Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance
Abstract
:1. Introduction
2. Materials and Methods
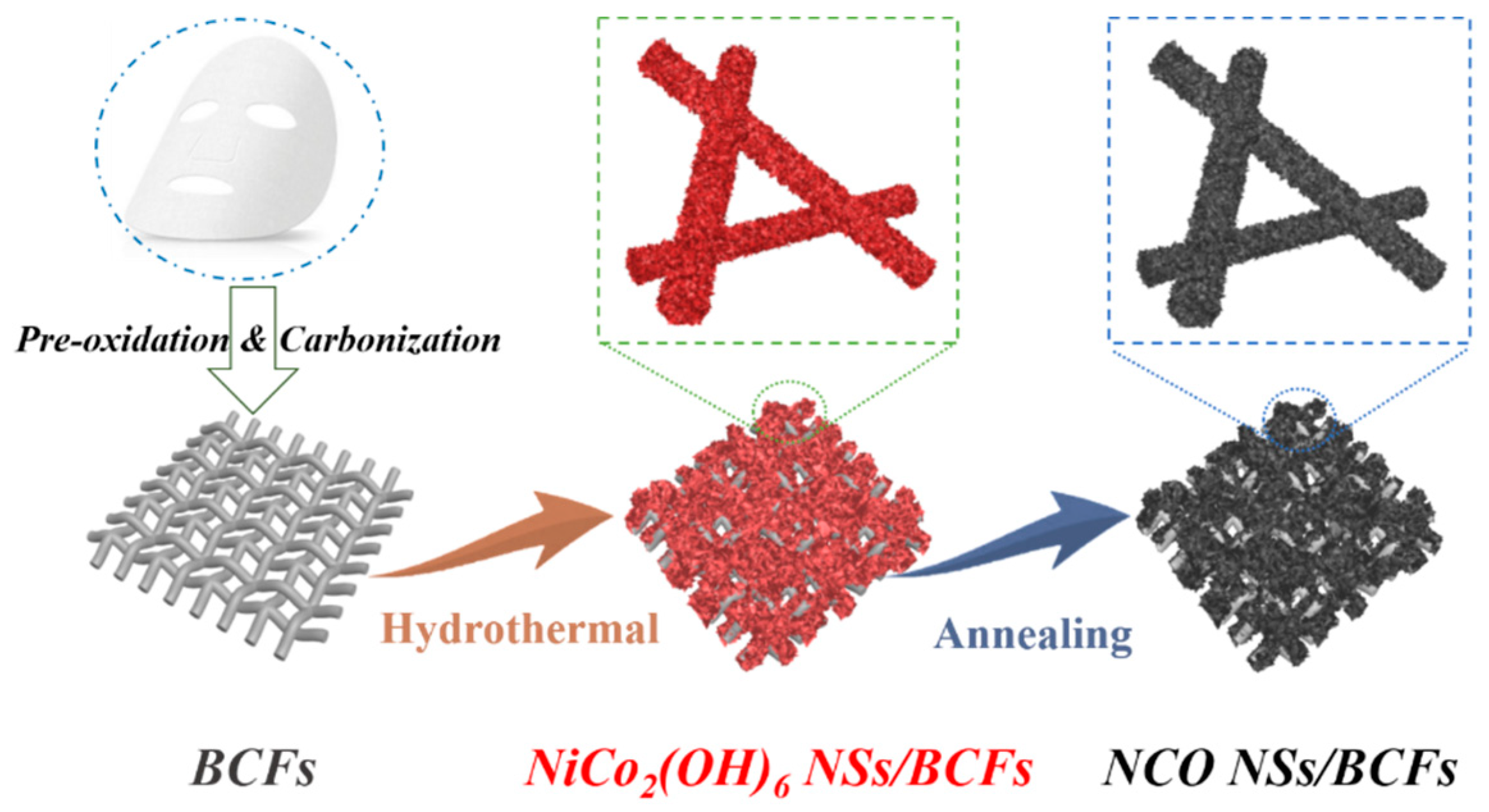
2.1. Synthesis of a Biomass-Derived Carbon-Fiber Film
2.2. Synthesis of NiCo2O4 NSs/BCF Composites
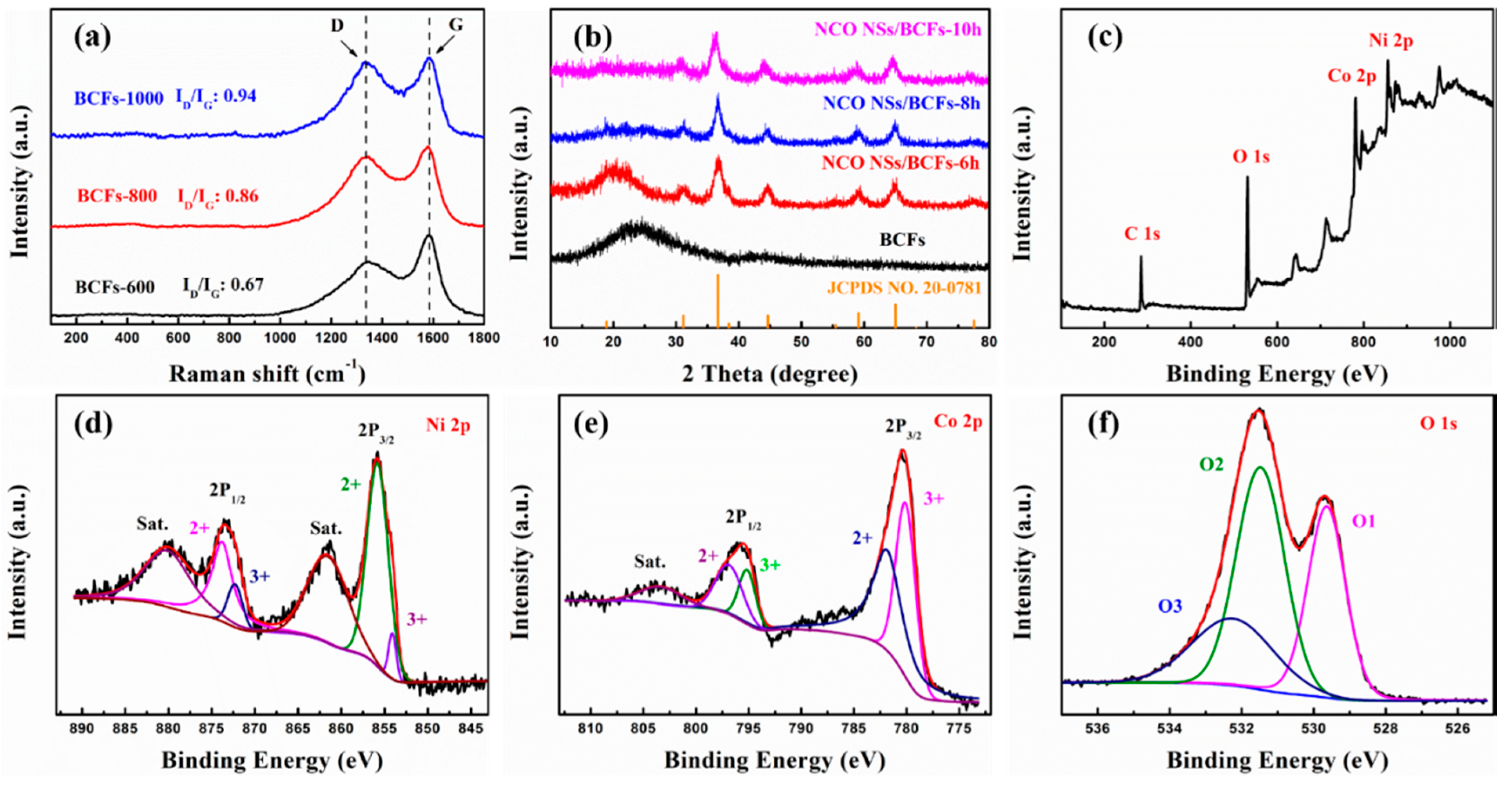
2.3. Characterization Methods
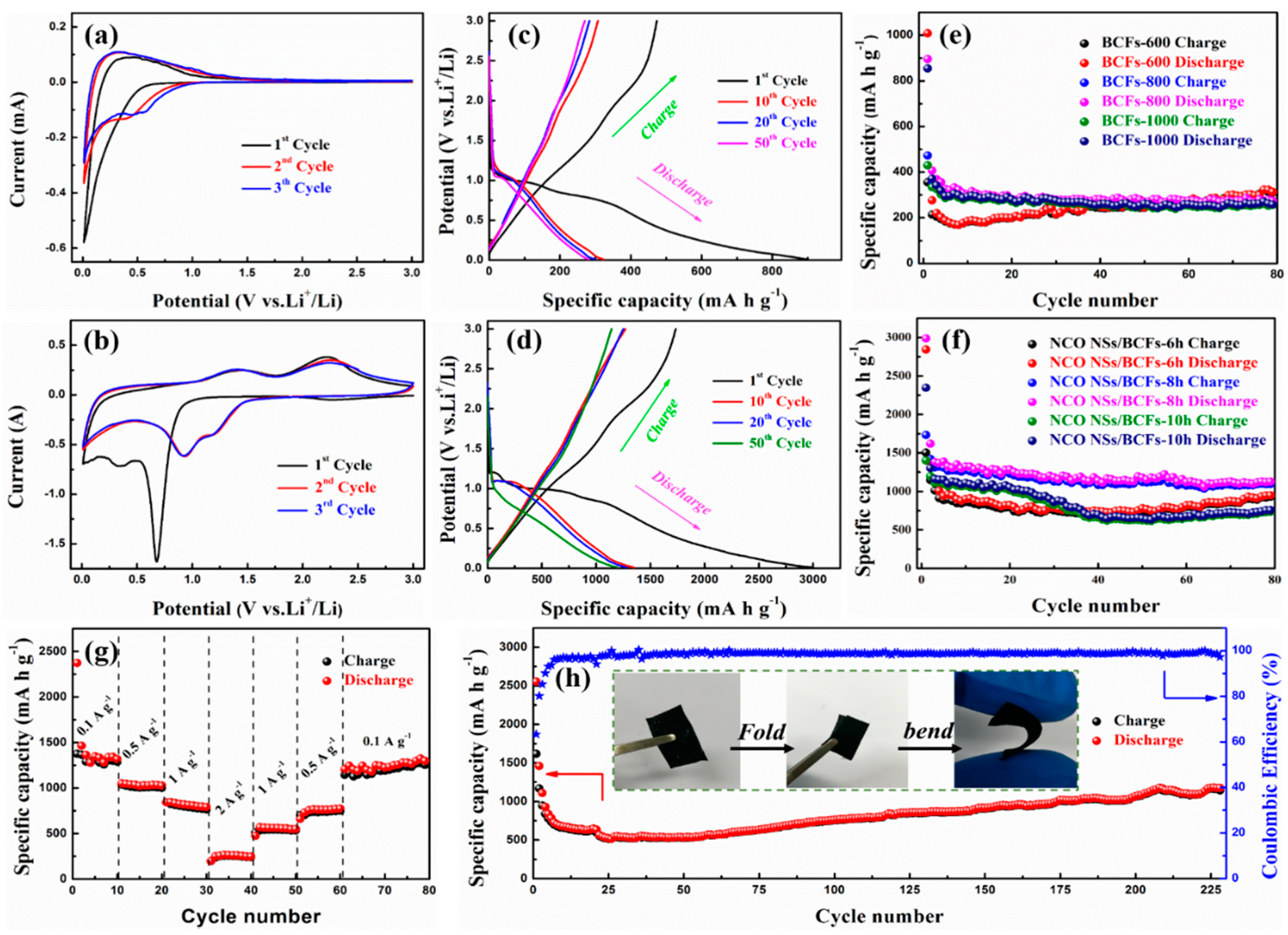
2.4. Electrochemical Measurement
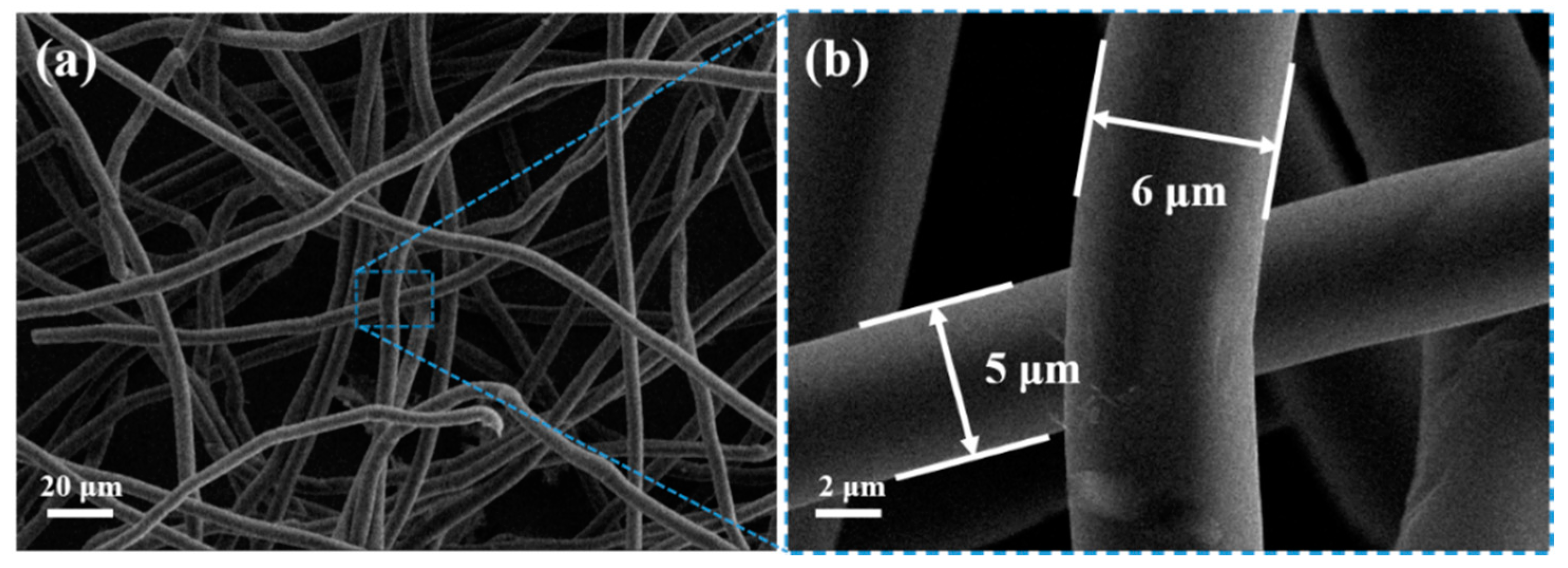
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, R.; Yan, C. Nitrogen-doped double-layer graphite supported CuCo2S4 electrode for high-performance asymmetric supercapacitors. Mater. Lett. 2019, 235, 6–10. [Google Scholar] [CrossRef]
- Lahiri, A.; Endres, F. Review—Electrodeposition of Nanostructured Materials from Aqueous, Organic and Ionic Liquid Electrolytes for Li-Ion and Na-Ion Batteries: A Comparative Review. J. Electrochem. Soc. 2017, 164, 597–612. [Google Scholar] [CrossRef]
- Cong, L.; Xie, H.; Li, J. Hierarchical Structures Based on Two-Dimensional Nanomaterials for Rechargeable Lithium Batteries. Adv. Energy Mater. 2017, 7, 1601906–1601941. [Google Scholar] [CrossRef]
- Tabassum, H.; Zou, R.; Mahmood, A.; Liang, Z.; Wang, Q.; Zhang, H.; Gao, S.; Qu, C.; Guo, W.; Guo, S. A Universal Strategy for Hollow Metal Oxide Nanoparticles Encapsulated into B/N Co-Doped Graphitic Nanotubes as High-Performance Lithium-Ion Battery Anodes. Adv. Mater. 2018, 30, 1705441–1705447. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Jin, Z.; Zhang, L.; Li, L.; Gao, Z.; Wang, C.; Xie, H.; Su, Z. Uniform Pomegranate-Like Nanoclusters Organized by Ultrafine Transition Metal Oxide@Nitrogen-Doped Carbon Subunits with Enhanced Lithium Storage Properties. Adv. Energy Mater. 2018, 8, 1702347–1702354. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Wang, L.; Lin, H.N.; Ma, L.B.; Zhao, P.Y.; Hu, Y.; Chen, T.; Chen, R.P.; Wang, Y.R.; Tie, Z.X.; et al. Walnut-Like Multicore-Shell MnO Encapsulated Nitrogen-Rich Carbon Nanocapsules as Anode Material for Long-Cycling and Soft-Packed Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1800003–1800009. [Google Scholar] [CrossRef]
- Cao, K.; Jin, T.; Yang, L.; Jiao, L. Recent progress in conversion reaction metal oxide anodes for Li-ion batteries. Mater. Chem. Front. 2017, 1, 2213–2242. [Google Scholar] [CrossRef]
- Zhang, G.; Xiao, X.; Li, B.; Gu, P.; Xue, H.; Pang, H. Transition metal oxides with one-dimensional/one-dimensional-analogue nanostructures for advanced supercapacitors. J. Mater. Chem. A 2017, 5, 8155–8186. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Su, H.; Huang, W.; Dong, X. Binary metal oxide: Advanced energy storage materials in supercapacitors. J. Mater. Chem. A 2015, 3, 43–59. [Google Scholar] [CrossRef]
- Qu, Z.C.; Shi, M.J.; Wu, H.Z.; Liu, Y.C.; Jiang, J.T.; Yan, C. An efficient binder-free electrode with multiple carbonized channels wrapped by NiCo2O4 nanosheets for high-performance capacitive energy storage. J. Power Sources 2019, 410, 179–187. [Google Scholar] [CrossRef]
- Fu, F.; Li, J.; Yao, Y.; Qin, X.; Dou, Y.; Wang, H.; Tsui, J.; Chan, K.Y.; Shao, M. Hierarchical NiCo2O4 Micro- and Nanostructures with Tunable Morphologies as Anode Materials for Lithium- and Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 16194–16201. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Ji, X. NiCo2O4-based materials for electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Wu, H.B.; Chen, J.S.; Hng, H.H.; Lou, X.W. Nanostructured metal oxide-based materials as advanced anodes for lithium-ion batteries. Nanoscale 2012, 4, 2526–2542. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qu, B.; Hu, L.; Xu, Z.; Li, Q.; Wang, T. High-performance supercapacitor and lithium-ion battery based on 3D hierarchical NH4F-induced nickel cobaltate nanosheet-nanowire cluster arrays as self-supported electrodes. Nanoscale 2013, 5, 9812–9820. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, P.; Zhu, K.; Wang, J.; Liu, J. Hierarchical bilayered hybrid nanostructural arrays of NiCo2O4 micro-urchins and nanowires as a free-standing electrode with high loading for high-performance lithium-ion batteries. Nanoscale 2017, 9, 14979–14989. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Tang, S.; Deng, M.; Du, Y. NiCo2O4@rGO hybrid nanostructures on Ni foam as high-performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 5912–5919. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General solution growth of mesoporous NiCo2O4 nanosheets on various conductive substrates as high-performance electrodes for supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, M.; Jiang, J.; Liu, Y.; Yan, C.; Liu, H.; Guo, Z. Heterogeneous interface induced formation of balsam pear-like PPy for high performance supercapacitors. Mater. Lett. 2019, 244, 27–30. [Google Scholar] [CrossRef]
- Mo, Y.; Ru, Q.; Chen, J.; Song, X.; Guo, L.; Hu, S.; Peng, S. Three-dimensional NiCo2O4 nanowire arrays: Preparation and storage behavior for flexible lithium-ion and sodium-ion batteries with improved electrochemical performance. J. Mater. Chem. A 2015, 3, 19765–19773. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Yu, P.; Tian, C.; Feng, H.; Diao, Z.; Fu, H. Hierarchical porous NiCo2O4 nanosheet arrays directly grown on carbon cloth with superior lithium storage performance. Dalton Trans. 2017, 46, 4717–4723. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Ru, Q.; Song, X.; Guo, L.; Chen, J.; Hou, X.; Hu, S. The sucrose-assisted NiCo2O4 @C composites with enhanced lithium-storage properties. Carbon 2016, 109, 616–623. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhu, K.; Wang, J.; Yan, K.; Liu, J. One-step fabrication of in situ carbon-coated NiCo2O4 @C bilayered hybrid nanostructural arrays as free-standing anode for high-performance lithium-ion batteries. Electrochim. Acta 2018, 273, 1–9. [Google Scholar] [CrossRef]
- Zhu, F.; Xia, H.; Feng, T. Nanowire interwoven NiCo2S4 nanowall arrays as promising anodes for lithium ion batteries. Mater. Technol. 2015, 30, 53–57. [Google Scholar] [CrossRef]
- Chen, S.; Wu, J.; Zhou, R.; Chen, Y.; Song, Y.; Wang, L. Controllable growth of NiCo2O4 nanoarrays on carbon fiber cloth and its anodic performance for lithium-ion batteries. RSC Adv. 2015, 5, 104433–104440. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Wang, S.; Wang, W.; Xiang, Y.; Dai, C.; Liu, Z.; He, Z.; Wu, X. Surfactant-assisted solvothermal synthesis of NiCo2O4 as an anode for lithium-ion batteries. RSC Adv. 2017, 7, 36909–36916. [Google Scholar] [CrossRef]
- Oh, S.H.; Kwon, O.H.; Kang, Y.C.; Kim, J.K.; Cho, J.S. Highly integrated and interconnected CNT hybrid nanofibers decorated with α-iron oxide as freestanding anodes for flexible lithium polymer batteries. J. Mater. Chem. A 2019, 7, 12480–12488. [Google Scholar] [CrossRef]
- Shen, L.; Che, Q.; Li, H.; Zhang, X. Mesoporous NiCo2O4 Nanowire Arrays Grown on Carbon Textiles as Binder-Free Flexible Electrodes for Energy Storage. Adv. Funct. Mater. 2014, 24, 2630–2637. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, W.; Jiu, H.; Ni, C.; Chang, J.; Qi, G. The synthesis of NiO and NiCo2O4 nanosheets by a new method and their excellent capacitive performance for asymmetric supercapacitor. Electrochim. Acta 2016, 215, 212–222. [Google Scholar] [CrossRef]
- Wang, L.; Shi, M.; Yang, C.; Liu, Y.; Jiang, J.; Dai, K.; Guo, Z.; Yan, C. Amorphous MnSiO3 confined in graphene sheets for superior lithium-ion batteries. J. Alloys Compd. 2019, 804, 243–251. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, M.; Yan, C.; Zhuo, Q.; Wu, H.; Wang, L.; Liu, H.; Guo, Z. Inspired cheese-like biomass-derived carbon with plentiful heteroatoms for high performance energy storage. J. Mater. Sci. Mater. Electron. 2019, 30, 6583–6592. [Google Scholar] [CrossRef]
- Pan, X.W.; Du, Q.C.; Zhou, Y.; Liu, L.C.; Xu, G.; Yan, C. Ammonia Borane Promoted Synthesis of Graphene Aerogels as High Efficient Dye Adsorbent. J. Nanosci. Nanotechnol. 2018, 18, 7231–7240. [Google Scholar] [CrossRef]
- Du, Q.; Zhou, Y.; Pan, X.; Zhang, J.; Zhuo, Q.; Chen, S.; Chen, G.; Liu, T.; Xu, F.; Yan, C. A graphene–melamine-sponge for efficient and recyclable dye adsorption. RSC Adv. 2016, 6, 54589–54596. [Google Scholar] [CrossRef]
- Fang, L.; Qiu, H.; Luo, P.; Li, W.; Zhang, H.; Wang, Y. Hierarchical flower-like carbon nanosheet assembly with embedded hollow NiCo2O4 nanoparticles for high- performance lithium ion batteries. Appl. Surf. Sci. 2017, 403, 35–42. [Google Scholar] [CrossRef]
- Xin, H.; Li, D.; Shi, L.; Ji, M.; Lin, Y.; Yu, J.; Yang, B.; Li, C.; Zhu, C. A simple approach to fabricate of Ni- NiCo2O4@ZnCo2O4 yolk-shell nano-tetrahedron composite as high-performance anode material for lithium-ion batteries. Chem. Eng. J. 2018, 341, 601–609. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Y.; Qiu, K.; Yan, H.; Xu, J.; Han, L.; Liu, X.; Luo, J.; Kim, J.K.; Luo, Y. Hierarchical Core/Shell NiCo2O4@NiCo2O4Nanocactus Arrays with Dual-functionalities for High Performance Supercapacitors and Li-ion Batteries. Sci. Rep. 2015, 5, 12099–12110. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, L.; Li, M.; Wang, P.; Zhang, J.; Lu, H. Ultra-high-rate, ultra-long-life asymmetric supercapacitors based on few-crystalline, porous NiCo2O4 nanosheet composites. J. Mater. Chem. A 2018, 6, 1412–1422. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.K.; Kang, Y.C. Three-dimensional macroporous CNTs microspheres highly loaded with NiCo2O4 hollow nanospheres showing excellent lithium-ion storage performances. Carbon 2018, 128, 191–200. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4Nanosheets Grown on Nitrogen-Doped Carbon Foams as an Advanced Electrode for Supercapacitors. Adv. Energy Mater. 2015, 5, 1400977–1400983. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.J.; Yuan, X.C.; Yang, Z.; Zhang, M.; Meng, A.; Li, Q.D. A High-Energy Density Asymmetric Supercapacitor Based on Fe2O3 Nanoneedle Arrays and NiCo2O4/Ni(OH)2 Hybrid Nanosheet Arrays Grown on SiC Nanowire Networks as Free-Standing Advanced Electrodes. Adv. Energy Mater. 2018, 8, 1702787–1702800. [Google Scholar] [CrossRef]
- Li, X.; Wu, H.J.; Elshahawy, A.M.; Wang, L.; Pennycook, S.J.; Guan, C.; Wang, J. Cactus-Like NiCoP/NiCo-OH 3D Architecture with Tunable Composition for High-Performance Electrochemical Capacitors. Adv. Funct. Mater. 2018, 28, 1800036–1800045. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High electrochemical performance of monodisperse NiCo2O4 mesoporous microspheres as an anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, J.; Qu, B.; Lu, B.; Xu, Z. Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays. Nano Energy 2014, 3, 88–94. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Wang, J.; Liu, S.; Li, C.; Jiang, Q.; Lu, J.; Zeng, X.; Peng, P.; Li, F. Fullerene-Based In Situ Doping of N and Fe into a 3D Cross-Like Hierarchical Carbon Composite for High-Performance Supercapacitors. Adv. Energy Mater. 2019, 9, 1802928–1802937. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Jiang, J.; Yuan, Y.; Jiang, Q.; Yan, C. Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance. Nanomaterials 2019, 9, 1336. https://doi.org/10.3390/nano9091336
Liu Y, Jiang J, Yuan Y, Jiang Q, Yan C. Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance. Nanomaterials. 2019; 9(9):1336. https://doi.org/10.3390/nano9091336
Chicago/Turabian StyleLiu, Yongchao, Jintian Jiang, Yanyan Yuan, Qinglong Jiang, and Chao Yan. 2019. "Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance" Nanomaterials 9, no. 9: 1336. https://doi.org/10.3390/nano9091336
APA StyleLiu, Y., Jiang, J., Yuan, Y., Jiang, Q., & Yan, C. (2019). Vertically Aligned NiCo2O4 Nanosheet-Encapsulated Carbon Fibers as a Self-Supported Electrode for Superior Li+ Storage Performance. Nanomaterials, 9(9), 1336. https://doi.org/10.3390/nano9091336




