Highly Dispersed Ag2S Nanoparticles: In Situ Synthesis, Size Control, and Modification to Mechanical and Tribological Properties towards Nanocomposite Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
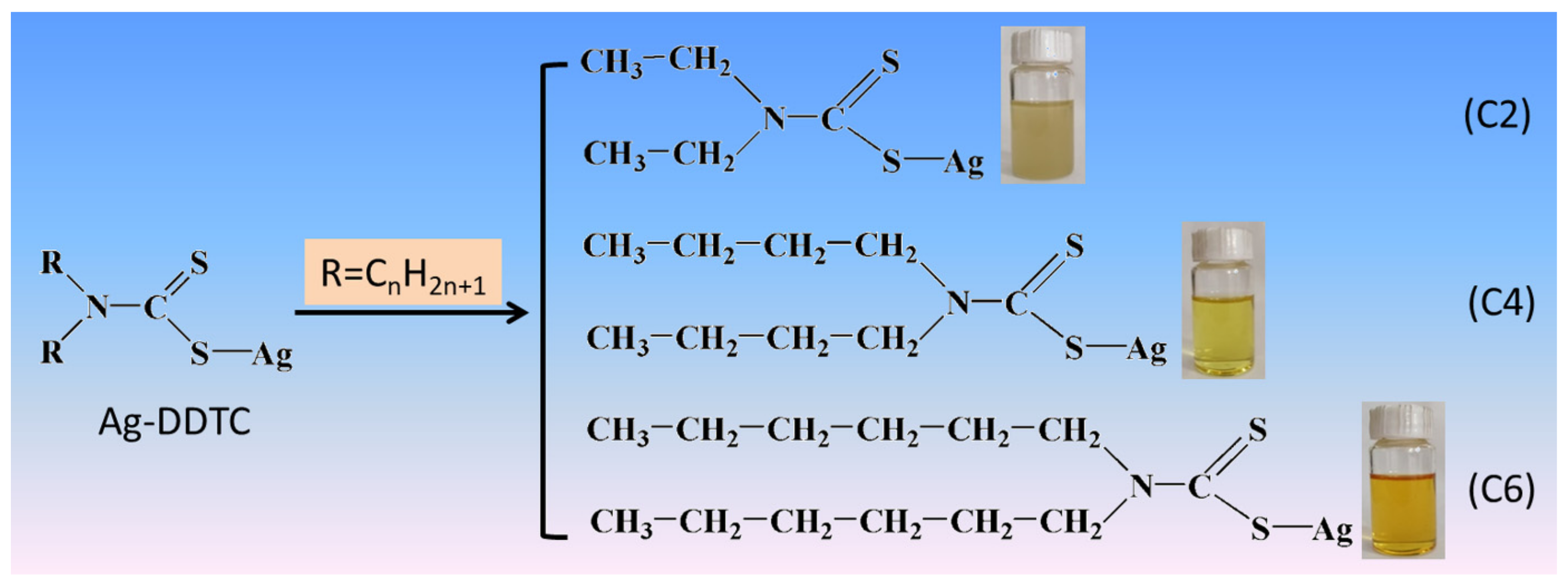
2.2. Controllable In Situ Synthesis of Ag2S Nanoparticles
2.3. Evaluation of Mechanical Properties
2.4. Friction and Wear Test
3. Results and Discussion
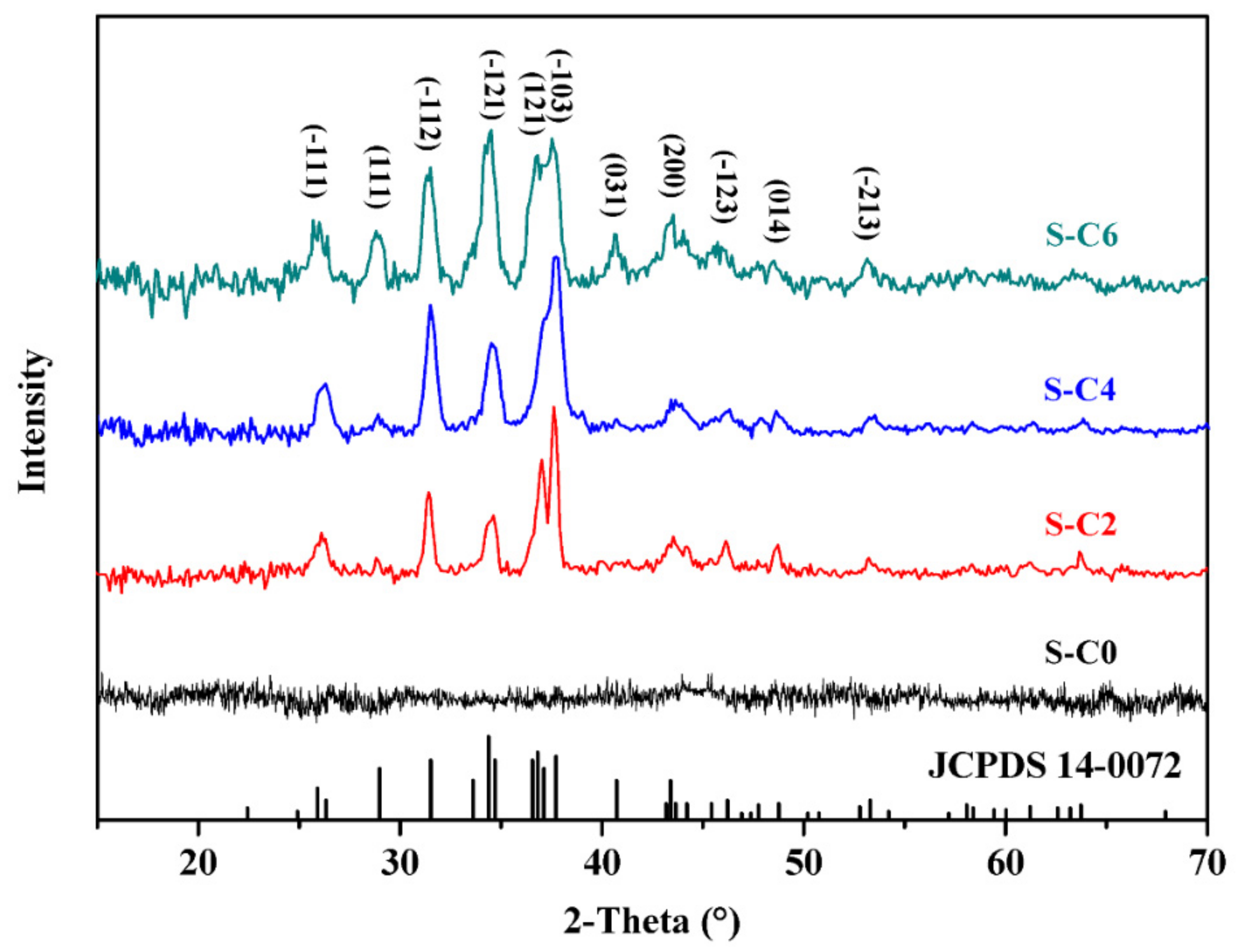
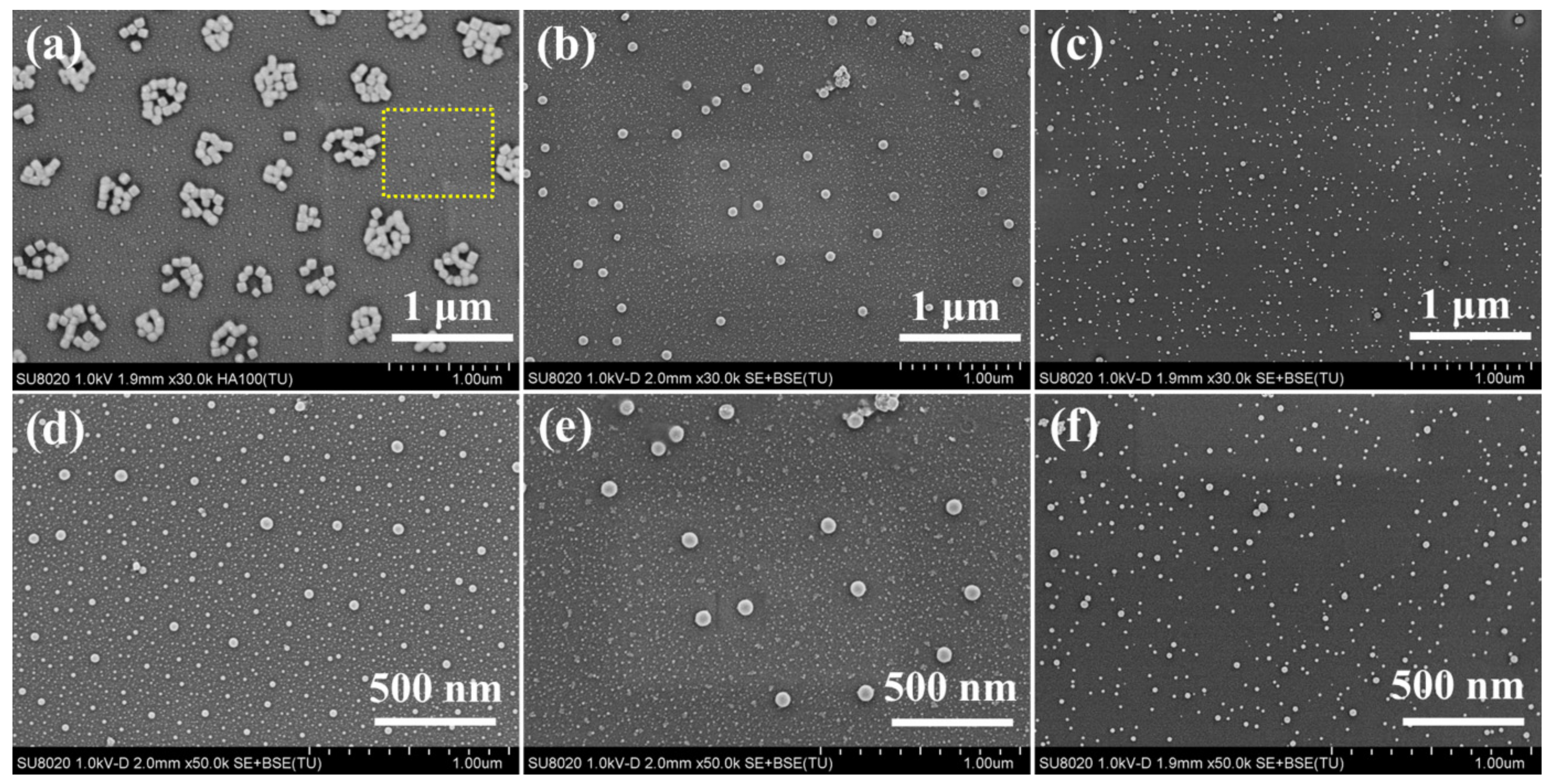
3.1. Size and Morphology of the In Situ Synthesized Ag2S Nanoparticles
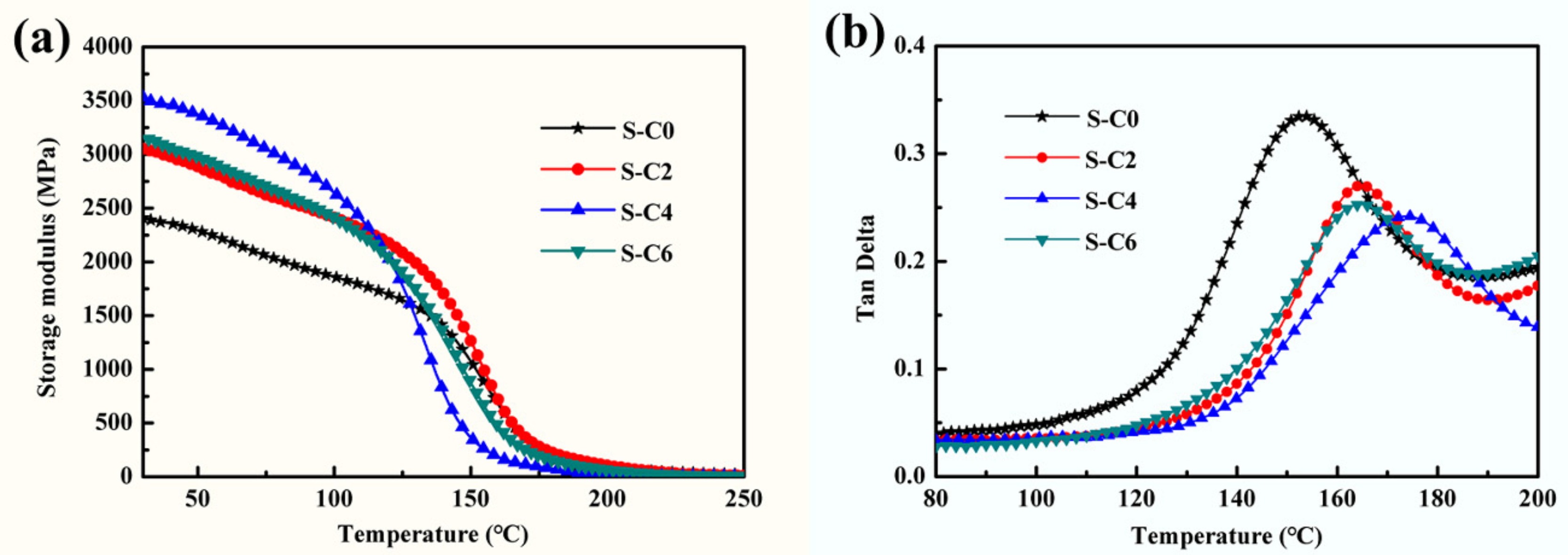
3.2. Effect of Ag2S Nanoparticles on Mechanical Properties of Nanocomposite Coatings
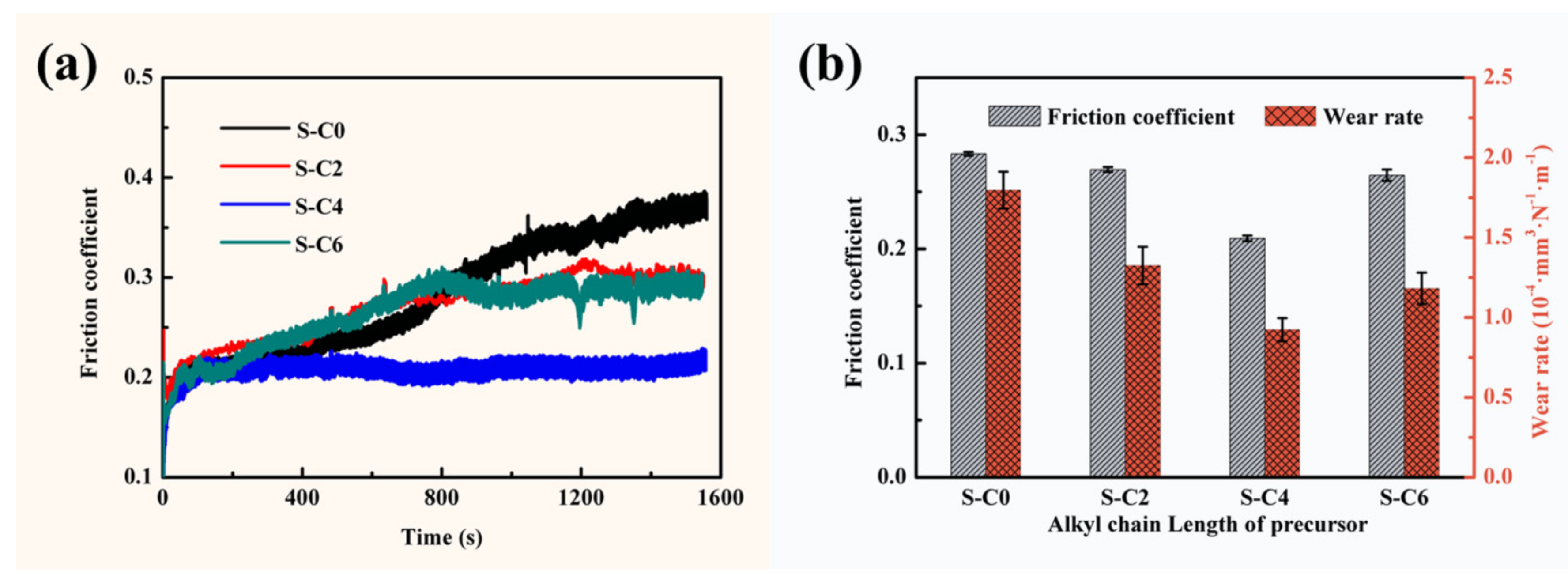
3.3. Effect of Ag2S Nanoparticles on Tribological Properties of Nanocomposite Coatings
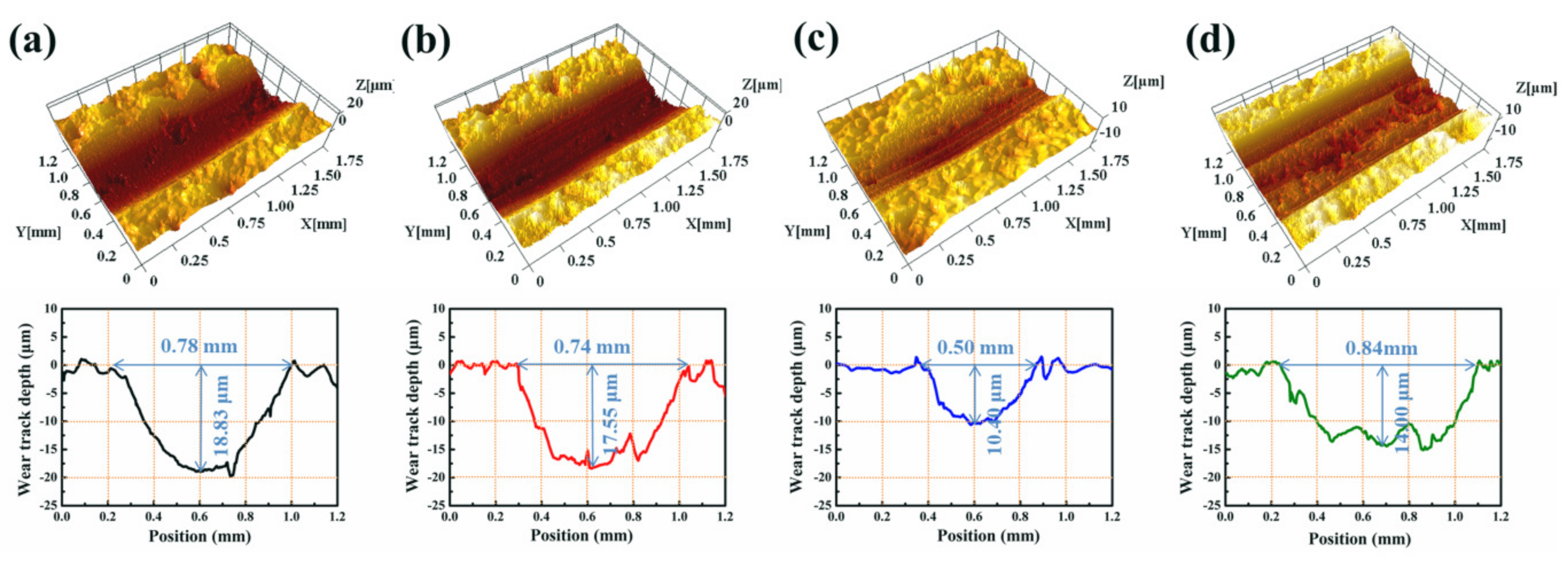
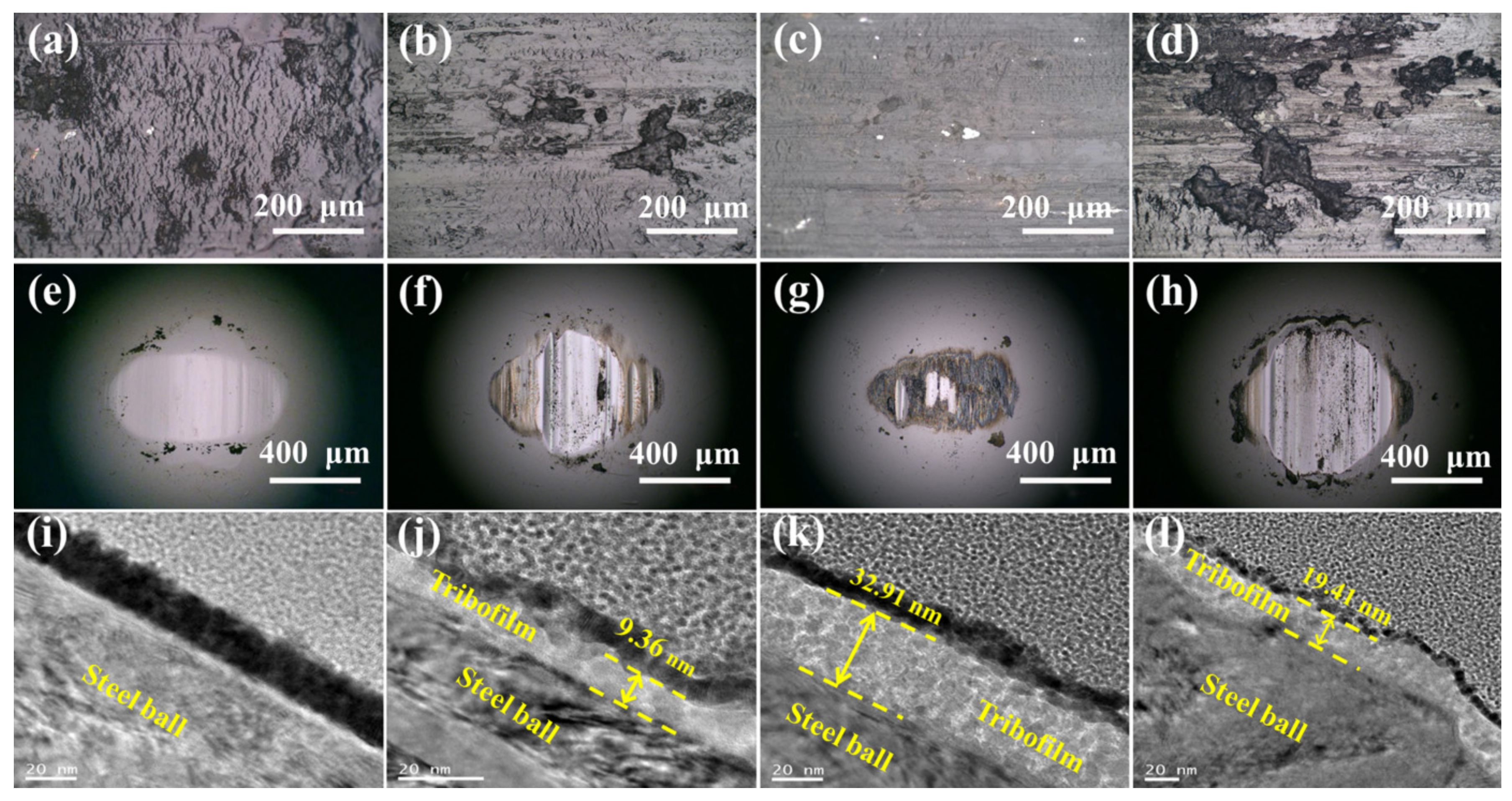
3.4. Analyses of Wear Track Morphology and Wear Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, B.; Wan, H.Q.; Ye, Y.P.; Chen, L.; Zhou, H.D.; Chen, J.M. Investigating the effect of LaF3 on the tribological performances of an environment friendly hydrophilic polyamide imide resin bonded solid lubricating coating. Tribol. Int. 2017, 116, 164–171. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.H.; Huang, J.; Li, Y.; Yang, J.; Song, H.J. Monodisperse Cu nanoparticles @ MoS2 nanosheets as a lubricant additive for improved tribological properties. Appl. Surf. Sci. 2019, 494, 430–439. [Google Scholar]
- Li, B.; Jiang, X.F.; Wan, H.Q.; Ye, Y.P.; Chen, L.; Zhou, H.D.; Chen, J.M. Fabrication and tribological behaviors of a novel environmental-friendly water-based PAI-PTFE-LaF3 bonded solid lubricating composite coating. Tribol. Int. 2018, 121, 400–409. [Google Scholar] [CrossRef]
- Song, H.J.; Wang, Z.Q.; Yang, J.; Jia, X.H.; Zhang, Z.Z. Facile synthesis of copper/polydopamine functionalized graphene oxide nanocomposites with enhanced tribological performance. Chem. Eng. J. 2017, 324, 51–62. [Google Scholar] [CrossRef]
- Wang, C.R.; Yin, J.J.; Wang, R.; Jiao, T.F.; Huang, H.M.; Zhou, J.X.; Zhang, L.X.; Peng, Q.M. Facile preparation of self-assembled polydopamine-modified electrospun fibers for highly effective removal of organic dyes. Nanomaterials 2019, 9, 116. [Google Scholar] [CrossRef] [PubMed]
- Yeap, S.P. Permanent agglomerates in powdered nanoparticles: Formation and future prospects. Powder Technol. 2018, 323, 51–59. [Google Scholar] [CrossRef]
- Salimi, M.N.; Bridson, R.H.; Grover, L.M.; Leeke, G.A. Effect of processing conditions on the formation of hydroxyapatite nanoparticles. Powder Technol. 2012, 218, 109–118. [Google Scholar] [CrossRef]
- Sato, K.; Li, J.G.; Kamiya, H.; Ishigaki, T. Ultrasonic dispersion of TiO2 nanoparticles in aqueous suspension. J. Am. Ceram. Soc. 2008, 91, 2481–2487. [Google Scholar]
- Sauter, C.; Emin, M.A.; Schuchmann, H.P.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef]
- Chen, Y.N.; Liang, W.Y.; Li, Y.P.; Wu, Y.X.; Chen, Y.R.; Xiao, W.; Zhao, L.; Zhang, J.C.; Li, H. Modification, application and reaction mechanisms of nano-sized iron sulfide particles for pollutant removal from soil and water: A review. Chem. Eng. J. 2019, 362, 144–159. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.N.; Yu, P.; Niu, J.Q.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.D.; Niu, X.B. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Ahmed, E.R.M.; Rohani, S. Modified TiO2 nanotube arrays (TNTAs): Progressive strategies towards visible light responsive photoanode, a review. Energy Environ. Sci. 2011, 4, 1065–1086. [Google Scholar]
- Eastman, J.A.; Choi, S.U.S.; Li, S.; Yu, W.; Thompson, L.J. Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 2001, 78, 718–720. [Google Scholar] [CrossRef]
- Tatarchuk, T.R.; Paliychuk, N.D.; Bououdina, M.; Al-Najar, B.; Pacia, M.; Macyk, W.; Shyichuk, A. Effect of cobalt substitution on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles. J. Alloys Compd. 2017, 731, 1256–1266. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Huang, J.P.; Shi, W.D.; Li, Y.; Wu, Y.Y.; Liu, Q.Q.; Zhu, J.; Zhao, N.; Zhang, L.L.; Yang, J.; et al. Ecofriendly and environment-friendly synthesis of size-controlled silver nanoparticles/graphene composites for antimicrobial and SERS actions. Appl. Surf. Sci. 2018, 457, 1000–1008. [Google Scholar]
- Peng, R.S.; Li, S.J.; Sun, X.B.; Ren, Q.M.; Chen, L.M.; Fu, M.L.; Wu, J.L.; Ye, D.Q. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2017, 220, 462–470. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Natarajan, M.; Santhanam, A.; Asokan, V.; Velauthapillal, D. Size controlled synthesis of TiO2 nanoparticles by modified solvothermal method towards effective photo catalytic and pholtovoltaic applications. Mater. Res. Bull. 2018, 97, 351–360. [Google Scholar] [CrossRef]
- Kalyani; Jaiswal, V.; Rastogi, R.B.; Kumar, D. The investigation of different particle size magnesium-doped zinc oxide (Zn0.92Mg0.08O) nanoparticles on the lubrication behavior of paraffin oil. Appl. Nanosci. 2017, 7, 275–281. [Google Scholar]
- Peng, D.X.; Chen, C.H.; Kang, Y.A.; Chang, Y.P.; Chang, S.Y. Size effects of SiO2 nanoparticles as oil additives on tribology of lubricant. Ind. Lubr. Tribol. 2013, 62, 111–120. [Google Scholar] [CrossRef]
- Zin, V.; Agresti, F.; Barison, S.; Colla, L.; Gondolini, A.; Fabrizio, M. The synthesis and effect of copper nanoparticles on the tribological properties of lubricant oils. IEEE Trans. Nanotechnol. 2013, 12, 751–759. [Google Scholar] [CrossRef]
- Xu, N.; Li, W.M.; Zhang, M.; Zhao, G.Q.; Wang, X.B. Reinforcing effect on the tribological behaviour of nanoparticles due to a bimodal grain size distribution. RSC Adv. 2014, 4, 55383–55387. [Google Scholar] [CrossRef]
- Echavarria, A.M.; Robledob, S.; Bejarano, G. Influence of Ag nanoparticles on the mechanical and tribological properties and on the cytotoxic and bactericidal effect of TaN (Ag) coatings. Rev. Metal. Madrid 2017, 53, 085. [Google Scholar]
- Li, R.P.; Cheng, Y.C.; Huang, W. Recent progress of Janus 2D transition metal chalcogenides: From theory to experiments. Small 2018, 14, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lai, Z.C.; Ma, Q.L.; Zhang, H. Novel structured transition metal dichalcogenide nanosheets. Chem. Soc. Rev. 2018, 47, 3301–3338. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Rempel, A.A.; Gusev, A.I. Nanostructured silver sulfide: Synthesis of various forms and their application. Russ. Chem. Rev. 2018, 87, 303–327. [Google Scholar] [CrossRef]
- Guo, W.J. Preparation and anti-wear properties of silver sulfide nanoparticles in microemulsion. J. Inorg. Mater. 2008, 23, 960–964. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I. Recent progress in nanostructured silver sulfide: From synthesis and nonstoichiometry to properties. J. Mater. Chem. A 2017, 5, 17676–17704. [Google Scholar] [CrossRef]
- Du, Y.P.; Xu, B.; Fu, T.; Cai, M.; Li, F.; Zhang, Y.; Wang, X.B. Near-infrared photoluminescent Ag2S quantum dots from a single source precursor. J. Am. Chem. Soc. 2010, 132, 1470–1471. [Google Scholar] [CrossRef]
- Shi, H.Q.; Fu, X.; Zhou, X.D.; Hu, Z.S. Preparation of organic fluids with high loading concentration of Ag2S nanoparticles using the extractant Cyanex 301. J. Mater. Chem. 2006, 16, 2097–2101. [Google Scholar] [CrossRef]
- Malik, M.A.; Revaprasadu, N.; O’Brien, P. Air-stable single-source precursors for the synthesis of chalcogenide semiconductor nanoparticles. Chem. Mater. 2001, 13, 913–920. [Google Scholar]
- Ma, Y.J.; Chen, L.; Ye, Y.P.; Wan, H.Q.; Zhou, H.D.; Chen, J.M. Preparation and tribological behaviors of a novel organic-inorganic hybrid resin bonded solid lubricating coating cured by ultraviolet radiation. Prog. Org. Coat. 2019, 127, 348–358. [Google Scholar] [CrossRef]
- Ye, Y.W.; Wang, Y.X.; Chen, H.; Li, J.L.; Zhou, S.G.; Xue, Q.J. Influences of bias voltage on the microstructures and tribological performances of Cr–C–N coatings in seawater. Surf. Coat. Technol. 2015, 270, 305–313. [Google Scholar] [CrossRef]
- Xu, F.; Xin, Y.; Li, T. Tribological enhancement effect of main-chain thermotropic liquid crystalline polymer. Compos. Part A Appl. Sci. Manuf. 2018, 108, 69–78. [Google Scholar] [CrossRef]
- Chatterjee, A. Effect of nano-TiO2 addition on poly(methyl methacrylate): An exciting nanocomposite. J. Appl. Polym. Sci. 2010, 116, 3396–3407. [Google Scholar]
- Lagashetty, A.; Vijayanand, H.; Basavaraja, S.; Bedre, M.D.; Venkataraman, A. Preparation, characterization, and thermal studies of γ-Fe2O3 and CuO dispersed polycarbonate nanocomposites. J. Therm. Anal. Calorim. 2010, 99, 577–581. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.Z.; Mai, Y.W. Fundamental aspects and recent progress on wear/scratch damage in polymer nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 31–80. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Rathod, A.P.; Sonawane, S.H.; Shimpi, N.G.; Parate, V.R. Copper-doped zinc oxide nanoparticles: Influence on thermal, thermo mechanical, and tribological properties of polycarbonate. Polym. Compos. 2017, 39, 1388–1406. [Google Scholar]
- Ratna, D.; Divekar, S.; Samui, A.B.; Chakraborty, B.C.; Banthia, A.K. Poly(ethylene oxide)/clay nanocomposite: Thermomechanical properties and morphology. Polymer 2006, 47, 4068–4074. [Google Scholar] [CrossRef]
- Le, M.T.; Huang, S.C. Thermal and mechanical behavior of hybrid polymer nanocomposite reinforced with graphene nanoplatelets. Materials 2015, 8, 5526–5536. [Google Scholar] [CrossRef] [PubMed]
- Rabaso, P.; Ville, F.; Dassenoy, F.; Diaby, M.; Afanasiev, P.; Cavoret, J.; Vacher, B.; Le Mogne, T. Boundary lubrication: Influence of the size and structure of inorganic fullerene-like MoS2 nanoparticles on friction and wear reduction. Wear 2014, 320, 161–178. [Google Scholar] [CrossRef]
- Scharf, T.W.; Prasad, S.V. Solid lubricants: A review. J. Mater. Sci. 2013, 48, 511–531. [Google Scholar] [CrossRef]
- Friedrich, K.; Zhang, Z.; Schlarb, A.K. Effects of various fillers on the sliding wear of polymer composites. Compos. Sci. Technol. 2005, 65, 2329–2343. [Google Scholar] [CrossRef]
- Myshkin, N.; Kovalev, A.; Spaltman, D.; Woydt, M. Contact mechanics and tribology of polymer composites. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
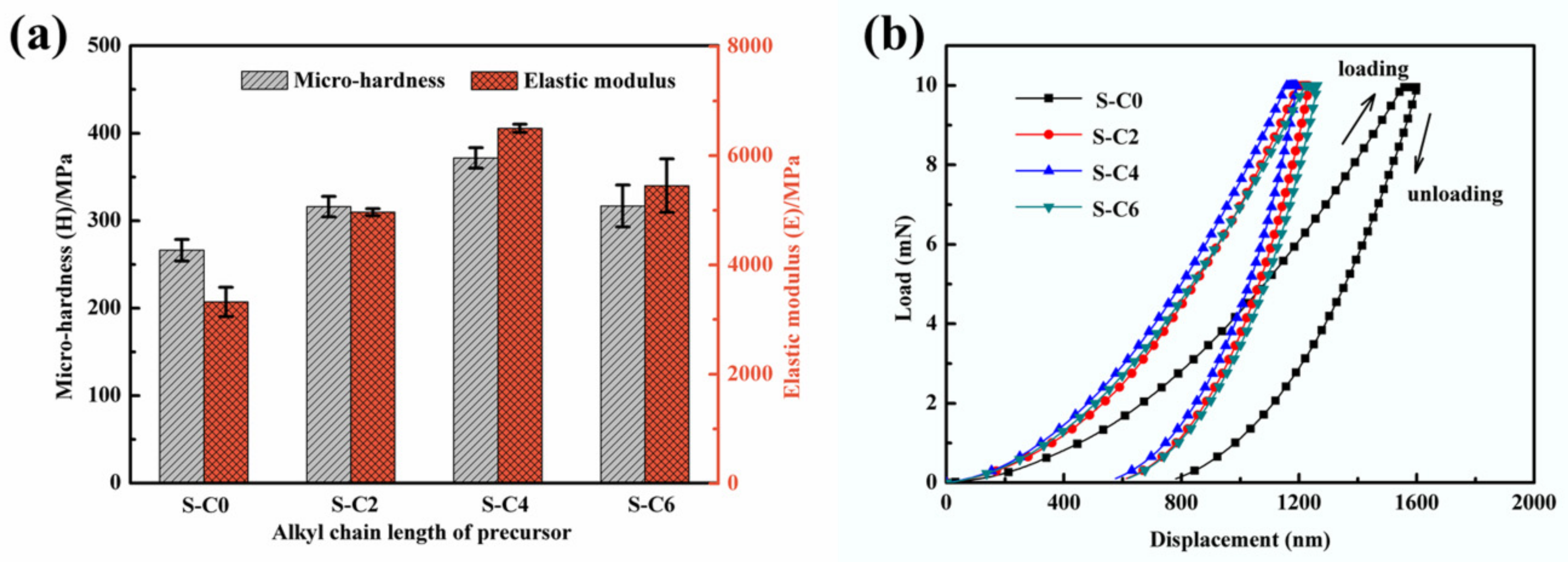

| Samples | S-C0 | S-C2 | S-C4 | S-C6 |
|---|---|---|---|---|
| Micro-hardness (H, Mpa) | 266 | 316 | 372 | 317 |
| Elastic modulus (E, Mpa) | 3322 | 4963 | 6497 | 5448 |
| Indentation depth (nm) | 1600.35 | 1233.36 | 1192.20 | 1261.70 |
| Storage modulus (E’, Mpa) | 2423.83 | 3065.64 | 3552.48 | 3143.89 |
| Loss factor (Tanδ) | 0.3348 | 0.2704 | 0.2413 | 0.2529 |
| Glass transition temperature (Tg, °C) | 153 | 165 | 175 | 164 |
| Critical load (Lc, N) | 7.76 ± 0.12 | 10.88 ± 0.25 | 12.64 ± 0.27 | 9.84 ± 0.08 |
| Samples | S-C0 | S-C2 | S-C4 | S-C6 |
|---|---|---|---|---|
| Average friction coefficient (µ) | 0.284 | 0.270 | 0.210 | 0.265 |
| Wear rate (W, mm3·N−1·m−1) | 1.80 × 10−4 | 1.32 × 10−4 | 9.24 × 10−5 | 1.18 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zhao, Z.; Xian, Y.; Wan, H.; Ye, Y.; Chen, L.; Zhou, H.; Chen, J. Highly Dispersed Ag2S Nanoparticles: In Situ Synthesis, Size Control, and Modification to Mechanical and Tribological Properties towards Nanocomposite Coatings. Nanomaterials 2019, 9, 1308. https://doi.org/10.3390/nano9091308
Ma Y, Zhao Z, Xian Y, Wan H, Ye Y, Chen L, Zhou H, Chen J. Highly Dispersed Ag2S Nanoparticles: In Situ Synthesis, Size Control, and Modification to Mechanical and Tribological Properties towards Nanocomposite Coatings. Nanomaterials. 2019; 9(9):1308. https://doi.org/10.3390/nano9091308
Chicago/Turabian StyleMa, Yanjun, Zhicheng Zhao, Yanbo Xian, Hongqi Wan, Yinping Ye, Lei Chen, Huidi Zhou, and Jianmin Chen. 2019. "Highly Dispersed Ag2S Nanoparticles: In Situ Synthesis, Size Control, and Modification to Mechanical and Tribological Properties towards Nanocomposite Coatings" Nanomaterials 9, no. 9: 1308. https://doi.org/10.3390/nano9091308
APA StyleMa, Y., Zhao, Z., Xian, Y., Wan, H., Ye, Y., Chen, L., Zhou, H., & Chen, J. (2019). Highly Dispersed Ag2S Nanoparticles: In Situ Synthesis, Size Control, and Modification to Mechanical and Tribological Properties towards Nanocomposite Coatings. Nanomaterials, 9(9), 1308. https://doi.org/10.3390/nano9091308







