Biotransformation of Pristine and Oxidized Carbon Nanotubes by the White Rot Fungus Phanerochaete chrysosporium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Degradation of MWCNTs
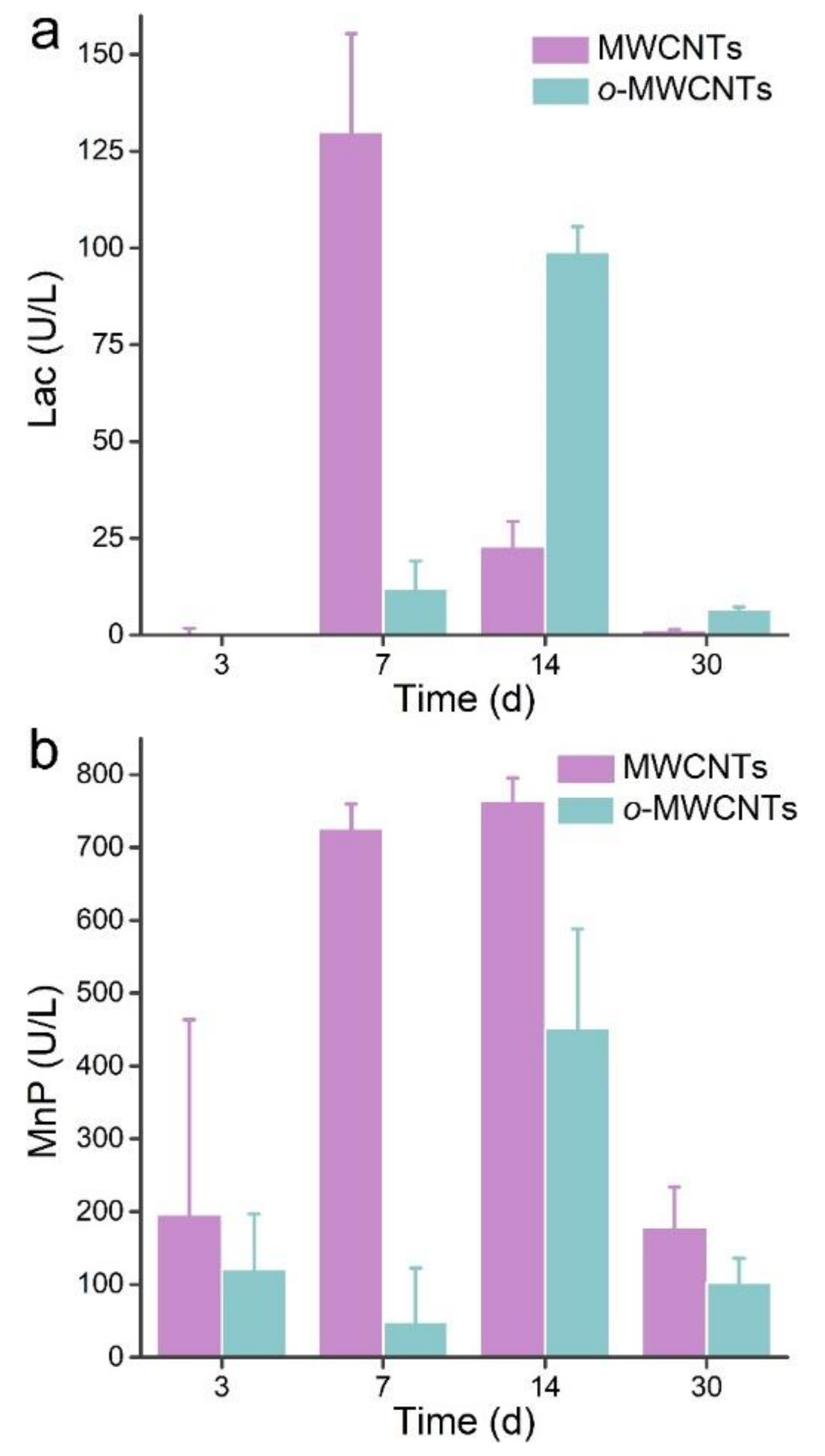
2.3. Enzyme Activities
2.4. Statistical Analysis
3. Results and Discussion
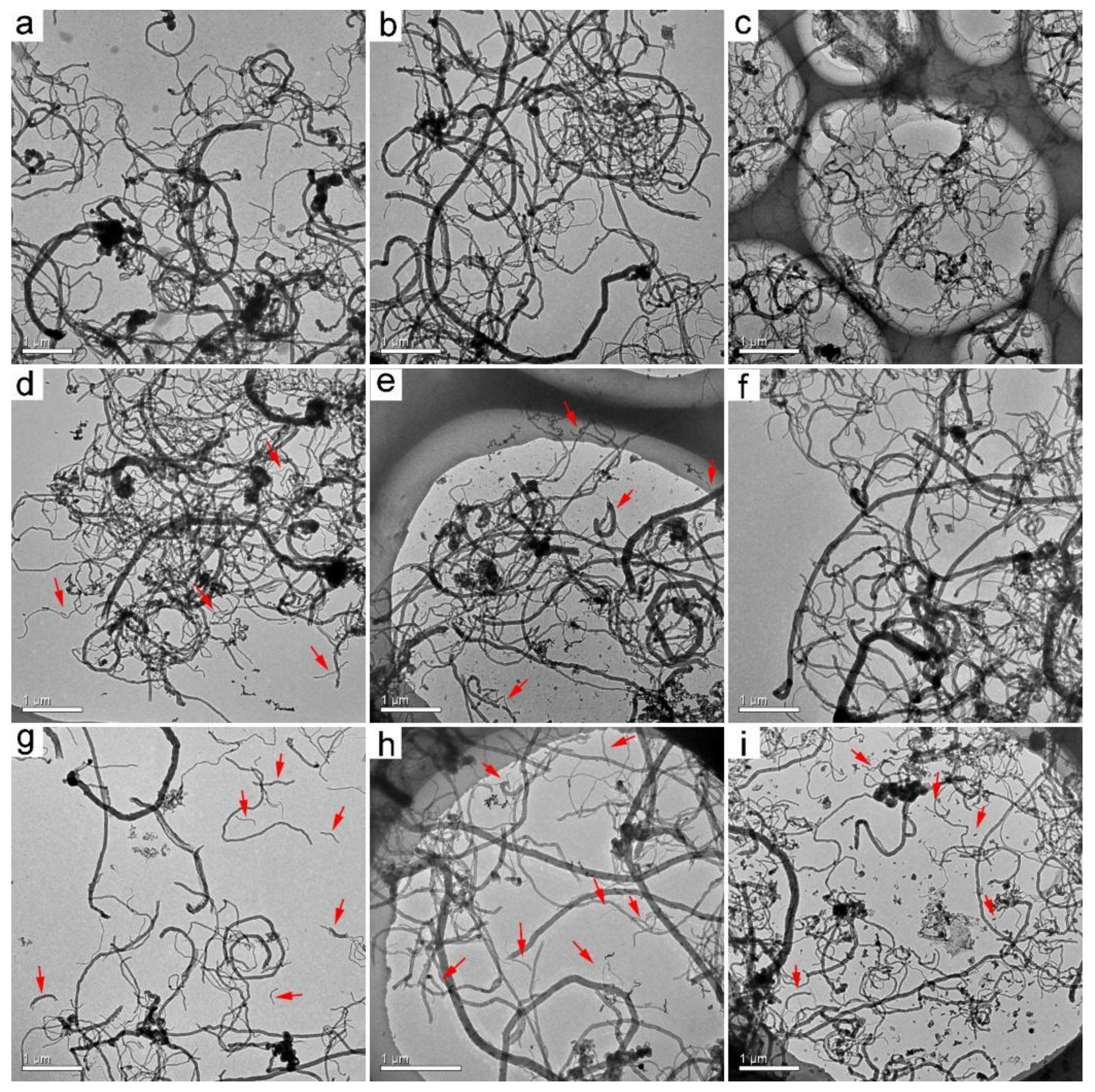
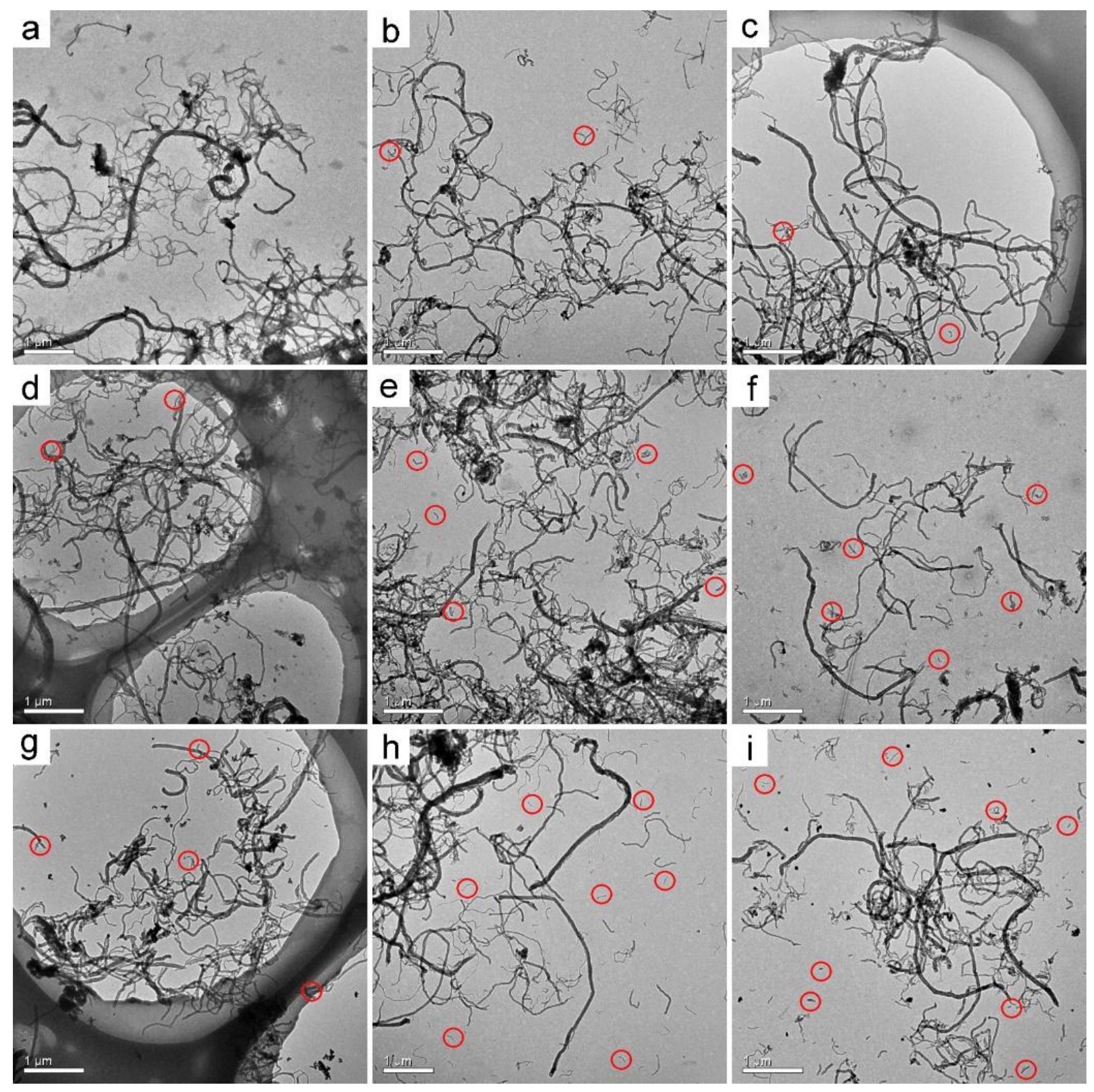
3.1. TEM Observation of MWCNTs after Transformation
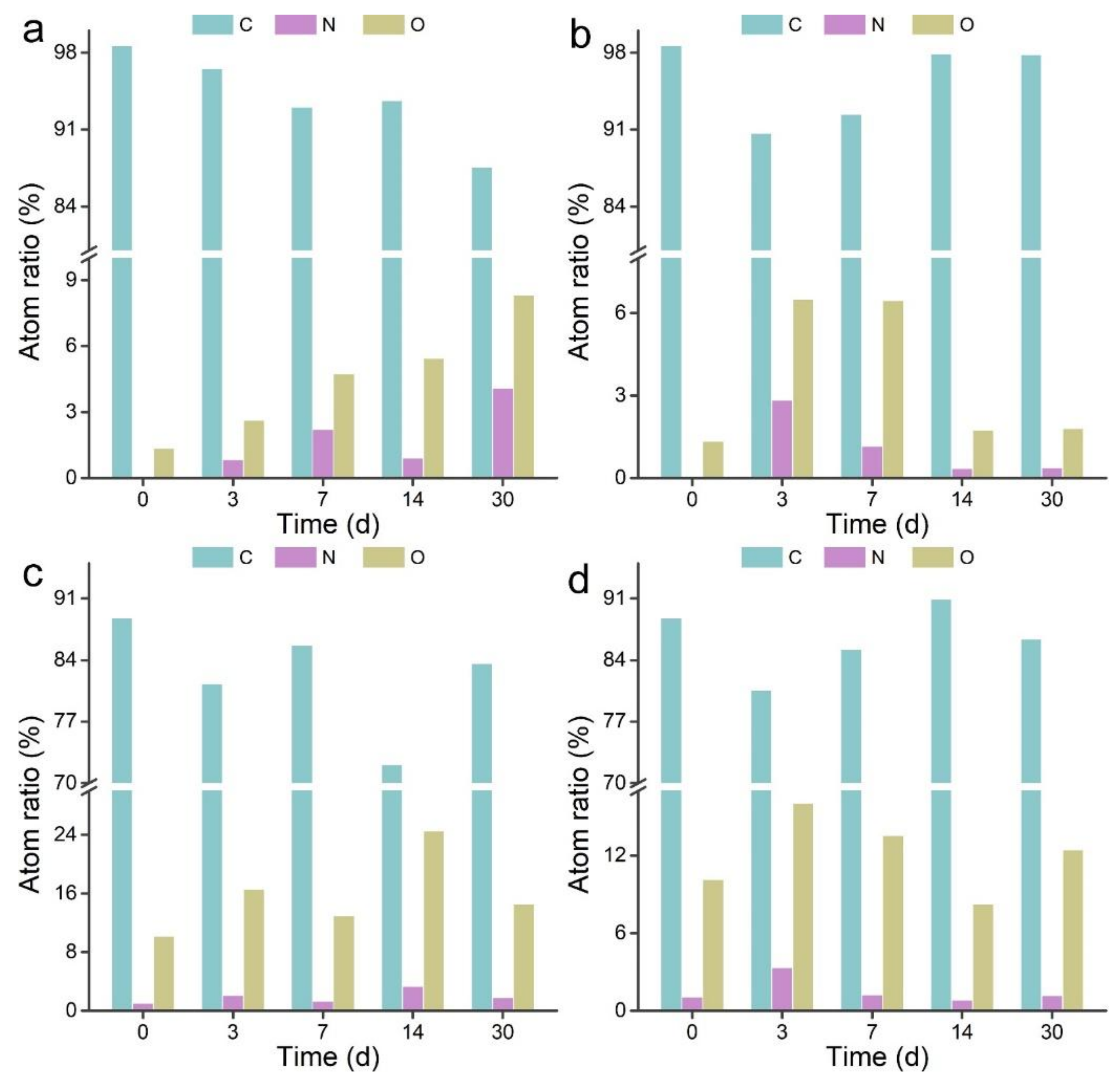
3.2. XPS Analyses of MWCNTs after Transformation
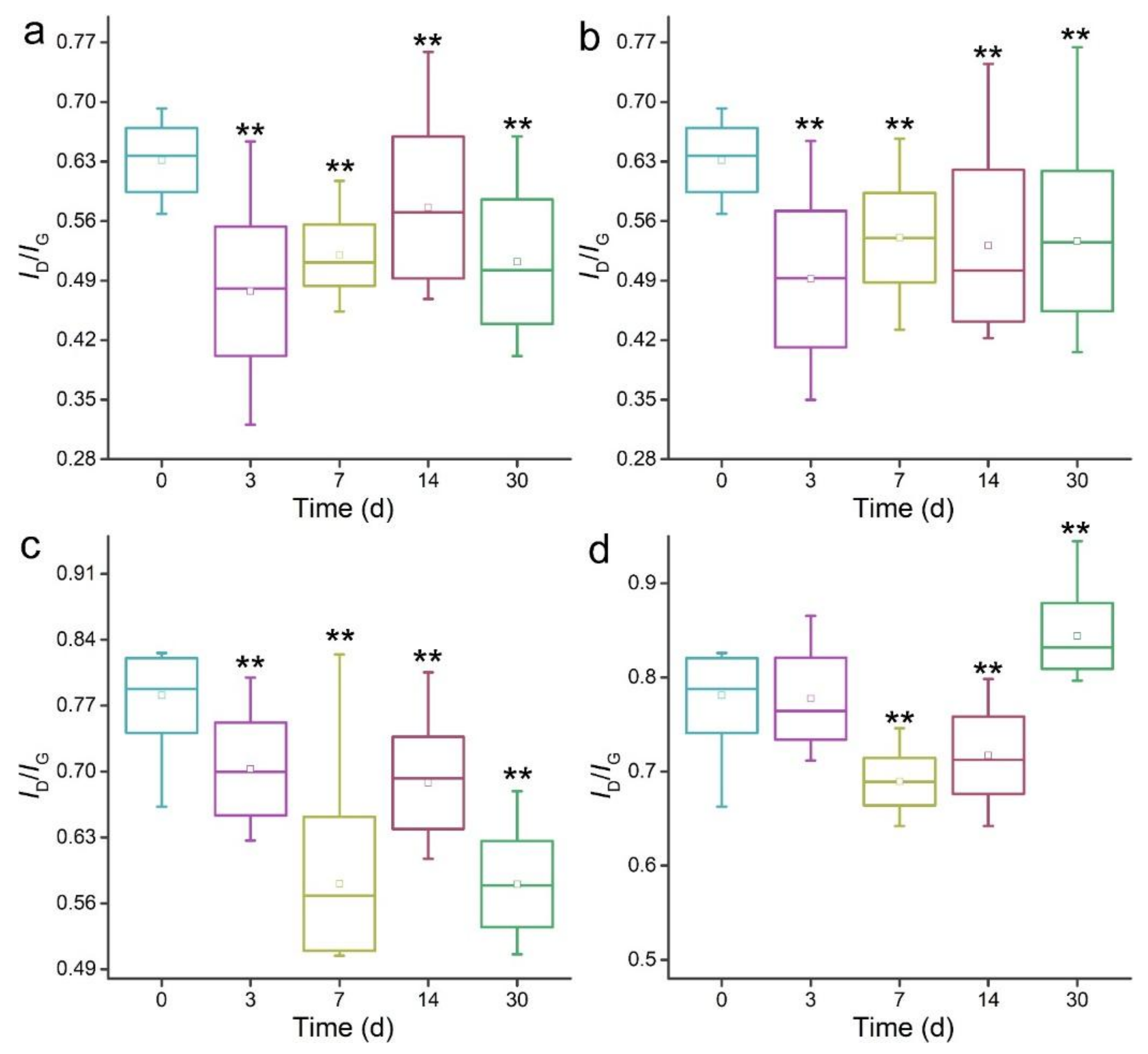
3.3. Raman Spectroscopy Analyses of MWCNTs after Transformation
3.4. IR Analyses of MWCNTs after Transformation
3.5. Transformation Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, L.; Chang, D.; Baek, J.B.; Lu, W. Carbon nanomaterials for advanced energy conversion and storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Dong, S. Graphene nanosheet: Synthesis molecular engineering thin film hybrids and energy and analytical applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [PubMed]
- Wang, Q.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Masciangioli, T.; Zhang, W. Peer reviewed: Environmental technologies at the nanoscale. Environ. Sci. Technol. 2003, 37, 102A–108A. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant. Sci. 2016, 7, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, C.; Li, H.; Qu, X.; Yang, S.-T.; Chang, X. Bioaccumulation and toxicity of 13C-skeleton labeled graphene oxide in wheat. Environ. Sci. Technol. 2017, 51, 10146–10153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, S.; Liu, Y.; Mo, M.; Guan, X.; Huang, L.; Sun, C.; Yang, S.-T.; Chang, X. Toxicity of graphene oxide to naked oats (Avena sativa L.) in hydroponic and soil cultures. RSC Adv. 2018, 8, 15336–15343. [Google Scholar] [CrossRef]
- Cimbaluk, G.V.; Ramsdorf, W.A.; Perussolo, M.C.; Santos, H.K.; De Assi, H.C.S.; Schnitzler, M.C.; Schnitzler, D.C.; Carneiro, P.G.; Cestari, M.M. Evaluation of multiwalled carbon nanotubes toxicity in two fish species. Ecotoxicol. Environ. Saf. 2017, 150, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Song, B.; Liang, H.; Liu, J.; Feng, X.; Deng, B.; Sun, T.; Shao, L. Toxicity of graphene-family nanoparticles: A general review of the origins and mechanisms. Part. Fibre Toxicol. 2016, 13, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Freixa, A.; Acuna, V.; Sanchís, J.; Farré, M.; Barceló, D.; Sabater, S. Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 2018, 619, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P.; Schaeffer, S.M. Microbial control over carbon cycling in soil. Front. Microbiol. 2012, 3, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Yu, B.; Xue, F.; Xie, J.; Zhang, X.; Wu, R.; Wang, R.; Hu, Z.; Yang, S.-T.; Luo, J. Facile hydrothermal preparation of recyclable S-doped graphene sponge for Cu2+ adsorption. J. Hazard. Mater. 2015, 286, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Backes, C.; Menard-Moyon, C.; Coleman, J.N.; Bianco, A. White graphene undergoes peroxidase degradation. Angew. Chem. Int. Ed. 2016, 55, 5506–5511. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, K.M.; Filley, T.R.; Blanchette, R.A.; Bowen, B.B.; Bolskar, R.D.; Hockaday, W.C.; Masiello, C.A.; Raebiger, J.W. White-rot basidiomycete-mediated decomposition of C60 fullerol. Environ. Sci. Technol. 2009, 43, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, C.; Fan, X.; Li, Y.; Song, M. Degradation of oxidized multi-walled carbon nanotubes in water via photo-Fenton method and its degradation mechanism. Chem. Eng. J. 2017, 323, 37–46. [Google Scholar] [CrossRef]
- Allen, B.L.; Kichambare, P.D.; Gou, P.; Vlasova, I.I.; Kapralov, A.A.; Konduru, N.; Kagan, V.E.; Star, A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008, 8, 3899–3903. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Ma, Q.; Shen, W.; Pei, X.; You, S.; Yin, Q.; Li, X. A novel environmental fate of graphene oxide: Biodegradation by a bacterium Labrys sp. WJW to support growth. Water Res. 2018, 143, 260–269. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, K.; Mao, L.; Guo, X.; Gao, S.; Petersen, E.J. Degradation of 14C-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects. Water Res. 2015, 84, 49–57. [Google Scholar] [CrossRef]
- Bai, H.; Jiang, W.; Kotchey, G.P.; Saidi, W.A.; Bythell, B.J.; Jarvis, J.M.; Marshall, A.G.; Robinson, R.A.S.; Star, A. Insight into the mechanism of graphene oxide degradation via the photo-fenton reaction. J. Phys. Chem. C 2014, 118, 10519–10529. [Google Scholar] [CrossRef]
- Flores-Cervantes, D.X.; Maes, H.M.; Schaffer, A.; Hollender, J.; Kohler, H.P.E. Slow biotransformation of carbon nanotubes by horseradish peroxidase. Environ. Sci. Technol. 2014, 48, 4826–4834. [Google Scholar] [CrossRef]
- Zhang, L.; Petersen, E.J.; Habteselassie, M.Y.; Mao, L.; Huang, Q. Degradation of multiwall carbon nanotubes by bacteria. Environ. Pollut. 2013, 181, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.N.; Chandler, G.T.; Ho, K.T.; Burgess, R.M.; Ferguson, P.L. Environmental biodegradability of [14C] single-walled carbon nanotubes by Trametes versicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge. Environ. Toxicol. Chem. 2015, 34, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Navarro, D.A.; Kookana, R.S.; McLaughlin, M.J.; Kirby, J.K. Fullerol as a potential pathway for mineralization of fullerene nanoparticles in biosolid-amended soils. Environ. Sci. Technol. Lett. 2016, 3, 7–12. [Google Scholar] [CrossRef]
- Avanasi, R.; Jackson, W.A.; Sherwin, B.; Mudge, J.F.; Anderson, T.A. C60 fullerene soil sorption, biodegradation, and plant uptake. Environ. Sci. Technol. 2014, 48, 2792–2797. [Google Scholar] [CrossRef] [PubMed]
- Berry, T.D.; Filley, T.R.; Clavijo, A.P.; Gray, M.B.; Turco, R. Degradation and microbial uptake of C60 fullerols in contrasting. Environ. Sci. Technol. 2017, 51, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Elgrabli, D.; Dachraoui, W.; Ménard-Moyon, C.; Liu, L.; Bégin, D.; Bégin-Colin, S.; Bianco, A.; Gazeau, F.; Alloyeau, D. Carbon nanotube degradation in macrophages: live nanoscale monitoring and understanding of biological pathway. ACS Nano 2015, 9, 10113–10124. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yokoyama, A.; Nodasaka, Y.; Kohgo, T.; Motomiya, K.; Matsumoto, H.; Nakazawa, E.; Numata, T.; Zhang, M.; Yudasaka, M.; et al. Long-term biopersistence of tangled oxidized carbon nanotubes inside and outside macrophages in rat subcutaneous tissue. Sci. Rep. 2013, 3, 2516. [Google Scholar] [CrossRef]
- Huang, D.; Guo, X.; Peng, Z.; Zeng, G.; Xu, P.; Gong, X.; Deng, R.; Xue, W.; Wang, R.; Yi, H.; et al. White rot fungi and advanced combined biotechnology with nanomaterials: Promising tools for endocrine-disrupting compounds biotransformation. Crit. Rev. Biotechnol. 2018, 38, 671–689. [Google Scholar] [CrossRef]
- Wong, D.W.S. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef]
- Lalwani, G.; Xing, W.; Sitharaman, B. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mater. Chem. B 2014, 2, 6354–6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, Z.; Feng, S.; Yilihamu, A.; Ma, Q.; Yang, S.; Yang, S.-T. Toxicity of pristine and chemically functionalized fullerenes to white rot fungus Phanerochaete Chrysosporium. Nanomaterials 2018, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Ming, Z.; Feng, S.; Yilihamu, A.; Yang, S.; Ma, Q.; Yang, H.; Bai, Y.; Yang, S.-T. Toxicity of carbon nanotubes to white rot fungus Phanerochaete Chrysosporium. Ecotoxicol. Environ. Saf. 2018, 162, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Russier, J.; Menard-Moyon, C.; Venturelli, E.; Gravel, E.; Marcolongo, G.; Meneghetti, M.; Doris, E.; Bianco, A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale 2011, 3, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; El-Sayed, R.; Andon, F.T.; Mukherjee, S.P.; Gregory, J.; Li, H.; Zhao, Y.; Seo, W.; Fornara, A.; Brandner, B.; et al. Lactoperoxidase-mediated degradation of single-walled carbon nanotubes in the presence of pulmonary surfactant. Carbon 2015, 91, 506–517. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, W.; Alvarez, P.J.J. Manganese peroxidase degrades pristine but not surface-oxidized (carboxylated) single-walled carbon nanotubes. Environ. Sci. Technol. 2014, 48, 7918–7923. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, L.; Fan, M.; Liu, L.; Dai, B.; Yang, J.; Sun, D. Microbial oxidation of graphite by Acidithiobacillus ferrooxidans CFMI-1. RSC Adv. 2014, 4, 55044–55047. [Google Scholar] [CrossRef]
- Kagan, V.E.; Kapralov, A.A.; St Croix, C.M.; Watkins, S.C.; Kisin, E.R.; Kotchey, G.P.; Balasubramanian, K.; Vlasova, I.I.; Yu, J.; Kim, K.; et al. Lung macrophages “digest” carbon nanotubes using a superoxide/peroxynitrite oxidative pathway. ACS Nano 2014, 8, 5610–5621. [Google Scholar] [CrossRef]
- Nunes, A.; Bussy, C.; Gherardini, L.; Meneghetti, M.; Herrero, M.A.; Bianco, A.; Prato, M.; Pizzorusso, T.; Al-Jamal, K.T.; Kostarelos, K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine 2012, 7, 1485–1494. [Google Scholar] [CrossRef]
- Yang, H.; Wu, X.; Ma, Q.; Yilihamu, A.; Yang, S.; Zhang, Q.; Feng, S.; Yang, S.-T. Fungal transformation of graphene by white rot fungus Phanerochaete Chrysosporium. Chemosphere 2019, 216, 9–18. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Sacchetti, C.; El-Sayed, R.; Fornara, A.; Kotchey, G.P.; Gaugler, J.A.; Star, A.; Bottini, M.; Fadeel, B. Enzymatic ‘stripping’ and degradation of PEGylated carbon nanotubes. Nanoscale 2014, 6, 14686–14690. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Hadad, C.; Prato, M.; Bianco, A.; Kostarelos, K. Intracellular degradation of chemically functionalized carbon nanotubes using a long-term primary microglial culture model. Nanoscale 2016, 8, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Feng, S.; Ma, Q.; Ming, Z.; Bai, Y.; Chen, L.; Yang, S.-T. Influence of reduced graphene oxide on the growth, structure and decomposition activity of white-rot fungus Phanerochaete Chrysosporium. RSC Adv. 2018, 8, 5026–5033. [Google Scholar] [CrossRef]
- Berry, T.D.; Filley, T.R.; Blanchette, R.A. Oxidative enzymatic response of white-rot fungi to single-walled carbon nanotubes. Environ. Pollut. 2014, 193, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, T.; Yuan, Y.; Xu, J. An efficient and environment-friendly method of removing graphene oxide in wastewater and its degradation mechanisms. Chemosphere 2016, 153, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Menard-Moyon, C.; Del Rio-Castillo, A.E.; Vazquez, E.; Samori, P.; Palermo, V.; Bianco, A. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Chowdhury, I.; Goodwin, D.G.; Henderson, W.M.; Fairbrother, D.H.; Bouchard, D.; Zepp, R.G. Photochemical transformation of graphene oxide in sunlight. Environ. Sci. Technol. 2015, 49, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ming, Z.; Li, H.; Yang, H.; Yu, B.; Wu, R.; Liu, X.; Bai, Y.; Yang, S.-T. Toxicity of graphene oxide to white rot fungus Phanerochaete Chrysosporium. Chemosphere 2016, 151, 324–331. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Yilihamu, A.; Ming, Z.; Yang, S.; Shi, M.; Ouyang, B.; Zhang, Q.; Guan, X.; Yang, S.-T. Biotransformation of Pristine and Oxidized Carbon Nanotubes by the White Rot Fungus Phanerochaete chrysosporium. Nanomaterials 2019, 9, 1340. https://doi.org/10.3390/nano9091340
Ma Q, Yilihamu A, Ming Z, Yang S, Shi M, Ouyang B, Zhang Q, Guan X, Yang S-T. Biotransformation of Pristine and Oxidized Carbon Nanotubes by the White Rot Fungus Phanerochaete chrysosporium. Nanomaterials. 2019; 9(9):1340. https://doi.org/10.3390/nano9091340
Chicago/Turabian StyleMa, Qiang, Ailimire Yilihamu, Zhu Ming, Shengnan Yang, Mengyao Shi, Bowei Ouyang, Qiangqiang Zhang, Xin Guan, and Sheng-Tao Yang. 2019. "Biotransformation of Pristine and Oxidized Carbon Nanotubes by the White Rot Fungus Phanerochaete chrysosporium" Nanomaterials 9, no. 9: 1340. https://doi.org/10.3390/nano9091340