Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component
Abstract
1. Introduction
- (1)
- The capability of the direct detection of the potential difference between the electrolyte and gate electrode due to the high input impedance of FETs, which enables continuous measurement free from electrolysis reactions.
- (2)
- The capability of rapid measurement due to the simple detection mechanism and small sensor size, which enables stress-free operation.
- (3)
- The capability of integration on a chip, which enables application to μ-total analysis systems.
2. Materials and Methods
2.1. Chemicals
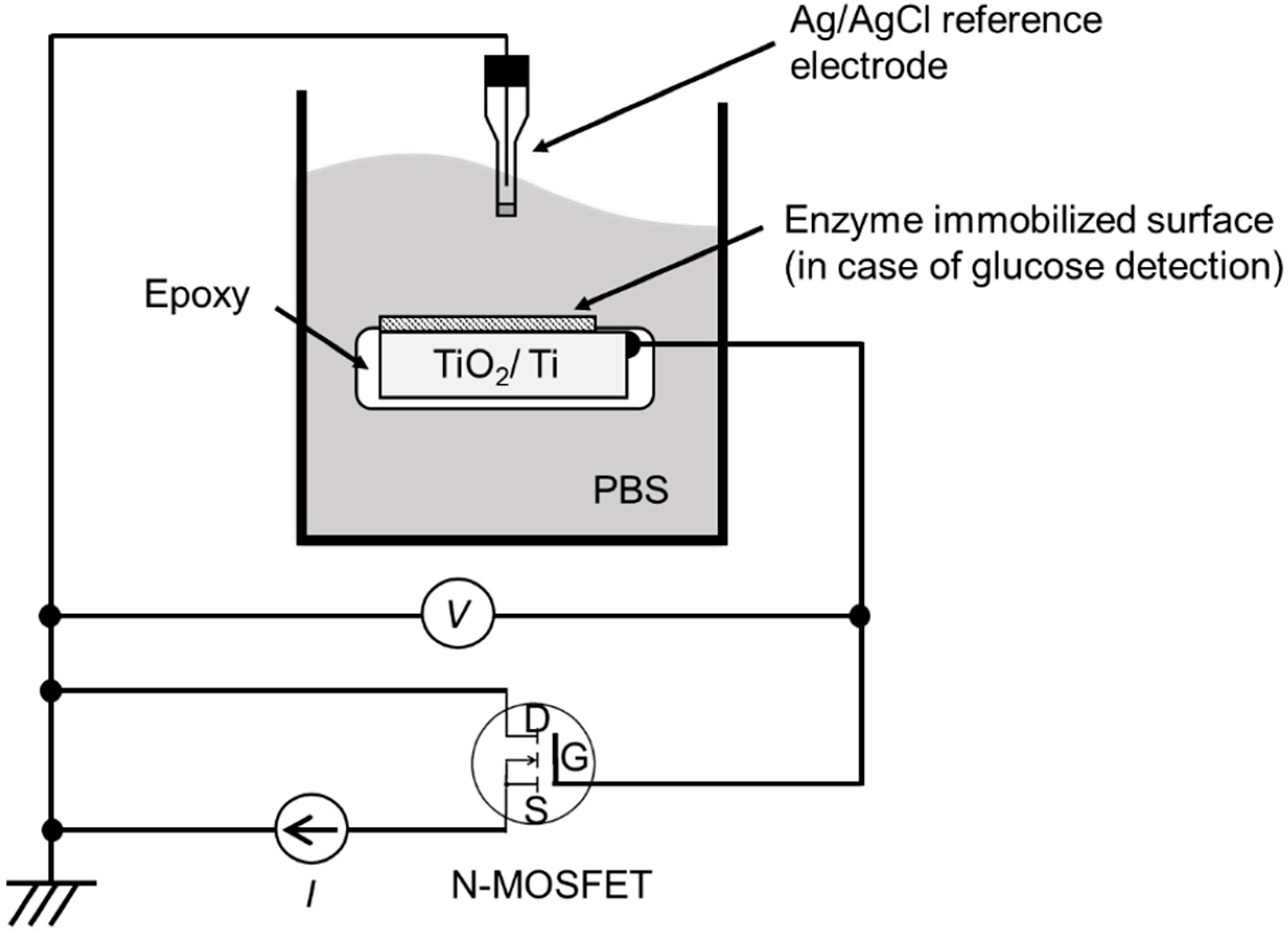
2.2. Experimental Apparatus
2.3. Device Setup and Measuring Conditions Used for pH and Glucose Sensing
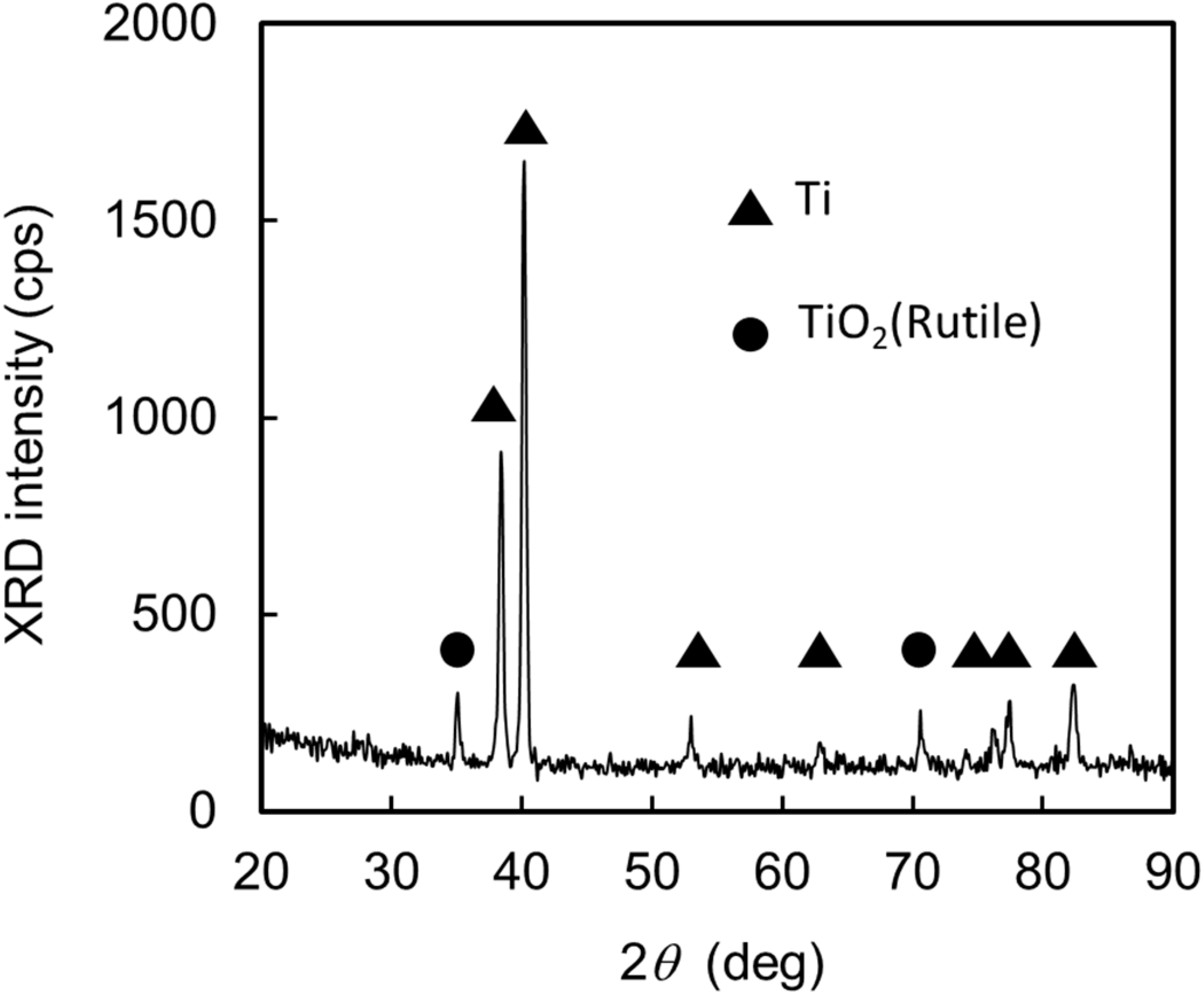
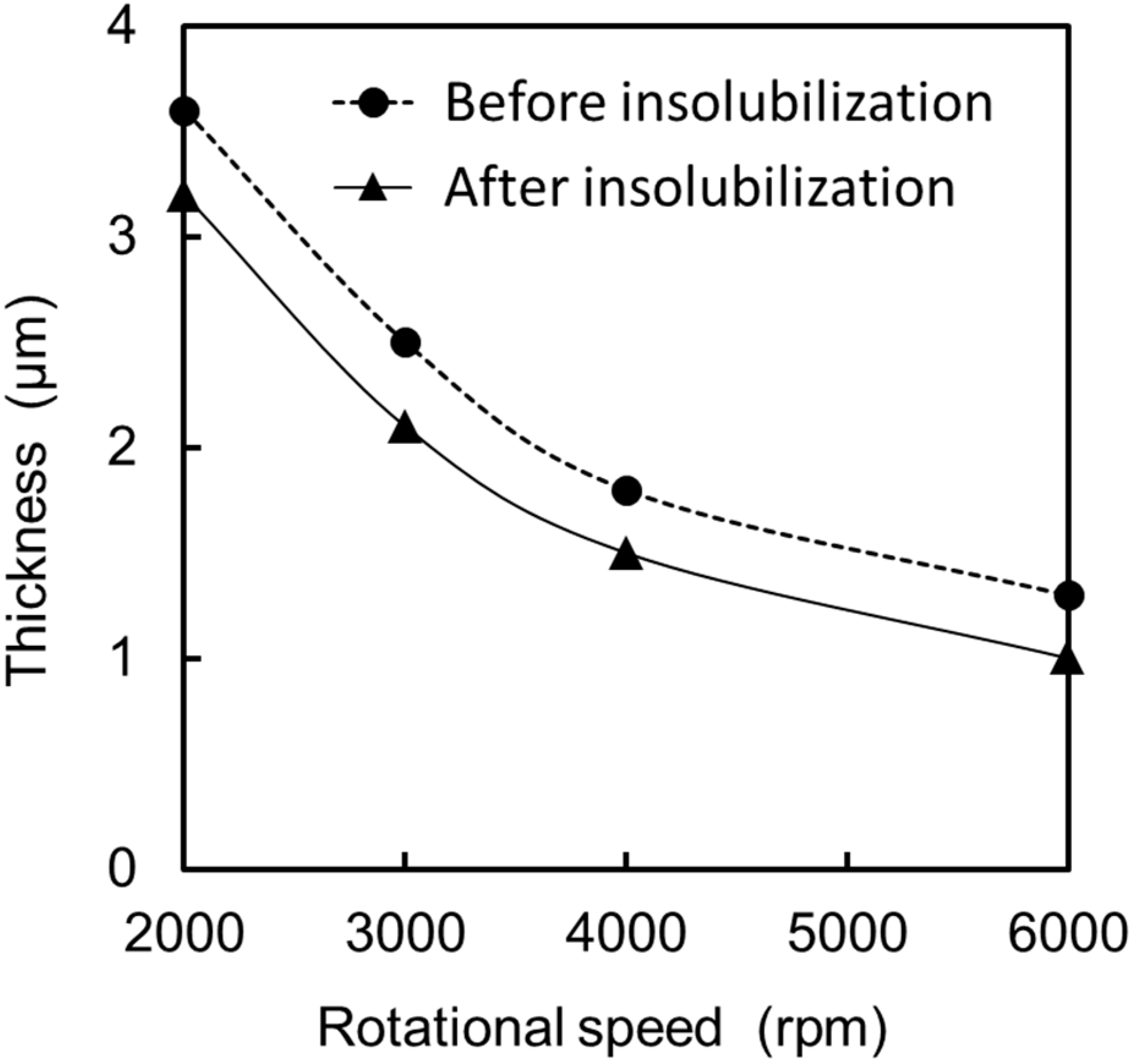
2.4. Modification Process for the TiO2 Surface and Preparation Process for the Silk Fibroin Membranes
- (1)
- Purify the TiO2 surface by ultrasonic washing in organic solvent followed by UV ozone cleaning.
- (2)
- Immerse in 2 vol % AOTMS solution in 2-propanol solvent at 35 °C and shake for 30 min.
- (3)
- Bake in air at 120 °C for 30 min.
- (1)
- Dissolve SF powder in deionized water with a specific ratio (0–40 wt %; it becomes difficult to drip at concentrations higher than 50 wt % due to the increase in viscosity with concentration).
- (2)
- Add a specific quantity of GOD powder (1–2 wt %) in the SF aqueous solution and stir at room temperature.
- (3)
- Apply the GOD-containing SF aqueous solution to the substrate surface by a spin-coating method.
- (4)
- Insolubilize the coated layer by immersing into 80 vol % ethanol aqueous solution at room temperature for 1 h.
- (5)
- Dry in air at room temperature after rinsing in deionized water.
3. Results and Discussion
3.1. Preparation of EGFET-Type Ion Sensor
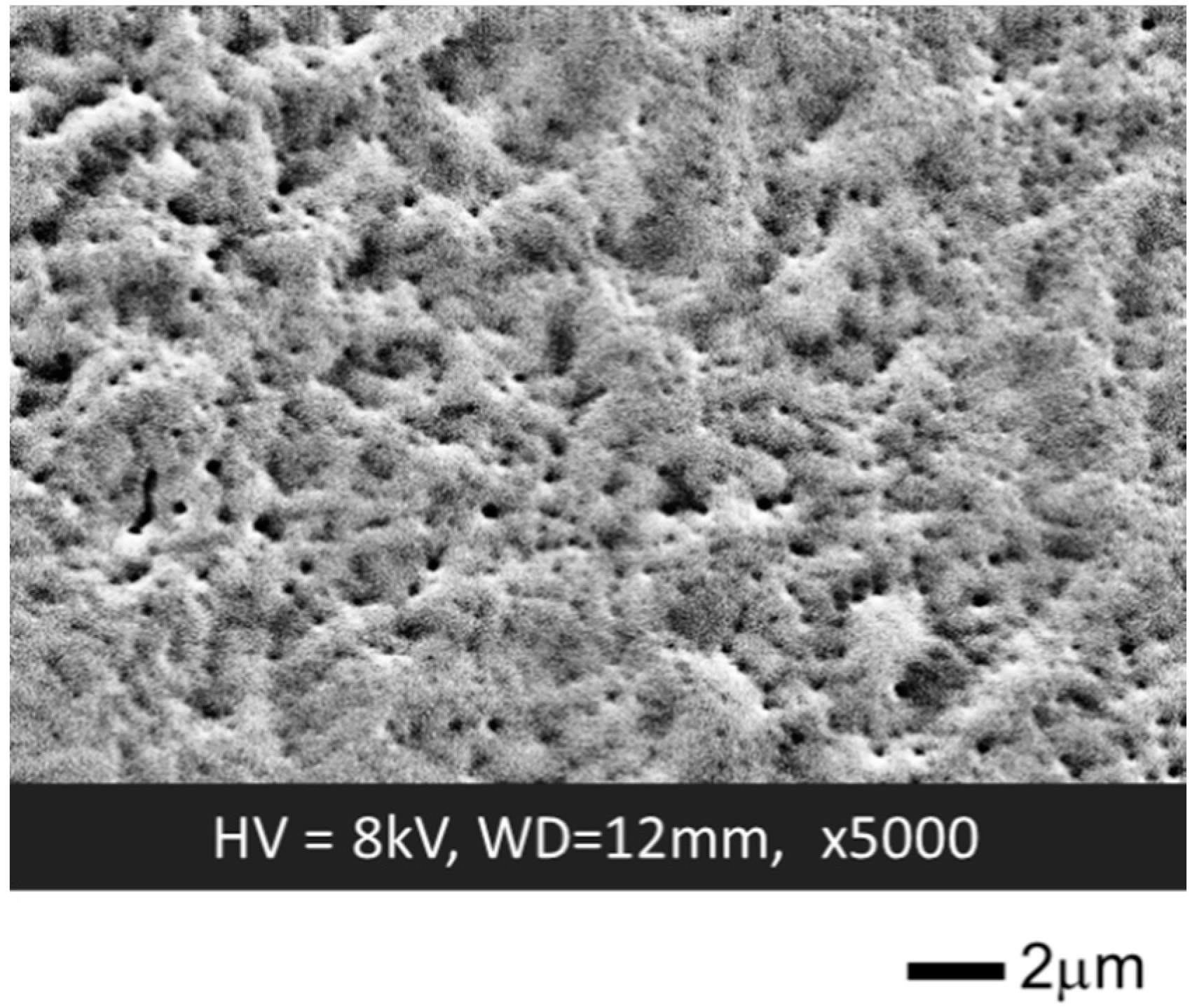
3.2. Formation of a GOD-Containing SF Membrane on the Extended Gate Electrode
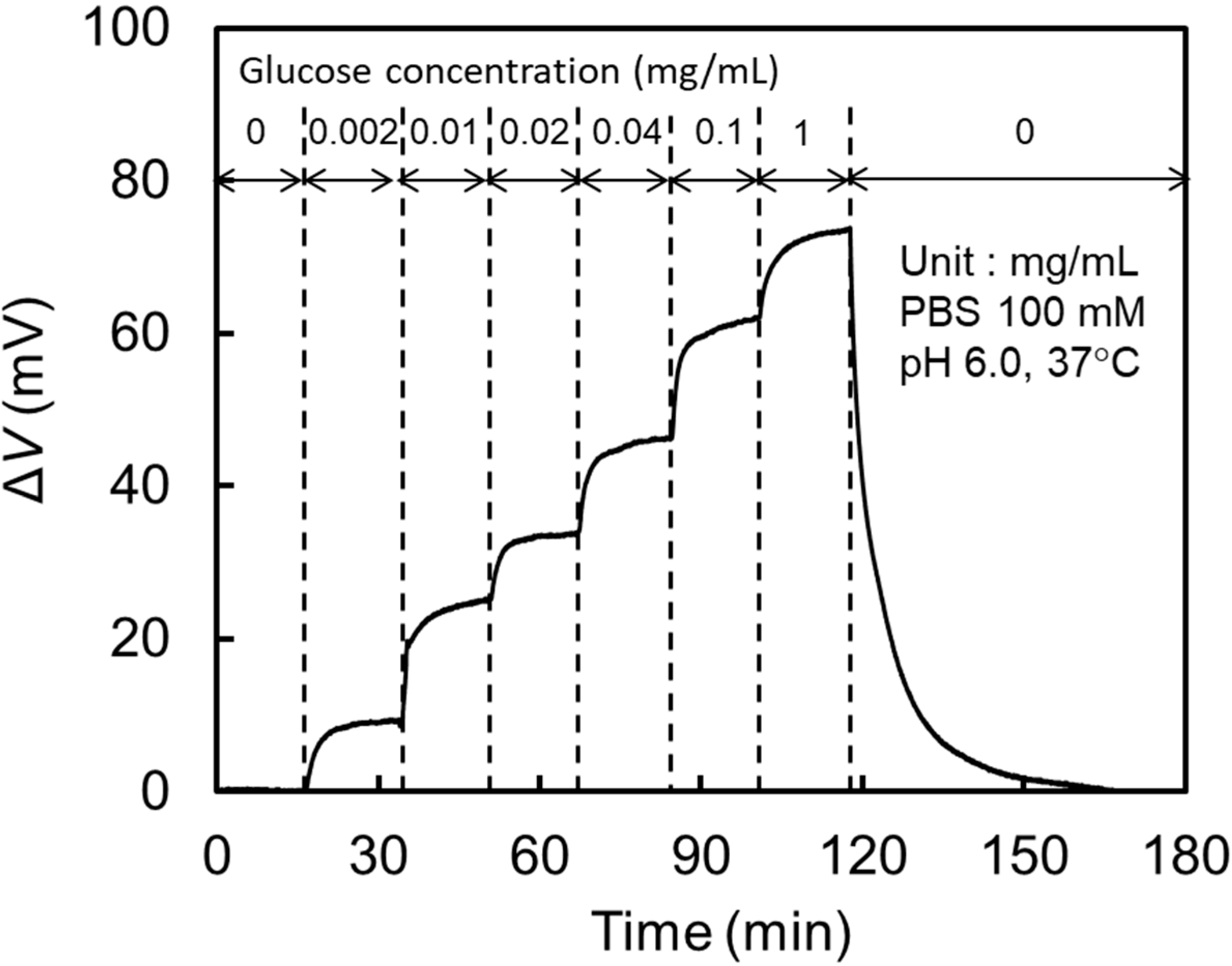
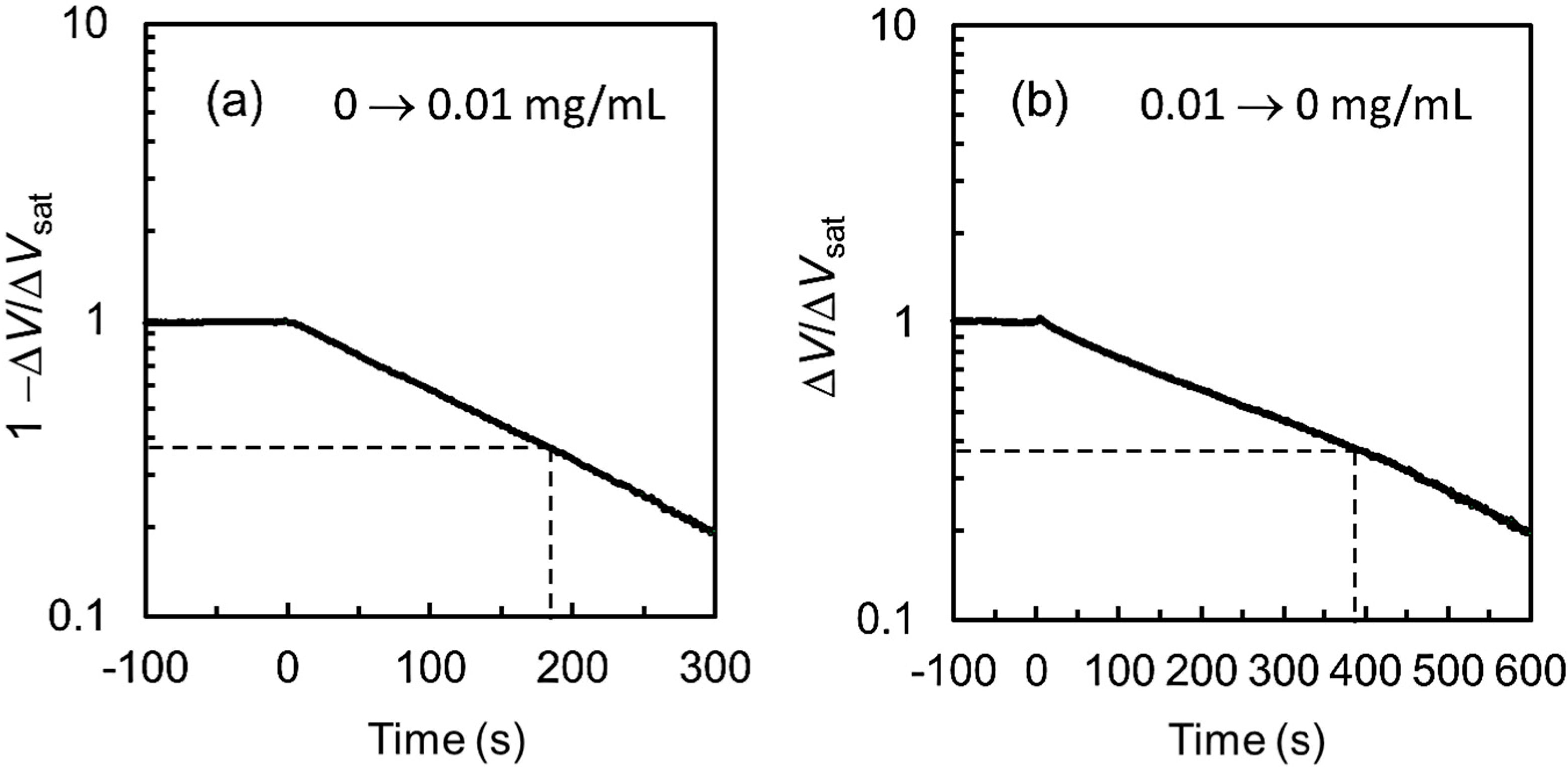
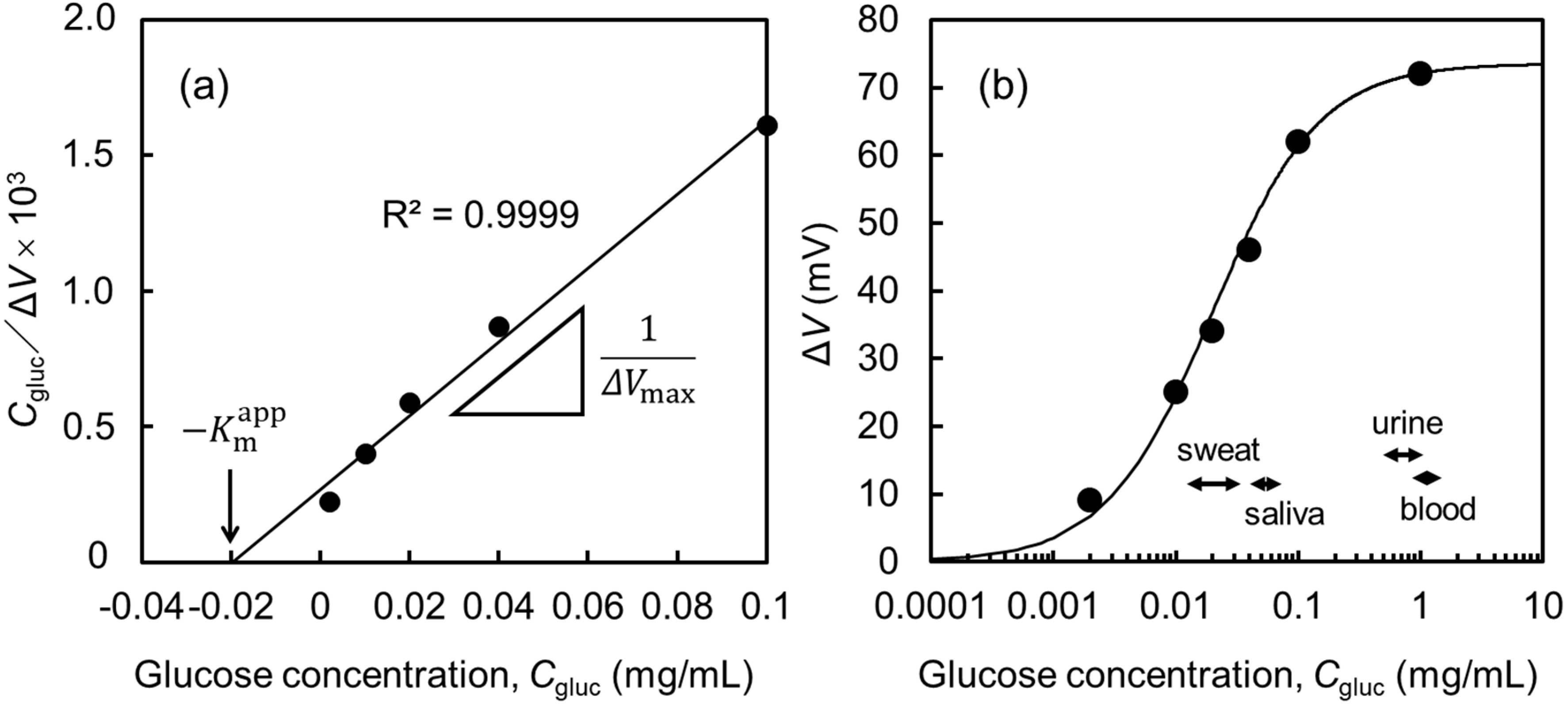
3.3. Characteristics of a Glucose Sensor
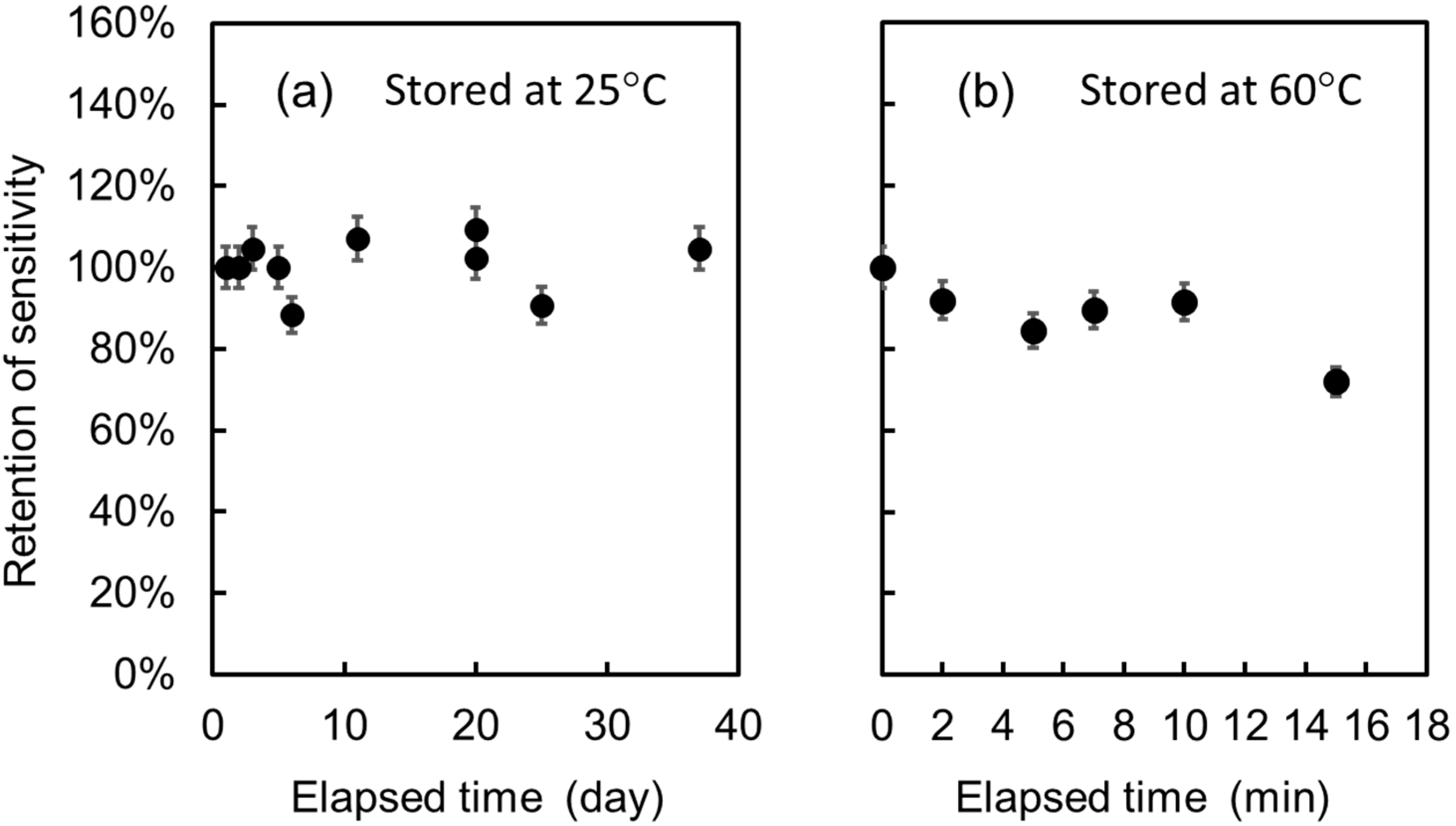
3.4. Endurance of the Sensor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, L.C., Jr.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. App. Physiol. 1953, 16, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular forgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Guilbault, G.G.; Lubrano, G.J. An enzyme electrode for the amperometric detection of glucose. Anal. Chim. Acta 1973, 64, 439–455. [Google Scholar] [PubMed]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Wise, K.D. An integrated field-effect electrode for biopotential recording. IEEE Trans. Biomed. Eng. 1974, BME-17, 485–487. [Google Scholar] [CrossRef]
- Caras, S.; Janata, J. Field effect transistor sensitive to penicillin. Anal. Chem. 1980, 52, 1935–1937. [Google Scholar] [CrossRef]
- Collins, S.; Janata, J. A critical evaluation of the mechanism of potential response of antigen polymer membranes to the corresponding antiserum. Anal. Chem. 1982, 136, 93–99. [Google Scholar]
- Anzai, J.; Kusano, T.; Osa, T.; Nakajima, H.; Matsuo, T. Urea sensor based on ion sensitive field effect transistor coated with cross-linked urease-albumin membrane. Bunseki Kagaku 1984, 33, E131–E136. [Google Scholar] [CrossRef]
- Nakano, M.; Hanazato, Y.; Maeda, M.; Shiono, S. Neutral lipid enzyme electrode based on ion-sensitive field effect transistors. Anal. Chim. Acta. 1986, 185, 179–185. [Google Scholar] [CrossRef]
- Iida, T.; Kawabe, T.; Noguchi, F.; Mitamura, T.; Nagata, K.; Tomita, K. ISFET-type L-glutamate sensor using thermophilic glutamine synthetase from a thermophile. J. Chem. Soc. Jpn. 1978, 1987, 1817–1821. [Google Scholar]
- Song, K.S.; Degawa, M.; Nakamura, Y.; Kanazawa, H.; Umezawa, H.; Kawarada, H. Surface-modified diamond field-effect transistors for enzyme-immobilized biosensors. Jpn. J. Appl. Phys. 2004, 43, L814–L817. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamaer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gate transistors. Sci. Rep. 2015, 5, 8311. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.S.; Wang, H.T.; Ren, F.; Pearton, J.; Morey, T.E.; Dennis, M.; Johnson, W.; Rajagopal, P.; Roberts, J.C.; Piner, E.L.; et al. Enzymatic glucose detection using ZnO nanorods on the gate region of AlGaN/GaN high electron mobility transistors. App. Phys. Lett. 2007, 91, 252103. [Google Scholar]
- Yano, M.; Koike, K.; Mukai, K.; Onaka, T.; Hirofuji, Y.; Ogata, K.; Omatsu, S.; Maemoto, T.; Sasa, S. Zinc oxide ion-sensitive field-effect transistors and biosensors. Phys. Status Solidi A 2014, 211, 2098–2104. [Google Scholar]
- Koike, K.; Mori, Y.; Sasa, S.; Hirofuji, Y.; Yano, M. Glucose sensing by an enzyme-modified ZnO-based FET. Procedia Eng. 2016, 168, 84–88. [Google Scholar] [CrossRef]
- Van der Spiegel, J.; Lauks, I.; Chan, P.; Babic, D. The extended gate chemically sensitive field effect transistor as multi-species microprobe. Sens. Actuators 1983, 4, 291–298. [Google Scholar]
- Ali, S.M.U.; Nur, O.; Willander, M.; Danielsson, B. Glucose detection with a commercial MOSFET using a ZnO nanowires extended gate. IEEE Trans. Nanotech. 2009, 8, 678–683. [Google Scholar]
- Li, S.K.; Chou, J.C.; Sun, T.P.; Hsiung, S.K.; Shieh, H.L. Study on potentiometric glucose biosensor based on separative extended gate field effect transistor. Sens. Lett. 2011, 9, 143–146. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, H.; Ji, Z.; Xu, M.; Zhang, Y. ZnO nano-array-based EGFET biosensor for glucose detection. Appl. Phys. A 2015, 119, 807–811. [Google Scholar]
- Koike, K.; Onishi, Y.; Ike, K.; Hirofuji, Y.; Nakamura, Y.; Yano, M. Glucose detection characteristics of an extended-gate field-effect transistor fabricated by the enzyme immobilization using a long-chain-aminosilane agent. IEEJ Trans. Sens. Micromach. 2019, 139, 143–148. [Google Scholar]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuators B Chem. 2014, 202, 1357–1365. [Google Scholar]
- Yao, F.; Zhong, S.Z.; Qi, D.J.; Yin, Y.T. Immobilization of glucose oxidase with bombyx mori silk fibroin and the preparation of glucose sensor. Chin. Sci. Bull. 1992, 37, 1436–1440. [Google Scholar]
- Zhang, Y.Q.; Zhu, J.; Gu, R.A. Improved biosensor for glucose based on glucose oxidase-immobilized silk fibroin membrane. Appl. Biochem. Biotechnol. 1998, 75, 215–233. [Google Scholar] [PubMed]
- Asakura, T.; Demura, M. The structure of silk fibroins and enzyme-immobilized membrane for biosensor using silk fibroins. Sen’i Gakkaishi 1989, 45, 252–257. [Google Scholar] [CrossRef]
- Demura, M.; Asakura, T.; Kuroo, T. Immobilization of biocatalysts with bombyx mori silk fibroin by several kinds of physical treatment and its application to glucose sensors. Biosensors 1989, 4, 361–372. [Google Scholar] [CrossRef]
- Matsuda, M. The future of sericulture industry spreading from silk fibroin liquefaction extraction technology. Agric. Biotechnol. 2017, 1, 33–39. [Google Scholar]
- Koike, K.; Ike, K.; Onishi, Y.; Sasaki, T.; Hirofuji, Y.; Yano, M. Fabrication and characterization of silk fibroin films by a spin-coating method toward the application to field-effect transistor-based biosensors. J. Soc. Mater. Sci. Jpn. 2019, 68, 751–756. [Google Scholar] [CrossRef]
- Products of Matsuda Silk Farm. Available online: http://www.nanofibroin.com/index.html (accessed on 28 May 2020).
- Chou, J.C.; Liao, L.P. Study of TiO2 thin films for ion sensitive field effect transistor application with rf sputtering deposition. Jpn. J. Appl. Phys. 2004, 43 Part 1, 61–65. [Google Scholar] [CrossRef]
- Yusof, K.A.; Rahman, R.A.; Zulkefle, M.A.; Herman, S.H.; Abdullah, W.F.H. EGFET pH sensor performance dependence on sputtered TiO2 sensing membrane deposition temperature. J. Sens. 2016, 2016, 2594531. [Google Scholar] [CrossRef]
- Zulkefle, M.A.; Rahman, R.A.; Yusoff, K.A.; Abdullar, W.F.H.; Rusop, M.; Herman, S.H. Annealing time dependence of the physical, electrical, and pH response characteristics of spin coated TiO2 thin films. Mater. Sci. Eng. 2015, 99, 012021. [Google Scholar]
- Guerra, E.M.; Mulato, M. Titanium oxide nanorods pH sensors: Comparison between voltammetry and extended gate field effect transistor measurements. Mater. Sci. Appl. 2014, 5, 459–466. [Google Scholar] [CrossRef][Green Version]
- Chou, J.C.; Li, Y.S.; Chiang, J.L. Simulation of Ta2O5-gate ISFET temperature characteristics. Sens. Actuators B Chem. 2000, 71, 73–76. [Google Scholar] [CrossRef]
- Yamada, K. Thermal transformation of secondary structure on ultrathin fibroin films. Netsu Sokutei 2009, 36, 271–276. [Google Scholar]
- Lu, S.; Wang, X.; Lu, Q.; Hu, X.; Uppal, N.; Omenetto, F.G.; Kaplan, D.L. Stabilization of enzymes in silk films. Biomacromolecules 2009, 10, 1032–1042. [Google Scholar]
- Michaelis, L.; Menten, M.L. Die kinetik der invertinwirkung. Biochem Z 1913, 49, 333–369. [Google Scholar]
- Ritchie, R.J.; Prvan, T. Current statistical methods for estimating the Km and Vmax of Michaelis-Menten Kinetics. Biochem. Edu. 1996, 24, 196–206. [Google Scholar]
- Bruen, D.; Delaney, C.; Florea, L.; Diamond, D. Glucose sensing for diabetes monitoring: Recent developments. Sensors 2017, 17, 1866. [Google Scholar] [CrossRef]

| Type | Structure | Detection Range (mg/mL) | Reference |
|---|---|---|---|
| ISFET(SGFET) | GOD/Diamond/Si | 0.018–1.8 | [11] |
| ISFET(SGFET) | GOD/Nafion/Pt NPs/Graphene/Glass | 0.00009–0.18 | [13] |
| ISFET(SGFET) | GOD/ZnO NRs/AlGaN/GaN | 0.00000009–0.023 | [14] |
| ISFET(SGFET) | GOD/InZnO/Glass | 0.036–7.2 | [15] |
| ISFET(SGFET) | SF-GOD/Graphene/SF | 0.018–1.8 | [22] |
| EGFET | GOD/ZnO NWs/Ag wires | 0.00018–0.018 | [18] |
| EGFET | GOD/SnO2/ITO/PET | 1–3 | [19] |
| EGFET | GOD/ZnO NRs/ITO/Glass | 0.0036–0.18 | [20] |
| EGFET | GOD/TiO2/Ti | 0.0045–0.45 | [21] |
| EGFET | SF-GOD/TiO2/Ti | 0.001–0.5 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koike, K.; Sasaki, T.; Hiraki, K.; Ike, K.; Hirofuji, Y.; Yano, M. Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component. Biosensors 2020, 10, 57. https://doi.org/10.3390/bios10060057
Koike K, Sasaki T, Hiraki K, Ike K, Hirofuji Y, Yano M. Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component. Biosensors. 2020; 10(6):57. https://doi.org/10.3390/bios10060057
Chicago/Turabian StyleKoike, Kazuto, Taihou Sasaki, Kenta Hiraki, Kodai Ike, Yuichi Hirofuji, and Mitsuaki Yano. 2020. "Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component" Biosensors 10, no. 6: 57. https://doi.org/10.3390/bios10060057
APA StyleKoike, K., Sasaki, T., Hiraki, K., Ike, K., Hirofuji, Y., & Yano, M. (2020). Characteristics of an Extended Gate Field-Effect Transistor for Glucose Sensing Using an Enzyme-Containing Silk Fibroin Membrane as the Bio-Chemical Component. Biosensors, 10(6), 57. https://doi.org/10.3390/bios10060057





