Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
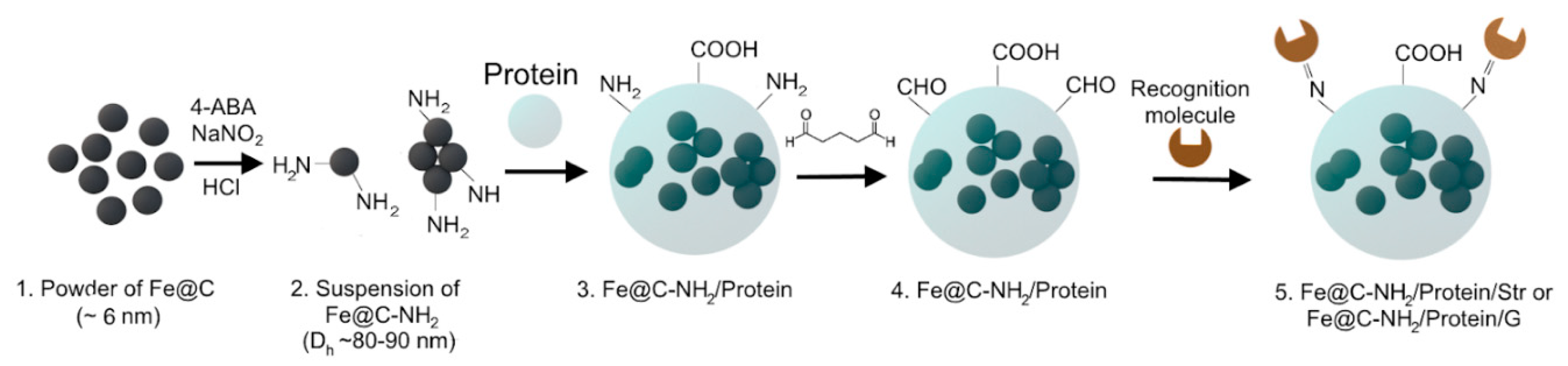
2.2. Preparation of Aminated Fe@C (Fe@C-NH2)
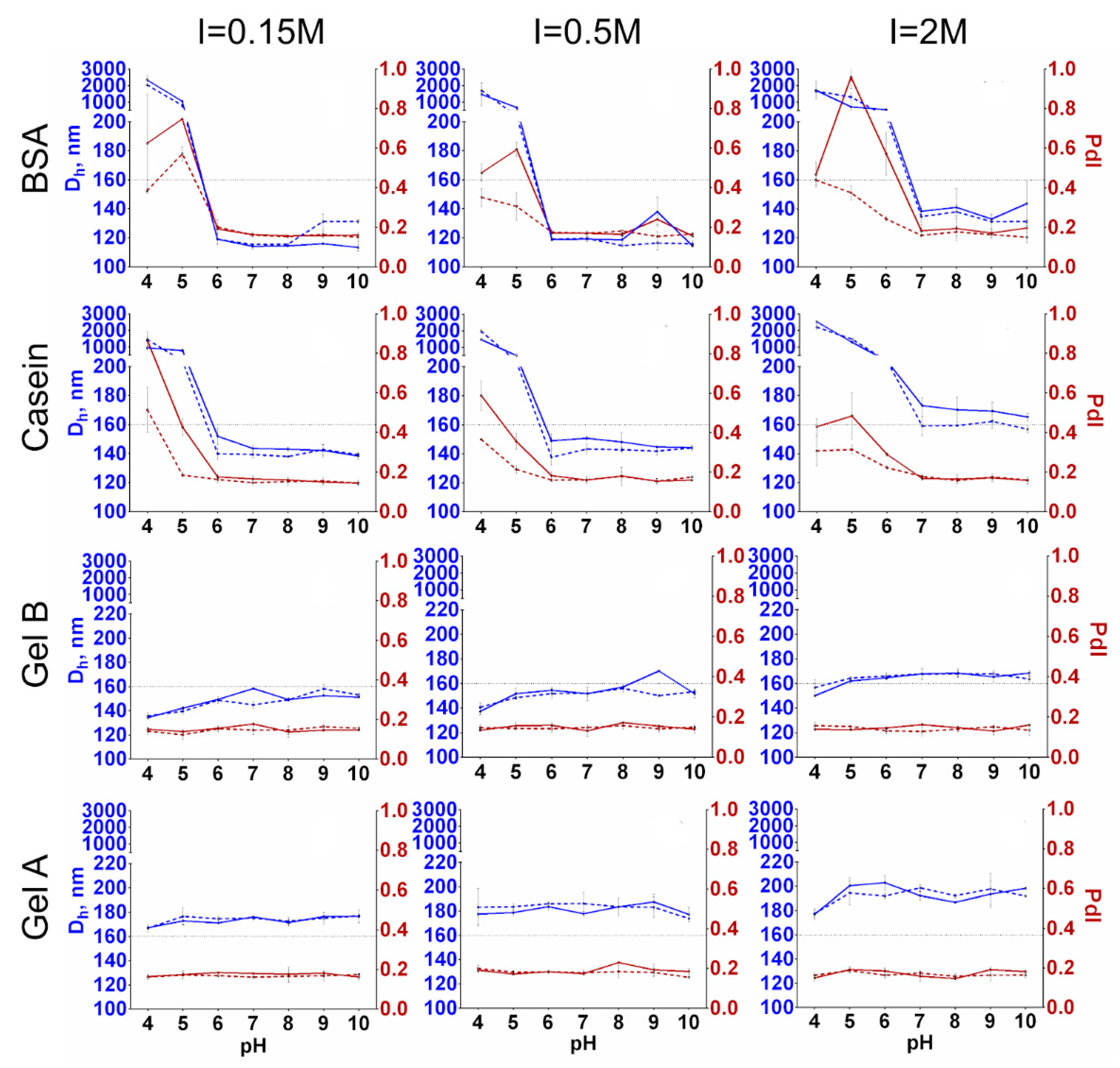
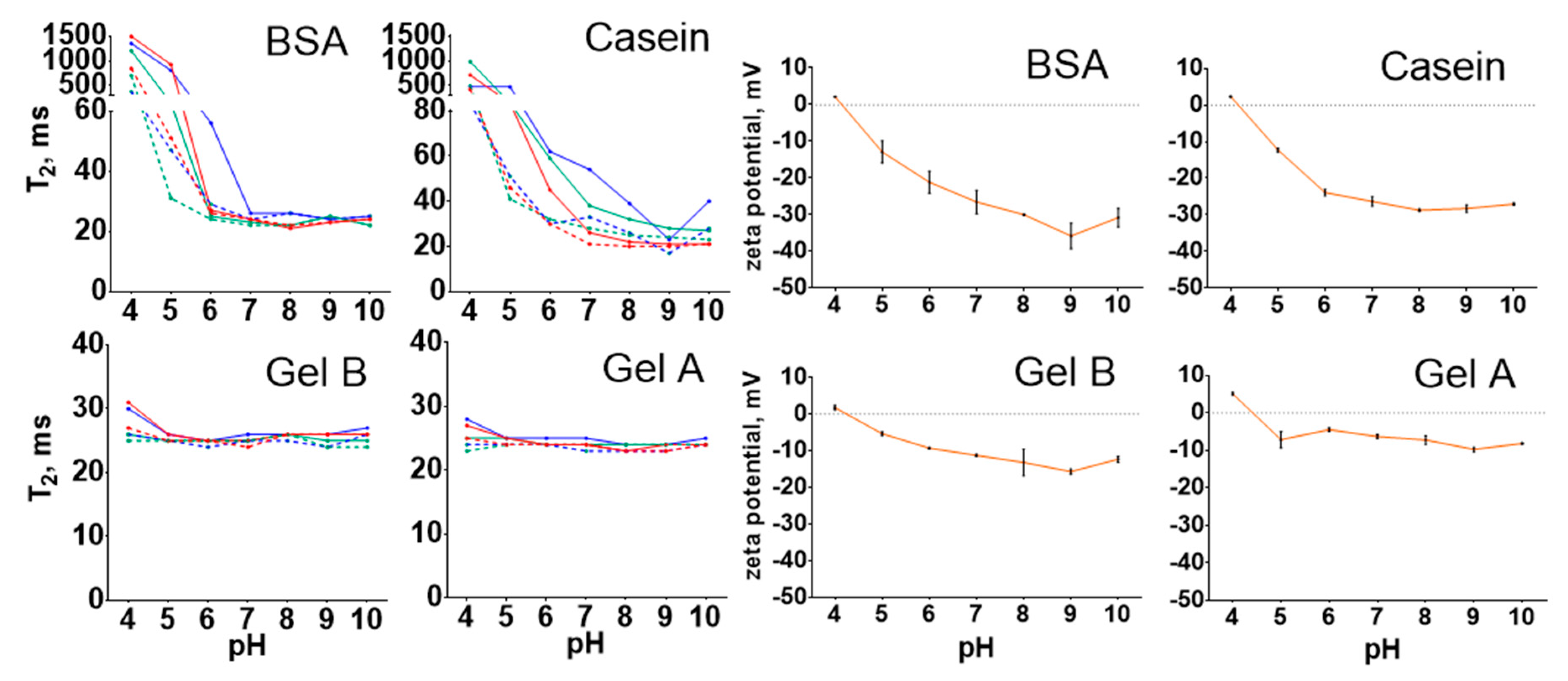
2.3. Influence of pH, Ionic Strength and Protein-to-Nanoparticle Ratio on the Size of Protein-Coated Nanoclusters
2.4. Synthesis of Protein-Coated Fe@C-NH2 Nanoclusters Conjugated with Streptavidin and Streptococcal Protein G
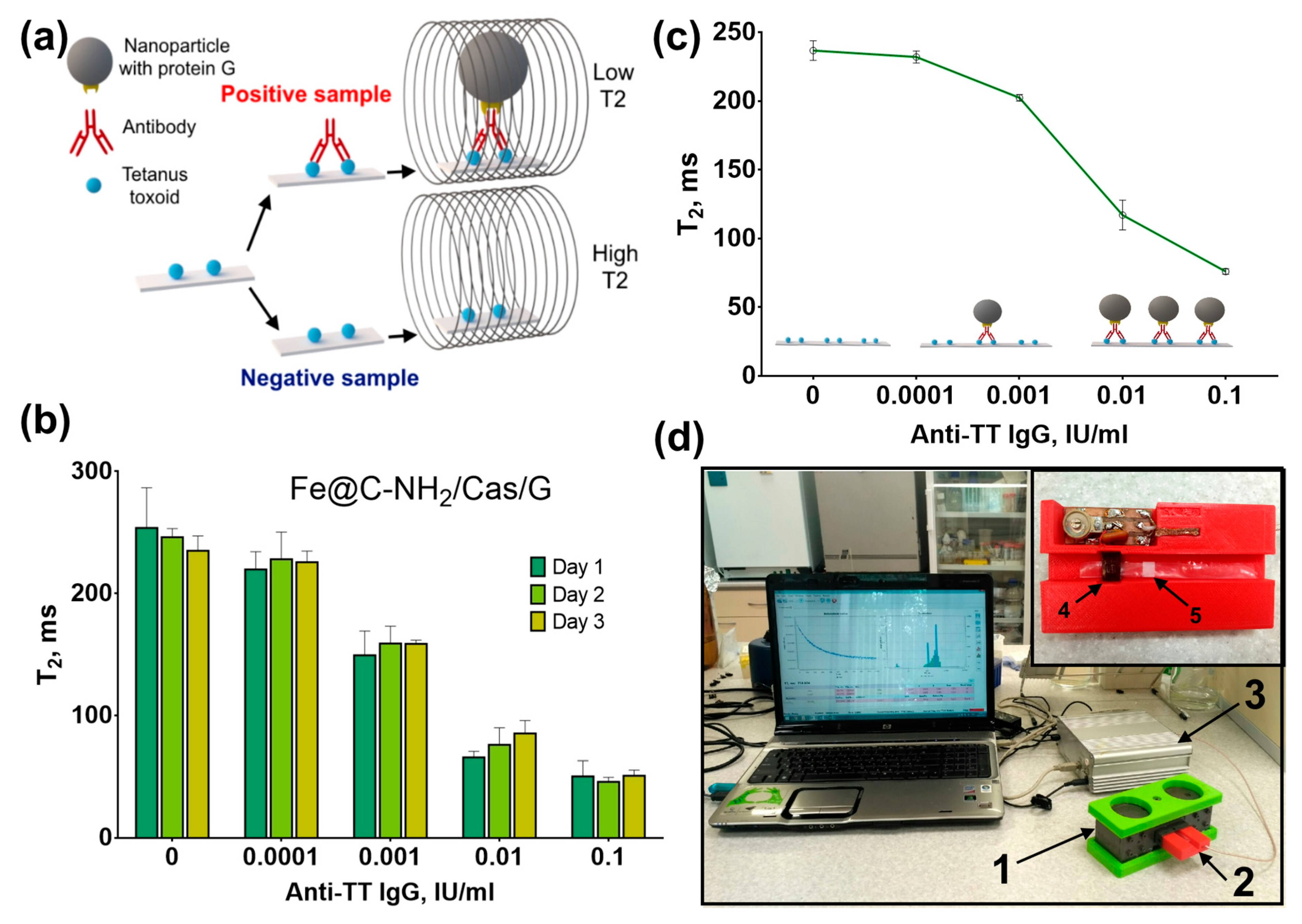
2.5. Assessment of Functional Activity of Protein-Coated Nanoclusters and Determination of Anti-Tetanus Toxoid in Serum Samples by NMR-Based Assay
2.6. Agarose Gel Electrophoresis
2.7. Protein Corona Study
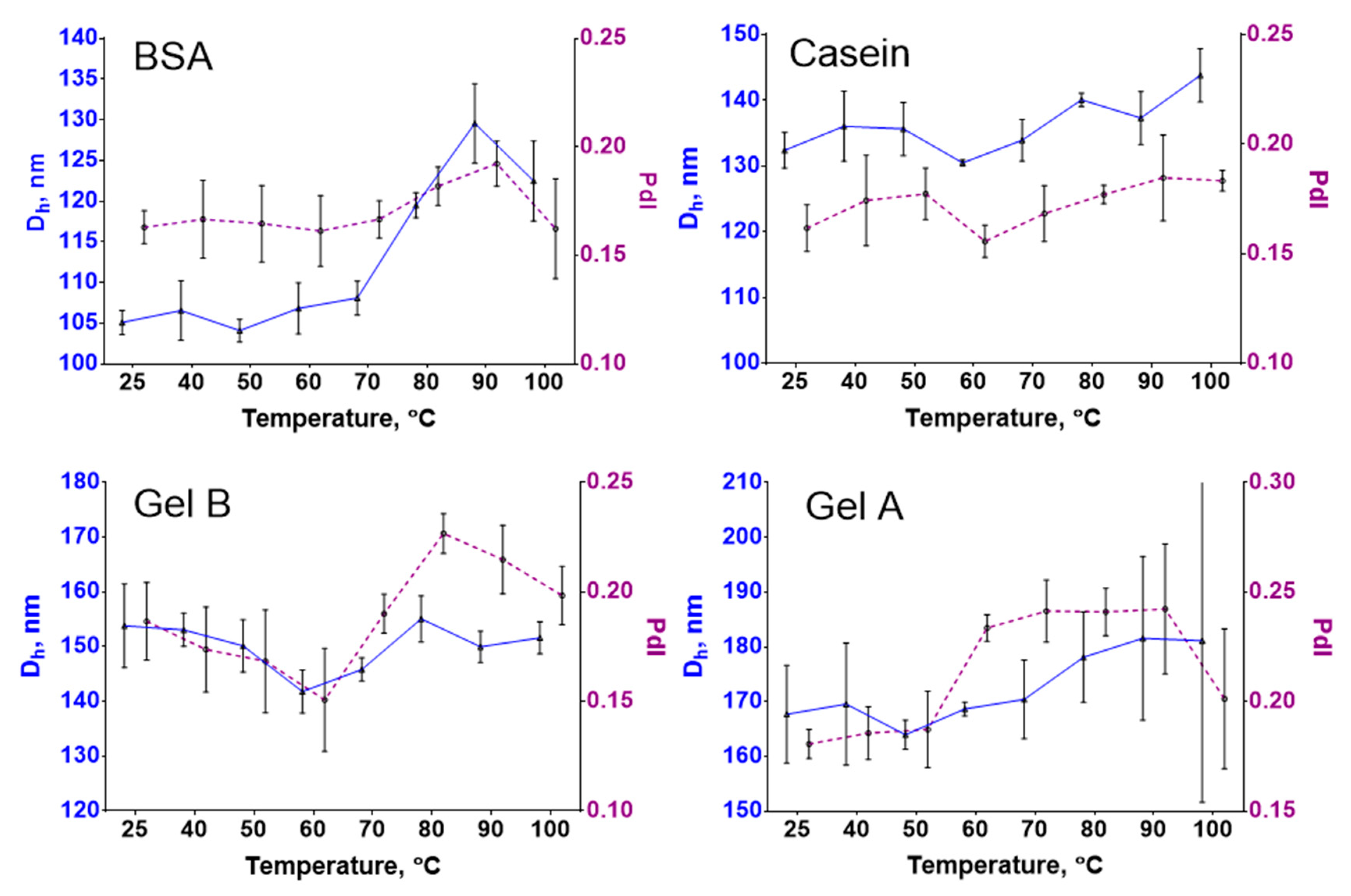
2.8. Thermal Stability of Nanoclusters
2.9. UV-VIS Spectrophotometry
2.10. Thermogravimetric Analysis
2.11. Dynamic Light Scattering (DLS)
2.12. Atomic Force Microscopy (AFM)
2.13. Colloidal Stability of Nanoclusters
2.14. Storage Stability of Protein-Coated Nanoclusters
2.15. Stability of Nanoclusters in Complex Media
2.16. Stability of Nanoclusters to Proteolytic Digestion
2.17. Determination of Nanoparticles’ Relaxivity
3. Results and Discussion
3.1. Influence of pH, Ionic Strength, and Protein-to-Nanoparticle Ratio on the Size of Protein-Coated Nanoclusters
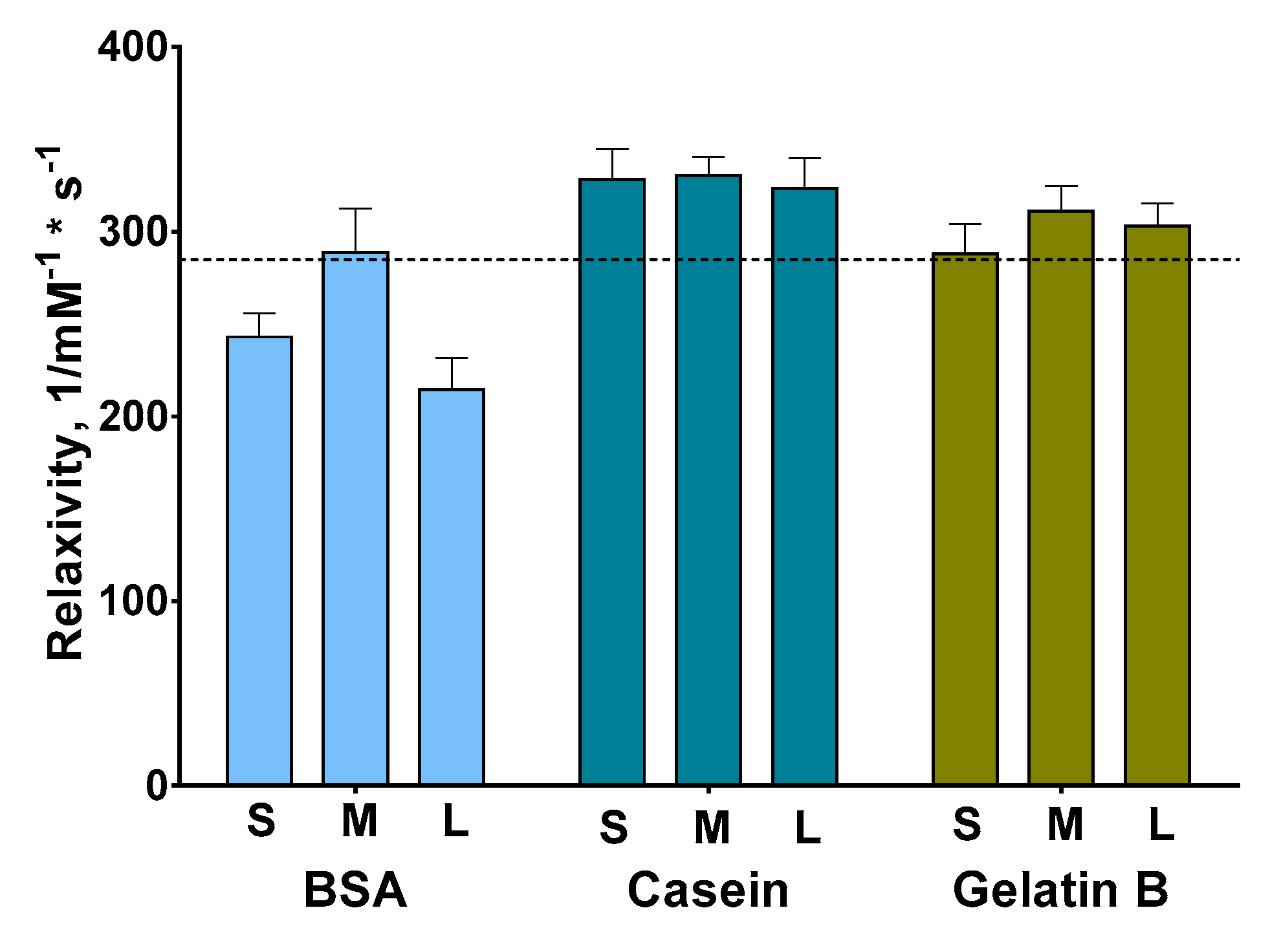
3.2. Synthesis and Relaxivity Study of Protein-Coated Nanoclusters with Tunable Size
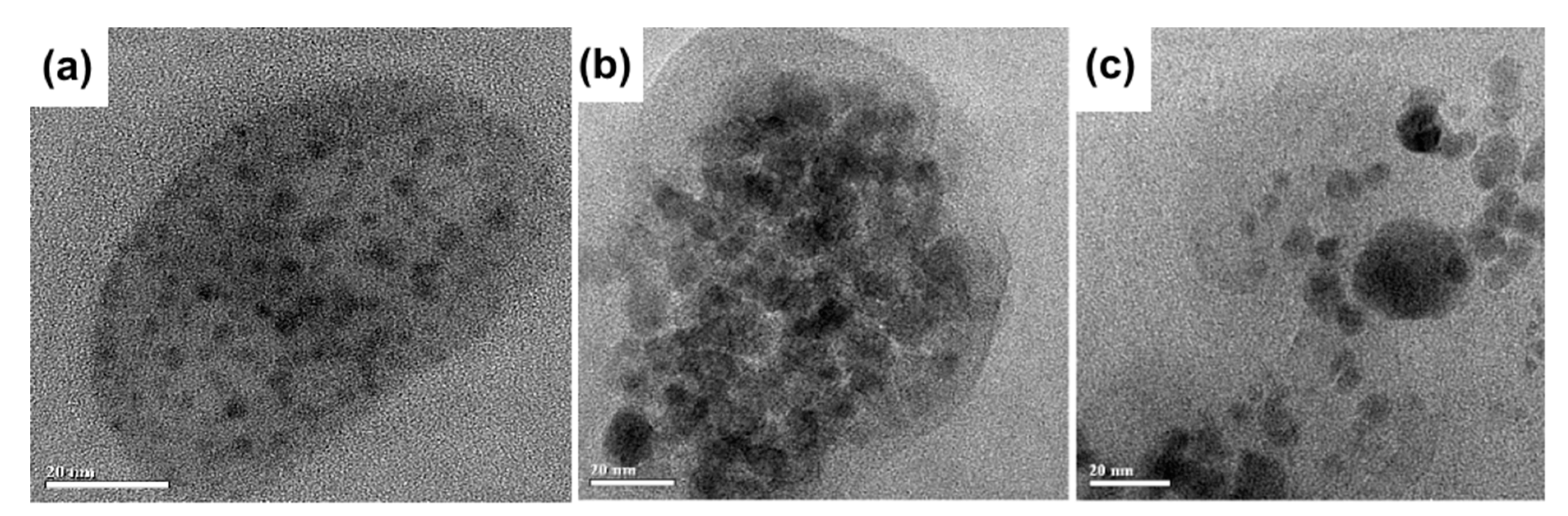
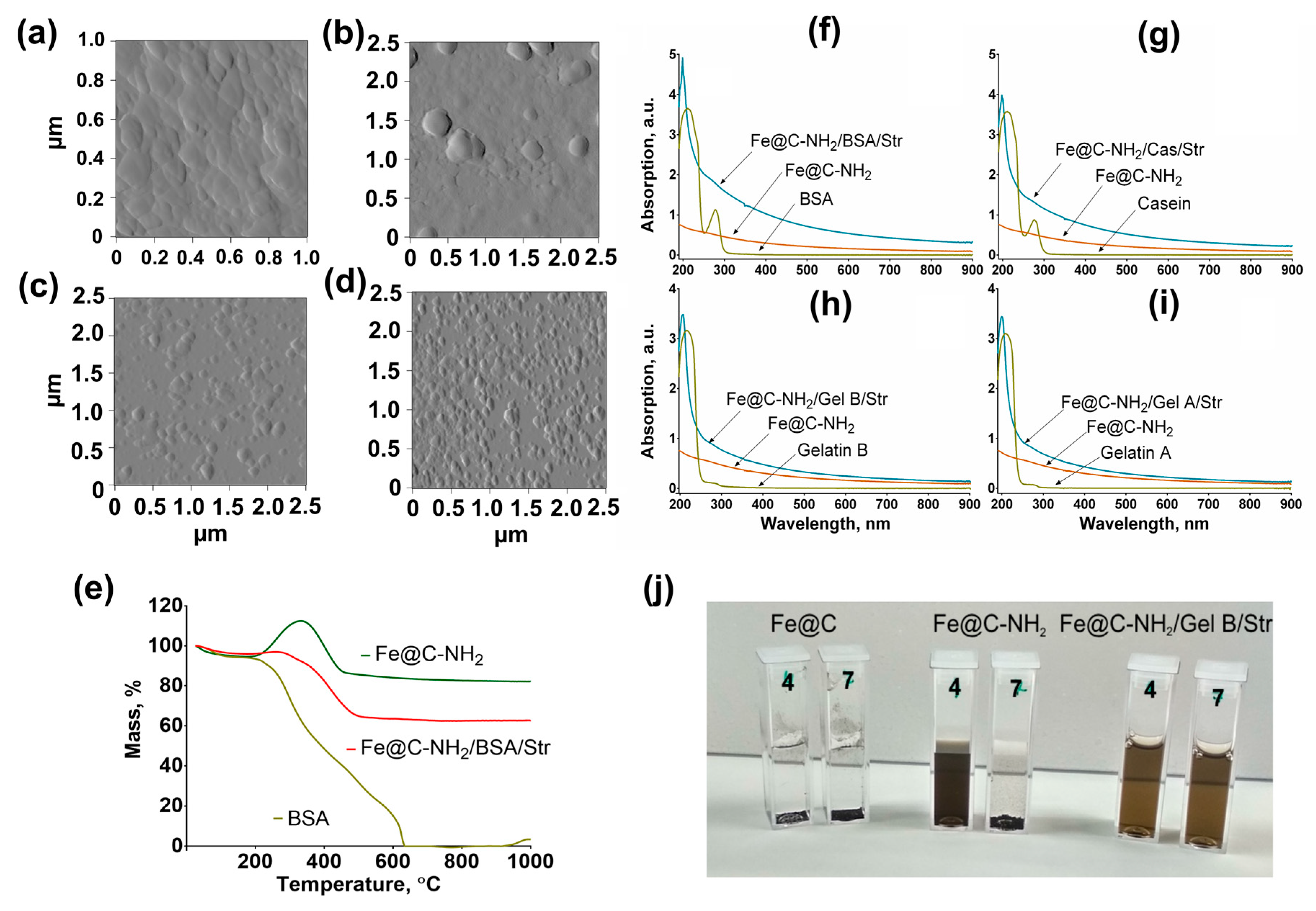
3.3. Characterization of Nanoclusters
3.4. NMR-Assay of Anti-Tetanus Antibodies
3.5. Thermal Stability Study
3.6. Colloidal Stability and Shelf Life
3.7. Storage Stability
3.8. Stability of Nanoclusters in Complex Media
3.9. Protein Corona
3.10. The Resistance of Nanoclusters to Proteolysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Hu, S.; Xiong, Y.; Wei, H.; Xu, H.; Duan, H.; Lai, W. Application and development of superparamagnetic nanoparticles in sample pretreatment and immunochromatographic assay. Trends Anal. Chem. 2019, 114, 151–170. [Google Scholar] [CrossRef]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Shi, X.; Shen, M. Aqueous-phase synthesis of iron oxide nanoparticles and composites for cancer diagnosis and therapy. Adv. Colloid Interface Sci. 2017, 249, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, H.; Zhou, Z.; Huang, K.; Yang, S.; Han, G. Recent Advances on Magnetic Relaxation Switching Assay-Based Nanosensors. Bioconj. Chem. 2017, 28, 869–879. [Google Scholar] [CrossRef]
- Shao, H.; Min, C.; Issadore, D.; Liong, M.; Yoon, T.-J.; Weissleder, R.; Lee, H. Magnetic nanoparticles and micronmr for diagnostic applications. Theranostics 2012, 2, 55–65. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Magnetic nanoparticle biosensors. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 291–304. [Google Scholar] [CrossRef]
- Alcantara, D.; Lopez, S.; García-Martin, M.L.; Pozo, D. Iron oxide nanoparticles as magnetic relaxation switching (MRSw) sensors: Current applications in nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1253–1262. [Google Scholar] [CrossRef]
- Luo, Z.-X.; Fox, L.; Cummings, M.; Lowery, T.J.; Daviso, E. New frontiers in in vitro medical diagnostics by low field T2 magnetic resonance relaxometry. Trends Anal. Chem. 2016, 83, 94–102. [Google Scholar] [CrossRef]
- Fung, F.; Wang, H.-S.; Menon, S. Food safety in the 21st century. Biomed. J. 2018, 41, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure–Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef] [PubMed]
- Xianyu, Y.; Wang, Q.; Chen, Y. Magnetic particles-enabled biosensors for point-of-care testing. Trends Anal. Chem. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Biehl, P.; von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, characterization, and applications of magnetic nanoparticles featuring polyzwitterionic coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Hemasa, A.L.; Freag, M.S. Hybrid protein-inorganic nanoparticles: From tumor-targeted drug delivery to cancer imaging. J. Control. Release 2016, 243, 303–322. [Google Scholar] [CrossRef] [PubMed]
- An, F.-F.; Zhang, X.-H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, L.; Alvarez-Puebla, R.A.; Pazos-Perez, N. Surface modifications of nanoparticles for stability in biological fluids. Materials 2018, 11, 1154. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating matters: Review on colloidal stability of nanoparticles with biocompatible coatings in biological media, living cells and organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef]
- Gou, Y.; Miao, D.; Zhou, M.; Wang, L.; Zhou, H.; Su, G. Bio-inspired protein-based nanoformulations for cancer theranostics. Front. Pharmacol. 2018, 9, 421. [Google Scholar] [CrossRef]
- Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Bioinspired Shielding Strategies for Nanoparticle Drug Delivery Applications. Mol. Pharm. 2018, 15, 2900–2909. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Jumeaux, C.; Gupta, B.; Stevens, M.M. Peptide and protein nanoparticle conjugates: Versatile platforms for biomedical applications. Chem. Soc. Rev. 2018, 47, 3574–3620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, L.; Chen, H.; Hu, K.; Delahunty, I.; Gao, S.; Xie, J. Surface impact on nanoparticle-based magnetic resonance imaging contrast agents. Theranostics 2018, 8, 2521–2548. [Google Scholar] [CrossRef] [PubMed]
- Foox, M.; Zilberman, M. Drug delivery from gelatin-based systems. Expert Opin. Drug Deliv. 2015, 12, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Abo El-Fotoh, W.S.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Tang, M. Review of the effects of manufactured nanoparticles on mammalian target organs. J. Appl. Toxicol. 2018, 38, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Tatara, A.M.; Mikos, A.G. Gelatin carriers for drug and cell delivery in tissue engineering. J. Control. Release 2014, 190, 210–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikhalev, K.N.; Germov, A.Y.; Uimin, M.A.; Yermakov, A.E.; Konev, A.S.; Novikov, S.I.; Gaviko, V.S.; Ponosov, Y.S. Magnetic state and phase composition of carbon-encapsulated Co@C nanoparticles according to 59Co, 13C NMR data and Raman spectroscopy. Mater. Res. Express 2018, 5, 055033. [Google Scholar] [CrossRef]
- Pitkowski, A.; Durand, D.; Nicolai, T. Structure and dynamical mechanical properties of suspensions of sodium caseinate. J. Colloid Interface Sci. 2008, 326, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Acharya, D.; Dhar, T.K. Method for Homogeneous Spotting of Antibodies on Membranes: Application to the Sensitive Detection of Ochratoxin A. Anal. Bioanal. Chem. 2006, 385, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, M.; Pierrat, S.; Zins, I.; Lotz, A.; Sönnichsen, C. Separation of nanoparticles by gel electrophoresis according to size and shape. Nano Lett. 2007, 7, 2881–2885. [Google Scholar] [CrossRef] [PubMed]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Khramtsov, P.; Kropaneva, M.; Byzov, I.; Minin, A.; Mysik, A.; Timganova, V.; Bochkova, M.; Uimin, M.; Zamorina, S.; Yermakov, A.; et al. Conjugation of carbon coated-iron nanoparticles with biomolecules for NMR-based assay. Colloids Surf. B 2019, 176, 256–264. [Google Scholar] [CrossRef]
- Leskinen, T.; Witos, J.; Valle-Delgado, J.J.; Lintinen, K.; Kostiainen, M.; Wiedmer, S.K.; Österberg, M.; Mattinen, M.L. Adsorption of Proteins on Colloidal Lignin Particles for Advanced Biomaterials. Biomacromolecules 2017, 18, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, J.; Philip, J. The interaction, stability and response to an external stimulus of iron oxide nanoparticle-casein nanocomplexes. Colloids Surf. A 2012, 406, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wei, M.; Gu, H.; Xu, Q.; Zhu, W. Study of superparamagnetic nanoparticles as labels in the quantitative lateral flow immunoassay. Mater. Sci. Eng. C 2009, 29, 714–718. [Google Scholar] [CrossRef]
- Koh, I.; Hong, R.; Weissleder, R.; Josephson, L. Sensitive NMR sensors detect antibodies to influenza. Angew. Chem. Int. Ed. 2008, 47, 4119–4121. [Google Scholar] [CrossRef] [PubMed]
- Cowger, T.A.; Tang, W.; Zhen, Z.; Hu, K.; Rink, D.E.; Todd, T.J.; Wang, G.D.; Zhang, W.; Chen, H.; Xie, J. Casein-coated Fe5C2 nanoparticles with superior r2 relaxivity for liver-specific magnetic resonance imaging. Theranostics 2015, 5, 1225–1232. [Google Scholar] [CrossRef]
- Shamsutdinova, N.; Zairov, R.; Nizameev, I.; Gubaidullin, A.; Mukhametshina, A.; Podyachev, S.; Ismayev, I.; Kadirov, M.; Voloshina, A.; Mukhametzyanov, T.; et al. Tuning magnetic relaxation properties of “hard cores” in core-shell colloids by modification of “soft shell”. Colloids Surf. B 2018, 162, 52–59. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, G.T.; Kim, H.-K.; Sung, B.; Lee, Y.; Kim, M.; Chang, Y.; Seo, J.H. Surface Design of Eu-Doped Iron Oxide Nanoparticles for Tuning the Magnetic Relaxivity. ACS Appl. Mater. Interfaces 2018, 10, 25080–25089. [Google Scholar] [CrossRef]
- Roca, A.G.; Veintemillas-Verdaguer, S.; Port, M.; Robic, C.; Serna, C.J.; Morales, M.P. Effect of nanoparticle and aggregate size on the relaxometric properties of MR contrast agents based on high quality magnetite nanoparticles. J. Phys. Chem. B 2009, 113, 7033–7039. [Google Scholar] [CrossRef] [PubMed]
- WHO. Tetanus vaccines: WHO position paper—February 2017. Relev. Epidemiol. Hebd. 2017, 92, 53–76. [Google Scholar]
- Kristiansen, M.; Aggerbeck, H.; Heron, I. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 1997, 105, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Van Gageldonk, P.G.M.; van Schaijk, F.G.; van der Klis, F.R.; Berbers, G.A.M. Development and validation of a multiplex immunoassay for the simultaneous determination of serum antibodies to Bordetella pertussis, diphtheria and tetanus. J. Immunol. Methods 2008, 335, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Li, Z.; Meng, H.; Zhang, L.; Wang, H.; Li, J.; Qu, L. A lateral flow assay for the determination of human tetanus antibody in whole blood by using gold nanoparticle labeled tetanus antigen. Microchim. Acta 2018, 185, 110. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, S.; Molaeirad, A.; Sadri, M.; Nejad, H.R. Detection of Anti-tetanus Toxoid Monoclonal Antibody by Using Modified Polycarbonate Surface. Plasmonics 2018, 13, 1555–1567. [Google Scholar] [CrossRef]
- Jain, S.; Chattopadhyay, S.; Jackeray, R.; Zainul Abid, C.K.V.; Kumar, M.; Singh, H. Detection of anti-tetanus toxoid antibody on modified polyacrylonitrile fibers. Talanta 2010, 82, 1876–1883. [Google Scholar] [CrossRef]
- Golberg, A.; Yarmush, M.L.; Konry, T. Picoliter droplet microfluidic immunosorbent platform for point-of-care diagnostics of tetanus. Microchim. Acta 2013, 180, 855–860. [Google Scholar] [CrossRef]
- Tian, B.; Liao, X.; Svedlindh, P.; Strömberg, M.; Wetterskog, E. Ferromagnetic Resonance Biosensor for Homogeneous and Volumetric Detection of DNA. ACS Sens. 2018, 3, 1093–1101. [Google Scholar] [CrossRef]
- Jansaento, W.; Jangpatarapongsa, K.; Polpanich, D.; Wonglumsom, W. Detection of Campylobacter DNA using magnetic nanoparticles coupled with PCR and a colorimetric end-point system. Food Sci. Biotechnol. 2016, 25, 193–198. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Capomaccio, R.; Osório, I.; Ojea-Jiménez, I.; Ceccone, G.; Colpo, P.; Gilliland, D.; Hussain, R.; Siligardi, G.; Rossi, F.; Ricard-Blum, S.; et al. Gold nanoparticles increases UV and thermal stability of human serum albumin. Biointerphases 2016, 11, 04B310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canpean, V.; Gabudean, A.M.; Astilean, S. Enhanced thermal stability of gelatin coated gold nanorods in water solution. Colloids Surf. A 2013, 433, 9–13. [Google Scholar] [CrossRef]
- Moriyama, Y.; Watanabe, E.; Kobayashi, K.; Harano, H.; Lnui, E.; Takeda, K. Secondary structural change of bovine serum albumin in thermal denaturation up to 130 °C and protective effect of sodium dodecyl sulfate on the change. J. Phys. Chem. B 2008, 112, 16585–16589. [Google Scholar] [CrossRef] [PubMed]
- Van Der Lee, R.; Buljan, M.; Lang, B.; Weatheritt, R.J.; Daughdrill, G.W.; Dunker, A.K.; Fuxreiter, M.; Gough, J.; Gsponer, J.; Jones, D.T.; et al. Classification of intrinsically disordered regions and proteins. Chem. Rev. 2014, 114, 6589–6631. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Y.; Kuo, W.-T.; Huang, H.-Y.; Chou, M.-J.; Wu, M.-C. Surface modification of gelatin nanoparticles with polyethylenimine as gene vector. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Gaihre, B.; Aryal, S.; Khil, M.S.; Kim, H.Y. Encapsulation of Fe3O4 in gelatin nanoparticles: Effect of different parameters on size and stability of the colloidal dispersion. J. Microencapsul. 2008, 25, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Sivera, M.; Kvitek, L.; Soukupova, J.; Panacek, A.; Prucek, R.; Vecerova, R.; Zboril, R. Silver nanoparticles modified by gelatin with extraordinary pH stability and long-term antibacterial activity. PLoS ONE 2014, 9, e103675. [Google Scholar] [CrossRef]
- Gaihre, B.; Parajuli, D.C.; Seo, H.C.; Khil, M.S.; Kim, H.Y. Effect of different parameters on gelatin adsorption and stability of the colloidal dispersion of gelatin-coated magnetic iron oxide nano-particles. Adsorpt. Sci. Technol. 2008, 26, 279–290. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Maniu, D.; Astilean, S. Gelatin-nanogold bioconjugates as effective plasmonic platforms for SERS detection and tagging. Colloids Surf. B 2013, 103, 475–481. [Google Scholar] [CrossRef]
- Huang, J.; Wang, L.; Lin, R.; Wang, A.Y.; Yang, L.; Kuang, M.; Qian, W.; Mao, H. Casein-coated iron oxide nanoparticles for high MRI contrast enhancement and efficient cell targeting. ACS Appl. Mater. Interfaces 2013, 5, 4632–4639. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Islam, M.S.; Podaralla, S.; Kaushik, R.S.; Reineke, J.; Woyengo, T.; Perumal, O. Food Protein Based Core-Shell Nanocarriers for Oral Drug Delivery: Effect of Shell Composition on in Vitro and in Vivo Functional Performance of Zein Nanocarriers. Mol. Pharm. 2017, 14, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Abbasi, A.Z.; Pfeiffer, C.; Hussain, S.Z.; Khalid, Z.M.; Gil, P.R.; Parak, W.J.; Hussain, I. Protein-mediated synthesis, pH-induced reversible aggregation, toxicity and cellular interaction of silver nanoparticles. Colloids Surf. B 2013, 102, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.Z.; Ou, K.-L.; Chen, J.-K.; Ho, M.-H.; Tzing, S.-H.; Chang, J.-Y. Development of bovine serum albumin-modified hybrid nanoclusters for magnetofluorescence imaging and drug delivery. RSC Adv. 2014, 4, 32762–32772. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Liu, F.; Cheng, Y.; Shi, D. Effective reduction of nonspecific binding by surface engineering of quantum dots with bovine serum albumin for cell-targeted imaging. Langmuir 2012, 28, 16605–16613. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, X.; Zhang, B.; Liu, F.; Cheng, Y.; Shi, D. Surface engineered antifouling optomagnetic SPIONs for bimodal targeted imaging of pancreatic cancer cells. Int. J. Nanomed. 2014, 9, 1601–1615. [Google Scholar] [CrossRef]
- Strozyk, M.S.; Chanana, M.; Pastoriza-Santos, I.; Pérez-Juste, J.; Liz-Marzán, L.M. Protein/polymer-based dual-responsive gold nanoparticles with pH-dependent thermal sensitivity. Adv. Funct. Mater. 2012, 22, 1436–1444. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Ortega-Vinuesa, J.L. How Proteins Stabilize Colloidal Particles by Means of Hydration Forces. Langmuir 1999, 15, 2644–2653. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [Green Version]
- Laurentius, L.B.; Owens, N.A.; Park, J.; Crawford, A.C.; Porter, M.D. Advantages and limitations of nanoparticle labeling for early diagnosis of infection. Expert Rev. Mol. Diagn. 2016, 16, 883–895. [Google Scholar] [CrossRef]
- Crowther, J.R. The ELISA Guidebook; Humana Press: New York, NY, USA, 2009; pp. 43–111. [Google Scholar]
- Jain, S.; Mittal, A.; Jain, A.K.; Mahajan, R.R.; Singh, D. Cyclosporin a loaded PLGA nanoparticle: Preparation, optimization, In-Vitro characterization and stability studies. Curr. Nanosci. 2010, 6, 422–431. [Google Scholar] [CrossRef]
- Bai, Y.; Song, M.; Cui, Y.; Shi, C.; Wang, D.; Paoli, G.C.; Shi, X. A rapid method for the detection of foodborne pathogens by extraction of a trace amount of DNA from raw milk based on amino-modified silica-coated magnetic nanoparticles and polymerase chain reaction. Anal. Chim. Acta 2013, 787, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Wiogo, H.T.R.; Lim, M.; Bulmus, V.; Gutiérrez, L.; Woodward, R.C.; Amal, R. Insight into serum protein interactions with functionalized magnetic nanoparticles in biological media. Langmuir 2012, 28, 4346–4356. [Google Scholar] [CrossRef] [PubMed]
- Matta, L.L.; Alocilja, E.C. Emerging nano-biosensing with suspended MNP microbial extraction and EANP labeling. Biosens. Bioelectron. 2018, 117, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Zhang, S.; Yang, Q.; Zhang, T.; Wei, X.-Q.; Jiang, L.; Zhang, C.-L.; Chen, Q.-M.; Zhang, Z.-R.; Lin, Y.-F. Preformed albumin corona, a protective coating for nanoparticles based drug delivery system. Biomaterials 2013, 34, 8521–8530. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, G.; Aguilar, Z.P.; Xiong, Y.; Xu, H. Affordable and simple method for separating and detecting ovarian cancer circulating tumor cells using BSA coated magnetic nanoprobes modified with folic acid. Sens. Actuators B Chem. 2018, 262, 611–618. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- Mirshafiee, V.; Kim, R.; Park, S.; Mahmoudi, M.; Kraft, M.L. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials 2016, 75, 295–304. [Google Scholar] [CrossRef]
- García-Álvarez, R.; Hadjidemetriou, M.; Sánchez-Iglesias, A.; Liz-Marzán, L.M.; Kostarelos, K. In vivo formation of protein corona on gold nanoparticles. The effect of their size and shape. Nanoscale 2018, 10, 1256–1264. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, H.; Zhang, Y.; Wu, R.; Zou, H. Nanoparticle size matters in the formation of plasma protein coronas on Fe3O4 nanoparticles. Colloids Surf. B 2014, 121, 354–361. [Google Scholar] [CrossRef]
- Simpson, R.J. Stabilization of proteins for storage. Cold Spring Harb. Protoc. 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Borgstrom, B.; Dahlqvist, A.; Lundh, G.; Sjovall, J. Studies of intestinal digestion and absorption in the human. J. Clin. Investig. 1957, 36, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Langer, K.; Anhorn, M.G.; Steinhauser, I.; Dreis, S.; Celebi, D.; Schrickel, N.; Faust, S.; Vogel, V. Human serum albumin (HSA) nanoparticles: Reproducibility of preparation process and kinetics of enzymatic degradation. Int. J. Pharm. 2008, 347, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Helmy, M.W.; Samy, W.M.; Elgindy, N.A. Novel ionically crosslinked casein nanoparticles for flutamide delivery: Formulation, characterization, and in vivo pharmacokinetics. Int. J. Nanomed. 2013, 8, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Ninan, G.; Joseph, J.; Abubacker, Z. Physical, Mechanical, and Barrier Properties of Carp and Mammalian Skin Gelatin Films. J. Food Sci. 2010, 75, E620–E626. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.M.; Salama, O.A.; El-Saied, K.M. Changes in amino acids profile of camel milk protein during the early lactation. Int. J. Dairy Sci. 2007, 2, 226–234. [Google Scholar] [CrossRef]
- Stein, W.H.; Moore, S. Amino acid composition of beta-lactoglobulin and bovine serum albumin. J. Biol. Chem. 1949, 178, 79–91. [Google Scholar] [PubMed]
- Silva, C.J.S.M.; Sousa, F.; Gübitz, G.; Cavaco-Paulo, A. Chemical Modifications on Proteins Using Glutaraldehyde. Food Technol. Biotechnol. 2004, 42, 51–56. [Google Scholar]
- Cao, X.; Han, Y.; Li, F.; Li, Z.; McClements, D.J.; He, L.; Decker, E.A.; Xing, B.; Xiao, H. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: Not just simple protein corona effects. NanoImpact 2019, 13, 37–43. [Google Scholar] [CrossRef]
- Chanana, M.; Rivera-gil, P.; Correa-Duarte, M.A.; Liz-Marzán, L.M.; Parak, W.J. Physicochemical properties of protein-coated gold nanoparticles in biological fluids and cells before and after proteolytic digestion. Angew. Chem. Int. Ed. 2013, 52, 4179–4183. [Google Scholar] [CrossRef]


| Coating | Fe@C-NH2/BSA/Str | Fe@C-NH2/Casein/Str | Fe@C-NH2/Gelatin B/Str | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | “Small” | “Medium” | “Large” | “Small” | “Medium” | “Large” | “Small” | “Medium” | “Large” |
| Dh, nm | 114–121 | 172–186 | 211–233 | 114–131 | 194–198 | 235–274 | 142–160 | 212–243 | 279–309 |
| PdI | 0.188–0.208 | 0.158–0.175 | 0.247–0.258 | 0.178–0.201 | 0.132–0.169 | 0.202–0.243 | 0.211–0.248 | 0.200–0.260 | 0.227–0.257 |
| Zeta potential, mv | −24–−25 | −23–−25 | −24–−25 | −25–−27 | −25–−26 | −24–−26 | −10–−12 | −9–−10 | −9–−10 |
| Relaxivity, 1/mM−1 × s−1 | 234–265 | 262–322 | 186–230 | 310–354 | 318–340 | 303–345 | 266–306 | 296–377 | 282–324 |
| Assay | Label | LOD, mIU/mL | Assay duration, h | Reference |
|---|---|---|---|---|
| ELISA | horseradish peroxidase | 0.01 | 4 | [43] |
| Multiplex immunoassay | fluorescent beads | 0.01 | 1.25 | [44] |
| Lateral flow assay | gold nanoparticles | 10 | 0.25 | [45] |
| Surface plasmon resonance assay | gold nanoparticles | 5 | 2.15 | [46] |
| ELISA on polymer fibers | horseradish peroxidase | 0.5 | 2.15 | [47] |
| Microfluidic assay | fluorescent dye | 100 | Less than 1 | [48] |
| NMR assay | carbon-coated iron nanoparticles | 0.52 | 3–4 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khramtsov, P.; Barkina, I.; Kropaneva, M.; Bochkova, M.; Timganova, V.; Nechaev, A.; Byzov, I.; Zamorina, S.; Yermakov, A.; Rayev, M. Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay. Nanomaterials 2019, 9, 1345. https://doi.org/10.3390/nano9091345
Khramtsov P, Barkina I, Kropaneva M, Bochkova M, Timganova V, Nechaev A, Byzov I, Zamorina S, Yermakov A, Rayev M. Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay. Nanomaterials. 2019; 9(9):1345. https://doi.org/10.3390/nano9091345
Chicago/Turabian StyleKhramtsov, Pavel, Irina Barkina, Maria Kropaneva, Maria Bochkova, Valeria Timganova, Anton Nechaev, Il’ya Byzov, Svetlana Zamorina, Anatoly Yermakov, and Mikhail Rayev. 2019. "Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay" Nanomaterials 9, no. 9: 1345. https://doi.org/10.3390/nano9091345
APA StyleKhramtsov, P., Barkina, I., Kropaneva, M., Bochkova, M., Timganova, V., Nechaev, A., Byzov, I., Zamorina, S., Yermakov, A., & Rayev, M. (2019). Magnetic Nanoclusters Coated with Albumin, Casein, and Gelatin: Size Tuning, Relaxivity, Stability, Protein Corona, and Application in Nuclear Magnetic Resonance Immunoassay. Nanomaterials, 9(9), 1345. https://doi.org/10.3390/nano9091345





