Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus and Screen-Printed Electrodes
2.3. Preparation of CB–Prussian Blue (PB)-Modified SPEs
2.4. Preparation of TEMPO-CNC
2.5. Immobilization of Glucose Oxidase (GOx) onto TEMPO-CNC
2.6. Preparation of the TEMPO-CNC Glucose Sensor
2.7. Preparation of the Glutaraldehyde-Based Glucose Sensor (GA Sensor)
2.8. Characterization of the Modification Step of the TEMPO-CNC Glucose Sensor
2.9. Calibration of the TEMPO-CNC Glucose Sensor in Buffer Solution
2.10. Selectivity Testing of the TEMPO-CNC Glucose Sensor
2.11. Operational Stability of the TEMPO-CNC Glucose Sensor
2.12. Shelf life of the TEMPO-CNC Glucose Sensor
2.13. NIH 3T3 Cell Culture
2.14. Investigation of the Matrix Effect of Cell Culture Medium
2.15. Glucose Concentration Monitoring in Fibroblast Cultures
3. Results and Discussion
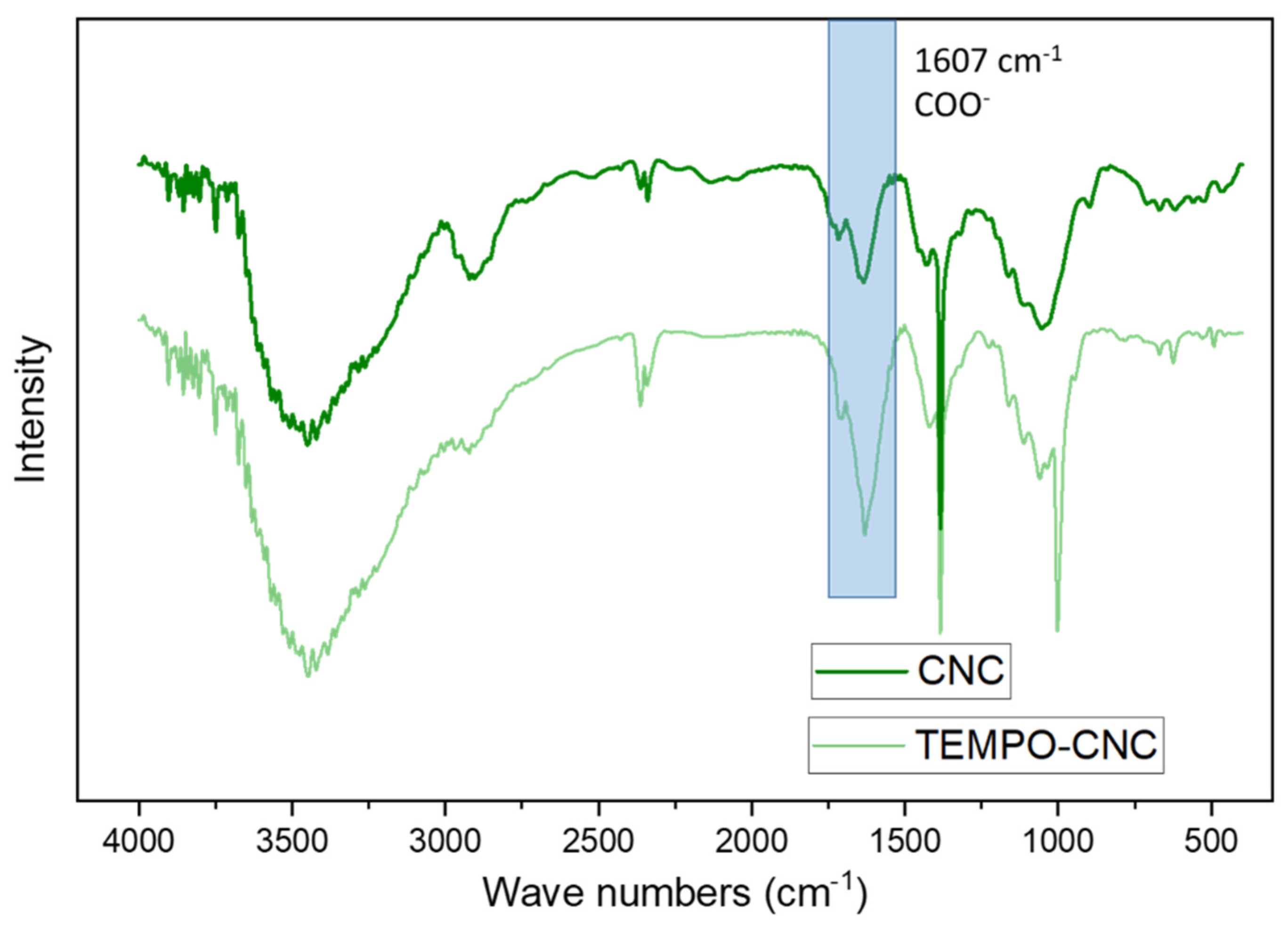
3.1. Characterization of TEMPO-CNCs with Fourier Transform Infrared Spectrometry (FTIR)
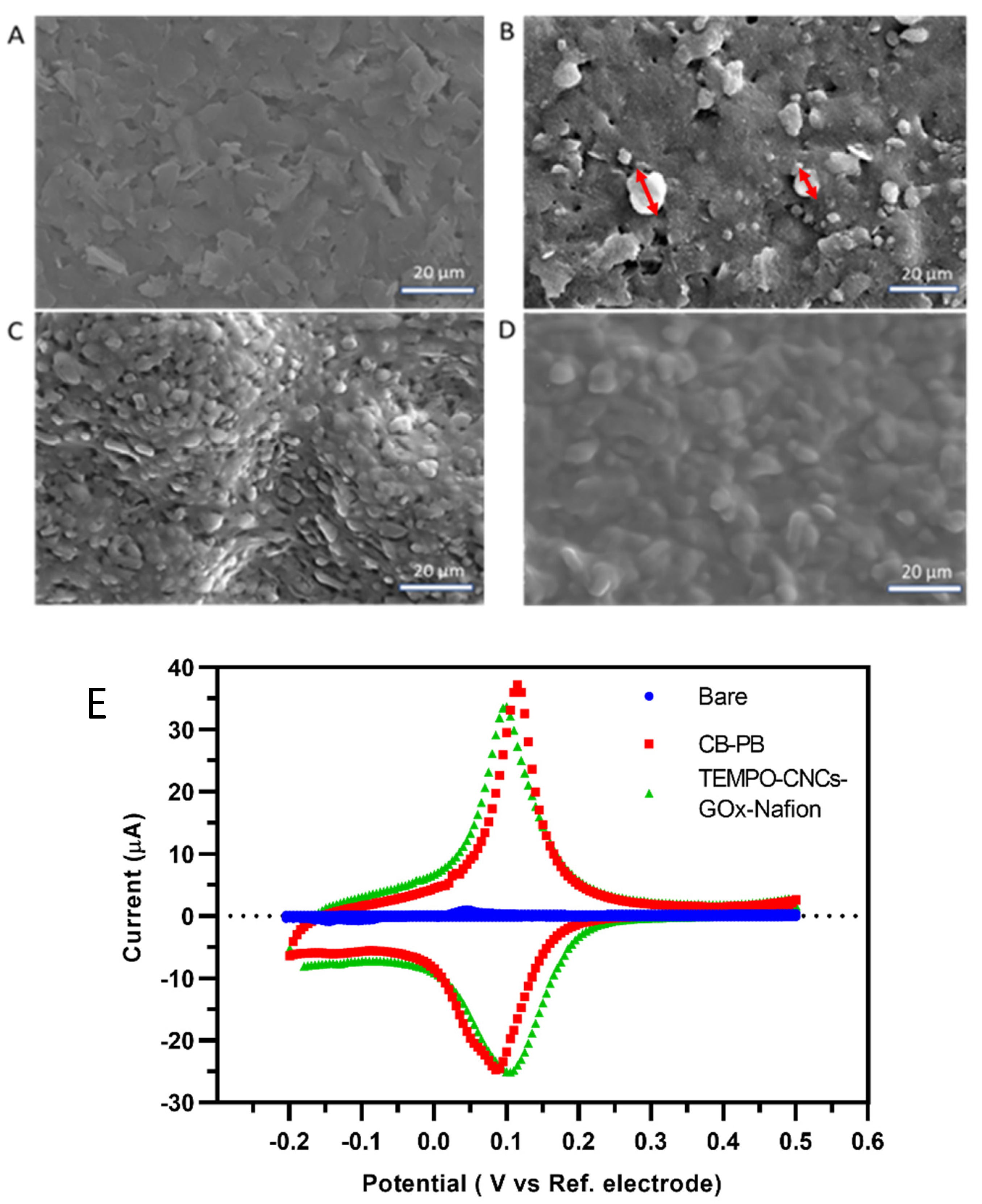
3.2. Characterization of the Sensor Functionalization
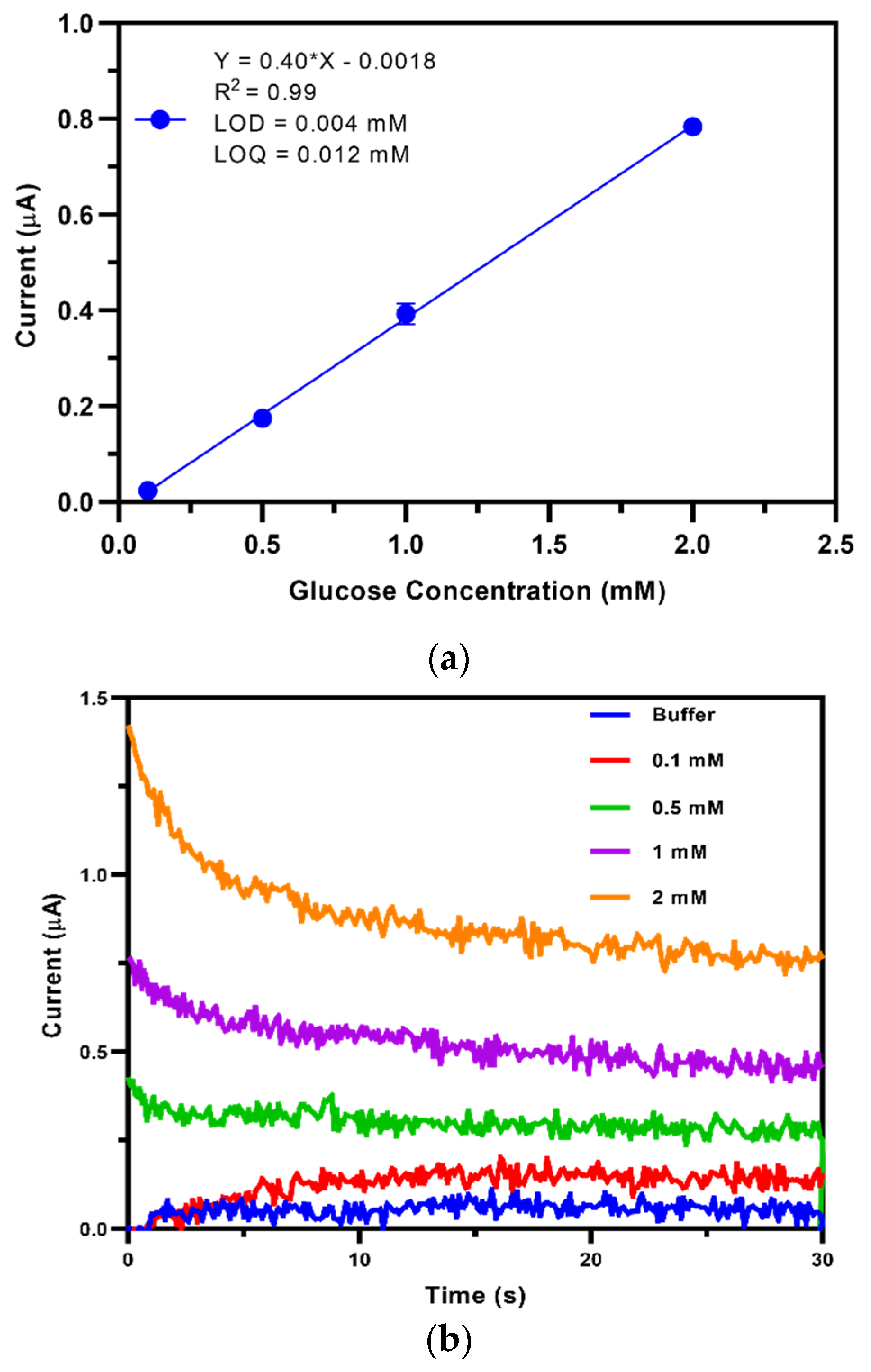
3.3. Calibration of the TEMPO-CNC Glucose Sensor in Buffer Solution
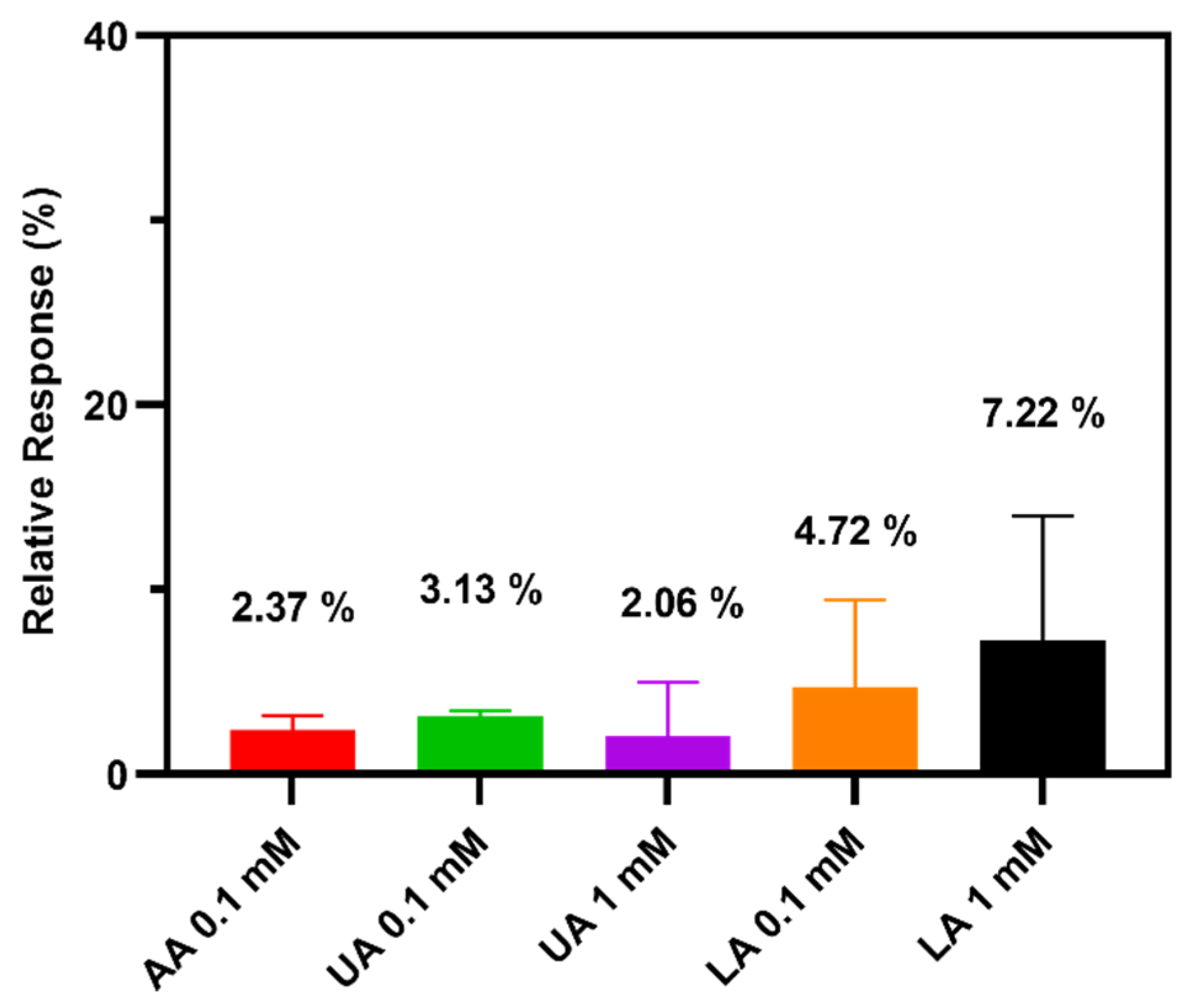
3.4. Selectivity Test of the TEMPO-CNC Glucose Sensor
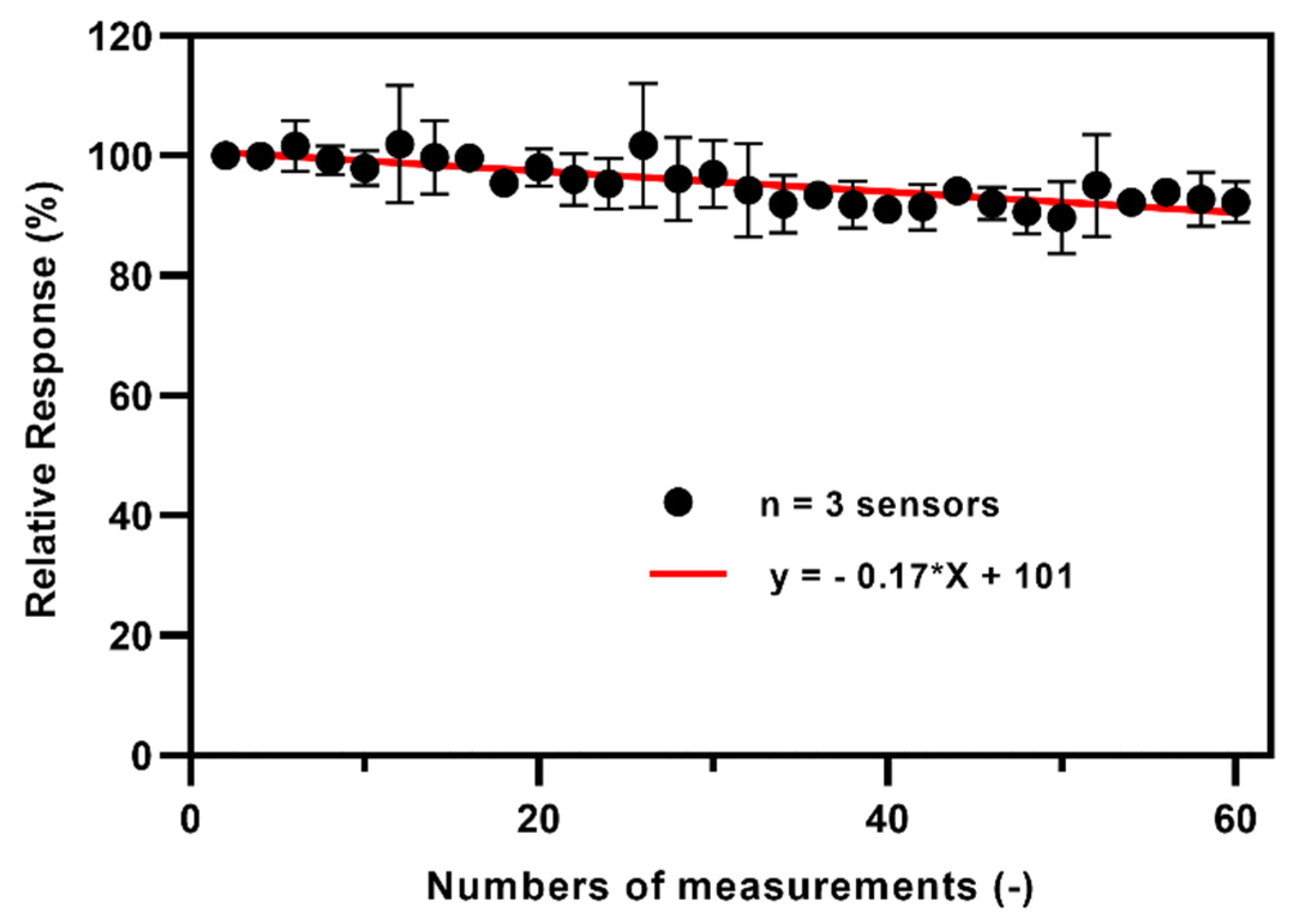
3.5. Operational Stability of the TEMPO-CNC Glucose Sensor
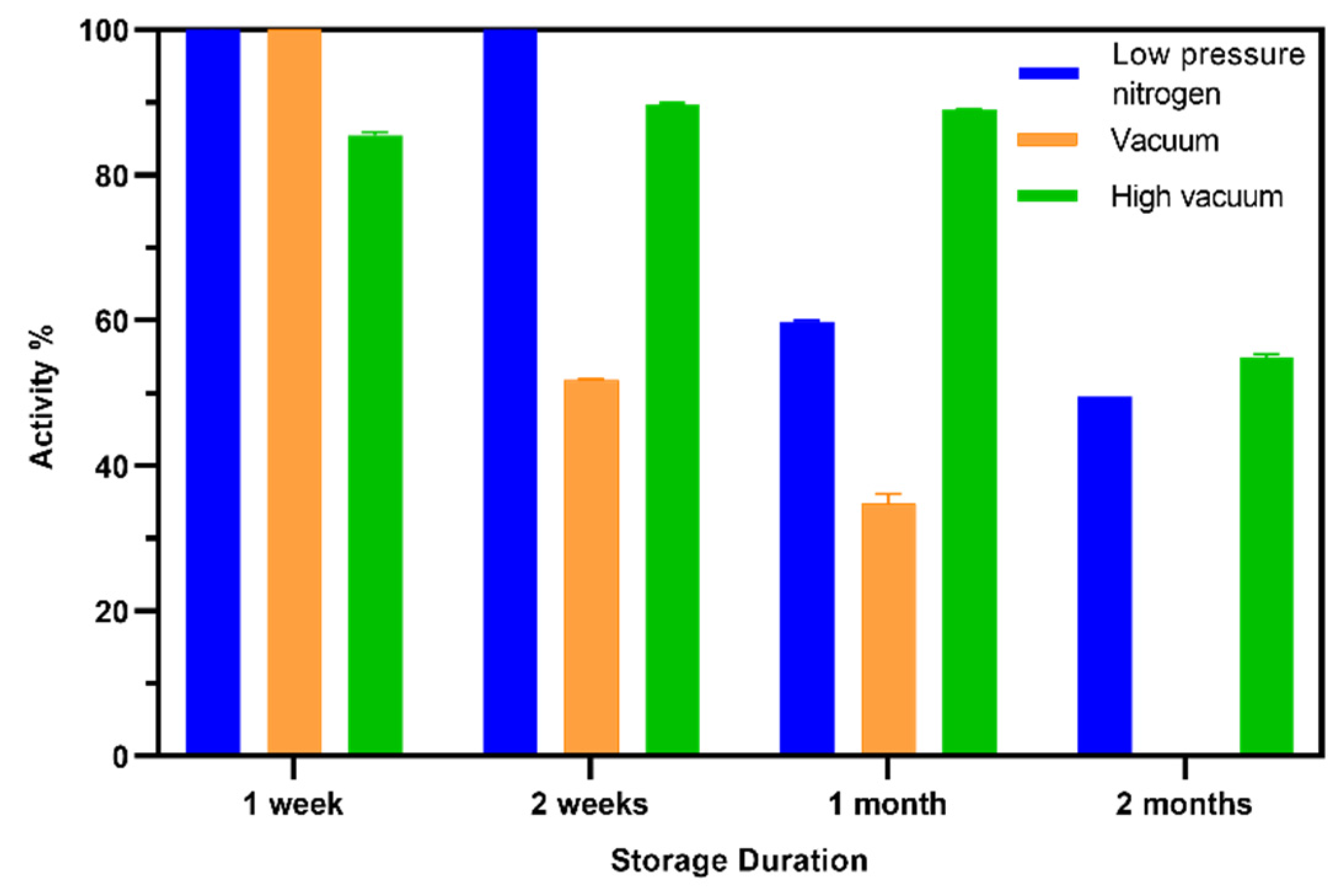
3.6. Shelf Life of the TEMPO-CNC Glucose Sensor
3.7. Comparing the Performance of the TEMPO-CNC Glucose Sensor with Those of Previously Reported Sensors
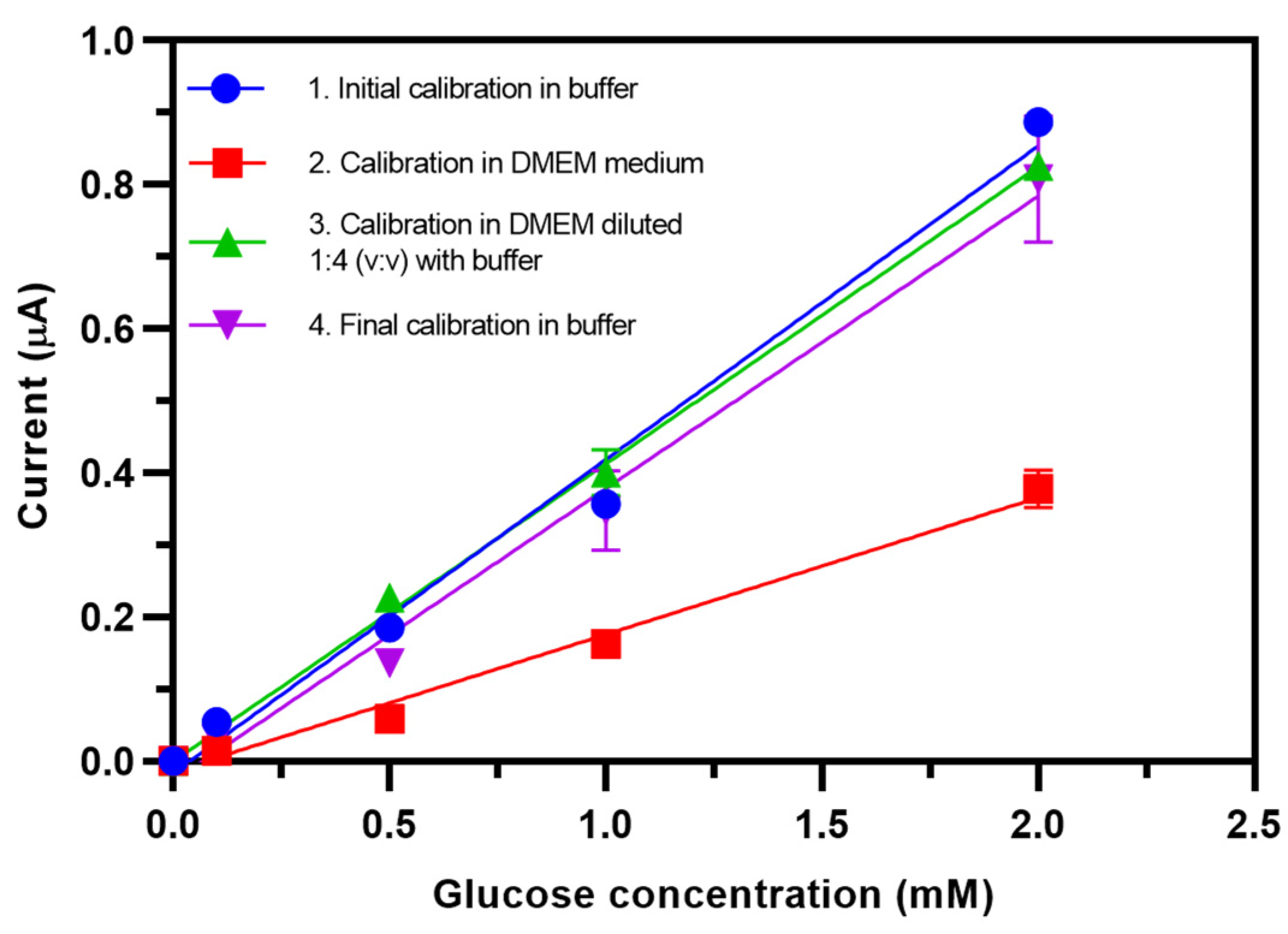
3.8. Investigation of the Matrix Effect of Cell Culture Medium
3.9. Glucose Concentration Monitoring in Fibroblast Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Gupta, S.; Sandhu, S.V.; Bansal, H.; Sharma, D. Comparison of salivary and serum glucose levels in diabetic patients. J. Diabetes Sci. Technol. 2015, 9, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Corrie, S.R.; Coffey, J.W.; Islam, J.; Markey, K.A.; Kendall, M.A.F. Blood, sweat, and tears: Developing clinically relevant protein biosensors for integrated body fluid analysis. Analyst 2015, 140, 4350–4364. [Google Scholar] [CrossRef] [PubMed]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.H.; Lee, S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors 2010, 10, 4558–4576. [Google Scholar] [CrossRef]
- Jina, A.; Tierney, M.J.; Tamada, J.A.; McGill, S.; Desai, S.; Chua, B.; Chang, A.; Christiansen, M. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 2014, 8, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, Y.; Wang, M.L. On-chip highly sensitive saliva glucose sensing using multilayer films composed of single-walled carbon nanotubes, gold nanoparticles, and glucose oxidase. Sens. Bio-Sens. Res. 2015, 4, 96–102. [Google Scholar] [CrossRef]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and limitations of non-invasive blood glucose monitoring using near-infrared spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.S.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, M.; Wang, Z.; Chen, Z.; Zhao, X.; Yuan, Y.; Li, Y.; Wang, C. Portable real-time ringdown breath acetone analyzer: Toward potential diabetic screening and management. Sensors 2016, 16, 1199. [Google Scholar] [CrossRef]
- Yao, H.; Shum, A.J.; Cowan, M.; Lähdesmäki, I.; Parviz, B.A. A contact lens with embedded sensor for monitoring tear glucose level. Biosens. Bioelectron. 2011, 26, 3290–3296. [Google Scholar] [CrossRef] [PubMed]
- Alp, B.; Mutlu, S.; Mutlu, M. Glow-discharge-treated cellulose acetate (CA) membrane for a high linearity single-layer glucose electrode in the food industry. Food Res. Int. 2000, 33, 107–112. [Google Scholar] [CrossRef]
- Boero, C.; Carrara, S.; Del Vecchio, G.; Calzà, L.; De Micheli, G. Highly Sensitive Carbon Nanotube-Based Sensing for Lactate and Glucose Monitoring in Cell Culture. IEEE Trans. Nanobiosci. 2011, 10, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Tric, M.; Lederle, M.; Neuner, L.; Dolgowjasow, I.; Wiedemann, P.; Wölfl, S.; Werner, T. Optical biosensor optimized for continuous in-line glucose monitoring in animal cell culture. Anal. Bioanal. Chem. 2017, 409, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, S.; Bones, J.; Ray, S.; Cha, S.; Karger, B.L.; Li, J.J.; Wilson, L.; Hinckle, G.; Rossomando, A. A quantitative proteomic analysis of cellular responses to high glucose media in Chinese hamster ovary cells. Biotechnol. Prog. 2015, 31, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Delgado, D.; Narváez-Cuenca, C.E.; Restrepo-Sánchez, L.P.; Kushalappa, A.; Mosquera-Vásquez, T. Development and validation of a liquid chromatographic method to quantify sucrose, glucose, and fructose in tubers of Solanum tuberosum Group Phureja. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 975, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.; Lewis, C.B.; Coté, G.L.; McNichols, R.J. Investigation of Near-Infrared Spectroscopy for Periodic Determination of Glucose in Cell Culture Media in Situ. Appl. Spectrosc. 2000, 54, 1453–1457. [Google Scholar]
- Berry, B.N.; Dobrowsky, T.M.; Timson, R.C.; Kshirsagar, R.; Ryll, T.; Wiltberger, K. Quick generation of Raman spectroscopy based in-process glucose control to influence biopharmaceutical protein product quality during mammalian cell culture. Biotechnol. Prog. 2016, 32, 224–234. [Google Scholar] [CrossRef]
- Matthews, T.E.; Smelko, J.P.; Berry, B.; Romero-Torres, S.; Hill, D.; Kshirsagar, R.; Wiltberger, K. Glucose monitoring and adaptive feeding of mammalian cell culture in the presence of strong autofluorescence by near infrared Raman spectroscopy. Biotechnol. Prog. 2018, 34, 1574–1580. [Google Scholar] [CrossRef]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Hariri, M.A.; Lee, Y.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Harris, J.M.; Reyes, C.; Lopez, G.P. Common causes of glucose oxidase instability in in vivo biosensing: A brief review. J. Diabetes Sci. Technol. 2013, 7, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.; Correa, S.; Betancor, L. Cellulose-Based Nanosupports for Enzyme Immobilization. In Cellulose-Based Superabsorbent Hydrogels; Springer International Publishing: Basel, Switzerland, 2019; pp. 1235–1253. [Google Scholar]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Mohaiyiddin, M.S.; Ong, H.L.; Othman, M.B.H.; Julkapli, N.M.; Villagracia, A.R.C.; Akil, H.M. Swelling behavior and chemical stability of chitosan/nanocellulose biocomposites. Polym. Compos. 2018, 39, E561–E572. [Google Scholar] [CrossRef]
- Sulaiman, S.; Mokhtar, M.N.; Naim, M.N.; Baharuddin, A.S.; Sulaiman, A. A Review: Potential Usage of Cellulose Nanofibers (CNF) for Enzyme Immobilization via Covalent Interactions. Appl. Biochem. Biotechnol. 2014, 175, 1817–1842. [Google Scholar] [CrossRef] [PubMed]
- Schyrr, B.; Pasche, S.; Voirin, G.; Weder, C.; Simon, Y.C.; Foster, E.J. Biosensors based on porous cellulose nanocrystal–poly (vinyl alcohol) scaffolds. ACS Appl. Mater. Interfaces 2014, 6, 12674–12683. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, R.; Siqueira, G.; Schubert, M.; Tingaut, P.; Maniura-Weber, K.; Zimmermann, T.; Thöny-Meyer, L.; Faccio, G.; Ihssen, J. TEMPO-Oxidized Nanofibrillated Cellulose as a High Density Carrier for Bioactive Molecules. Biomacromolecules 2015, 16, 3640–3650. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Lapasin, R.; Grassi, M.; Zanconati, F.; Chiarappa, G.; Forte, G.; Perrone, F.; Dapas, B.; Halib, N.; Fiotti, N.; Grassi, G.; et al. Potential Applications of Nanocellulose-Containing Materials in the Biomedical Field. Materials 2017, 10, 977. [Google Scholar] [CrossRef]
- Golmohammadi, H.; Morales-Narváez, E.; Naghdi, T.; Merkoçi, A. Nanocellulose in Sensing and Biosensing. Chem. Mater. 2017, 29, 5426–5446. [Google Scholar] [CrossRef]
- Shin, S.R.; Kilic, T.; Zhang, Y.S.; Avci, H.; Hu, N.; Kim, D.; Branco, C.; Aleman, J.; Massa, S.; Silvestri, A.; et al. Label-Free and Regenerative Electrochemical Microfluidic Biosensors for Continual Monitoring of Cell Secretomes. Adv. Sci. 2017, 4, 1–14. [Google Scholar] [CrossRef]
- Esmaeili, C.; Abdi, M.M.; Mathew, A.P.; Jonoobi, M.; Oksman, K.; Rezayi, M. Synergy effect of nanocrystalline cellulose for the biosensing detection of glucose. Sensors 2015, 15, 24681–24697. [Google Scholar] [CrossRef] [PubMed]
- Elina Faradilla Wan Khalid, W.; Yook Heng, L.; Nasir Mat Arip, M. Surface Modification of Cellulose Nanomaterial for Urea Biosensor Application. Sains Malaysiana 2018, 47, 941–949. [Google Scholar] [CrossRef]
- Yang, X.; Johansson, G.; Gorton, L. A glucose sensor made by chemically crosslinking glucose oxidase directly on the surface of a carbon electrode modified with Pd/Au forhydrogen peroxide electrocatalysis. Mikrochim. Acta 1989, 97, 9–16. [Google Scholar] [CrossRef]
- Miao, Y.; Chia, L.S.; Goh, N.K.; Tan, S.N. Amperometric Glucose Biosensor Based on Immobilization of Glucose Oxidase in Chitosan Matrix Cross-Linked with Glutaraldehyde. Electroanalysis 2001, 13, 347–349. [Google Scholar] [CrossRef]
- Qi, W.; Yan, X.; Duan, L.; Cui, Y.; Yang, Y.; Li, J. Glucose-sensitive microcapsules from glutaraldehyde cross-linked hemoglobin and glucose oxidase. Biomacromolecules 2009, 10, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Piermarini, S.; Migliorelli, D.; Volpe, G.; Massoud, R.; Pierantozzi, A.; Cortese, C.; Palleschi, G. Uricase biosensor based on a screen-printed electrode modified with Prussian blue for detection of uric acid in human blood serum. Sensors Actuators B Chem. 2013, 179, 170–174. [Google Scholar] [CrossRef]
- Mohammad, A.; Yang, Y.; Khan, M.A.; Faustino, P.J. Long-term stability study of Prussian blue—A quality assessment of water content and cyanide release. Clin. Toxicol. 2015, 53, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose nanofibers prepared by TEMPO-mediated oxidation of native cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Orelma, H.; Filpponen, I.; Johansson, L.-S.; Österberg, M.; Rojas, O.J.; Laine, J. Surface Functionalized Nanofibrillar Cellulose (NFC) Film as a Platform for Immunoassays and Diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef]
- Florescu, M.; Brett, C.M.A. Development and evaluation of electrochemical glucose enzyme biosensors based on carbon film electrodes. Talanta 2005, 65, 306–312. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian Blue-Based First-Generation Biosensor. A Sensitive Amperometric Electrode for Glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shaded, E.; Levine, M. Pharamacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissuse. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Huff, R.D.; Hsu, A.C.-Y.; Nichol, K.S.; Jones, B.; Knight, D.A.; Wark, P.A.B.; Hansbro, P.M.; Hirota, J.A. Regulation of xanthine dehydrogensase gene expression and uric acid production in human airway epithelial cells. PLoS ONE 2017, 12, e0184260. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Repo, E.; Song, Y.; Yin, D.; Hammouda, S.B.; Chen, L.; Kalliola, S.; Tang, J.; Tam, K.C.; Sillanpää, M. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green Chem. 2017, 19, 4816–4828. [Google Scholar] [CrossRef]
- Chu, N.; Wang, J.; Zhang, Y.; Yang, J.; Lu, J.; Yin, D. Nestlike hollow hierarchical MCM-22 microspheres: Synthesis and exceptional catalytic properties. Chem. Mater. 2010, 22, 2757–2763. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Vellucci, G.; Cacciotti, I.; Nanni, F.; Moscone, D. Carbon black assisted tailoring of Prussian Blue nanoparticles to tune sensitivity and detection limit towards H2O2 by using screen-printed electrode. Electrochem. Commun. 2014, 47, 63–66. [Google Scholar] [CrossRef]
- Snyder, S.L.; McAuley, K.B.; McLellan, P.J.; Brouwer, E.B.; McCaw, T. Modeling the thermal stability of enzyme-based in vitro diagnostics biosensors. Sensors Actuators B Chem. 2011, 156, 621–630. [Google Scholar] [CrossRef]
- Hwa, K.Y.; Subramani, B. Synthesis of zinc oxide nanoparticles on graphene-carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014, 62, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Tian, R.; Yan, W.; Yao, C. Glucose biosensor based on three dimensional ordered macroporous self-doped polyaniline/Prussian blue bicomponent film. Anal. Chim. Acta 2012, 723, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Wustoni, S.; Pappa, A.M.; Salama, K.N.; Baran, D.; Inal, S. A fully inkjet-printed disposable glucose sensor on paper. Npj Flex. Electron. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Chandra Sekar, N.; Mousavi Shaegh, S.A.; Ng, S.H.; Ge, L.; Tan, S.N. A paper-based amperometric glucose biosensor developed with Prussian Blue-modified screen-printed electrodes. Sens. Actuators B Chem. 2014, 204, 414–420. [Google Scholar] [CrossRef]


| Modification of Electrode | Detection Method | Linear Range [mM] | LOD [mM] | Shelf Life (Remaining Activity) | Storage Conditions | Reference |
|---|---|---|---|---|---|---|
| TEMPO-CNC (SPE) | Amperometric | 0.1–2.0 | 0.004 | 90 % after (2 weeks) 90 % (1 month) 60 % (2 months) | 4 °C under dry high-vacuum conditions | this work |
| GA sensor (SPE) | Amperometric | 0.1–2.0 | 0.004 | 70 % (2 weeks) <10 % (1 month) <10 % (2 months) | 4 °C under low-pressure nitrogen | this work |
| GR–CNT–ZnO (GCE) | Amperometric | 0.01–6.5 | 4.5 × 10−3 | 94.6 % (1 month) | 4 °C | [51] |
| CNCs/PPY (SPE) | DPV | 1.0–20 | 0.05 | 95 % (2.4 weeks) | 4 °C under dry high vacuum conditions | [32] |
| PANI/PB (SPE) | Amperometric | 0.002–1.6 | 0.4 × 10−3 | 95 % (2 weeks) | 4 °C | [52] |
| PEDOT:PSS (IPE) | Amperometric | 0.02-1.0 | N/A | 80 % (1 month) | 4 °C | [53] |
| Paper disk (SPE) | Amperometric | 0.25–2.0 | N/A | 72 % (1.5 months) | 4 °C | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Petropoulos, K.; Kurth, F.; Gao, H.; Migliorelli, D.; Guenat, O.; Generelli, S. Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring. Biosensors 2020, 10, 125. https://doi.org/10.3390/bios10090125
Tang Y, Petropoulos K, Kurth F, Gao H, Migliorelli D, Guenat O, Generelli S. Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring. Biosensors. 2020; 10(9):125. https://doi.org/10.3390/bios10090125
Chicago/Turabian StyleTang, Ye, Konstantinos Petropoulos, Felix Kurth, Hui Gao, Davide Migliorelli, Olivier Guenat, and Silvia Generelli. 2020. "Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring" Biosensors 10, no. 9: 125. https://doi.org/10.3390/bios10090125
APA StyleTang, Y., Petropoulos, K., Kurth, F., Gao, H., Migliorelli, D., Guenat, O., & Generelli, S. (2020). Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring. Biosensors, 10(9), 125. https://doi.org/10.3390/bios10090125





