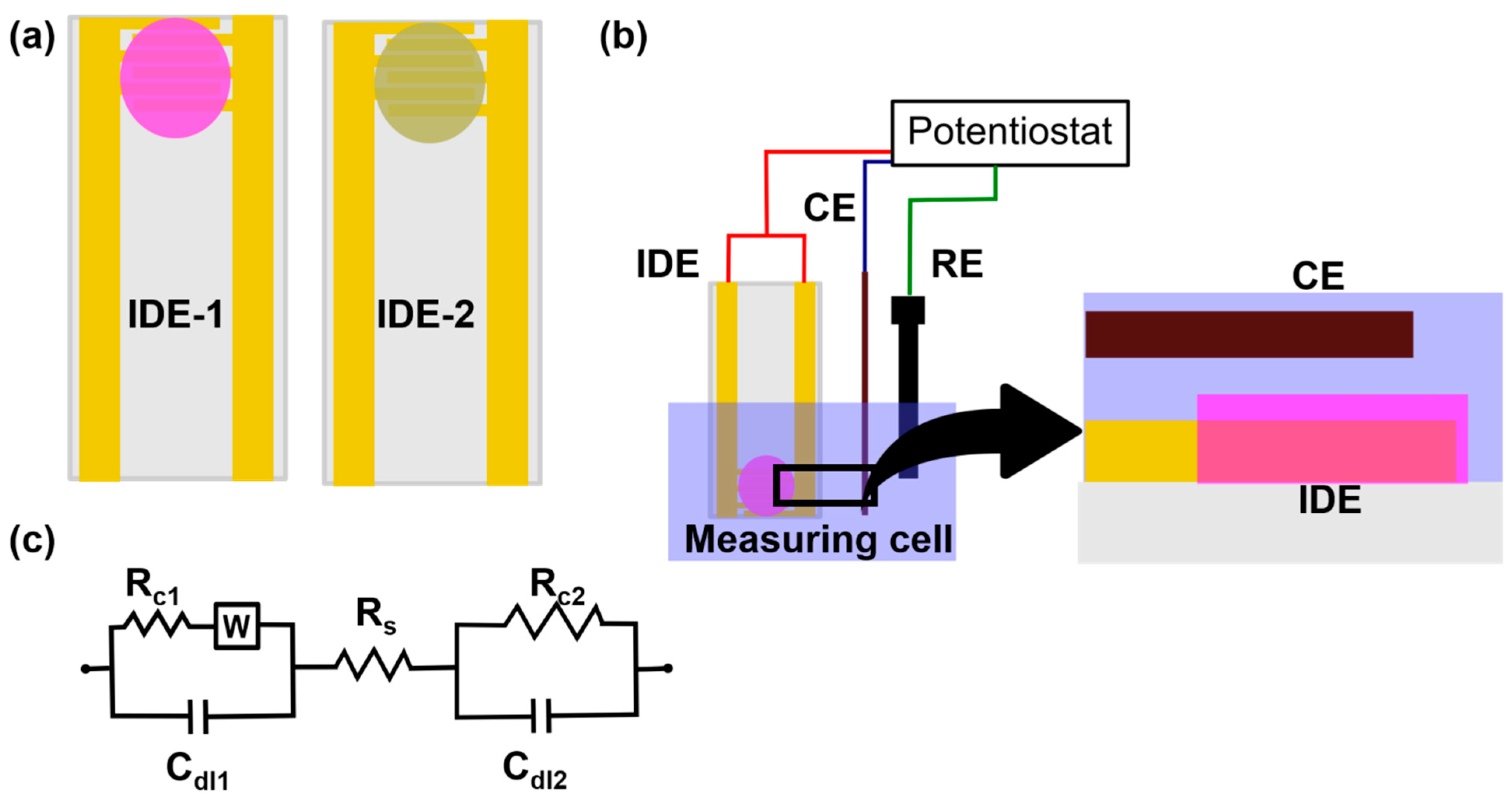
2.2. Conductometric Sensors
2.3. FET
3. Chemiresistive Sensors
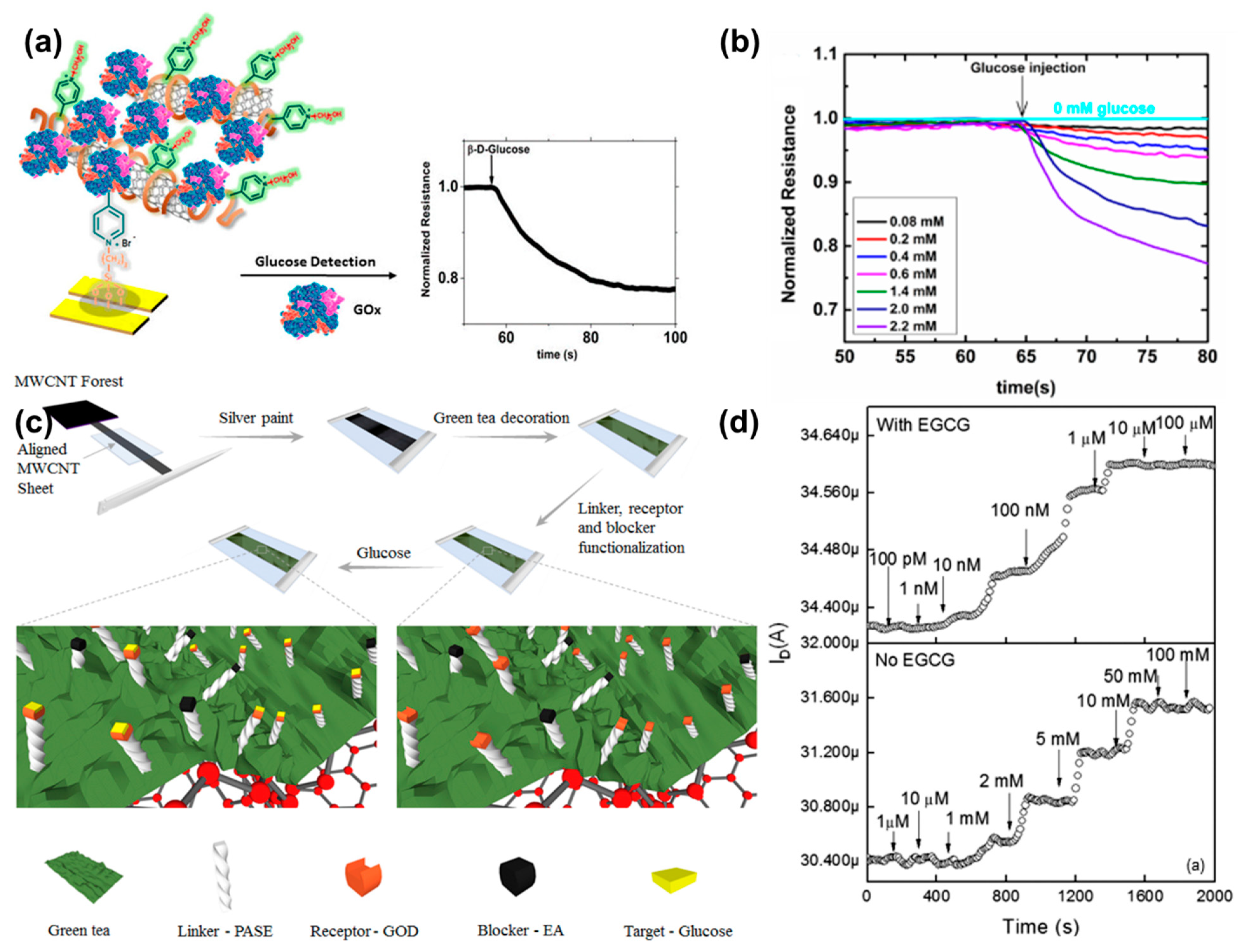
3.1. Chemiresistive Sensors Based on CNTs
3.2. Chemiresistive Sensors Based on Conducting Polymers
| Substrate | Target Analyte | Ligand/ Enzyme | LOD (mM) | Measuring Range (mM) | Voltage Bias (mV) | Response Time (s) | Buffer/ Working pH | Comments | Interference Tested | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Carbon nanotube based | ||||||||||
| PPy-MWCNT | H2O2/ Glucose | Dodecyl benzene sulfonate | NR | 0–20 | 1 | NR | NR | Investigated the sensitivity of temperature humidity etc. | No | [48] |
| CNT | Glucose | EGCG-GOD | 8.7 nM | 10 nM–1 μM | 100 | <400 (est.) | Working pH 7.4 Buffer: PBS | Sensor responds to all reactive oxidative species | Yes | [49] |
| SWCNT-PVP | Glucose | GOD | 0.08 | 0.02–2 | 100 | 3 | Working pH 5.5 Buffer: Acetate | Tested in juice & iced tea Stable for 5 consecutive tests | Yes | [19] |
| Conducting polymer based | ||||||||||
| Au-PANI nanowires | H2O2 | AgNPs | 5 | 5–40 | 20 | 25 | Working pH 5 Buffer: Phosphate (200 mM) | Stable response for 36 h Reusable sensor | Yes | [20] |
| MWCNT-PANI nanowires | H2O2 | AgNPs | 1 | 1–20 | NR | 180 | NR | Inkjet printed sensors | No | [51] |
| MWCNT-PANI nanowire | H2O2 Glucose | PtNPs | 2 | 2–10 | 500 | 240 | NR | Inkjet printed | No | [50] |
| Others | ||||||||||
| Alumina | Glucose | SnO2-GOD | 0.5 (est.) | 0.5–20 | NR | 50 | Working pH 7.2 Buffer: Phosphate | Sensor sensitivity increases with deposition temperature of SnO2 | No | [54] |
3.3. Chemiresistive Sensors Based on Other Materials
4. Conductometric Sensors
4.1. Conductometric Sensors Based on Metal Electrodes
4.2. Conductometric Sensors Based on Metal Nanoparticles
4.3. Conductometric Sensors Using Other Materials
5. FET Sensors
5.1. FET Sensors Based on Silicon Nitride
5.2. FET Sensors Based on Conducting Polymers
5.2.1. Polyaniline
5.2.2. Polypyrrole
5.2.3. PEDOT
5.3. FET Sensors Based on Metal Oxides
5.4. FET Sensors Based on Carbon Nanomaterials
| Substrate | Target Analyte | Ligand/ Enzyme | LOD (µM) | Sensitivity | Measuring Range (mM) | OC | Response Time (s) | Working pH & Buffer | Comments | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Silicon nitride FET | ||||||||||
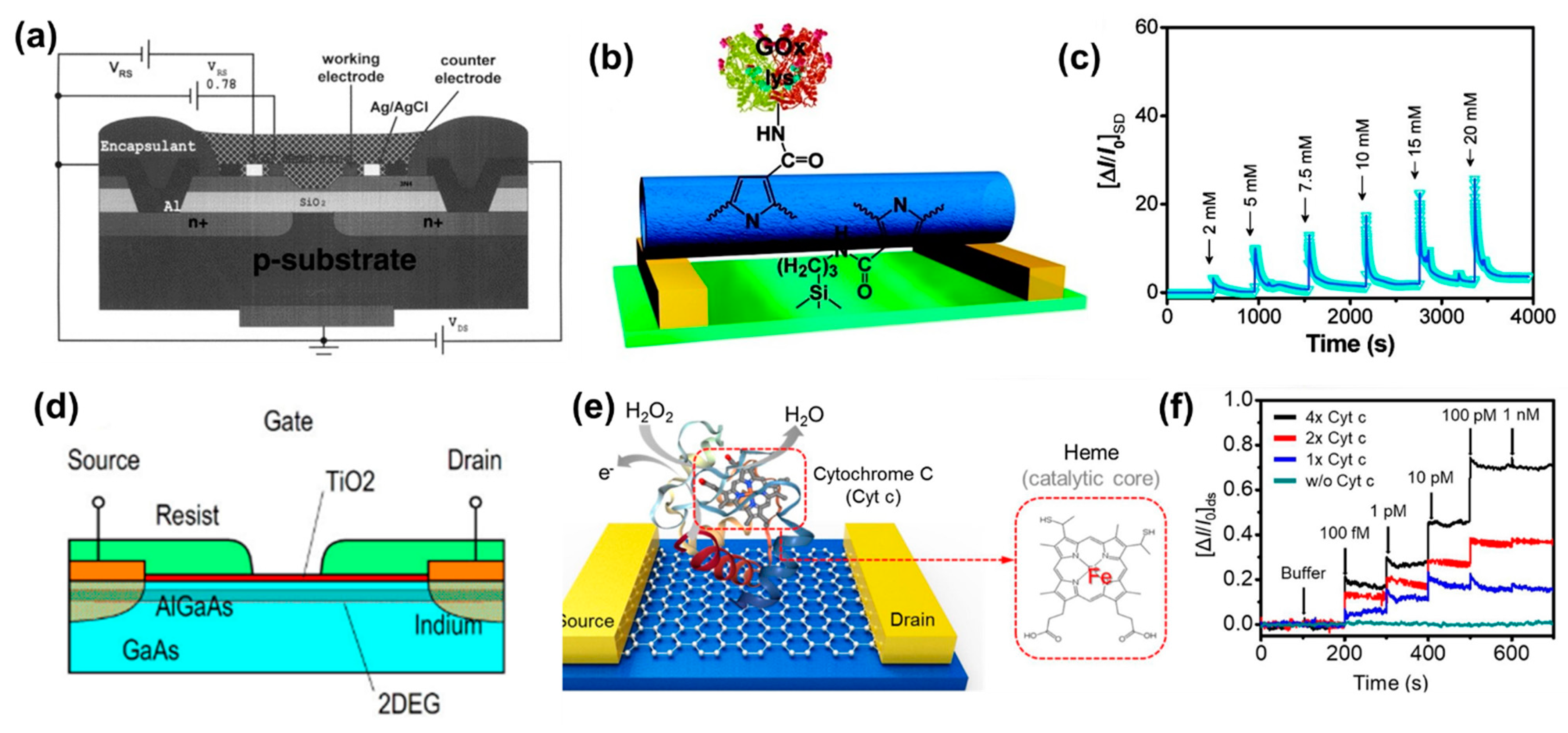
| Si3N4-FET | H2O2 | HRP | 5 | ~15 mV/mM (est.) | <2 | Is: 300 μA Vds: 2 V | 30–90 | Buffer: Phosphate (10 mM) Working pH: 6 | <10% reduction in enzyme activity after 1000 measurements | [41] |
| Si3N4-FET/Pt electrode | Glucose | GOD | NR | ~40 mV/mM | <5 | Vbias: 0.64 V | ~480 | Buffer: Phosphate (5–20 mM) Working pH: 7.4 | Baseline established by removing the potential bias | [40] |
| Si3N4-FET/Pt electrode | Glucose & sucrose | GOD & Invertase-mutarotase-GOD | ~50 (est) | NR | 1.67–16.67 | Vbias: 0.7 V | 180–300 | Buffer: Phosphate (10 mM) Working pH: 7.4 | Greater Pt area, increases sensitivity | [42] |
| Si3N4-FET/Pt electrode | Glucose | GOD | 1000 (est.) | ~11 mV/mM (est.) | 1–10 | Vbias: 0.7 V | ~60 | Buffer: Phosphate (10 mM) Working pH: 7.4 | Ladder shape Pt electrode was used for potential bias | [45] |
| Si3N4-FET | H2O2 | Pt | 10000 | 5 mV/mM | 10–100 | Ids: 0.1 mA Vds: IV | 300 | Buffer: Phosphate (100 mM) Working pH: 7.2 | Used for glucose and lactate | [46] |
| Conducting polymers | ||||||||||
| Carbon | H2O2 | PANI-pDAB-HRP | 100 | NR | <0.5 | Vg: 200 mV Vd: 20 mV | ~100 s | Buffer: citrate-phosphate-Na2SO4 Working pH: 5 | HRP inhibition at H2O2 concentration > 0.5 mM. | [75] |
| Kapton-Carbon | H2O2 | PSPANI-HRP | 25 | 0.126 μA/s | 0.025–1 | Vg: 0 V Vd: 20 mV | 100–300 s | Buffer: HEPES-KNO3 (100 mM) Working pH: 7 | Sultonation improves the PANI conductivity at pH 7 | [76] |
| Si3N4-FET | Glucose | PANI-PAA-GOD | NR | 1 nA/mM | 0–9 | Vg: 20 mV Vds: 10 mV | <1 s | Buffer: McIlvaine Working pH: 5 | PANI-PAA film was deposition by electropolymerization | [61] |
| PEDOT-TFT | H2O2 & Glucose | GOD | 100 | NR | 0.1–1 | Vd: 0.2 V Vg: 0–0.6 V | ~60 s | Buffer: PBS Working pH: 7.14 | pH independent response from pH 5 to 9 | [83] |
| PEDOT-TFT | Glucose | GOD | 1 | 0.1 V/decade | <1 | Vds: −0.2 V | NR | Buffer: PBS (15 mM) Working pH: 6.8 | Sensitivity can be improved by increasing Vg | [63] |
| PEDOT-TFT | Glucose | GOD | <1000 | 1.65 μA/mM | 1.1 to 16.5 | Vds: −1.5 V Vg: 0.0 V | 10–20 s | NR | Sensor was encapsulated in cellulose acetate membrane | [64] |
| Liquid gate-FET | H2O2 & Glucose | PPyNT-GOD | 500 (est.) | 3.75%/mM (est.) | 2–20 | Vds: −0.01 V Vg: 0.01 V | 5–10 s | Buffer: PBS (10 mM) Working pH: 7.0 | High enzyme loading was achieved | [65] |
| TFT | H2O2 & Glucose | PEDOT-GOD | 1 | 0.79–3 μA/mM | 0.001–5 | Vds: −0.4 V Vg: 0.4 V | <20 s | Buffer: PBS Working pH: 7.4 | Used as both optical and electrochemical | [84] |
| TFT | Glucose | PEDOT-GOD | 10 | NR | 0.01–100 | Vds: −0.7 V Vg: 0.7 V | ~360 s | Buffer: PBS (120 mM) | Stable for 100 days with covalently immobilized GOD | [86] |
| TFT | H2O2 & Glucose | PEDOT-TiO2-GOD | 1 | 0.126%/decade | 0.001–5 | Vds: −0.1 V Vg: 0.4 V | ~1000 s (est.) | Buffer: PBS (10 mM) Working pH: 7.0 | Stable for 10 days with intermittent testing | [85] |
| Liquid gate FET | H2O2 | rGO-PPy NTs | 0.1 nM | 2%/decade | 0.1–100 nM | Vg: 0.1 V Vds: −0.01 V | <1 s | Buffer: PBS Working pH: 7.4 | Stable up to 1 month, when stored in air | [66,67] |
| Metal oxides | ||||||||||
| Glass-ITO-SnO2 | Glucose | GOD-MnO2 | 2700 | 2.35 mV/mM | <20 | No bias | 720 | Buffer: Phosphate-KOH (5 mM) Working pH: 8.1 | Dynamic range strongly depends on pH value | [68] |
| Si3N4-FET | Glucose | GOD-MnO2 NPs | 20 | NR | 0.025–1.9 | No bias | ~140 s | Buffer: Tris (10 mM) Working pH: 7.4 | Repeatability: 1.9% (RSD) for 7 measurements | [62] |
| FET | H2O2 | Iridium oxide | 100 | 400 mV/dec | 0.1–10 | Ibias: 25 nA | NR | Working pH: 3.5–9 | - | [69] |
| Prussian blue | 10 | 290 mV/dec | 0.01–1 | Ibias: 50 nA | Working pH: 4.5–6 | |||||
| Os-PVP-HRP | 0.1 | 700 mV/dec | 10−7–10−5 M | Ibias: 25 nA | Working pH: 4.5–6 | |||||
| Ta2O5-FET-Pt | H2O2 | Perovskite oxide | 4 | 35 mV/dec | 0.005–0.2 | Ibias: 25 nA | 1800 | Buffer: Phosphate Working pH: 7 | Change in stoichiometry of oxide can result in lower detection limit | [44] |
| FET | H2O2 | TiO2 | NR | 4.5 mV/μM (DMEM media) | NR | Ids: 0.1 mA Vds: 1 V | 300 (est.) | Buffer: Phosphate | DMEM media | [71] |
| FET | Glucose | ZnO-NiO quantum dots | 26 | 13.14 μA mM−1(0.001–10 mM) | 0.001–50 | Vg: 1.2–2 V Vds: 0.0 V | NR | Buffer: PBS (10 mM) Working pH: 7.4 | Tested in whole blood and serum | [94] |
| Liquid gate FET | Glucose | ZnO rod-GOD | 0.07 | 32.27 μA mM−1cm−2 | 0.05–70 | Vg: 0–2 V | NR | Buffer: PBS (50 mM) Working pH: 7.4 | Mice blood, serum | [74] |
| Cholesterol | ZnO rod-COD | 0.04 | 17.1 μA mM−1cm−2 | 0.01–45 | Vg: 2–3 V | NR | ||||
| Carbon nanomaterials | ||||||||||
| Graphene-FET | Glucose | GOD | 100 | ~1 μA/mM (est.) | <10 | Vds: 0.1 V Vg: 0 V | <200 s (est.) | Buffer: PBS (10 mM) Working pH: 7.2 | Glutamate was also detected using the sensor with GluD | [77] |
| OTFT | Glucose | Graphene-Chitosan-GOD | 0.01 | 370 mV/dec | 0.01–1 μM | Vg: 0.4 V Vds: 0.05 V | ~500 s | Buffer: PBS Working pH: 7.4 | Investigated the effect of interference of UA and AA | [98] |
| Graphene-FET | Glucose | Silk fibroin-GOD | 100 | 2.5 μA/mM | 0.1–10 | Vg: 0 V Vds:0.1 V | ~100 s | Buffer: PBS (10 mM) Working pH: 7.4 | Stable for 10 months at room temperature | [79] |
| FET | H2O2 & Glucose | Graphene-Chitosan-PtNPs-GOD | 0.03 | 91.7 mV/dec | 30 nM–1 mM | Vg: 0.7 V Vds: 0.05 V | ~100 s (est.) | Buffer: PBS Working pH: 7.2 | No interference was observed from AA and UA | [99] |
| rGO-FET | H2O2 | MoS2 | 1 pM | 0.46%/dec | 1 pM–100 nM | Vg: 0.1 V Vds: 0.01 V | ~1 s | Buffer: PBS Working pH: 7.4 | HeLa Cells | [25] |
| FET | H2O2 | Graphene-Cyt-c | 0.1 pM | 14%/dec | 0.1–100 pM | Vg: 1.75 V Vds: 0.001 V | <1 s | Buffer: PBS Working pH: 7.4 | No interference from UA, AA, dopamine, and glutamate | [24] |
| Others | ||||||||||
| SiO2-MOSC | Glucose | HRP-GOD | 5000 | 1.76 nA/cm2M | <2 M | Vg: 5 V | 1200 | Dry sensor so no need for a buffer solution | - | [70] |
| Polysilicon wire-ISFET | H2O2 & Glucose | APTES-SiNPs-UV treatment | 32 pM | 12 AmM−1cm−2 | 10−10–10−3 M | Vds: 5 V | NR | Tested solution volume: 0.03 pL (Dry sensor) | Serum | [73] |
6. Outlook
6.1. Contact Resistance and Its Engineering
6.2. Real Sample Testing
6.3. In Vivo Applications
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Dickinson, B.C.; Chang, C.J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 15681–15686. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive Oxygen Species as a Signal in Glucose-Stimulated Insulin Secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidatives stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Rao, A.V.; Balachandran, B. Role of Oxidative Stress and Antioxidants in Neurodegenerative Diseases. Nutr. Neurosci. 2002, 5, 291–309. [Google Scholar] [CrossRef]
- Schöning, M.J.; Poghossian, A. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst 2002, 127, 1137–1151. [Google Scholar] [CrossRef]
- Andersen, B.M.; Rasch, M.; Hochlin, K.; Jensen, F.H.; Wismar, P.; Fredriksen, J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 2006, 62, 149–155. [Google Scholar] [CrossRef]
- Burmistrova, N.A.; Kolontaeva, O.A.; Duerkop, A. New Nanomaterials and Luminescent Optical Sensors for Detection of Hydrogen Peroxide. Chemosensors 2015, 3, 253–273. [Google Scholar] [CrossRef]
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-Layton, R.E.; Ratan, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent advances in electrochemical sensing for hydrogen peroxide: A review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Weng, L.; Yang, C. A review on nanomaterial-based electrochemical sensors for H2O2, H2S and NO inside cells or released by cells. Microchim. Acta 2017, 184, 1267–1283. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.-W. Review—Non-Enzymatic Hydrogen Peroxide Electrochemical Sensors Based on Reduced Graphene Oxide. J. Electrochem. Soc. 2020, 167, 037531. [Google Scholar] [CrossRef]
- Aziz, A.; Asif, M.; Ashraf, G.; Azeem, M.; Majeed, I.; Ajmal, M.; Wang, J.; Liu, H. Advancements in electrochemical sensing of hydrogen peroxide, glucose and dopamine by using 2D nanoarchitectures of layered double hydroxides or metal dichalcogenides. A review. Microchim. Acta 2019, 186, 671. [Google Scholar] [CrossRef]
- Chen, L.; Compton, R.G. Reference Electrodes for Electrochemical Sensors Based on Redox Couples Immobilized within Nafion Films. ACS Sens. 2019, 4, 1716–1723. [Google Scholar] [CrossRef]
- Aydoğdu, G.; Zeybek, D.K.; Pekyardımcı, Ş.; Kilic, E. A novel amperometric biosensor based on ZnO nanoparticles-modified carbon paste electrode for determination of glucose in human serum. Artif. Cells Nanomed. Biotechnol. 2013, 41, 332–338. [Google Scholar] [CrossRef]
- Soylemez, S.; Yoon, B.; Toppare, L.; Swager, T.M. Quaternized Polymer–Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor. ACS Sens. 2017, 2, 1123–1127. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. A selective hydrogen peroxide sensor based on chemiresistive polyaniline nanowires modified with silver catalytic nanoparticles. J. Micromech. Microeng. 2014, 24, 24. [Google Scholar] [CrossRef]
- Li, L.; Guo, W.; Lin, Y.; Tang, D.; Liu, J. Facile and feasible conductometric immunoanalytical assay for alpha-fetoprotein using platinum-functionalized graphitic carbon nitride nanosheets. Anal. Methods 2018, 10, 4886–4893. [Google Scholar] [CrossRef]
- Valencia, G.A.; De Oliveira Vercik, L.C.; Vercik, A. A new conductometric biosensor based on horseradish peroxidase immobilized on chitosan and chitosan/gold nanoparticle films. J. Polym. Eng. 2014, 34, 633–638. [Google Scholar] [CrossRef]
- Nouira, W.; Maaref, A.; Elaissari, H.; Vocanson, F.; Siadat, M.; Jaffrezic-Renault, N. Comparative study of conductometric glucose biosensor based on gold and on magnetic nanoparticles. Mater. Sci. Eng. C 2013, 33, 298–303. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, K.H.; Seo, S.E.; Kim, M.I.; Park, S.J.; Kwon, O.S. Cytochrome C-decorated graphene field-effect transistor for highly sensitive hydrogen peroxide detection. J. Ind. Eng. Chem. 2020, 83, 29–34. [Google Scholar] [CrossRef]
- Zheng, C.; Jin, X.; Li, Y.; Mei, J.; Sun, Y.; Xiao, M.; Zhang, H.; Zhang, Z.; Zhang, G.J. Sensitive Molybdenum Disulfide Based Field Effect Transistor Sensor for Real-time Monitoring of Hydrogen Peroxide. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Shan, J.; Li, J.; Chu, X.; Xu, M.; Jin, F.; Wang, X.; Ma, L.; Fang, X.; Wei, Z.; Wang, X. High sensitivity glucose detection at extremely low concentrations using a MoS2-based field-effect transistor. RSC Adv. 2018, 8, 7942–7948. [Google Scholar] [CrossRef]
- Sugiyasu, K.; Swager, T.M. Conducting-Polymer-Based Chemical Sensors: Transduction Mechanisms. Bull. Chem. Soc. Jpn. 2007, 80, 2074–2083. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Narwal, V. Quantitative analysis of hydrogen peroxide with special emphasis on biosensors. Bioprocess Biosyst. Eng. 2018, 41, 313–329. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, W. Recent advances in graphene-based nanomaterials for fabricating electrochemical hydrogen peroxide sensors. Biosens. Bioelectron. 2017, 89, 249–268. [Google Scholar] [CrossRef]
- Olenin, A.Y. Methods of nonenzymatic determination of hydrogen peroxide and related reactive oxygen species. J. Anal. Chem. 2017, 72, 243–255. [Google Scholar] [CrossRef]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of Advanced Nanomaterials in the Non-Enzymatic Electrochemical Sensing of Glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Kruse, P. Review on water quality sensors. J. Phys. D Appl. Phys. 2018, 51, 203002. [Google Scholar] [CrossRef]
- Moonoosawmy, K.R.; Kruse, P. Cause and Consequence of Carbon Nanotube Doping in Water and Aqueous Media. J. Am. Chem. Soc. 2010, 132, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Fennell, J.F.; Liu, S.F.; Azzarelli, J.M.; Weis, J.G.; Rochat, S.; Mirica, K.A.; Ravnsbaek, J.B.; Swager, T.M. Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chem. Int. Ed. 2016, 55, 1266–1281. [Google Scholar] [CrossRef]
- Tang, R.; Shi, Y.; Hou, Z.; Wei, L. Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V.M. Chemiresistors based on conducting polymers: A review on measurement techniques. Anal. Chim. Acta 2011, 687, 105–113. [Google Scholar] [CrossRef]
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef]
- Watson, L.D.; Maynard, P.; Cullen, D.C.; Sethi, R.S.; Brettle, J.; Lowe, C.R. A microelectronic conductimetric biosensor. Top. Catal. 1987, 3, 101–115. [Google Scholar] [CrossRef]
- Hwa-II, S.; Chang-Soo, K.; Byung-Ki, S.; Terence, Y.; Son Mun-Tak, H.M. ISFET glucose sensor based on a new prinicple using the electrolysis of hydrogen peroixde. Sens. Actuators B Chem. 1997, 40, 1–5. [Google Scholar]
- Shul’Ga, A.A.; Gibson, T.D. An alternative microbiosensor for hydrogen peroxide based on an enzyme field effect transistor with a fast response. Anal. Chim. Acta 1994, 296, 163–170. [Google Scholar] [CrossRef]
- Sohn, B.-K.; Cho, B.-W.; Kim, C.-S.; Kwon, D.-H. ISFET glucose and sucrose sensors by using platinum electrode and photo-crosslinkable polymers. Sens. Actuators B Chem. 1997, 41, 7–11. [Google Scholar] [CrossRef]
- Sergeyeva, T.A.; Lavrik, N.V.; Rachkov, A.E.; Kazantseva, Z.I.; Piletsky, S.A.; El’Skaya, A.V. Hydrogen peroxide—Sensitive enzyme sensor based on phthalocyanine thin film. Anal. Chim. Acta 1999, 391, 289–297. [Google Scholar] [CrossRef]
- Dam, V.-A.T.; Oithuis, W.; Bergveld, P.; ven den Berg, A. Catalytic Hydrogen Peroxide decompositon on pervoskite oxide. In Proceedings of the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Seoul, South Korea, 22 August 2005; Volume 2, pp. 1840–1843. [Google Scholar] [CrossRef]
- Kim, C.S.; Seo, H.I.; Lee, C.H.; Sohn, B.K. Miniaturized ISFET glucose sensor including a new structure actuation system. In Proceedings of the International Solid State Sensors and Actuators Conference (Transducers ‘97), Chicago, IL, USA, 19 June 1997; Volume 2, pp. 911–914. [Google Scholar] [CrossRef]
- Diallo, A.K.; Djeghlaf, L.; Mazenq, L.; Launay, J.; Sant, W.; Temple-Boyer, P. Development of pH-based ElecFET biosensors for lactate ion detection. Biosens. Bioelectron. 2013, 40, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemically sensitive field effect transistor as multi-species microprobe. Sens. Actuators 1983, 4, 291–298. [Google Scholar] [CrossRef]
- Teh, K.-S.; Lin, L. MEMS sensor material based on polypyrrole–carbon nanotube nanocomposite: Film deposition and characterization. J. Micromech. Microeng. 2005, 15, 2019–2027. [Google Scholar] [CrossRef]
- Salila Vijayalal Mohan, H.K.; Hansen Varghese, R.; Wong, C.H.; Zheng, L.; Yang, J. Epigallocatechin gallate decorated carbon nanotube chemiresistors for ultrasensitive glucose detection. Org. Electron. 2016, 28, 210–216. [Google Scholar] [CrossRef]
- Song, E.; Da Costa, T.H.; Choi, J.W. A chemiresistive glucose sensor fabricated by inkjet printing. Microsyst. Technol. 2017, 23, 3505–3511. [Google Scholar] [CrossRef]
- Song, E.; Tortorich, R.P.; Da Costa, T.H.; Choi, J.W. Inkjet printing of conductive polymer nanowire network on flexible substrates and its application in chemical sensing. Microelectron. Eng. 2015, 145, 143–148. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Multi-analyte detection of chemical species using a conducting polymer nanowire-based sensor array platform. Sens. Actuators B Chem. 2015, 215, 99–106. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.W. Self-calibration of a polyaniline nanowire-based chemiresistive pH sensor. Microelectron. Eng. 2014, 116, 26–32. [Google Scholar] [CrossRef]
- Ansari, S.G.; Ansari, Z.A.; Wahab, R.; Kim, Y.S.; Khang, G.; Shin, H.S. Glucose sensor based on nano-baskets of tin oxide templated in porous alumina by plasma enhanced CVD. Biosens. Bioelectron. 2008, 23, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Hnaien, M.; Lagarde, F.; Jaffrezic-Renault, N. A rapid and sensitive alcohol oxidase/catalase conductometric biosensor for alcohol determination. Talanta 2010, 81, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Bouyahia, N.; Hamlaoui, M.L.; Hnaien, M.; Lagarde, F.; Jaffrezic-Renault, N. Impedance spectroscopy and conductometric biosensing for probing catalase reaction with cyanide as ligand and inhibitor. Bioelectrochemistry 2011, 80, 155–161. [Google Scholar] [CrossRef]
- Nguyen-Boisse, T.T.; Saulnier, J.; Jaffrezic-Renault, N.; Lagarde, F. Highly sensitive conductometric biosensors for total lactate, d- and l-lactate determination in dairy products. Sens. Actuators B Chem. 2013, 179, 232–239. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Chen, P.; Zhong, Z. Enhanced conductometric immunoassay for hepatitis B surface antigen using double-codified nanogold particles as labels. Biochem. Eng. J. 2009, 45, 107–112. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J. Conductometric glucose biosensor made with cellulose and tin oxide hybrid nanocomposite. Sens. Actuators B Chem. 2011, 157, 177–182. [Google Scholar] [CrossRef]
- Volotovsky, V.; Kim, N. Cyanide determination by an ISFET-based peroxidase biosensor. Biosens. Bioelectron. 1998, 13, 1029–1033. [Google Scholar] [CrossRef]
- Forzani, E.S.; Zhang, H.; Nagahara, L.A.; Amlani, I.; Tsui, R.; Tao, N. A Conducting Polymer Nanojunction Sensor for Glucose Detection. Nano Lett. 2004, 4, 1785–1788. [Google Scholar] [CrossRef]
- Luo, X.L.; Xu, J.J.; Zhao, W.; Chen, H.Y. A novel glucose ENFET based on the special reactivity of MnO2 nanoparticles. Biosens. Bioelectron. 2004, 19, 1295–1300. [Google Scholar] [CrossRef]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Liu, J.; Agarwal, M.; Varahramyan, K. Glucose sensor based on organic thin film transistor using glucose oxidase and conducting polymer. Sens. Actuators B Chem. 2008, 135, 195–199. [Google Scholar] [CrossRef]
- Yoon, H.; Ko, S.; Jang, J. Field-Effect-Transistor Sensor Based on Enzyme-Functionalized Polypyrrole Nanotubes for Glucose Detection. J. Phys. Chem. B 2008, 112, 9992–9997. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, S.J.; Kwon, O.S.; Lee, C.; Jang, J. Polypyrrole Nanotube Embedded Reduced Graphene Oxide Transducer for Field-Effect Transistor-Type H2O2 Biosensor. Anal. Chem. 2014, 86, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, C.; Jang, J. High-performance field-effect transistor-type glucose biosensor based on nanohybrids of carboxylated polypyrrole nanotube wrapped graphene sheet transducer. Sens. Actuators B Chem. 2015, 208, 532–537. [Google Scholar] [CrossRef]
- Yin, L.T.; Chou, J.C.; Chung, W.Y.; Sun, T.P.; Hsiung, K.P.; Hsiung, S.K. Glucose ENFET doped with MnO2 powder. Sens. Actuators B Chem. 2001, 76, 187–192. [Google Scholar] [CrossRef]
- Anh, D.T.V.; Olthuis, W.; Bergveld, P. Electroactive gate materials for a hydrogen peroxide sensitive eMOSFET. IEEE Sens. J. 2002, 2, 26–33. [Google Scholar] [CrossRef]
- Lin, J.J.; Wu, Y.L.; Hsu, P.Y. Novel Dry-Type Glucose Sensor Based on a Metal–Oxide–Semiconductor Capacitor Structure with Horseradish Peroxidase + Glucose Oxidase Catalyzing Layer. Jpn. J. Appl. Phys. Part 1 Regul. Pap. Short Notes Rev. Pap. 2007, 46, 6871–6874. [Google Scholar] [CrossRef]
- Ozasa, K.; Nemoto, S.; Li, Y.; Hara, M.; Maeda, M.; Mochitate, K. Contact angle and biocompatibility of sol-gel prepared TiO2 thin films for their use as semiconductor-based cell-viability sensors. Surf. Interface Anal. 2008, 40, 579–583. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, Y.D.; Vedala, H.; Allen, B.L.; Star, A. Exploring the Chemical Sensitivity of a Carbon Nanotube/Green Tea Composite. ACS Nano 2010, 4, 6854–6862. [Google Scholar] [CrossRef]
- Wu, Y.L.; Hsu, P.Y.; Lin, J.J. Polysilicon wire glucose sensor highly immune to interference. Biosens. Bioelectron. 2011, 26, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Tripathy, N.; Park, J.H.; Hahn, Y.B. A comprehensive biosensor integrated with a ZnO nanorod FET array for selective detection of glucose, cholesterol and urea. Chem. Commun. 2015, 51, 11968–11971. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.N.; Birkin, P.R.; Wang, J.H.; Palmisano, F.; De Benedetto, G. An Enzyme Switch Employing Direct Electrochemical Communication between Horseradish Peroxidase and a Poly(aniline) Film. Anal. Chem. 1998, 70, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Raffa, D.; Leung, K.T.; Battaglini, F. A Microelectrochemical Enzyme Transistor Based on an N-Alkylated Poly(Aniline) and Its Application to Determine Hydrogen Peroxide at Neutral pH. Anal. Chem. 2003, 75, 4983–4987. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, X.; Shi, Y.; Li, C.M.; Li, L.J.; Chen, P. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale 2010, 2, 1485–1488. [Google Scholar] [CrossRef]
- Kwak, Y.H.; Choi, D.S.; Kim, Y.N.; Kim, H.; Yoon, D.H.; Ahn, S.S.; Yang, J.W.; Yang, W.S.; Seo, S. Flexible glucose sensor using CVD-grown graphene-based field effect transistor. Biosens. Bioelectron. 2012, 37, 82–87. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Van Der Schoot, B.H.; Bergveld, P. ISFET based enzyme sensors. Biosensors 1987, 3, 161–186. [Google Scholar] [CrossRef]
- Lee, C.H.; Seo, H.I.; Lee, Y.C.; Cho, B.W.; Jeong, H.; Sohn, B.K. All solid type ISFET glucose sensor with fast response and high sensitivity characteristics. Sens. Actuators B Chem. 2000, 64, 37–41. [Google Scholar] [CrossRef]
- Zhang, Q.; Kaisti, M.; Prabhu, A.; Yu, Y.; Song, Y.A.; Rafailovich, M.H.; Rahman, A.; Levon, K. Polyaniline-functionalized ion-sensitive floating-gate FETs for the on-chip monitoring of peroxidase-catalyzed redox reactions. Electrochim. Acta 2018, 261, 256–264. [Google Scholar] [CrossRef]
- Zhu, Z.; Mabeck, J.T.; Zhu, C.; Cady, N.C.; Batt, A.; Malliaras, G.G. A simple PEDOT:PSS transistor for glucose sensing at neutral pH. Chem. Commun. 2004, 13, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Do, J.; Kim, J.; Yang, S.Y.; Malliaras, G.G.; Ober, C.K.; Kim, E. A Glucose Sensor Based on an Organic Electrochemical Transistor Structure Using a Vapor Polymerized Poly(3,4-ethylenedioxythiophene) Layer. Jpn. J. Appl. Phys. 2010, 49. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Yang, Y.; Liu, K.; Du, W. Highly selective and sensitive glucose sensors based on organic electrochemical transistors using TiO2 nanotube arrays-based gate electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Welch, M.E.; Doublet, T.; Bernard, C.; Malliaras, G.G.; Ober, C.K. A glucose sensor via stable immobilization of the GOx enzyme on an organic transistor using a polymer brush. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 372–377. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Epstein, A.J. The Polyanilines: A Novel Class of Conducting Polymers. MRS Proc. 1989, 173. [Google Scholar] [CrossRef]
- Ray, A.; Asturias, G.E.; Kershner, D.L.; Richter, A.F.; MacDiarmid, A.G.; Epstein, A.J. Polyaniline: Doping, structure and derivatives. Synth. Met. 1989, 29, 141–150. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Wang, J.H. Electroactivity, stability and application in an enzyme switch at pH 7 of poly(aniline)-poly(styrenesulfonate) composite films. J. Chem. Soc. Faraday Trans. 1996, 92, 4137–4143. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. Conducting Polyaniline Nanowire and Its Applications in Chemiresistive Sensing. Nanomaterials 2013, 3, 498–523. [Google Scholar] [CrossRef]
- Keiji Kanazawa, K.; Diaz, A.F.; Gill, W.D.; Grant, P.M.; Street, G.B.; Piero Gardini, G.; Kwak, J.F. Polypyrrole: An electrochemically synthesized conducting organic polymer. Synth. Met. 1980, 1, 329–336. [Google Scholar] [CrossRef]
- Lu, J.; Pinto, N.J.; MacDiarmid, A.G. Apparent dependence of conductivity of a conducting polymer on an electric field in a field effect transistor configuration. J. Appl. Phys. 2002, 92, 6033–6038. [Google Scholar] [CrossRef]
- Nilsson, B.D.; Chen, M.; Kugler, T.; Remonen, T.; Armgarth, M.; Berggren, M. Bi-stable and Dynamic Current Modulation in Electrochemical Organic Transistors. Adv. Mater. 2002, 14, 51–54. [Google Scholar] [CrossRef]
- Jung, D.-U.-J.; Ahmad, R.; Hahn, Y.B. Nonenzymatic flexible field-effect transistor based glucose sensor fabricated using NiO quantum dots modified ZnO nanorods. J. Colloid Interface Sci. 2018, 512, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, Z. Potentiometric determination of hydrogen peroxide at MnO2-doped carbon paste electrode. Talanta 2000, 50, 1157–1162. [Google Scholar] [CrossRef]
- Manso, M.; Ogueta, S.; García, P.; Pérez-Rigueiro, J.; Jiménez, C.; Martínez-Duart, J.M.; Langlet, M. Mechanical and in vitro testing of aerosol-gel deposited titania coatings for biocompatible applications. Biomaterials 2002, 23, 349–356. [Google Scholar] [CrossRef]
- Tang, H.; Yan, F.; Lin, P.; Xu, J.; Chan, H.L.W. Highly Sensitive Glucose Biosensors Based on Organic Electrochemical Transistors Using Platinum Gate Electrodes Modified with Enzyme and Nanomaterials. Adv. Funct. Mater. 2011, 21, 2264–2272. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly selective and sensitive glucose sensors based on organic electrochemical transistors with graphene-modified gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon Nanotube Field-Effect-Transistor-Based Biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X. Carbon nanomaterials field-effect-transistor-based biosensors. NPG Asia Mater. 2012, 4, 1–10. [Google Scholar] [CrossRef]
- Tran, T.T.; Mulchandani, A. Carbon nanotubes and graphene nano field-effect transistor-based biosensors. TrAC Trends Anal. Chem. 2016, 79, 222–232. [Google Scholar] [CrossRef]
- Wang, C.; Takei, K.; Takahashi, T.; Javey, A. Carbon nanotube electronics—Moving forward. Chem. Soc. Rev. 2013, 42, 2592–2609. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, G.; Narimatsu, K.; Niidome, Y.; Nakashima, N. Green Tea Solution Individually Solubilizes Single-walled Carbon Nanotubes. Chem. Lett. 2007, 36, 1140–1141. [Google Scholar] [CrossRef]
- Schulman, D.S.; Arnold, A.J.; Das, S. Contact engineering for 2D materials and devices. Chem. Soc. Rev. 2018, 47, 3037–3058. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Chen, H.Y.; Penumatcha, A.V.; Appenzeller, J. High Performance Multilayer MoS2 Transistors with Scandium Contacts. Nano Lett. 2013, 13, 100–105. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, G.; Hein, R.; Liu, N.; Luo, X.; Davis, J.J. Antifouling Strategies for Selective In Vitro and In Vivo Sensing. Chem. Rev. 2020, 120, 3852–3889. [Google Scholar] [CrossRef]

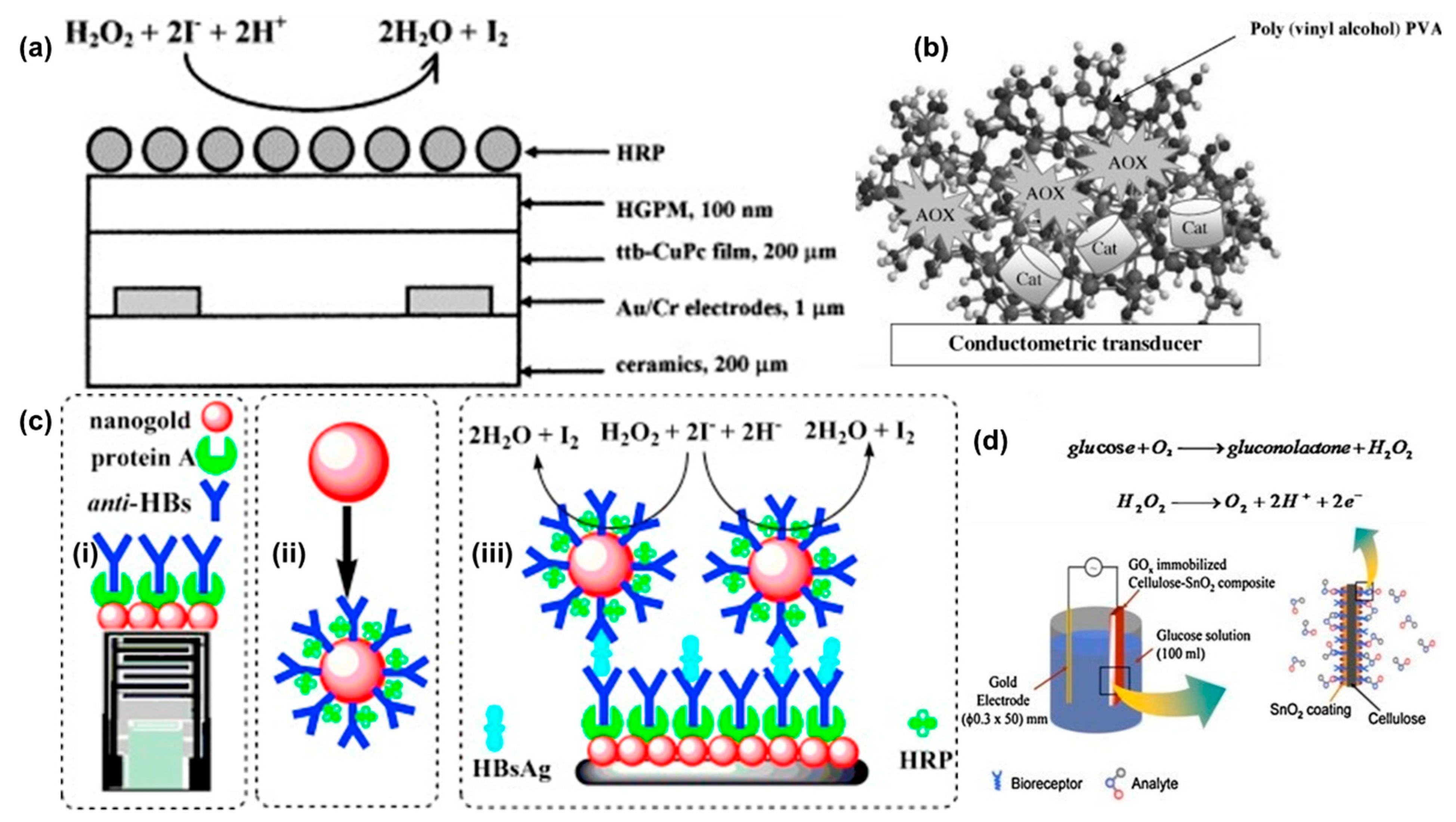
| Substrate | Target Analyte | Ligand/ Enzyme | LOD (µM) | Measuring Range (mM) | Voltage Bias (mV) (Frequency) | Response Time (Minutes) | Buffer/ Working pH | Comments | Interference Tested | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Metal interdigitated electrodes | ||||||||||
| Ceramic-Au | H2O2 | Pthalocyanine | NR | 0.005–0.3 | 60 | 10 | Working pH 6.0 Buffer: Phosphate (20 mM) | Storage stability for 90 days at 4 °C | No | [43] |
| Silicon-Au | H2O2/ Cyanide | PVA-Catalase | 6 | 0–100 | 10 (100 kHz) | 5 | Working pH 7.2 Buffer: Phosphate (5 mM) | Inhibitory assay for cyanide detection | No | [56] |
| Au | Methanol | AOX-Catalase | 0.5 | <0.075 | 10 (100 kHz) | <10 | Working pH 7.2 Buffer: Phosphate (5 mM) | Alcoholic beverages | Yes | [55] |
| Ethanol | 1 | <0.070 | ||||||||
| Propanol | 3 | <0.065 | ||||||||
| Ceramic-Au | Lactate | LOD-HRP | 0.05 | 0–0.21 | 10 (100 kHz) | ~20 (est.) | Working pH 6 Buffer: Phosphate (5 mM) | Diluted yogurt samples Storage stability for 40 days at 4 °C | Yes | [57] |
| Metal nanoparticles | ||||||||||
| AuNPs | Hepatitis B (HB) | HRP/Anti-HBs | 0.01 ng/mL | 0.1–600 ng/mL | 10 (100 kHz) | >30 | Working pH 7.0 Buffer: Phosphate (10 mM) | Tested with serum samples Assay stable for 16 days when stored at 4 °C | Yes | [58] |
| Ceramic-Au & magnetic NPs | Glucose | GOD | 3 | 0.04–3 | 10 (100 kHz) | <10 | Working pH 7.3 Buffer: Phosphate (5 mM) | Stable for 12 days when stored at 4 °C | No | [23] |
| g-C3N4 | AFP/H2O2 | Pt NPs | 0.01 ng/mL | 0.01–100 ng/mL | 10 (100 kHz) | 5–6 | Working pH 6.5 Buffer: PBS (10 mM) | Tested with human serum Inhibitory Immunoassay | Yes | [21] |
| Others | ||||||||||
| Cellulose- SnO2 | H2O2/ Glucose | GOD | 500 | 0.5–12 | 0–3 V (dc) | NR | Working pH 7.2 Buffer: Phosphate | Storage stability > 10 days Flexible one-time use sensor | No | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, V.; Kruse, P.; Selvaganapathy, P.R. Solid State Sensors for Hydrogen Peroxide Detection. Biosensors 2021, 11, 9. https://doi.org/10.3390/bios11010009
Patel V, Kruse P, Selvaganapathy PR. Solid State Sensors for Hydrogen Peroxide Detection. Biosensors. 2021; 11(1):9. https://doi.org/10.3390/bios11010009
Chicago/Turabian StylePatel, Vinay, Peter Kruse, and Ponnambalam Ravi Selvaganapathy. 2021. "Solid State Sensors for Hydrogen Peroxide Detection" Biosensors 11, no. 1: 9. https://doi.org/10.3390/bios11010009
APA StylePatel, V., Kruse, P., & Selvaganapathy, P. R. (2021). Solid State Sensors for Hydrogen Peroxide Detection. Biosensors, 11(1), 9. https://doi.org/10.3390/bios11010009







