Study of the Dielectric Properties of Artificial Sweat Mixtures at Microwave Frequencies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Tested Solutions
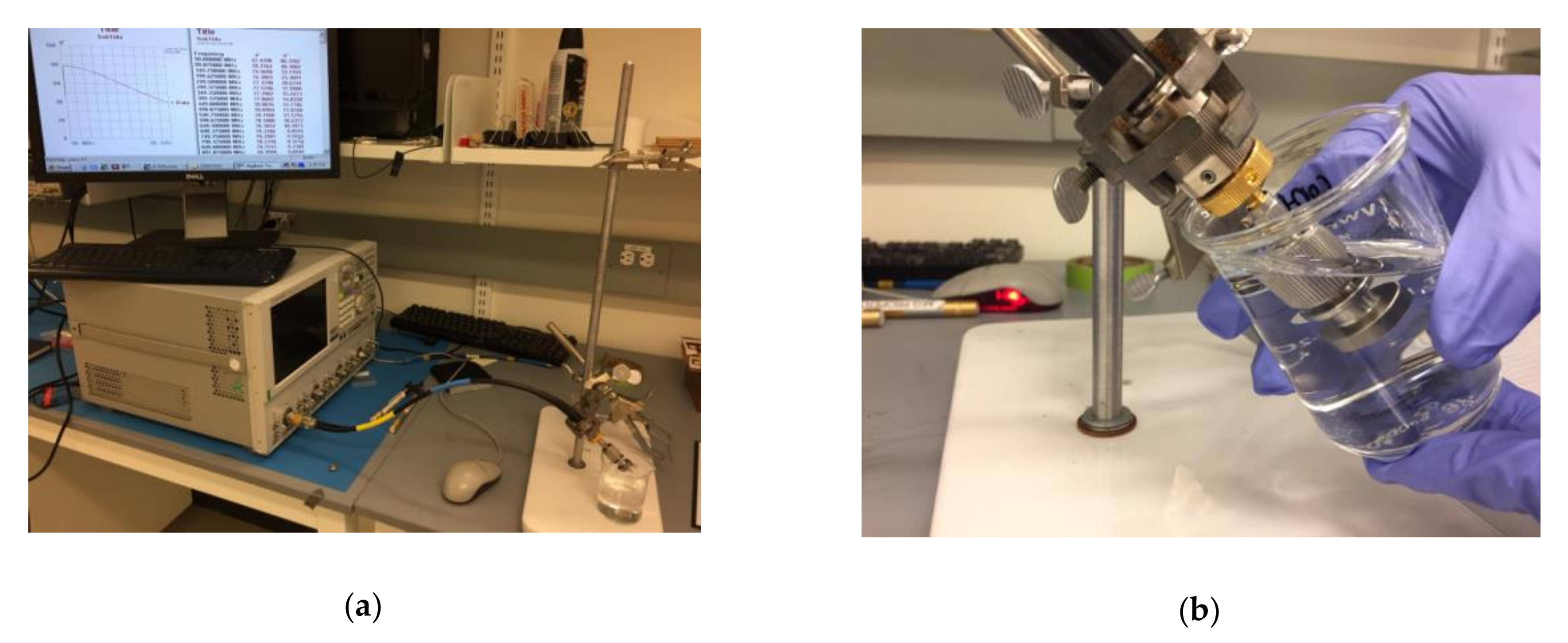
2.2. Measurement Setup
2.3. Validation of Measurements Using NaCl Aqueous Solutions
3. Experimental Results
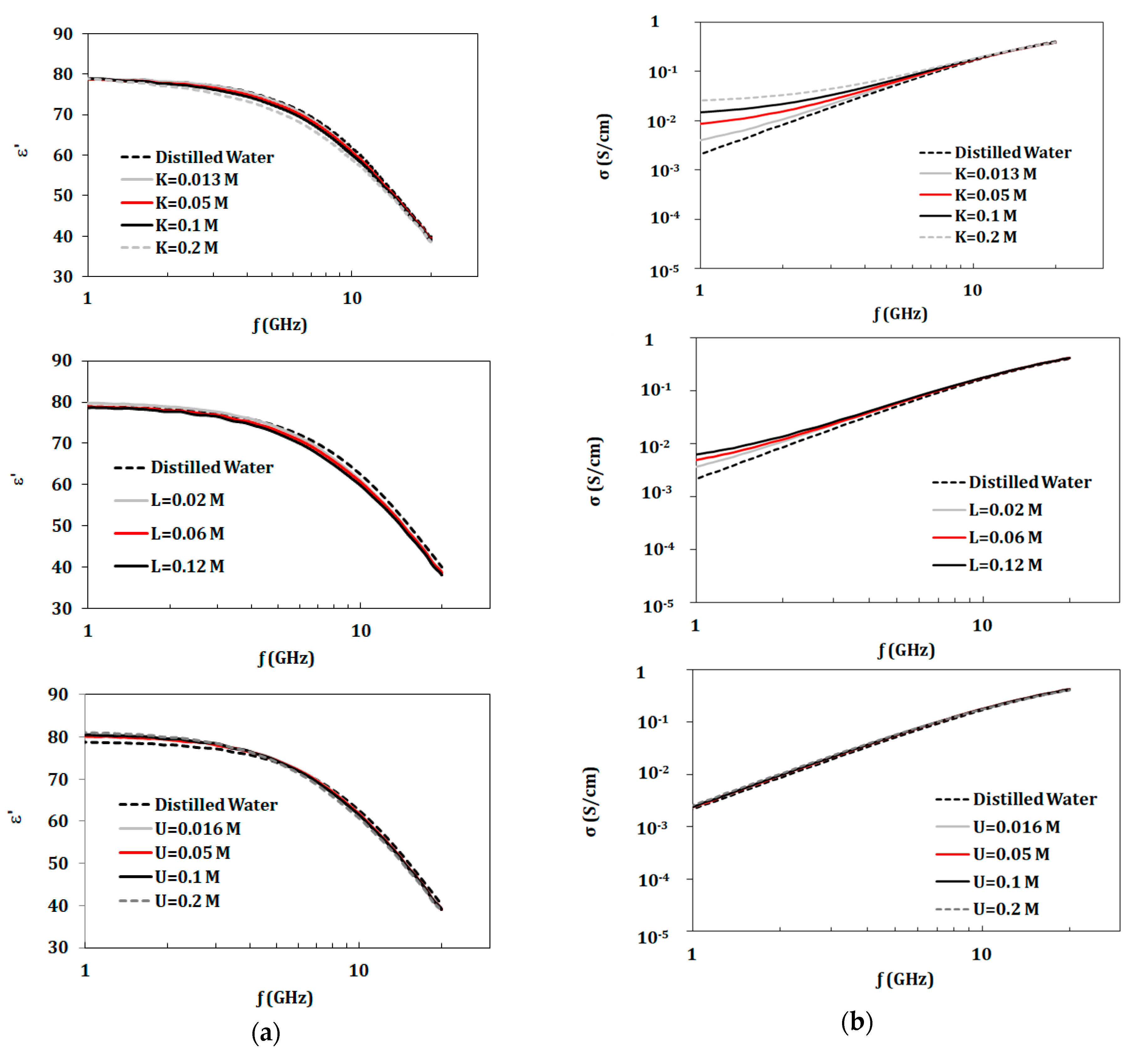
3.1. Single Component Solutions (KCl, Urea, and Lactic Acid)
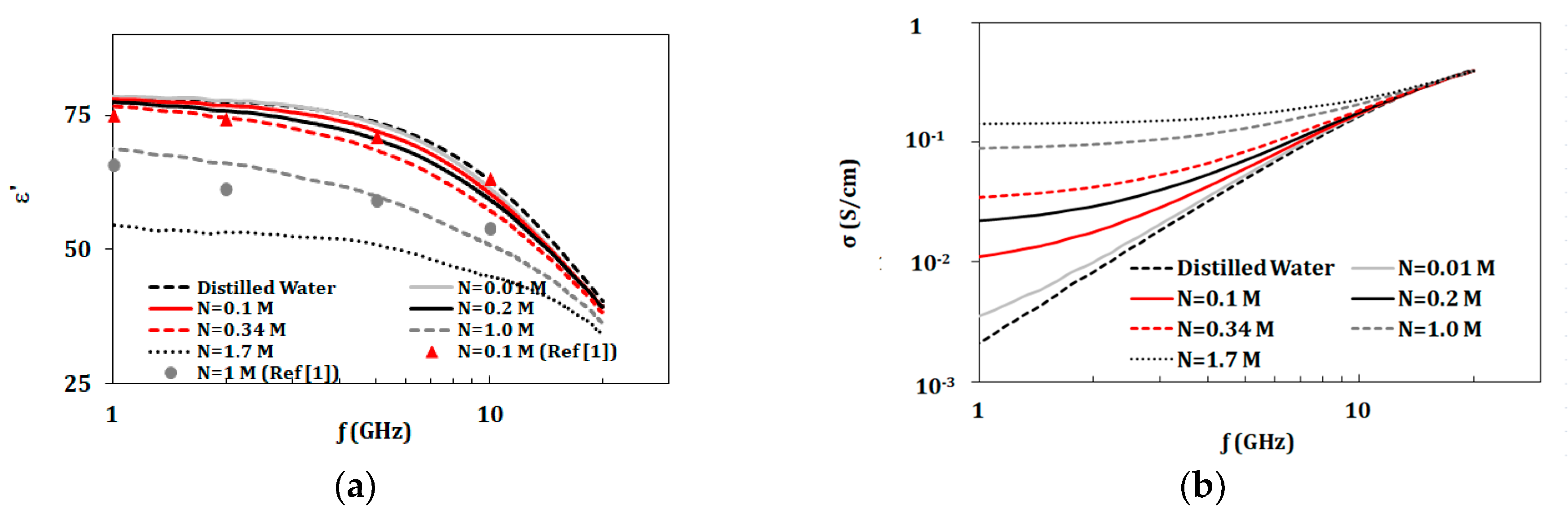
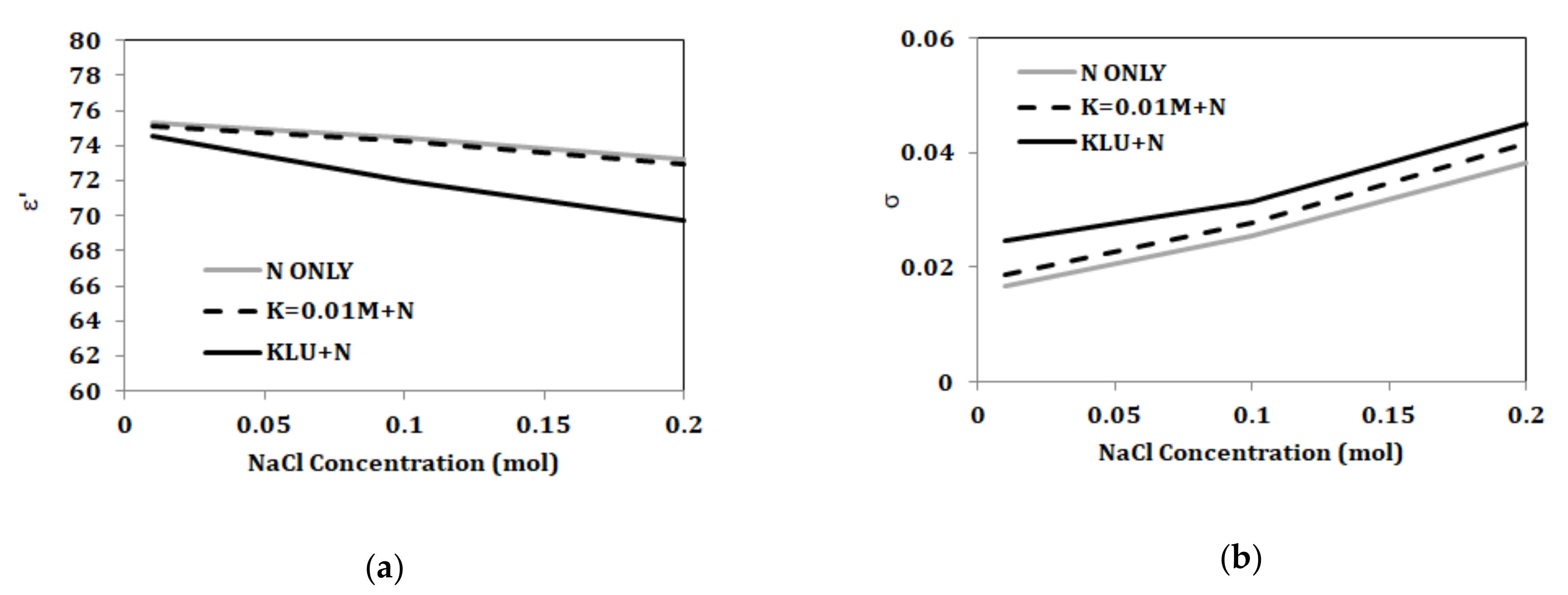
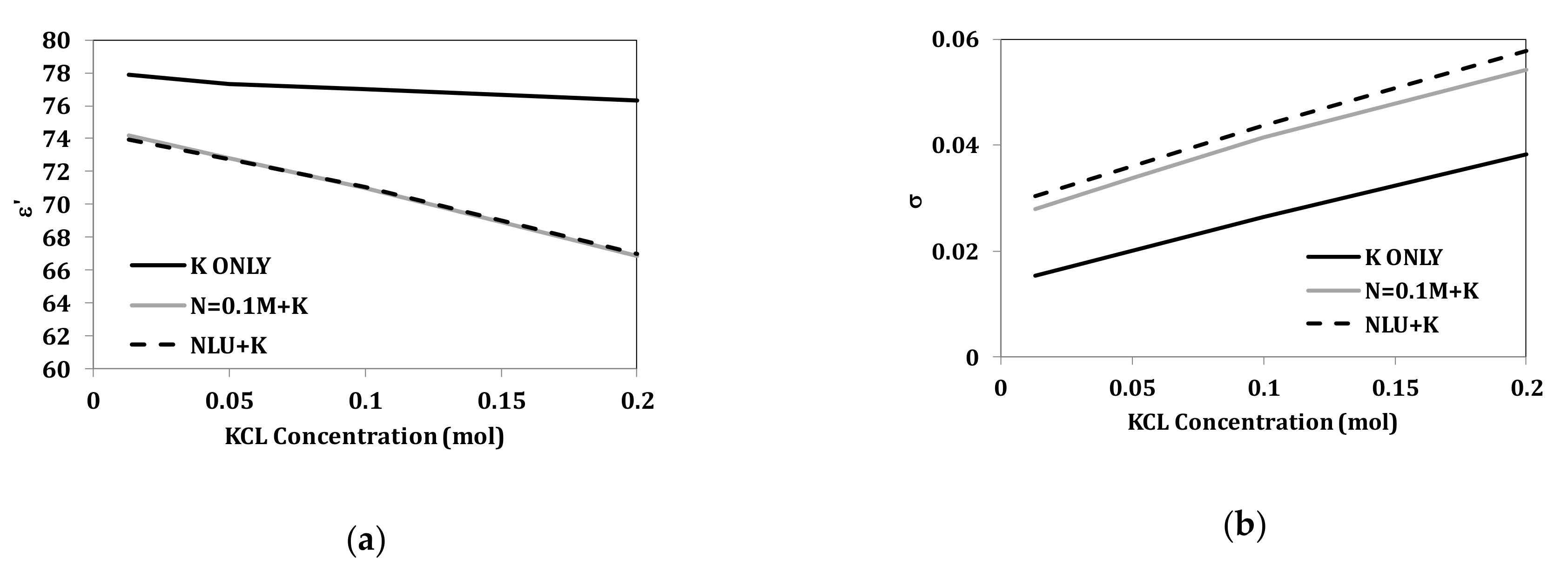
3.2. Dual Component Solutions (NaCl and KCl)
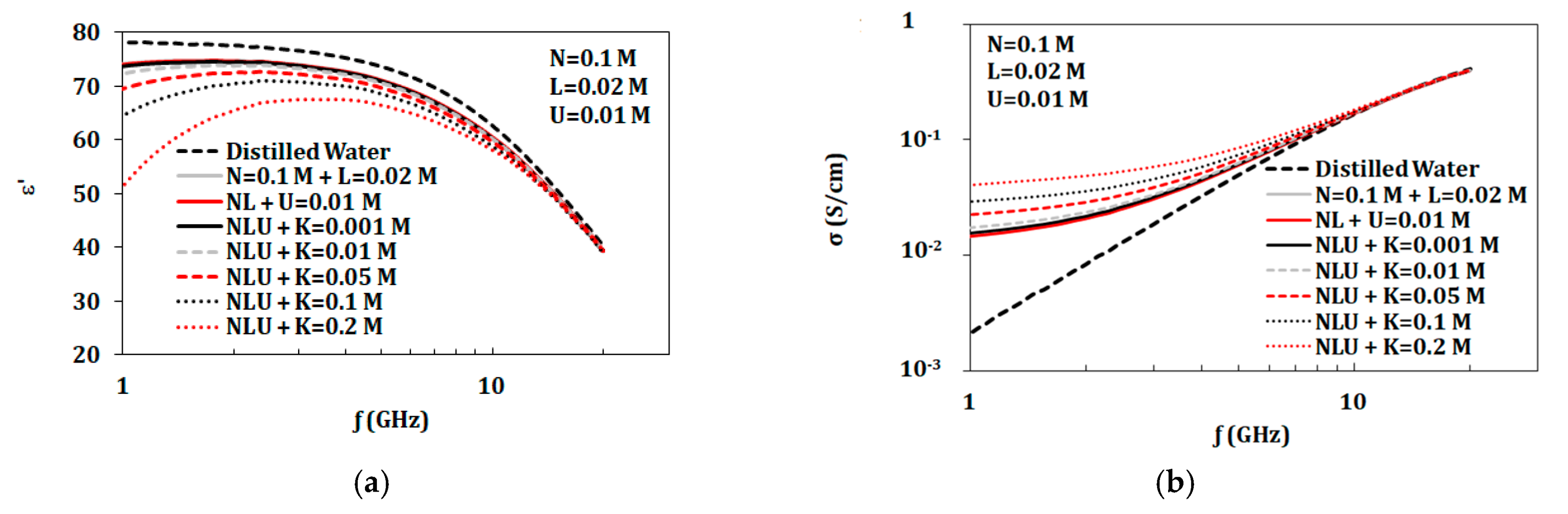
3.3. Artificial Sweat Mixtures
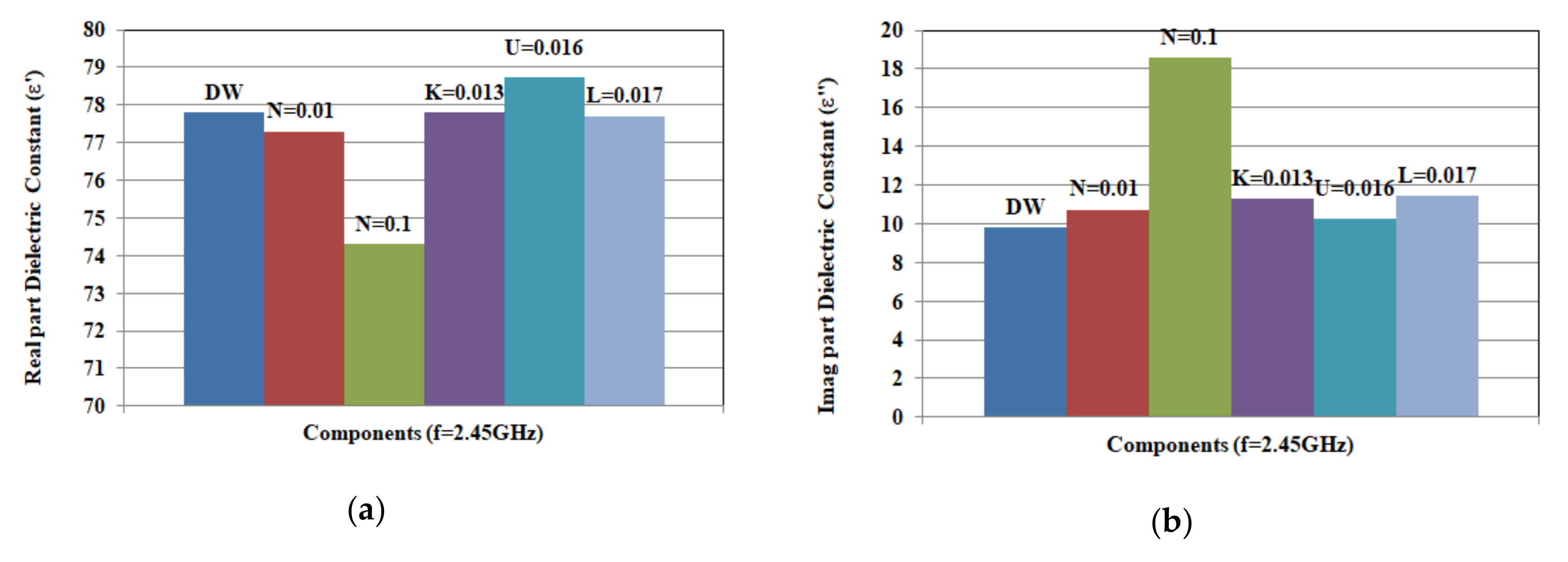
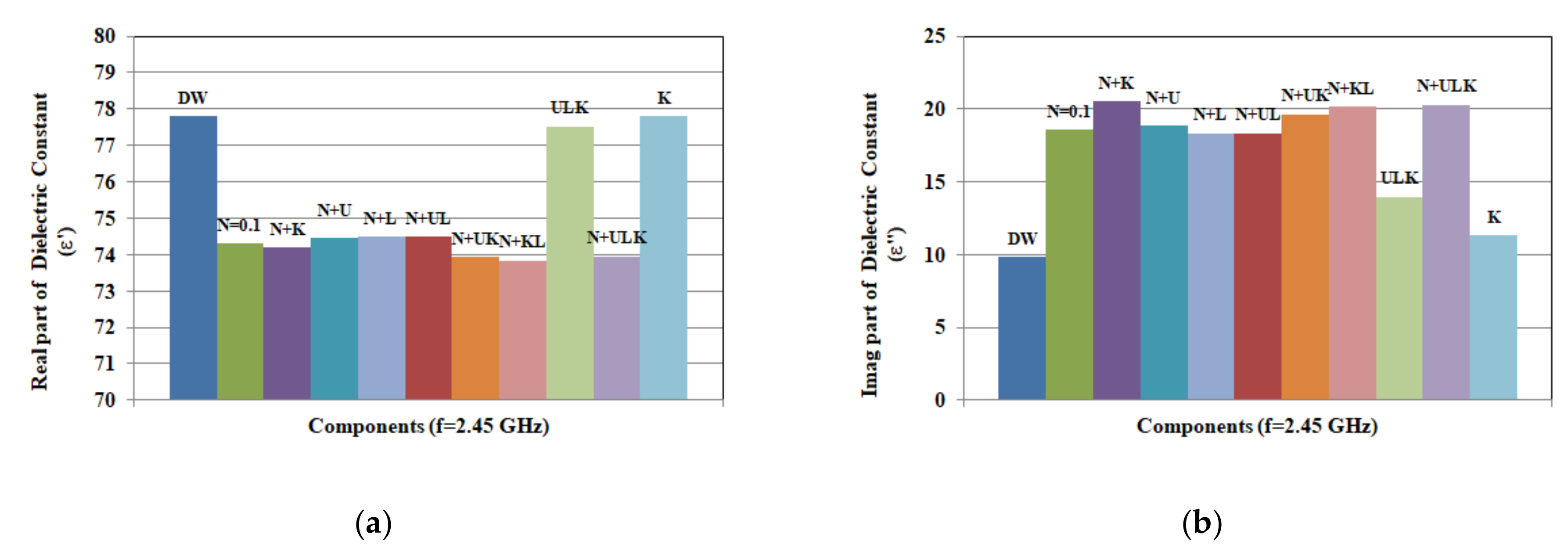
3.3.1. Artificial Sweat Dielectric Properties
3.3.2. Effect of pH
3.3.3. Cole–Cole Model for Artificial Sweat Mixtures
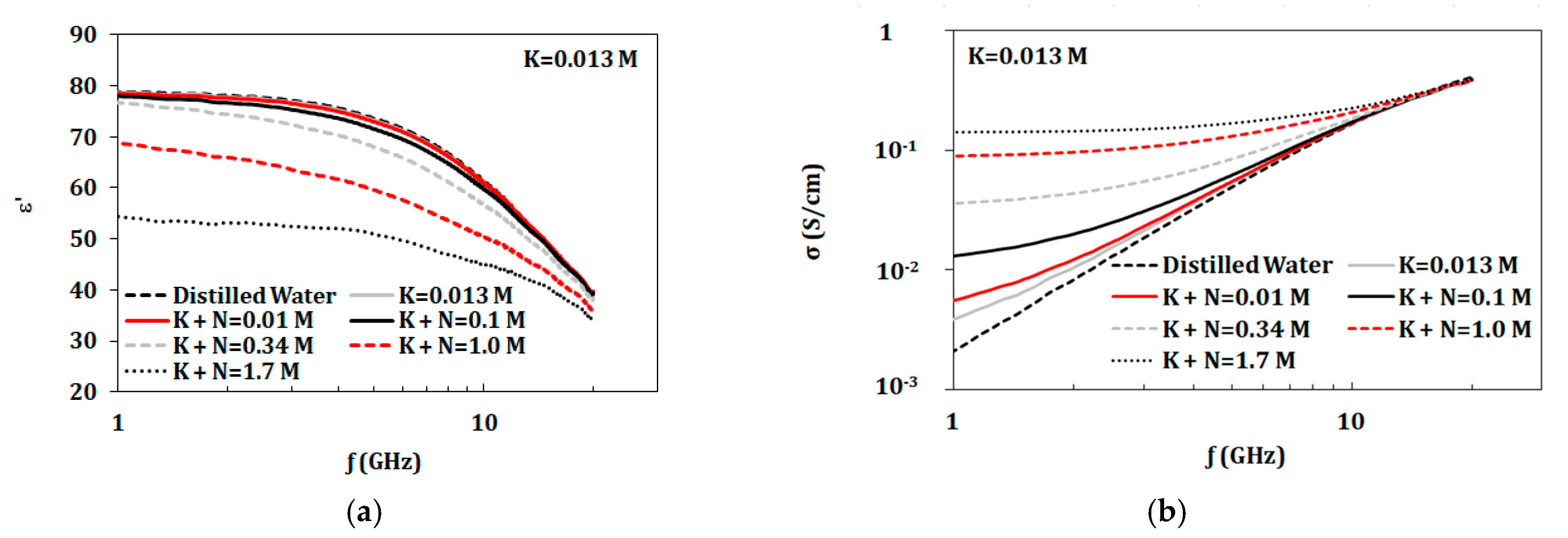
3.3.4. Variation of Components Concentrations
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gulich, R.; Köhler, M.; Lunkenheimer, P.; Loidl, A. Dielectric spectroscopy on aqueous electrolytic solutions. Radiat. Environ. Biophys. 2009, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Peyman, A.; Gabriel, C.; Grant, E.H. Complex permittivity of sodium chloride solutions at microwave frequencies. Bioelectromagnetics 2007, 28, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Nörtemann, K.; Hilland, J.; Kaatze, U. Dielectric Properties of Aqueous NaCl Solutions at Microwave Frequencies. J. Phys. Chem. A 1997, 101, 6864–6869. [Google Scholar] [CrossRef]
- Romanov, A.N. Dielectric properties of human sweat fluid in the microwave range. Biophysics 2010, 55, 473–476. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; Qin, H.; Xie, Y.; Liu, Y.; Wang, H.; Zhao, W.; Zhang, M.; et al. Flexible, Stretchable Sensors for Wearable Health Monitoring: Sensing Mechanisms, Materials, Fabrication Strategies and Features. Sensors 2018, 18, 645. [Google Scholar] [CrossRef]
- White Papers—Pickering Test Solutions . Available online: https://www.pickeringtestsolutions.com/white-papers/ (accessed on 2 April 2020).
- Midander, K.; Julander, A.; Kettelarij, J.; Lidén, C. Testing in artificial sweat—Is less more? Comparison of metal release in two different artificial sweat solutions. Regul. Toxicol. Pharmacol. 2016, 81, 381–386. [Google Scholar] [CrossRef]
- Brennan, D.; Galvin, P. Flexible substrate sensors for multiplex biomarker monitoring. MRS Commun. 2018, 8, 627–641. [Google Scholar] [CrossRef]
- Callewaert, C.; Buysschaert, B.; Vossen, E.; Fievez, V.; Van de Wiele, T.; Boon, N. Artificial sweat composition to grow and sustain a mixed human axillary microbiome. J. Microbiol. Methods 2014, 103, 6–8. [Google Scholar] [CrossRef]
- Liu, G.; Alomari, M.; Sahin, B.; Snelgrove, S.E.; Edwards, J.; Mellinger, A.; Kaya, T. Real-time sweat analysis via alternating current conductivity of artificial and human sweat. Appl. Phys. Lett. 2015, 106, 133702. [Google Scholar] [CrossRef]
- Liu, G.; Ho, C.; Slappey, N.; Zhou, Z.; Snelgrove, S.E.; Brown, M.; Grabinski, A.; Guo, X.; Chen, Y.; Miller, K. A wearable conductivity sensor for wireless real-time sweat monitoring. Sens. Actuators B Chem. 2016, 227, 35–42. [Google Scholar] [CrossRef]
- Hoekstra, R.; Blondeau, P.; Andrade, F.J. IonSens: A Wearable Potentiometric Sensor Patch for Monitoring Total Ion Content in Sweat. Electroanalysis 2018, 30, 1536–1544. [Google Scholar] [CrossRef]
- Baker, L.B. Sweating Rate and Sweat Sodium Concentration in Athletes: A Review of Methodology and Intra/Interindividual Variability. Sports Med. 2017, 47, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.M.; Patterson, M.J.; Nimmo, M.A. Acute effects of dehydration on sweat composition in men during prolonged exercise in the heat. Acta Physiol. 2004, 182, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schazmann, B.; Morris, D.; Slater, C.; Beirne, S.; Fay, C.; Reuveny, R.; Moyna, N.; Diamond, D. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods 2010, 2, 342–348. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdés-Ramírez, G.; Andrade, F.J.; Schöning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelley-Loughnane, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID sensor patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Anastasova, S.; Crewther, B.; Bembnowicz, P.; Curto, V.; Ip, H.M.; Rosa, B.; Yang, G.-Z. A wearable multisensing patch for continuous sweat monitoring. Biosens. Bioelectron. 2017, 93, 139–145. [Google Scholar] [CrossRef]
- Choi, D.-H.; Thaxton, A.; Jeong, I.C.; Kim, K.; Sosnay, P.R.; Cutting, G.R.; Searson, P.C. Sweat test for cystic fibrosis: Wearable sweat sensor vs. standard laboratory test. J. Cyst. Fibros. 2018, 17, e35–e38. [Google Scholar] [CrossRef]
- Lamkaouchi, K.; Balana, A.; Delbos, G.; Ellison, W.J. Permittivity Measurements of Lossy Liquids in the Range 26–110 GHz. Meas. Sci. Technol. 2003, 14, 444–450. [Google Scholar] [CrossRef]
- Maribo-Mogensen, B.; Kontogeorgis, G.M.; Thomsen, K. Modeling of Dielectric Properties of Aqueous Salt Solutions with an Equation of State. J. Phys. Chem. B 2013, 117, 10523–10533. [Google Scholar] [CrossRef]
- Jensen, P.D.; Meaney, P.M.; Epstien, N.R.; Paulsen, K.D. Cole–Cole Parameter Characterization of Urea and Potassium for Improving Dialysis Treatment Assessment. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1598–1601. [Google Scholar] [CrossRef]
- De los Reyes, R.; Heredia, A.; Fito, P.; De los Reyes, E.; Andrés, A. Dielectric spectroscopy of osmotic solutions and osmotically dehydrated tomato products. J. Food Eng. 2007, 80, 1218–1225. [Google Scholar] [CrossRef]
- Eldamak, A.R.; Fear, E.C. Conformal and Disposable Antenna-Based Sensor for Non-Invasive Sweat Monitoring. Sensors 2018, 18, 4088. [Google Scholar] [CrossRef]
- La Gioia, A.; Porter, E.; Merunka, I.; Shahzad, A.; Salahuddin, S.; Jones, M.; O’Halloran, M. Open-Ended Coaxial Probe Technique for Dielectric Measurement of Biological Tissues: Challenges and Common Practices. Diagnostics 2018, 8, 40. [Google Scholar] [CrossRef]
- Kellomaki, T.; Whittow, W.G.; Heikkinen, J.; Kettunen, L. 2.4 GHz plaster antennas for health monitoring. In Proceedings of the 2009 3rd European Conference on Antennas and Propagation, Berlin, Germany, 23–27 March 2009; pp. 211–215. [Google Scholar]
- Patel, M.; Wang, J. Applications, challenges, and prospective in emerging body area networking technologies. IEEE Wirel. Commun. 2010, 17, 80–88. [Google Scholar] [CrossRef]
- Mercuri, M.; Soh, P.J.; Pandey, G.; Karsmakers, P.; Vandenbosch, G.A.E.; Leroux, P.; Schreurs, D. Analysis of an Indoor Biomedical Radar-Based System for Health Monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2061–2068. [Google Scholar] [CrossRef]
- Soh, P.J.; Vandenbosch, G.A.E.; Mercuri, M.; Schreurs, D.M.M. Wearable Wireless Health Monitoring: Current Developments, Challenges, and Future Trends. IEEE Microw. Mag. 2015, 16, 55–70. [Google Scholar] [CrossRef]
- Lai, Y.-L.; Chen, C.-L.; Chang, C.-H.; Hsu, C.-Y.; Lai, Y.-K.; Tseng, K.-K.; Chen, C.-C.; Zheng, C.-Y. An intelligent health monitoring system using radio-frequency identification technology. Technol. Health Care 2016, 24, S421–S431. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef] [PubMed]
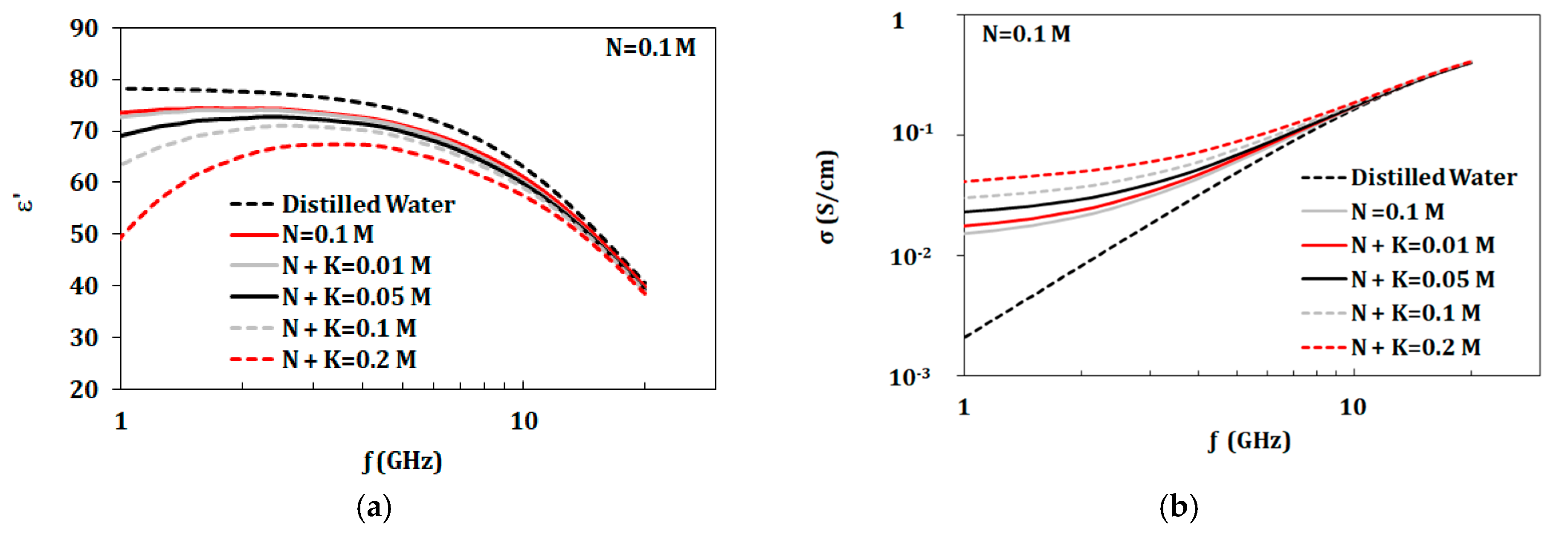
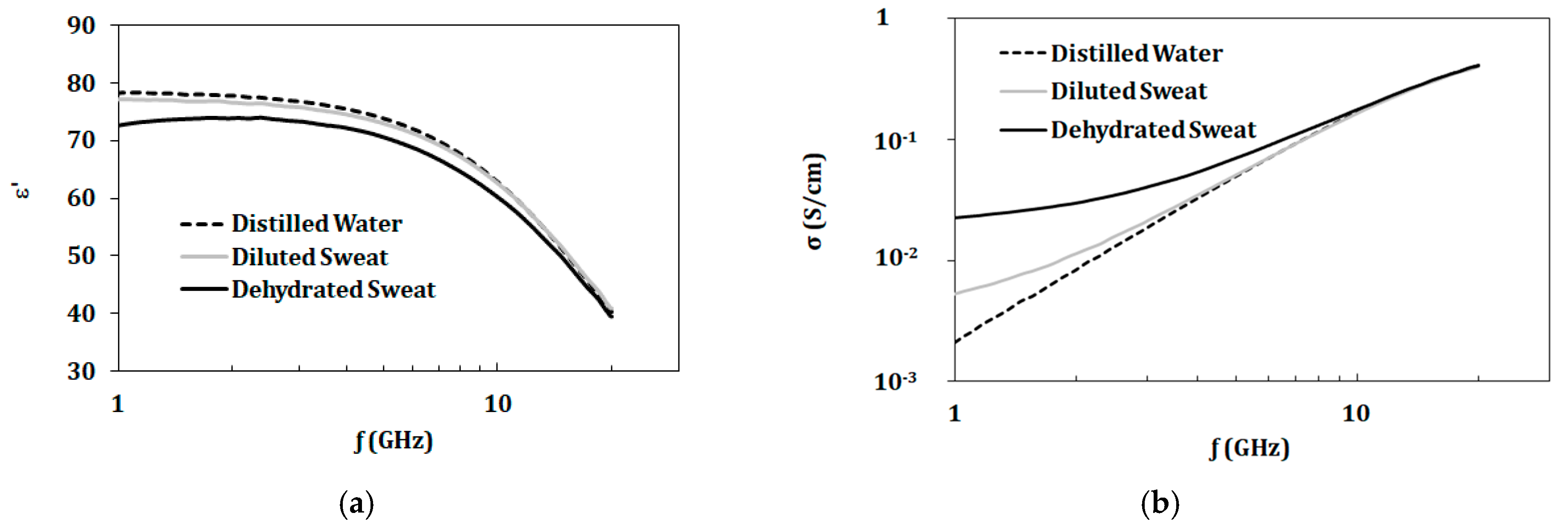
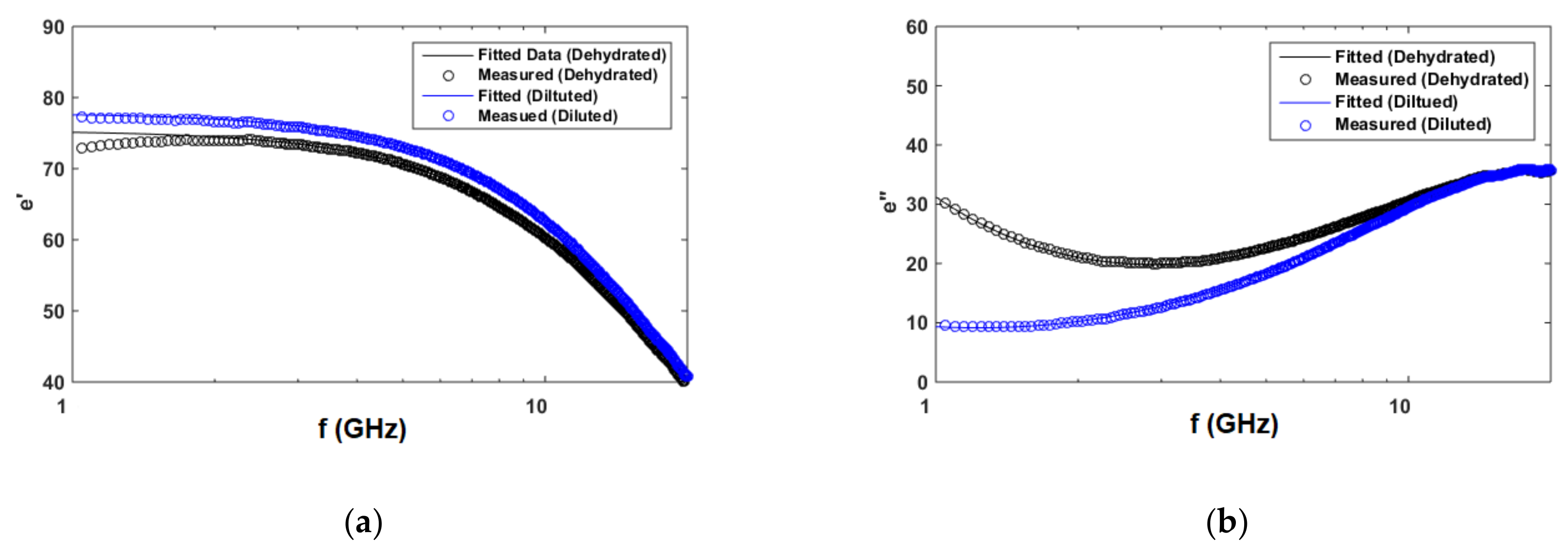
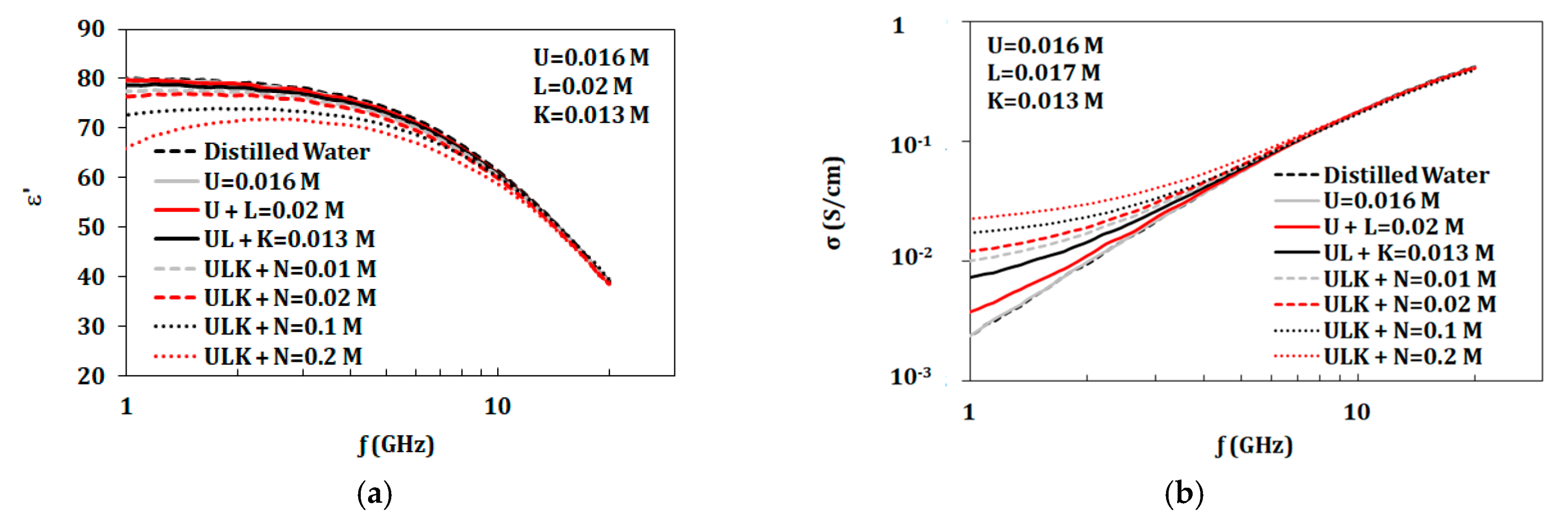
| Tested Component | Concentrations of Components (mol/L) | ||||
|---|---|---|---|---|---|
| NaCl (“N”) | 0.01 | 0.1 | 0.2 | 0.34 | 1.7 |
| KCl (“K”) | 0.01 | 0.05 | 0.1 | 0.2 | |
| Lactic Acid (“L”) | 0.02 | 0.06 | 0.12 | ||
| Urea (“U”) | 0.016 | 0.05 | 0.1 | 0.2 | |
| Base Component | Concentrations of Tested Components (mol/L) | |||||
|---|---|---|---|---|---|---|
| K = 0.013 mol | N | 0.01 | 0.1 | 0.34 | 1 | 1.7 |
| N = 0.1 mol | K | 0.01 | 0.05 | 0.1 | 0.2 | |
| U = 0.016 mol, L = 0.02 mol, K = 0.013 mol | N | 0.01 | 0.02 | 0.1 | 0.2 | |
| N = 0.1 mol, L = 0.02 mol, U = 0.016 mol | K | 0.01 | 0.05 | 0.1 | 0.2 | |
| N = 0.1 mol, K = 0.013 mol, U = 0.016 mol | L | 0.02 | 0.06 | 0.12 | ||
| N = 0.1 mol, K = 0.013 mol, L = 0.02 mol | U | 0.016 | 0.05 | 0.1 | 0.2 | |
| Tested Solution | ε’ | ε” | σ |
|---|---|---|---|
| Distilled water | 77.8 | 9.8 | 0.012599 |
| Diluted sweat (13 mmol/L) | 76.4218 | 11.2541 | 0.015295 |
| Dehydrated sweat (131 mmol/L) | 73.9557 | 20.29 | 0.03443 |
| Tested Solution | Concentration | εs | τ | α | σi |
|---|---|---|---|---|---|
| Diluted sweat | 13.1 mmol/L | 77.8 | 8.15 ps | 0.005 | 0.31 |
| Dehydrated sweat | 131 mmol/L | 75.4 | 8.1 ps | 0.015 | 1.53 |
| Component (N = 0.1 mol, K = 0.013 mol, U = 0.016 mol, and L = 0.017 mol) |
|---|
| Single component solutions: N = 0.01 mol, N = 0.1 mol, K, L, U |
| Dual component solutions: N + K, N + L, N + K |
| Three component solutions: N + KL, N + UL, N + KU, UKL |
| Four component solution: N + UKL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldamak, A.R.; Thorson, S.; Fear, E.C. Study of the Dielectric Properties of Artificial Sweat Mixtures at Microwave Frequencies. Biosensors 2020, 10, 62. https://doi.org/10.3390/bios10060062
Eldamak AR, Thorson S, Fear EC. Study of the Dielectric Properties of Artificial Sweat Mixtures at Microwave Frequencies. Biosensors. 2020; 10(6):62. https://doi.org/10.3390/bios10060062
Chicago/Turabian StyleEldamak, Angie R., Sarah Thorson, and Elise C. Fear. 2020. "Study of the Dielectric Properties of Artificial Sweat Mixtures at Microwave Frequencies" Biosensors 10, no. 6: 62. https://doi.org/10.3390/bios10060062
APA StyleEldamak, A. R., Thorson, S., & Fear, E. C. (2020). Study of the Dielectric Properties of Artificial Sweat Mixtures at Microwave Frequencies. Biosensors, 10(6), 62. https://doi.org/10.3390/bios10060062






