Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement
Abstract
1. Introduction
2. Experimental Section
2.1. Microorganisms and Growth Conditions
2.2. Electrode Fabrication and Electrochemical Characterisation
2.3. Legionella Pneumophila Concentration and Antibody Reaction
2.4. Electrochemical Measurements for Legionella Detection
3. Results and Discussion
3.1. Electrode and Redox Substrate Characterisation
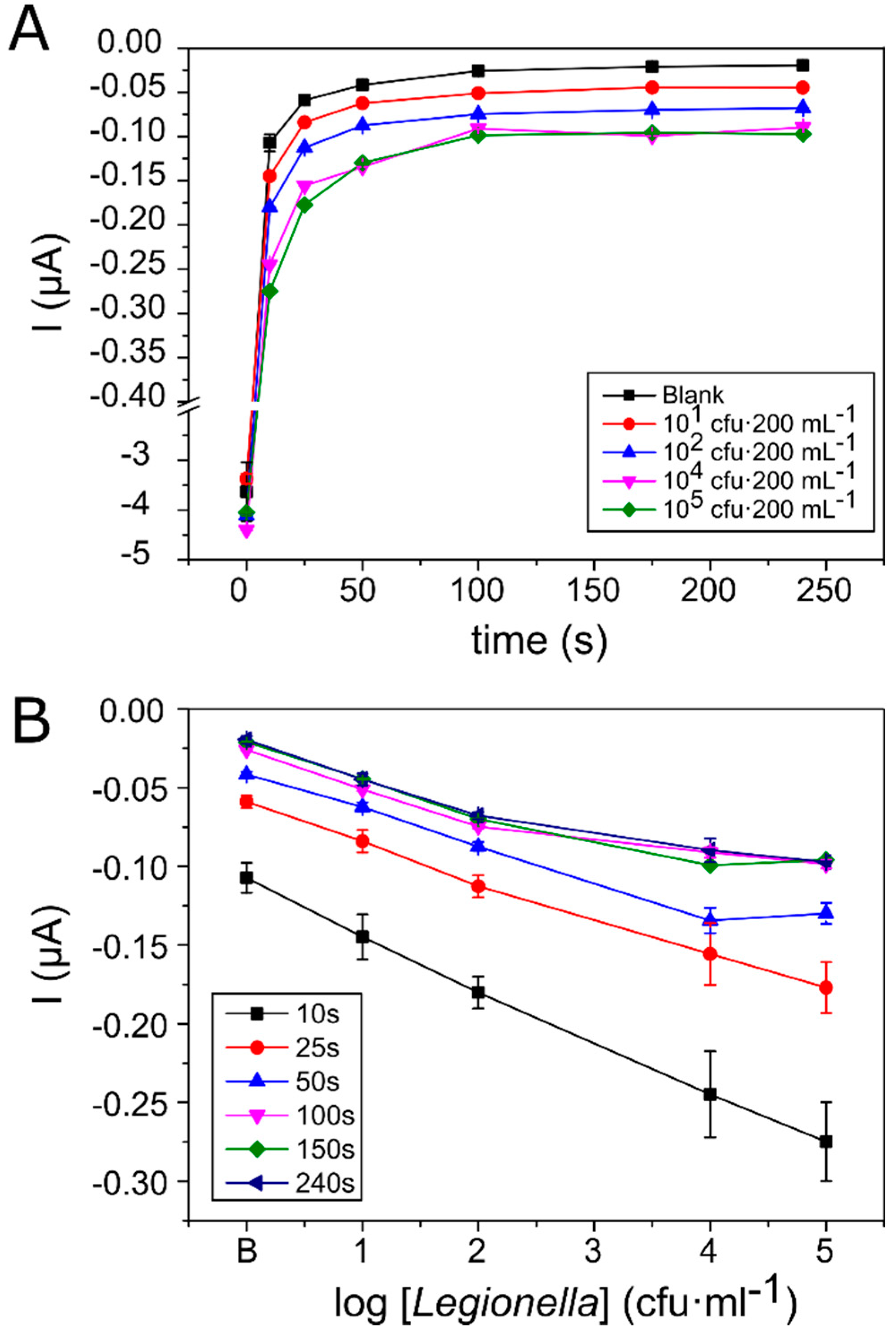
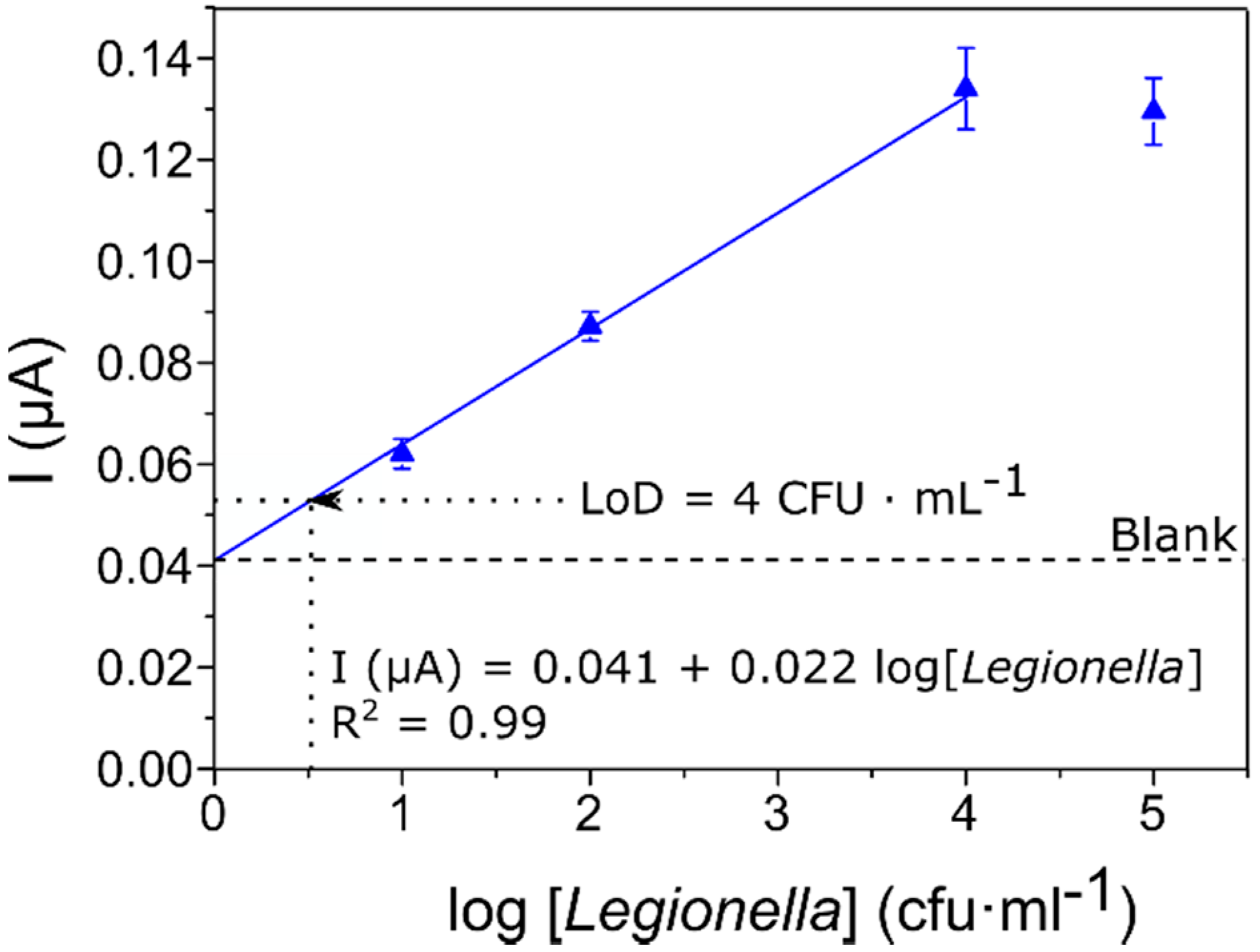
3.2. Calibration Curve for the Detection and Quantification of Legionella
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- McDade, J.E. Legionella and the Prevention of Legionellosis. Emerg. Infect. Dis. 2009, 14, 1006. [Google Scholar] [CrossRef]
- Yoshida, M.; Furuya, N.; Hosokawa, N.; Kanamori, H.; Kaku, M.; Koide, M.; Higa, F.; Fujita, J. Legionella pneumophila contamination of hospital dishwashers. Am. J. Infect. Control 2018, 46, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Flanders, W.D.; Kirkland, K.H.; Shelton, B.G. Effects of holding time and measurement error on culturing Legionella in environmental water samples. Water Res. 2014, 62, 293–301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Atlas, R.M. Legionella: From environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1999, 1, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Whiley, H. Legionella risk management and control in potable water systems: Argument for the abolishment of routine testing. Int. J. Environ. Res. Public Health 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, C.; Rosenwinkel, K.H.; Exner, M.; Verstraete, W.; Suchenwirth, R.; Hartemann, P.; Nogueira, R. Legionella occurrence in municipal and industrial wastewater treatment plants and risks of reclaimed wastewater reuse: Review. Water Res. 2019, 149, 21–34. [Google Scholar] [CrossRef]
- Tronel, H.; Hartemann, P. Overview of diagnostic and detection methods for legionellosis and Legionella spp. Lett. Appl. Microbiol. 2009, 48, 653–656. [Google Scholar] [CrossRef]
- Jubete, E.; Loaiza, O.A.; Ochoteco, E.; Pomposo, J.A.; Grande, H.; Rodríguez, J. Nanotechnology: A Tool for Improved Performance on Electrochemical Screen-Printed (Bio)Sensors. J. Sens. 2009, 2009, 842575. [Google Scholar] [CrossRef]
- Torre, I.; Alfano, R.; Borriello, T.; De Giglio, O.; Iervolino, C.; Montagna, M.T.; Scamardo, M.S.; Pennino, F. Environmental surveillance and in vitro activity of antimicrobial agents against Legionella pneumophila isolated from hospital water systems in Campania, South Italy: A 5-year study. Environ. Res. 2018, 164, 574–579. [Google Scholar] [CrossRef]
- Sboui, D.; Souiri, M.; Reynaud, S.; Palle, S.; Ismail, M.B.; Epalle, T.; Mzoughi, R.; Girardot, F.; Allegra, S.; Riffard, S.; et al. Characterisation of electrochemical immunosensor for detection of viable not-culturable forms of Legionellla pneumophila in water samples. Chem. Pap. 2015, 69, 1402–1410. [Google Scholar] [CrossRef]
- Petrisek, R.; Hall, J. Evaluation of a most probable number method for the enumeration of Legionella pneumophila from North American potable and nonpotable water samples. J. Water Health 2018, 16, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Jaber, L.; Amro, M.; Tair, H.A.; Bahader, S.A.; Alalam, H.; Butmeh, S.; Hilal, D.A.; Brettar, I.; Höfle, M.G.; Bitar, D.M. Comparison of in situ sequence type analysis of Legionella pneumophila in respiratory tract secretions and environmental samples of a hospital in East Jerusalem. Epidemiol. Infect. 2018, 146, 2116–2121. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Párraga-Niño, N.; Quero, S.; Ventós-Alfonso, A.; Uria, N.; Castillo-Fernandez, O.; Ezenarro, J.J.; Muñoz, F.X.; Garcia-Nuñez, M.; Sabrià, M. New system for the detection of Legionella pneumophila in water samples. Talanta 2018, 189, 324–331. [Google Scholar] [CrossRef]
- Rapid Self-Test for Legionella—LegionellaFast. Available online: https://legionellacontrol.com/rapid-self-test-for-legionella-legionellafast/ (accessed on 11 March 2019).
- Albalat, G.R.; Broch, B.B.; Bono, M.J. Method Modification of the Legipid® Legionella Fast Detection Test Kit. J. AOAC Int. 2014, 97, 1403–1409. [Google Scholar] [CrossRef][Green Version]
- Ramírez-Castillo, F.; Loera-Muro, A.; Jacques, M.; Garneau, P.; Avelar-González, F.; Harel, J.; Guerrero-Barrera, A. Waterborne Pathogens: Detection Methods and Challenges. Pathogens 2015, 4, 307–334. [Google Scholar] [CrossRef]
- Bridle, H.; Desmulliez, M. Biosensors for the Detection of Waterborne Pathogens. In Waterborne Pathogens: Detection Methods and Applications; Academic Press: Amsterdam, The Netherlands, 2013; pp. 189–229. ISBN 9780444595430. [Google Scholar]
- Connelly, J.T.; Baeumner, A.J. Biosensors for the detection of waterborne pathogens. Anal. Bioanal. Chem. 2012, 402, 117–127. [Google Scholar] [CrossRef]
- EC. Council Directive 2009/54/EC of 18 June 2009 on the exploitation and marketing of natural mineral waters. Off. J. Eur. Union 2009, 164, 45–58. [Google Scholar]
- EC. Council Directive 2006/7/EC of 15 February 2006 concerning the man- agement of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 64, 37–51. [Google Scholar]
- EC. Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. 1998, 330, 32–53. [Google Scholar]
- Turner, A.P.F. Principles of Bacterial Detection: Biosensors, Recognition Receptors and Microsystems; Zourob, M., Elwary, S., Turner, A.P.F., Eds.; Springer: New York, NY, USA, 2008. [Google Scholar]
- Zhang, Y.; Yan, C.; Yang, H.; Yu, J.; Wei, H. Rapid and selective detection of E. coli O157:H7 combining phagomagnetic separation with enzymatic colorimetry. Food Chem. 2017, 234, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zheng, L.; Zhang, H.; Jin, X.; Lin, J. An ultrasensitive fluorescent biosensor using high gradient magnetic separation and quantum dots for fast detection of foodborne pathogenic bacteria. Sens. Actuators B Chem. 2018, 265, 318–325. [Google Scholar] [CrossRef]
- Clark, K.D.; Purslow, J.A.; Pierson, S.A.; Nacham, O.; Anderson, J.L. Rapid preconcentration of viable bacteria using magnetic ionic liquids for PCR amplification and culture-based diagnostics. Anal. Bioanal. Chem. 2017, 409, 4983–4991. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kwon, D.; Chung, B.; Jung, G.Y.; Au, A.; Folch, A.; Jeon, S. Ultrarapid detection of pathogenic bacteria using a 3D immunomagnetic flow assay. Anal. Chem. 2014, 86, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Wiederoder, M.S.; Smith, S.; Madzivhandila, P.; Mager, D.; Moodley, K.; DeVoe, D.L.; Land, K.J. Novel functionalities of hybrid paper-polymer centrifugal devices for assay performance enhancement. Biomicrofluidics 2017, 11, 054101. [Google Scholar] [CrossRef]
- Wu, X.; Huang, X.; Zhu, Y.; Li, J.; Hoffmann, M.R. Synthesis and application of superabsorbent polymer microspheres for rapid concentration and quantification of microbial pathogens in ambient water. Sep. Purif. Technol. 2020, 239, 116540. [Google Scholar] [CrossRef]
- Martín, M.; Salazar, P.; Jiménez, C.; Lecuona, M.; Ramos, M.J.; Ode, J.; Alcoba, J.; Roche, R.; Villalonga, R.; Campuzano, S.; et al. Rapid Legionella pneumophila determination based on a disposable core–shell Fe3O4@poly(dopamine) magnetic nanoparticles immunoplatform. Anal. Chim. Acta 2015, 887, 51–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Guo, T.; Hong, L. An automated bacterial concentration and recovery system for pre-enrichment required in rapid Escherichia coli detection. Sci. Rep. 2018, 8, 17808. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth, J.V.; Hirst, N.A.; Millner, P.A. Biosensors for whole-cell bacterial detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Párraga-Niño, N.; Quero, S.; Uria, N.; Castillo-Fernandez, O.; Jimenez-Ezenarro, J.; Muñoz, F.X.; Sabrià, M.; Garcia-Nuñez, M. Antibody test for Legionella pneumophila detection. Diagn. Microbiol. Infect. Dis. 2018, 90, 85–89. [Google Scholar] [CrossRef]
- Ezenarro, J.J.; Uria, N.; Castillo-Fernández, Ó.; Párraga, N.; Sabrià, M.; Muñoz Pascual, F.X. Development of an integrated method of concentration and immunodetection of bacteria. Anal. Bioanal. Chem. 2018, 410, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Amperometric detection in TMB/HRP-based assays. In Analytical and Bioanalytical Chemistry; Springer Natuer: Cham, Switzeland, 2005; Volume 382, pp. 297–302. [Google Scholar]
- Marquez, L.A.; Dunford, H.B. Mechanism of the oxidation of 3,5,3′,5′-tetramethylbenzidine by myeloperoxidase determined by transient- and steady-state kinetics. Biochemistry 1997, 36, 9349–9355. [Google Scholar] [CrossRef] [PubMed]
- Baldrich, E.; del Campo, F.J.; Muñoz, F.X. Biosensing at disk microelectrode arrays. Inter-electrode functionalisation allows formatting into miniaturised sensing platforms of enhanced sensitivity. Biosens. Bioelectron. 2009, 25, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Superiore, I.; Elena, V.R.; Volpe, G.; Draisci, R.; Palleschi, G.; Compagnone, D. 3,3′,5,5′-Tetramethylbenzidine as electrochemical substrate for horseradish peroxidase based enzyme immunoassays. A comparative study. Analyst 1998, 123, 1303–1307. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Y.; Chen, Y.; Xie, Q.; Yao, S. EQCM and in situ FTIR spectroelectrochemistry study on the electrochemical oxidation of TMB and the effect of large-sized anions. J. Electroanal. Chem. 2008, 622, 184–192. [Google Scholar] [CrossRef]
- Kim, S.D.; Chung, J.W.; Kim, J.T.; Krause, H.; Pyun, J.C. Gold-film array-electrode for electrochemical ELISA. In Sensors and Actuators, B: Chemical; Elsevier: Amsterdam, The Netherlands, 2005; Volume 111–112, pp. 463–469. [Google Scholar]
- ISO 11731:2017(en). Water Quality—Enumeration of Legionella. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11731:ed-2:v1:en (accessed on 4 September 2019).
- World Health Organization. Legionella and the Prevention of Legionellosis; World Health Organization Press: Geneva, Switzerland, 2007; ISBN 9241562978. [Google Scholar]
- BOE. Documento BOE-A-2003-14408. Available online: https://www.boe.es/buscar/doc.php?id=BOE-A-2003-14408 (accessed on 4 September 2019).
- ISO. ISO/TS 12869:2019—Water Quality—Detection and Quantification of Legionella spp. and/or Legionella pneumophila by Concentration and Genic Amplification by Quantitative Polymerase Chain Reaction (qPCR). Available online: https://www.iso.org/standard/70756.html (accessed on 4 September 2019).
- BOE-A-2003-14408. Real Decreto 865/2003, de 4 de Julio, por el Que se Establecen los Criterios Higiénico-Sanitarios para la Prevención y Control de la Legionelosis. In Boletín Oficial del Estado; BOE: Madrid, Spain, 2009. (In Spanish)
- Rainbow, J.; Sedlackova, E.; Jiang, S.; Maxted, G.; Moschou, D.; Richtera, L.; Estrela, P. Integrated electrochemical biosensors for detection of waterborne pathogens in low-resource settings. Biosensors 2020, 10, 36. [Google Scholar] [CrossRef]
- Moschou, D.; Vourdas, N.; Filippidou, M.K.; Tsouti, V.; Kokkoris, G.; Tsekenis, G.; Zergioti, I.; Chatzandroulis, S.; Tserepi, A. Integrated biochip for PCR-based DNA amplification and detection on capacitive biosensors. In Bio-MEMS and Medical Microdevices; SPIE: Bellingham, WA, USA, 2013; Volume 8765, p. 87650L. [Google Scholar]
- Ma, Y.D.; Li, K.H.; Chen, Y.H.; Lee, Y.M.; Chou, S.T.; Lai, Y.Y.; Huang, P.C.; Ma, H.P.; Lee, G.B. A sample-to-answer, portable platform for rapid detection of pathogens with a smartphone interface. Lab Chip 2019, 19, 3804–3814. [Google Scholar] [CrossRef]
- Rompré, A.; Servais, P.; Baudart, J.; De-Roubin, M.-R.; Laurent, P. Detection and enumeration of coliforms in drinking water: Current methods and emerging approaches. J. Microbiol. Methods 2002, 49, 31–54. [Google Scholar] [CrossRef]
- Lazcka, O.; Del Campo, F.J.; Muñoz, F.X. Pathogen detection: A perspective of traditional methods and biosensors. Biosens. Bioelectron. 2007, 22, 1205–1217. [Google Scholar] [CrossRef]
- Shah, J.; Chemburu, S.; Wilkins, E.; Abdel-Hamid, I. Rapid Amperometric Immunoassay for Escherichia coli Based on Graphite Coated Nylon Membranes. Electroanalysis 2003, 15, 1809–1814. [Google Scholar] [CrossRef]
- Miranda-Castro, R.; De-Los-Santos-Álvarez, P.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Hairpin-DNA probe for enzyme-amplified electrochemical detection of Legionella pneumophila. Anal. Chem. 2007, 79, 4050–4055. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Nyine, Y.T.; Hapuarachchi, H.C.; Yap, H.M.; Ng, L.C.; Toh, C.S. Electrochemically amplified molecular beacon biosensor for ultrasensitive DNA sequence-specific detection of Legionella sp. Biosens. Bioelectron. 2012, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Deng, J.; Toh, C.S. Electrochemical nanoporous alumina membrane-based label-free DNA biosensor for the detection of Legionella sp. Talanta 2012, 98, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A.M.; Brassard, D.; Tabrizian, M.; Veres, T. Rapid and multiplex detection of Legionella’s RNA using digital microfluidics. Lab Chip 2015, 15, 1609–1618. [Google Scholar] [CrossRef] [PubMed]
- Foudeh, A.M.; Daoud, J.T.; Faucher, S.P.; Veres, T.; Tabrizian, M. Sub-femtomole detection of 16s rRNA from Legionella pneumophila using surface plasmon resonance imaging. Biosens. Bioelectron. 2014, 52, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Olabarria, G.; Eletxigerra, U.; Rodriguez, I.; Bilbao, A.; Berganza, J.; Merino, S. Highly sensitive and fast Legionella spp. in situ detection based on a loop mediated isothermal amplification technique combined to an electrochemical transduction system. Talanta 2020, 217, 121061. [Google Scholar] [CrossRef]
- Mobed, A.; Hasanzadeh, M.; Agazadeh, M.; Mokhtarzadeh, A.; Rezaee, M.A.; Sadeghi, J. Bioassays: The best alternative for conventional methods in detection of Legionella pneumophila. Int. J. Biol. Macromol. 2019, 121, 1295–1307. [Google Scholar] [CrossRef]
- Lei, K.F.; Leung, P.H.M. Microelectrode array biosensor for the detection of Legionella pneumophila. Microelectron. Eng. 2012, 91, 174–177. [Google Scholar] [CrossRef]
- Meneghello, A.; Sonato, A.; Ruffato, G.; Zacco, G.; Romanato, F. A novel high sensitive surface plasmon resonance Legionella pneumophila sensing platform. Sens. Actuators B Chem. 2017, 250, 351–355. [Google Scholar] [CrossRef]
- Oh, B.K.; Kim, Y.K.; Lee, W.; Bae, Y.M.; Lee, W.H.; Choi, J.W. Immunosensor for detection of Legionella pneumophila using surface plasmon resonance. In Biosensors and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2003; Volume 18, pp. 605–611. [Google Scholar]
- Lin, H.Y.; Tsao, Y.C.; Tsai, W.H.; Yang, Y.W.; Yan, T.R.; Sheu, B.C. Development and application of side-polished fiber immunosensor based on surface plasmon resonance for the detection of Legionella pneumophila with halogens light and 850 nm-LED. Sens. Actuators A Phys. 2007, 138, 299–305. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ezenarro, J.J.; Párraga-Niño, N.; Sabrià, M.; Del Campo, F.J.; Muñoz-Pascual, F.-X.; Mas, J.; Uria, N. Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement. Biosensors 2020, 10, 102. https://doi.org/10.3390/bios10090102
Ezenarro JJ, Párraga-Niño N, Sabrià M, Del Campo FJ, Muñoz-Pascual F-X, Mas J, Uria N. Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement. Biosensors. 2020; 10(9):102. https://doi.org/10.3390/bios10090102
Chicago/Turabian StyleEzenarro, Josune J., Noemí Párraga-Niño, Miquel Sabrià, Fancisco Javier Del Campo, Francesc-Xavier Muñoz-Pascual, Jordi Mas, and Naroa Uria. 2020. "Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement" Biosensors 10, no. 9: 102. https://doi.org/10.3390/bios10090102
APA StyleEzenarro, J. J., Párraga-Niño, N., Sabrià, M., Del Campo, F. J., Muñoz-Pascual, F.-X., Mas, J., & Uria, N. (2020). Rapid Detection of Legionella pneumophila in Drinking Water, Based on Filter Immunoassay and Chronoamperometric Measurement. Biosensors, 10(9), 102. https://doi.org/10.3390/bios10090102





