Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis
Abstract
:1. Introduction
| Company name | Product name 2 | Technology | Limit of detection [ng/cm2] | Number of parallel sensors | Sample volume 6 | Sample volume per sensor/pixel 7 | Web address | Comments | |
|---|---|---|---|---|---|---|---|---|---|
| SPR 1 | GE Healthcare | Biacore 4000 | optical | 0.01 | 16 | 60 µL (For 4 flow cells) | 4 µL | www.biacore.com | |
| Biacore T100 | 0.01 | 4 | 20 to 50 µL | 21 to 50 µL | |||||
| SPRi 3 | Horiba | SPRi-Plex™ | optical | 0.5 | up to 1,000 | 1.6 mL | 2 µL (target)/1.6 mL (ligand) | www.horiba.com | Up to 1,000 substances can be spotted, only one substance can be measured in flow |
| BLI | ForteBio | Octet RED384 | optical | 0.1 | 16 | n/a | 200 µL | www.fortebio.com | |
| Diffraction Grating Based 4 | SRU Biosystems | BIND | optical | 0.01 | 96-, 384- and 1,536-well microplate | n/a | down to 5 µL | www.srubiosystems.com | |
| Corning | Epic | 0.5 | 384-well microplate | n/a | 15–30 µL typical | www.corning.com | |||
| MicroVacuum Ltd. | OWLS 210 | optical | 0.5 | 1 | n/a | 20 to 250 µL | www.owls-sensors.com | ||
| Farfield | AnaLight 4D | 0.01 | 1 | n/a | 50 µL | www.farfield-group.com | |||
| ELM | Maven Biotechnologies | LFIRE | optical | 0.1 | 1 | n/a | n/a | www.mavenbiotech.com | |
| QCM 5 | Q-Sense | E4 Auto | acoustic | 0.5 | 4 | n/a | 400 µL | www.q-sense.com | |
| SAW | SAW instruments GmbH | sam5 | acoustic | 0.05 | 5 | 40 to 80 µL | 8 to 16 µL | www.saw-instruments.de | |
| n/a | n/a | n/a | n/a | www.ecochemie.nl | |||||
| ITC | MicroCal | iTC200 | calorimetric | n/a | 1 | n/a | n/a (at least 10 µg protein) | www.microcal.com | in-solution, no immobilization needed |
2. Transducer Principles
2.1. Acoustic Sensors
2.1.1. Quartz Crystal Microbalance (QCM) and Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)

 where η is the viscosity, m the mass, ρ the density, ω the angular frequency, h the thickness of the adsorbent, G’ the storage and G’’ the loss modulus of the adsorbent. The index ‘1’ corresponds to the adsorbed layer, the index ‘q’ to the quartz and the index ‘2’ to the bulk liquid [25]. This model assumes that the viscosity of the adsorbent is constant over frequency, which is most likely not the case for most materials and should be therefore be carefully used especially if the measurement covers a broad range of frequencies [26].
where η is the viscosity, m the mass, ρ the density, ω the angular frequency, h the thickness of the adsorbent, G’ the storage and G’’ the loss modulus of the adsorbent. The index ‘1’ corresponds to the adsorbed layer, the index ‘q’ to the quartz and the index ‘2’ to the bulk liquid [25]. This model assumes that the viscosity of the adsorbent is constant over frequency, which is most likely not the case for most materials and should be therefore be carefully used especially if the measurement covers a broad range of frequencies [26]. 
2.1.2. Surface Acoustic Wave (SAW) Devices

2.1.3. Film Bulk Acoustic Resonator (FBAR)
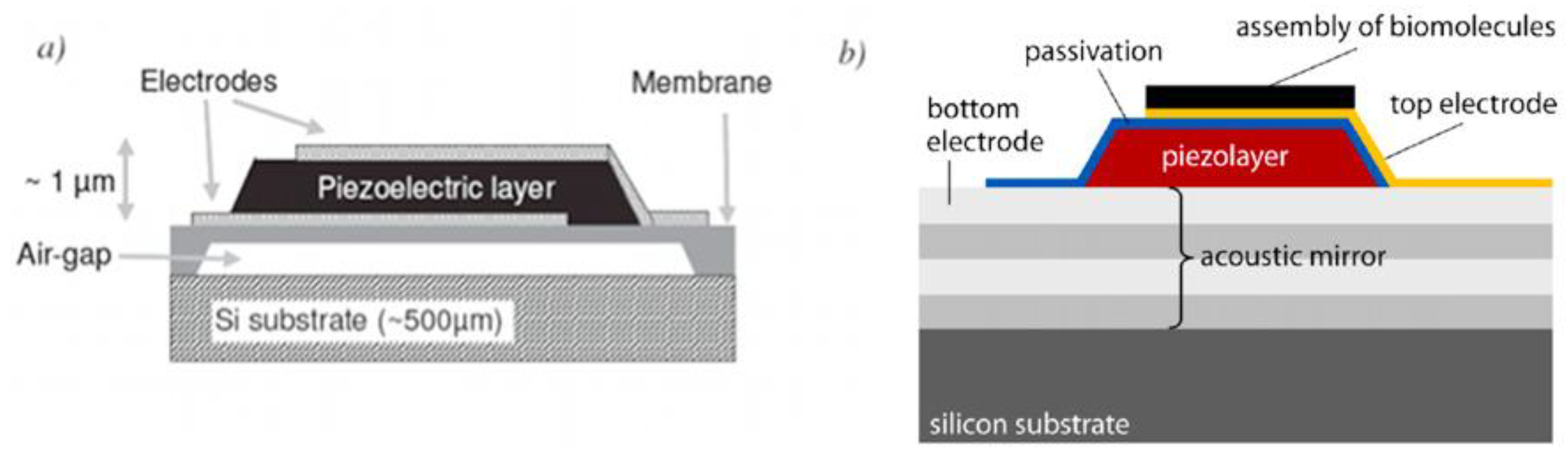
2.2. Optical Sensors
2.2.1. Surface Plasmon Resonance (SPR)
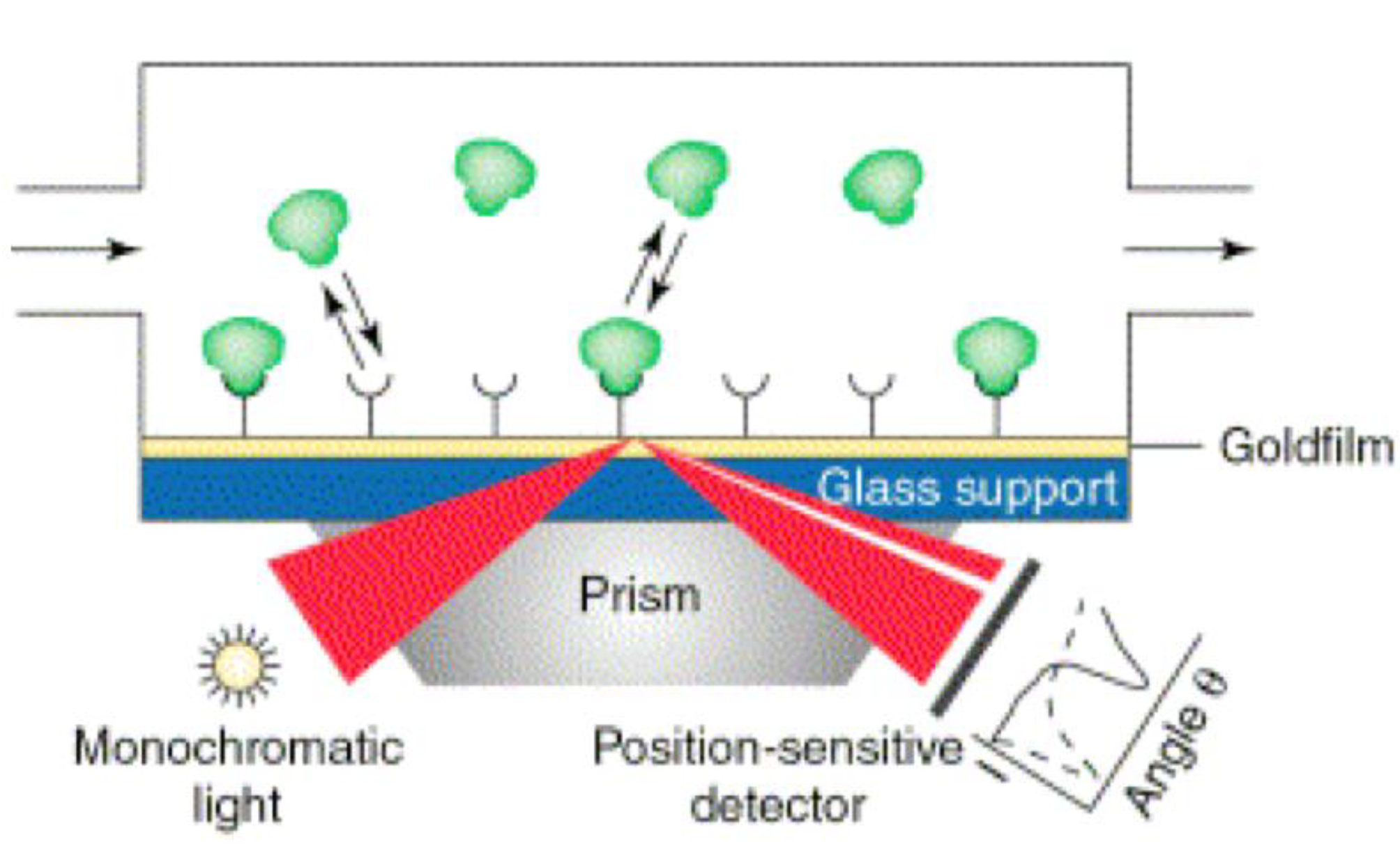
2.2.2. Surface Plasmon Resonance Imaging (SPRi)
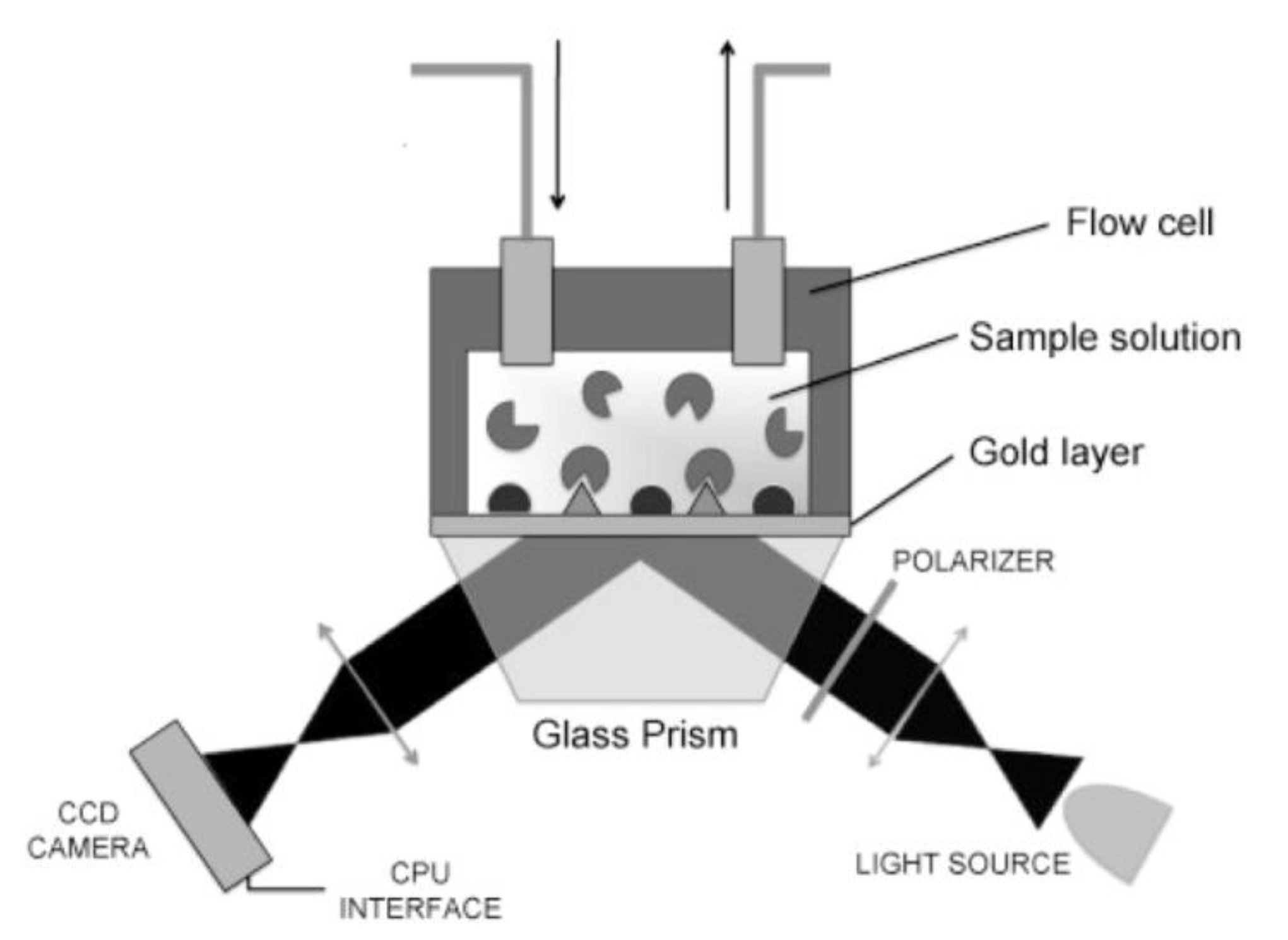
2.2.3. Biolayer Interferometry (BLI)

2.2.4. Diffraction Grating Based Sensors
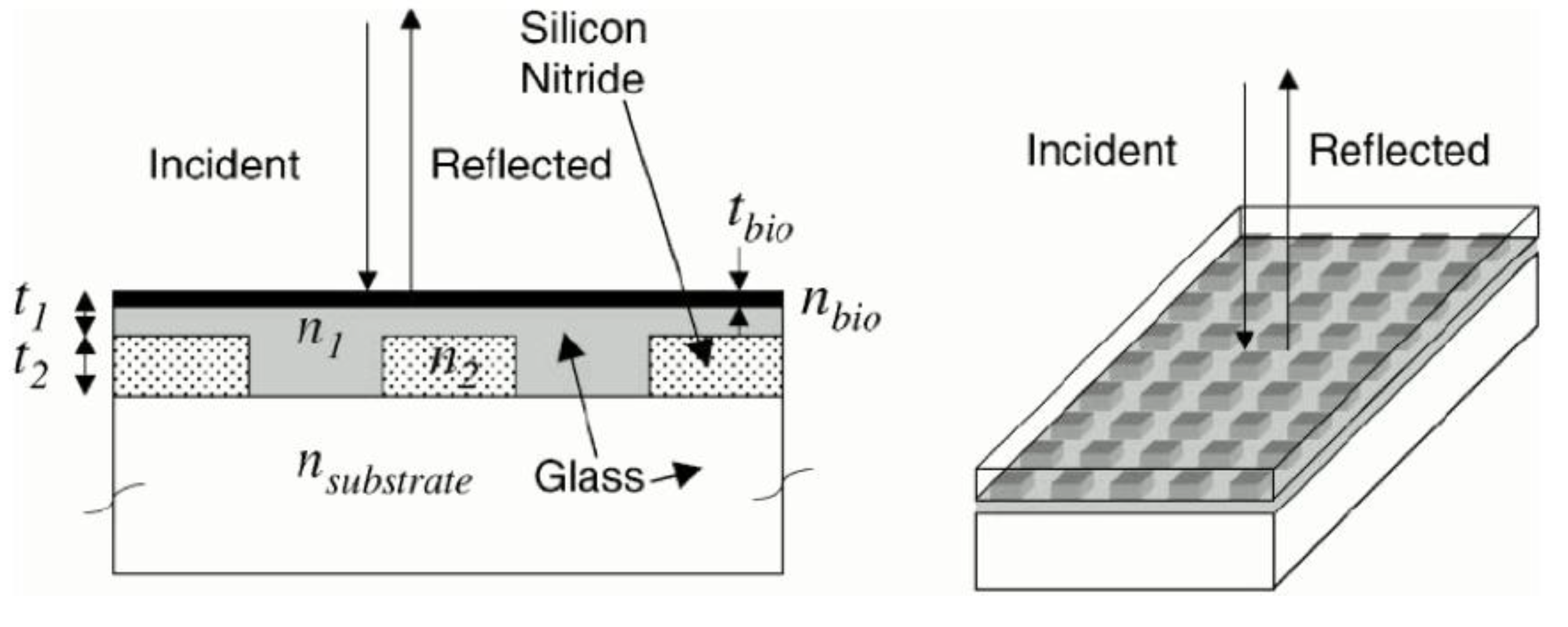
2.2.5. Optical-Waveguide-Based Transducers
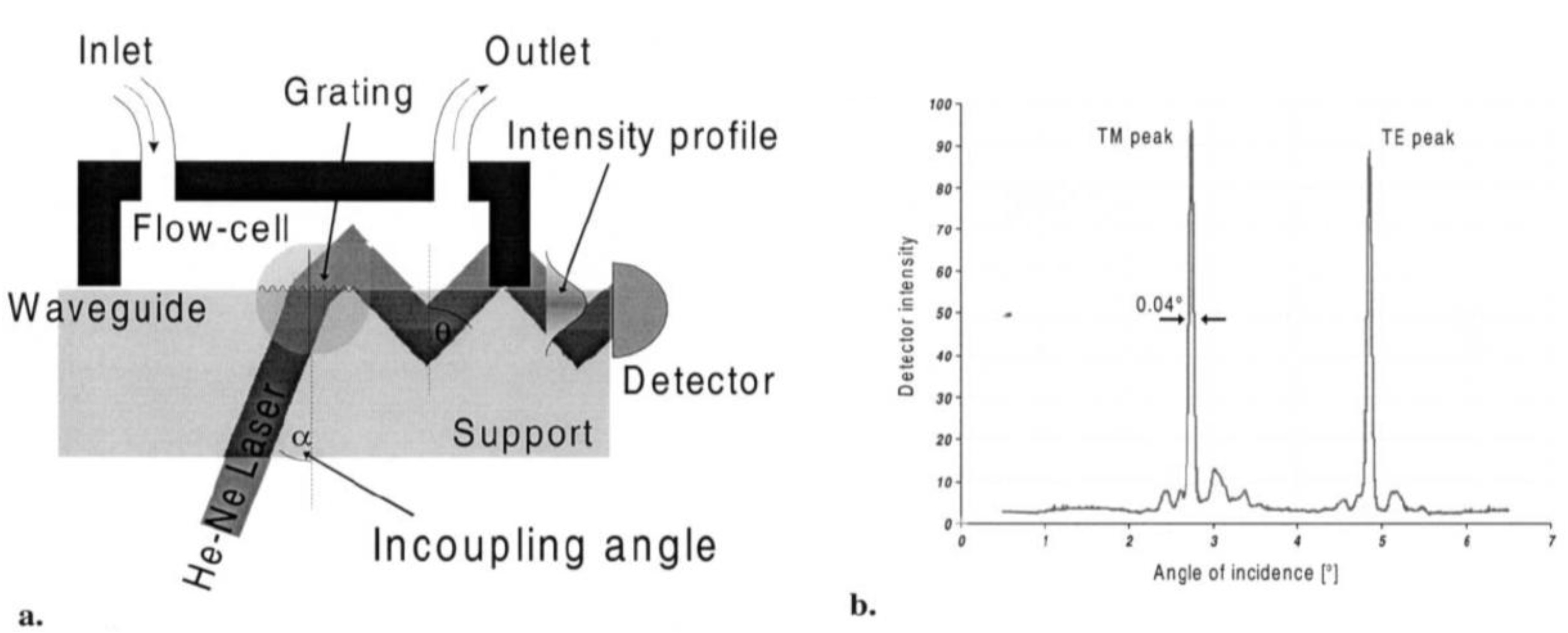
2.2.6. Ellipsometry (ELM)

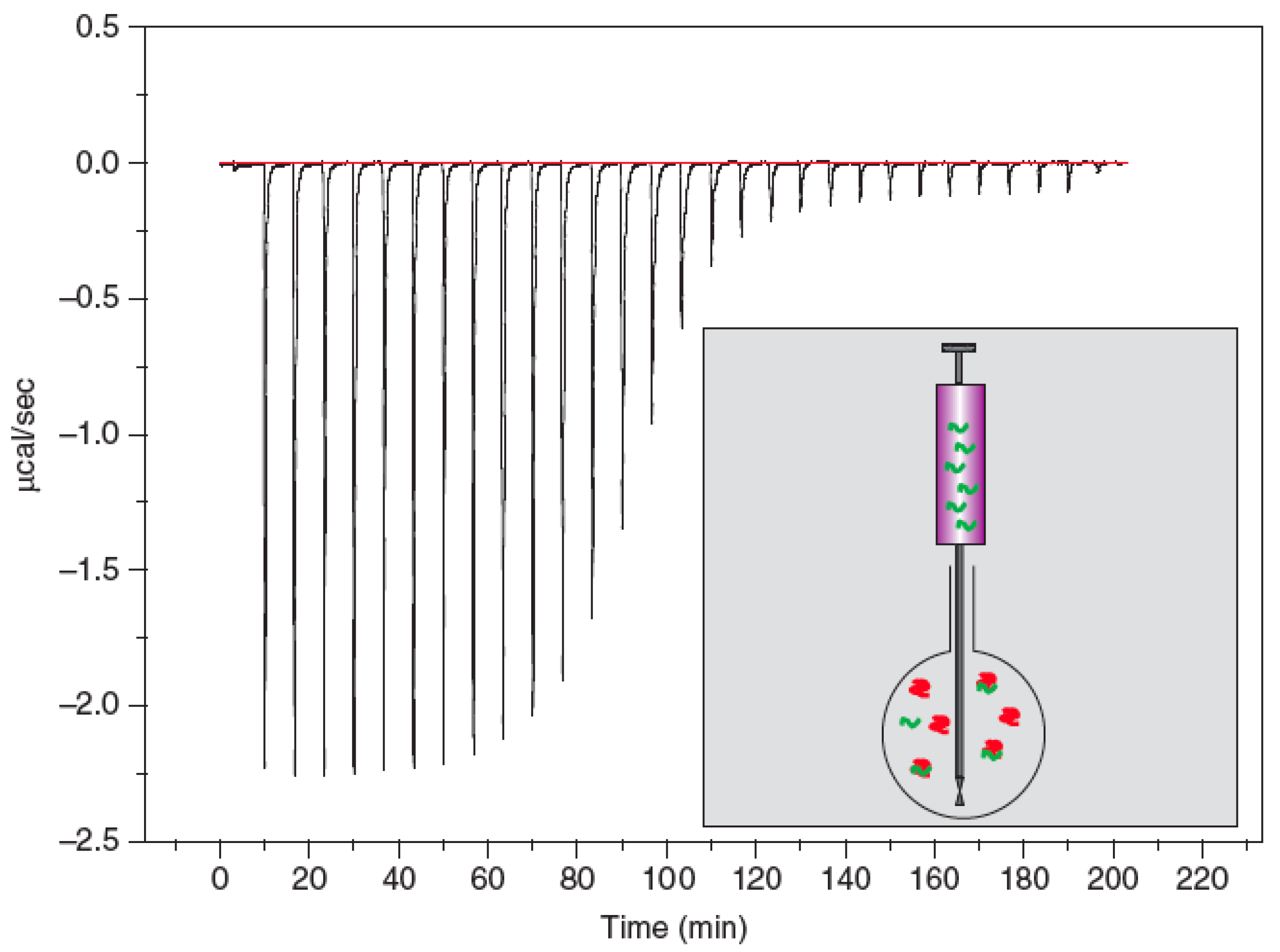
2.3. Isothermal Titration Calorimetry (ITC)
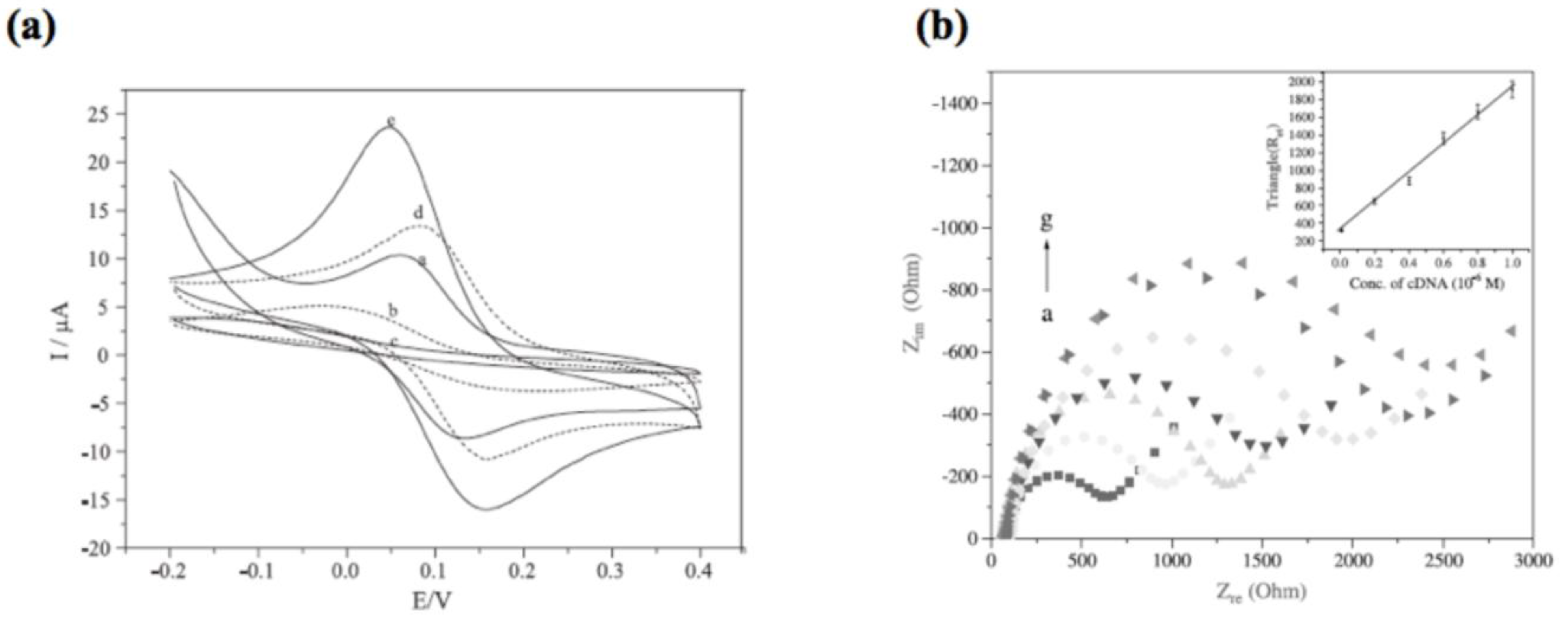
2.4. Electrochemical Sensors
2.5. Nanostructure Biosensors
2.5.1. Nanoplasmonics
2.5.2. Nanowire Biosensors
3. Conclusions
References
- Zimmermann, B.; Hahnefeld, C.; Herberg, F.W. Applications of biomolecular interaction analysis in drug development. TARGETS 2002, 1, 66–73. [Google Scholar] [CrossRef]
- Hintersteiner, M.; Buehler, C.; Uhl, V.; Schmied, M.; Müller, J.; Kottig, K.; Auer, M. Confocal Nanoscanning, Bead Picking (CONA): PickoScreen microscopes for automated and quantitative screening of one-bead one-compound libraries. J. Comb. Chem. 2009, 11, 886–894. [Google Scholar]
- Längee, K.; Rapp, B.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef]
- Milyutin, E.; Muralt, P. Thin film bulk acoustic wave resonators for gravimetric sensing. Nanosyst. Des. Technol. 2009. [Google Scholar] [CrossRef]
- Vörös, J.; Ramsden, J.; Csucs, G.; Szendr, I.; De Paul, S.; Textor, M.; Spencer, N. Optical grating coupler biosensors. Biomaterials 2002, 23, 3699–3710. [Google Scholar] [CrossRef]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Bally, M.; Halter, M.; Vörös, J.; Grandin, H. Optical microarray biosensing techniques. Surf. Interface Anal. 2006, 38, 1442–1458. [Google Scholar] [CrossRef]
- Grieshaber, D.; MacKenzie, R.; Voeroes, J.; Riemhult, E. Electrochemical biosensors—sensor principles and architectures. Sensors 2008, 8, 1440–1168. [Google Scholar]
- Stewart, M.; Anderton, C.; Thompson, L.; Maria, J.; Gray, S.; Rogers, J.; Nuzzo, R. Nanostructured plasmonic sensors. Chem. Rev. 2008, 108, 494–521. [Google Scholar] [CrossRef]
- Yan, R.; Gargas, D.; Yang, P. Nanowire photonics. Nat. Photonics 2009, 3, 569–576. [Google Scholar] [CrossRef]
- Rich, R.L.; Myszka, D.G. Higher-throughput, label-free, real-time molecular interaction analysis. Anal. Biochem. 2007, 361, 1–6. [Google Scholar] [CrossRef]
- Cooper, M.A. Label-free screening of bio-molecular interactions. Anal. Bioanal. Chem. 2003, 377, 834–842. [Google Scholar] [CrossRef]
- Cunningham, B.; Laing, L. Microplate-based, label-free detection of biomolecular interactions: Applications in proteomics. Expert Rev. Proteomics 2006, 3, 271–281. [Google Scholar] [CrossRef]
- Qavi, A.; Washburn, A.; Byeon, J.; Bailey, R. Label-free technologies for quantitative multiparameter biological analysis. Anal. Bioanal. Chem. 2009, 394, 121–135. [Google Scholar] [CrossRef]
- Barbulovic-Nad, I.; Lucente, M.; Sun, Y.; Zhang, M.; Wheeler, A.; Bussmann, M. Bio-microarray fabrication techniques—A review. Crit. Rev. Biotechnol. 2006, 26, 237–259. [Google Scholar] [CrossRef]
- Fang, Y. Label-free cell-based assays with optical biosensors in drug discovery. Assay Drug Dev. Technol. 2006, 4, 583–595. [Google Scholar] [CrossRef]
- Lucklum, R.; Hauptmann, P. Acoustic microsensors—the challenge behind microgravimetry. Anal. Bioanal. Chem. 2006, 384, 667–682. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. EPJA 1959, 155, 206–222. [Google Scholar]
- Kanazawa, K.K.; Gordon, J.G. Frequency of a quartz microbalance in contact with liquid. Anal. Chem. 1985, 57, 1770–1771. [Google Scholar] [CrossRef]
- Johannsmann, D.; Mathauer, K.; Wegner, G.; Knoll, W. Viscoelastic properties of thin films probed with a quartz-crystal resonator. Phys. Rev. B 1992, 46, 7808–7815. [Google Scholar] [CrossRef]
- Voinova, M.; Rodahl, M.; Jonson, M.; Kasemo, B. Viscoelastic acoustic response of layered polymer films at fluid-solid interfaces: Continuum mechanics approach. Phys. Scr. 1999, 59, 391–399. [Google Scholar] [CrossRef]
- Johannsmann, D. Viscoelastic, mechanical, and dielectric measurements on complex samples with the quartz crystal microbalance. Phys. Chem. Chem. Phys. 2008, 10, 4516–4534. [Google Scholar] [CrossRef]
- Domack, A.; Prucker, O.; Rühe, J.; Johannsmann, D. Swelling of a polymer brush probed with a quartz crystal resonator. Phys. Rev. E 1997, 56, 680–689. [Google Scholar] [CrossRef]
- Borovikov, A. Measurement of viscosity of media by means of shear vibration of plane piezoresonators. Instrum. Exp. Tech. 1976, 19, 223–224. [Google Scholar]
- Voinova, M.; Jonson, M.; Kasemo, B. Dynamics of viscous amphiphilic films supported by elastic solid substrates. J. Phys. Condens. Matter 1997, 9, 7799–7808. [Google Scholar] [CrossRef]
- Johannsmann, D. Studies of viscoelasticity with the QCM. In Piezoelectric Sensors; Janshoff, A., Steinem, C., Eds.; Springer: Heidelberg, Germany, 2007; Volume 5, pp. 49–109. [Google Scholar]
- Cooper, M.A.; Singleton, V.T. A survey of the 2001 to 2005 quartz crystal microbalance biosensor literature: Applications of acoustic physics to the analysis of biomolecular interactions. J. Mol. Recognit. 2007, 20, 154–184. [Google Scholar] [CrossRef]
- Wang, X.; Ellis, J.; Lyle, E.; Sundaram, P.; Thompson, M. Conformational chemistry of surface-attached calmodulin detected by acoustic shear wave propagation. Mol. BioSyst. 2006, 2, 184–192. [Google Scholar] [CrossRef]
- Furusawa, H.; Komatsu, M.; Okahata, Y. In situ monitoring of conformational changes of and peptide bindings to calmodulin on a 27 MHz quartz-crystal microbalance. Anal. Chem. 2009, 81, 1841–1847. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef]
- Keller, C.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef]
- Höök, F.; Kasemo, B.; Nylander, T.; Fant, C.; Sott, K.; Elwing, H. Variations in coupled water, viscoelastic properties, and film thickness of a Mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef]
- Weigel, R.; Morgan, D.; Owens, J.; Ballato, A.; Lakin, K.; Hashimoto, K.; Ruppel, C. Microwave acoustic materials, devices, and applications. IEEE Trans. Microw. Theory Tech. 2002, 50, 738–749. [Google Scholar] [CrossRef]
- Gizeli, E.; Goddard, N.; Lowe, C.; Stevenson, A. A Love plate biosensor utilising a polymer layer. Sens. Actuat. B 1992, 6, 131–137. [Google Scholar] [CrossRef]
- Kovacs, G.; Vellekoop, M.; Haueis, R.; Lubking, G.; Venema, A. A Love wave sensor for (bio) chemical sensing in liquids. Sens. Actuat. A 1994, 43, 38–43. [Google Scholar] [CrossRef]
- Länge, K.; Blaess, G.; Voigt, A.; Rapp, M.; Hansjosten, E.; Schygulla, U. Packaging of surface acoustic wave (SAW) based biosensors: An important issue for future biomedical applications. In Proceedings of the 2004 IEEE International Frequency Control Symposium and Exposition, Montreal, Canada, 23–27 August 2004; pp. 321–325.
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw Theory 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Gronewold, T. Surface acoustic wave sensors in the bioanalytical field: Recent trends and challenges. Anal. Chim. Acta 2007, 603, 119–128. [Google Scholar] [CrossRef]
- Benes, E.; Groschl, M.; Seifert, F.; Pohl, A. Comparison between BAW and SAW sensor principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1998, 45, 1314–1330. [Google Scholar] [CrossRef]
- El Gowini, M.M.; Moussa, W.A. A reduced three dimensional model for saw sensors using finite element analysis. Sensors 2009, 9, 9945–9964. [Google Scholar] [CrossRef]
- Bjurstrom, J.; Wingqvist, G.; Yantchev, V.; Katardjiev, I. 3I-5 design and fabrication of temperature compensated liquid FBAR sensors. In Proceedings of IEEE International Ultrasonics Symposium, Vancouver, Canada, 2–6 October 2006; pp. 898–901.
- Dickherber, A.; Corso, C.D.; Hunt, W. Lateral field excitation (LFE) of thickness shear mode (TSM) acoustic waves in thin film bulk acoustic resonators (FBAR) as a potential biosensor. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 1, 4590–4593. [Google Scholar] [CrossRef]
- Link, M.; Schreiter, M.; Weber, J.; Gabl, R.; Pitzer, D.; Primig, R.; Wersing, W.; Assouar, M.B.; Elmazria, O. C-axis inclined ZnO films for shear-wave transducers deposited by reactive sputtering using an additional blind. J. Vac. Sci. Technol. A 2006, 24, 218–222. [Google Scholar] [CrossRef]
- Weber, J.; Albers, W.M.; Tuppurainen, J.; Link, M.; Gabl, R.; Wersing, W.; Schreiter, M. Shear mode FBARs as highly sensitive liquid biosensors. Sens. Actuat. A 2006, 128, 84–88. [Google Scholar] [CrossRef]
- Lakin, K.M. Thin film resonators and filters. In Proceedings of IEEE Ultrasonics Symposium 1999, Lake Tahoe, NV, USA, 1999; Volume 892, pp. 895–906.
- Wang, J.S.; Lakin, K.M. Sputtered c-axis inclined ZnO films for shear wave resonators. In Proceedings of Ultrasonics Symposium 1982, San Diego, CA, USA, 27–29 October 1982; pp. 480–483.
- Carlotti, G.; Fioretto, D.; Socino, G.; Palmieri, L.; Petri, A.; Verona, E. Surface acoustic waves in c-axis inclined ZnO films. In Proceedings of IEEE Ultrasonics Symposium 1990, Honolulu, HI, USA, 4–7 December 1990; pp. 449–453.
- Fardeheb-Mammeri, A.; Assouar, M.B.; Elmazria, O.; Gatel, C.; Fundenberger, J.J.; Benyoucef, B. C-axis inclined AlN film growth in planar system for shear wave devices. Diamond Relat. Mater. 2008, 17, 1770–1774. [Google Scholar] [CrossRef]
- Link, M.; Schreiter, M.; Weber, J.; Pitzer, D.; Primig, R.; Assouar, M.B.; Elmazria, O. C-axis inclined ZnO films deposited by reactive sputtering using an additional blind for shear BAW devices. In Proceedings of IEEE Ultrasonics Symposium 2005, Rotterdam, The Netherlands, 18–21 September 2005; pp. 202–205.
- Akiyama, M.; Nagao, K.; Ueno, N.; Tateyama, H.; Yamada, T. Influence of metal electrodes on crystal orientation of aluminum nitride thin films. Vacuum 2004, 74, 699–703. [Google Scholar] [CrossRef]
- Martin, F.; Jan, M.E.; Rey-Mermet, S.; Su, D.; Muralt, P.; Cantoni, M. Shear mode coupling and tilted grain growth of AlN thin films in BAW resonators. In Proceedings of IEEE Ultrasonics Symposium 2005, Rotterdam, The Netherlands, 18–21 September 2005; pp. 333–336.
- Yanagitani, T.; Kiuchi, M.; Matsukawa, M.; Watanabe, Y. Characteristics of pure-shear mode BAW resonators consisting of (1120) textured ZnO films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 1680–1686. [Google Scholar] [CrossRef]
- Wingqvist, G.; Bjurstrom, J.; Katardjiev, I. Shear mode AlN thin film electroacoustic resonator for biosensor applications. In Proceedings of IEEE Ultrasonics Symposium 2005, Rotterdam, The Netherlands, 18–21 September 2005; pp. 50–53.
- Nirschl, M.; Rantala, A.; Tukkiniemi, K.; Auer, S.; Hellgren, A.C.; Pitzer, D.; Schreiter, M.; Vikholm-Lundin, I. CMOS-Integrated film bulk acoustic resonators for label-free biosensing. Sensors 2010, 10, 4180–4193. [Google Scholar] [CrossRef]
- Link, M. Study and Realization of Shear Wave Mode Solidly Mounted Film Bulk Acoustic Resonators (FBAR) Made of Caxis Inclined Zinc Oxide (Zno) Thin Films: Application as Gravimetric Sensors in Liquid Environments. Ph.D. Thesis, UniversitÈ Henri PoincarÈ, Nancy I, Nancy Cedex, France, 2006. [Google Scholar]
- Nirschl, M.; Blüher, A.; Erler, C.; Katzschner, B.; Vikholm-Lundin, I.; Auer, S.; Vörös, J.; Pompe, W.; Schreiter, M.; Mertig, M. Film bulk acoustic resonators for DNA and protein detection and investigation of in vitro bacterial S-layer formation. Sens. Actuat. A 2009, 156, 180–184. [Google Scholar] [CrossRef]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef]
- Harrison, D.; Kjellberg, H. Segmenting a market in the making: Industrial market segmentation as construction. Ind. Mark. Manage. 2010, 39, 784–792. [Google Scholar] [CrossRef]
- Siontorou, C.; Batzias, F. Innovation in biotechnology: Moving from academic research to product development-the case of biosensors. Crit. Rev. Biotechnol. 2010, 30, 79–98. [Google Scholar] [CrossRef]
- Bergstrom, J.; Lofaas, S.; Johnsson, B. Matrix Coating for Sensing Surfaces Capable of Selective Biomolecular Interactions, to be Used in Biosensor Systems. U.S. Patent 5,436,161, 25 July 1995. [Google Scholar]
- Löfås, S.; Johnsson, B. A novel hydrogel matrix on gold surfaces in surface plasmon resonance sensors for fast and efficient covalent immobilization of ligands. J. Chem. Soc. Chem. Commun. 1990, 1990, 1526–1528. [Google Scholar]
- Hearty, S.; Conroy, P.; Ayyar, B.; Byrne, B.; OKennedy, R. Surface plasmon resonance for vaccine design and efficacy studies: Recent applications and future trends. Expert Rev. Vaccines 2010, 9, 645–664. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Nelson, B.P.; Grimsrud, T.E.; Liles, M.R.; Goodman, R.M.; Robert, M. Surface plasmon resonance imaging measurements of DNA and RNA hybridization adsorption onto DNA microarrays. Anal. Chem. 2001, 73, 1–7. [Google Scholar] [CrossRef]
- Shumaker-Parry, J.S.; Campbell, C.T. Quantitative methods for spatially resolved adsorption/desorption measurements in real time by surface plasmon resonance microscopy. Anal. Chem. 2004, 76, 907–917. [Google Scholar] [CrossRef]
- Boozer, C.; Kim, G.; Cong, S.; Guan, H.W.; Londergan, T. Looking towards label-free biomolecular interaction analysis in a high-throughput format: A review of new surface plasmon resonance technologies. Curr. Opin. Biotechnol. 2006, 17, 400–405. [Google Scholar] [CrossRef]
- Shumaker-Parry, J.S.; Zareie, M.H.; Aebersold, R.; Campbell, C.T. Microspotting streptavidin and double-stranded DNA arrays on gold for high-throughput studies of protein-DNA interactions by surface plasmon resonance microscopy. Anal. Chem. 2004, 76, 918–929. [Google Scholar] [CrossRef]
- Scarano, S.; Scuffi, C.; Mascini, M.; Minunni, M. Surface plasmon resonance imaging (SPRi)-based sensing: A new approach in signal sampling and management. Biosens. Bioelectron. 2010, 26, 1380–1385. [Google Scholar] [CrossRef]
- Flournoy, P.; McClure, R.; Wyntjes, G. White-Light interferometric thickness gauge. Appl. Opt. 1972, 11, 1907–1915. [Google Scholar] [CrossRef]
- Do, T.; Ho, F.; Heidecker, B.; Witte, K.; Chang, L.; Lerner, L. A rapid method for determining dynamic binding capacity of resins for the purification of proteins. Protein Expr. Purif. 2008, 60, 147–150. [Google Scholar] [CrossRef]
- Abdiche, Y.; Malashock, D.; Pinkerton, A.; Pons, J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal. Biochem. 2008, 377, 209–217. [Google Scholar] [CrossRef]
- Cunningham, B.; Li, P.; Lin, B.; Pepper, J. Colorimetric resonant reflection as a direct biochemical assay technique. Sens. Actuat. B 2002, 81, 316–328. [Google Scholar] [CrossRef]
- Tiefenthaler, K.; Lukosz, W. Sensitivity of grating couplers as integrated-optical chemical sensors. J. Opt. Soc. Am. B 1989, 6, 209–220. [Google Scholar] [CrossRef]
- Ramsden, J. Review of new experimental techniques for investigating random sequential adsorption. J. Stat. Phys. 1993, 73, 853–877. [Google Scholar] [CrossRef]
- Brusatori, M.; Tie, Y.; Van Tassel, P. Protein adsorption kinetics under an applied electric field: An optical waveguide lightmode spectroscopy study. Langmuir 2003, 19, 5089–5097. [Google Scholar] [CrossRef]
- Höök, F.; Vörös, J.; Rodahl, M.; Kurrat, R.; Böni, P.; Ramsden, J.; Textor, M.; Spencer, N.; Tengvall, P.; Gold, J. A comparative study of protein adsorption on titanium oxide surfaces using in situ ellipsometry, optical waveguide lightmode spectroscopy, and quartz crystal microbalance/dissipation. Colloids Surf. B 2002, 24, 155–170. [Google Scholar] [CrossRef]
- Cross, G.; Reeves, A.; Brand, S.; Swann, M.; Peel, L.; Freeman, N.; Lu, J. The metrics of surface adsorbed small molecules on the Young's fringe dual-slab waveguide interferometer. J. Phys. D 2004, 37, 74–80. [Google Scholar]
- Cross, G.; Reeves, A.; Brand, S.; Popplewell, J.; Peel, L.; Swann, M.; Freeman, N. A new quantitative optical biosensor for protein characterisation. Biosens. Bioelectron. 2003, 19, 383–390. [Google Scholar] [CrossRef]
- Rothen, A. The ellipsometer, an apparatus to measure thicknesses of thin surface films. Rev. Sci. Instrum. 1945, 16, 26–30. [Google Scholar] [CrossRef]
- Tompkins, H.; Irene, E. Handbook of Ellipsometry; William Andrew: Mona Vale, Australia, 2005. [Google Scholar]
- Westphal, P.; Bornmann, A. Biomolecular detection by surface plasmon enhanced ellipsometry. Sens. Actuat. B 2002, 84, 278–282. [Google Scholar] [CrossRef]
- Kurrat, R.; Wälivaara, B.; Marti, A.; Textor, M.; Tengvall, P.; Ramsden, J.; Spencer, N. Plasma protein adsorption on titanium: Comparative in situ studies using optical waveguide lightmode spectroscopy and ellipsometry. Colloids Surf. B 1998, 11, 187–201. [Google Scholar] [CrossRef]
- Freire, E. Isothermal titration calorimetry. Curr. Protoc Cell Biol. 2004, 17, 11–24. [Google Scholar]
- Bruylants, G.; Wouters, J.; Michaux, C. Differential scanning calorimetry in life science: Thermodynamics, stability, molecular recognition and application in drug design. Curr. Med. Chem. 2005, 12, 2011–2020. [Google Scholar] [CrossRef]
- Brown, M.E. Introduction to Thermal Analysis: Techniques and Applications; Springer: Dordrecht, The Netherlands, 2001; Volume 1. [Google Scholar]
- Leavitt, S.; Freire, E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Current Opinion in Structural Biology 2001, 11, 560–566. [Google Scholar] [CrossRef]
- Sethi, R. Transducer aspects of biosensors. Biosens. Bioelectron. 1994, 9, 243–264. [Google Scholar] [CrossRef]
- Macdonald, J. Impedence Spectroscopy—Emphasizing Solid Materials and Systems; John Wiley and Sons: Hoboken, NJ, USA, 1987; pp. 1–346. [Google Scholar]
- Bravman, T.; Bronner, V.; Lavie, K.; Notcovich, A.; Papalia, G.; Myszka, D. Exploring one-shot kinetics and small molecule analysis using the ProteOn XPR36 array biosensor. Anal. Biochem. 2006, 358, 281–288. [Google Scholar] [CrossRef]
- Chang-Yen, D.; Myszka, D.; Gale, B. A novel PDMS microfluidic spotter for fabrication of protein chips and microarrays. J. Microelectromech. Syst. 2006, 15, 1145–1151. [Google Scholar] [CrossRef]
- Schöning, M.; Poghossian, A. Bio FEDs (field-effect devices): State-of-the-art and new directions. Electroanalysis 2006, 18, 1893–1900. [Google Scholar] [CrossRef]
- Bearinger, J.; Vörös, J.; Hubbell, J.; Textor, M. Electrochemical optical waveguide lightmode spectroscopy (EC-OWLS): A pilot study using evanescent-field optical sensing under voltage control to monitor polycationic polymer adsorption onto indium tin oxide (ITO)-coated waveguide chips. Biotechnol. Bioeng. 2003, 82, 465–473. [Google Scholar] [CrossRef]
- Brusatori, M.; Van Tassel, P. Biosensing under an applied voltage using optical waveguide lightmode spectroscopy. Biosens. Bioelectron. 2003, 18, 1269–1277. [Google Scholar] [CrossRef]
- Kang, X.; Cheng, G.; Dong, S. A novel electrochemical SPR biosensor. Electrochem. Commun. 2001, 3, 489–493. [Google Scholar] [CrossRef]
- Lavers, C.; Harris, R.; Hao, S.; Wilkinson, J.; O’Dwyer, K.; Brust, M.; Schiffrin, D. Electrochemically-controlled waveguide-coupled surface plasmon sensing. J. Electroanal. Chem. 1995, 387, 11–22. [Google Scholar] [CrossRef]
- Ying, P.; Viana, A.; Abrantes, L.; Jin, G. Adsorption of human serum albumin onto gold: A combined electrochemical and ellipsometric study. J. Colloid Interface Sci. 2004, 279, 95–99. [Google Scholar]
- Wang, Z.; Viana, A.; Jin, G.; Abrantes, L. Immunosensor interface based on physical and chemical immunoglobulin G adsorption onto mixed self-assembled monolayers. Bioelectrochemistry 2006, 69, 180–186. [Google Scholar] [CrossRef]
- Yu, Y.; Jin, G. Influence of electrostatic interaction on fibrinogen adsorption on gold studied by imaging ellipsometry combined with electrochemical methods. J. Colloid Interface Sci. 2005, 283, 477–481. [Google Scholar] [CrossRef]
- Marx, K.A. Quartz crystal microbalance: a useful tool for studying thin polymer films and complex biomolecular systems at the solution-surface interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef]
- Dong, Y. The frequency response of QCM in electrochemically characterizing the immobilization on gold electrode. Sens. Actuat. B 2005, 108, 622–626. [Google Scholar] [CrossRef]
- Fu, Y.; Yuan, R.; Xu, L.; Chai, Y.; Liu, Y.; Tang, D.; Zhang, Y. Electrochemical impedance behavior of DNA biosensor based on colloidal Ag and bilayer two-dimensional sol-gel as matrices. J. Biochem. Biophys. Methods 2005, 62, 163–174. [Google Scholar] [CrossRef]
- Feynman, R. There’s plenty of room at the bottom. Available online: http://www.zyvex.com/nanotech/feynman.html (accessed on 28 April 2011).
- Dahlin, A.; Chen, S.; Jonsson, M.; Gunnarsson, L.; Käll, M.; Höök, F. High-resolution microspectroscopy of plasmonic nanostructures for miniaturized biosensing. Anal. Chem. 2009, 81, 6572–6580. [Google Scholar]
- Larsson, C.; Rodahl, M.; Hook, F. Characterization of DNA immobilization and subsequent hybridization on a 2D arrangement of streptavidin on a biotin-modified lipid bilayer supported on SiO2. Anal. Chem. 2003, 75, 5080–5087. [Google Scholar] [CrossRef]
- Rindzevicius, T.; Alaverdyan, Y.; Dahlin, A.; Höök, F.; Sutherland, D.; Käll, M. Plasmonic sensing characteristics of single nanometric holes. Nano Lett. 2005, 5, 2335–2339. [Google Scholar] [CrossRef]
- MacKenzie, R.; Auzelyte, V.; Olliges, S.; Spolenak, R.; Solak, H.; Vörös, J. Nanowire development and characterization for applications in biosensing. Nanosyst. Des. Technol. 2009. [Google Scholar] [CrossRef]
- Sannomiya, T.; Sahoo, P.; Mahcicek, D.; Solak, H.; Hafner, C.; Grieshaber, D.; Vörös, J. Biosensing by densely packed and optically coupled plasmonic particle arrays. Small 2009, 5, 1889–1896. [Google Scholar] [CrossRef]
- Willets, K.; Van Duyne, R. Localized surface plasmon resonance spectroscopy and sensing. Annu Rev. Phys. Chem. 2007, 58, 267–297. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Nath, S.; Kundu, S.; Esumi, K.; Pal, T. Solvent and ligand effects on the localized surface plasmon resonance (LSPR) of gold colloids. J. Phys. Chem. B 2004, 108, 13963–13971. [Google Scholar]
- Hutter, E.; Fendler, J. Exploitation of localized surface plasmon resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Koehne, J.; Chen, H.; Cassell, A.; Ye, Q.; Han, J.; Meyyappan, M.; Li, J. Miniaturized multiplex label-free electronic chip for rapid nucleic acid analysis based on carbon nanotube nanoelectrode arrays. Clin. Chem. 2004, 50, 1886–1893. [Google Scholar] [CrossRef]
- Svedendahl, M.; Chen, S.; Dmitriev, A.; Käll, M. Refractometric sensing using propagating versus localized surface plasmons: A direct comparison. Nano Lett. 2009, 9, 4428–4433. [Google Scholar] [CrossRef]
- Karlsson, R.; Kullman-Magnusson, M.; Hämäläinen, M.; Remaeus, A.; Andersson, K.; Borg, P.; Gyzander, E.; Deinum, J. Biosensor analysis of drug-target interactions: Direct and competitive binding assays for investigation of interactions between thrombin and thrombin inhibitors. Anal. Biochem. 2000, 278, 1–13. [Google Scholar]
- Gao, Z.; Agarwal, A.; Trigg, A.; Singh, N.; Fang, C.; Tung, C.; Fan, Y.; Buddharaju, K.; Kong, J. Silicon nanowire arrays for label-free detection of DNA. Anal. Chem. 2007, 79, 3291–3297. [Google Scholar] [CrossRef]
- Elfström, N.; Juhasz, R.; Sychugov, I.; Engfeldt, T.; Karlström, A.; Linnros, J. Surface charge sensitivity of silicon nanowires: Size dependence. Nano Lett. 2007, 7, 2608–2612. [Google Scholar]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.; Lieber, C. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Stern, E.; Wagner, R.; Sigworth, F.; Breaker, R.; Fahmy, T.; Reed, M. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 2007, 7, 3405–3409. [Google Scholar] [CrossRef]
- Wanekaya, A.; Chen, W.; Myung, N.; Mulchandani, A. Nanowire-based electrochemical biosensors. Electroanalysis 2006, 18, 533–550. [Google Scholar] [CrossRef]
- Karlsson, R. Affinity analysis of non-steady-state data obtained under mass transport limited conditions using BIAcore technology. J. Mol. Recognit. 1999, 12, 285–292. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nirschl, M.; Reuter, F.; Vörös, J. Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis. Biosensors 2011, 1, 70-92. https://doi.org/10.3390/bios1030070
Nirschl M, Reuter F, Vörös J. Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis. Biosensors. 2011; 1(3):70-92. https://doi.org/10.3390/bios1030070
Chicago/Turabian StyleNirschl, Martin, Florian Reuter, and Janos Vörös. 2011. "Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis" Biosensors 1, no. 3: 70-92. https://doi.org/10.3390/bios1030070
APA StyleNirschl, M., Reuter, F., & Vörös, J. (2011). Review of Transducer Principles for Label-Free Biomolecular Interaction Analysis. Biosensors, 1(3), 70-92. https://doi.org/10.3390/bios1030070




