Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
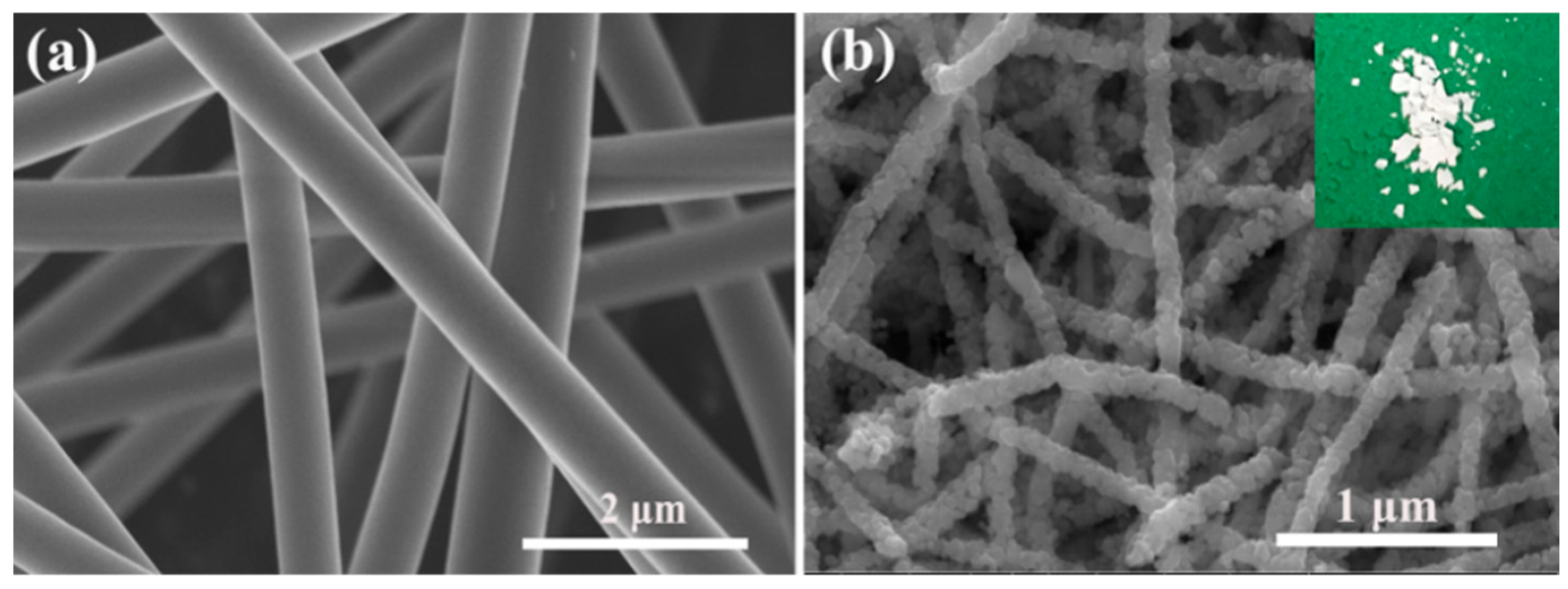
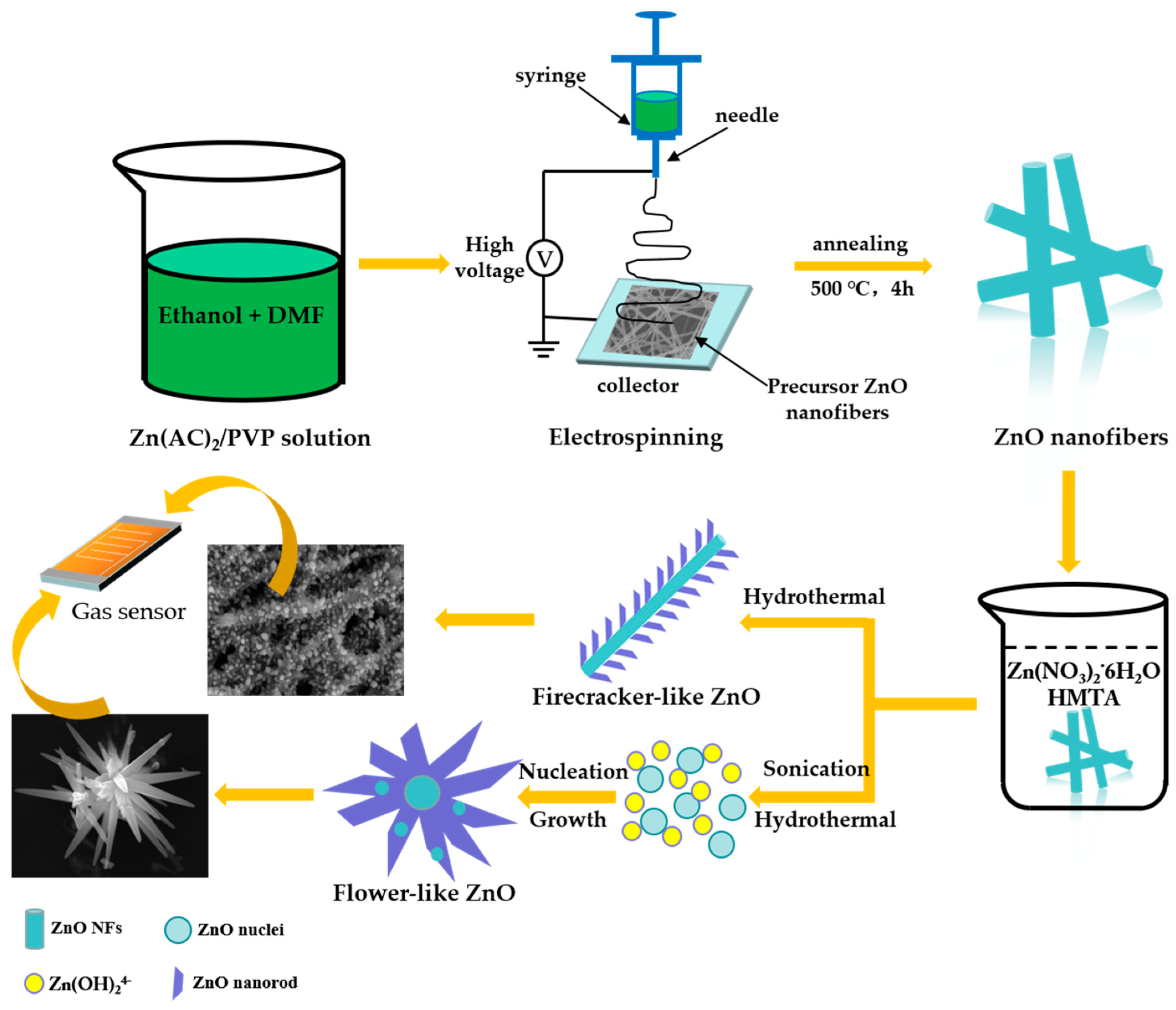
2.2. Synthesis of ZnO NFs
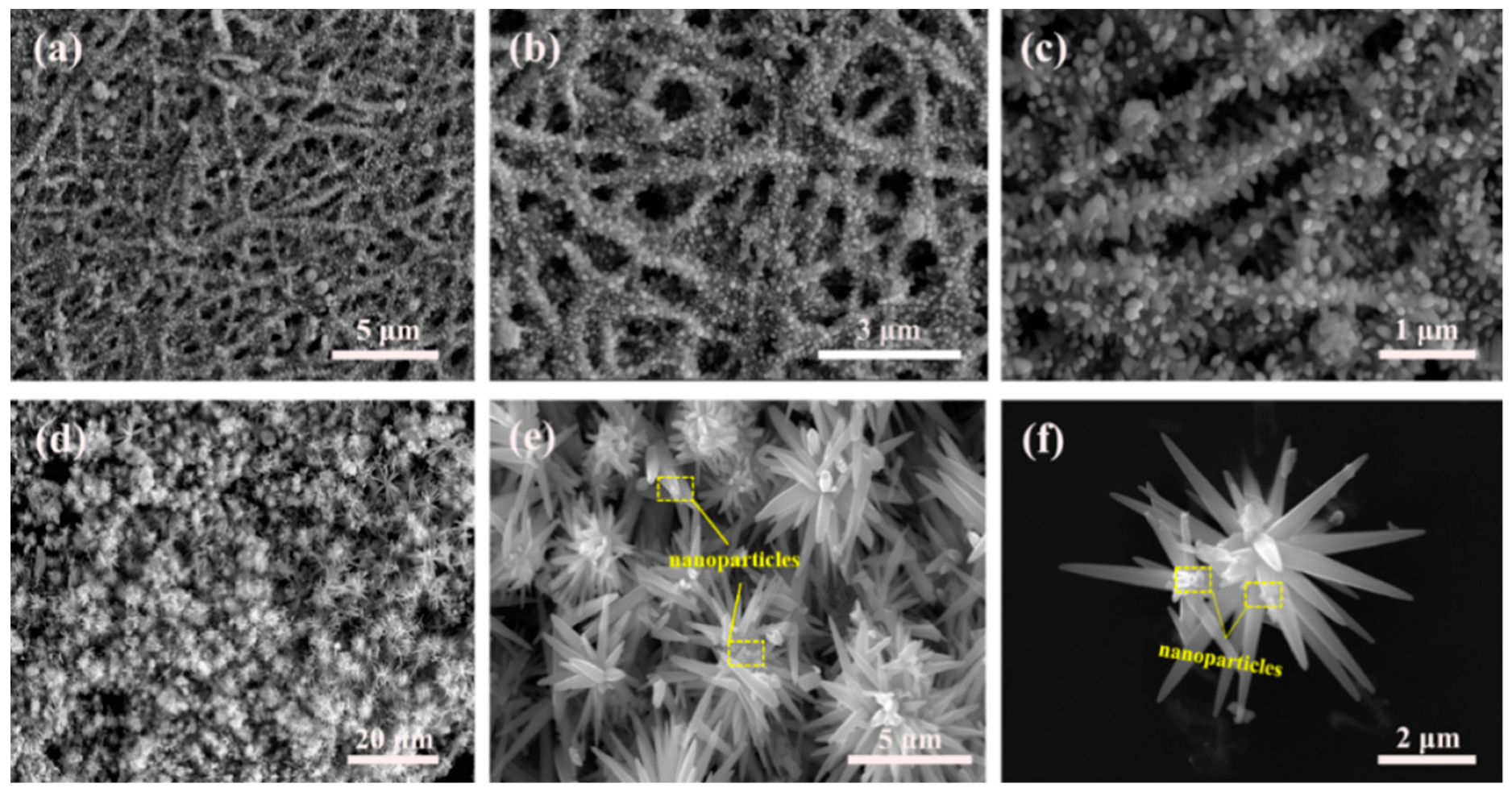
2.3. Synthesis of Firecracker and Flower Morphological ZnO Hierarchical Structures
2.4. Characterizations
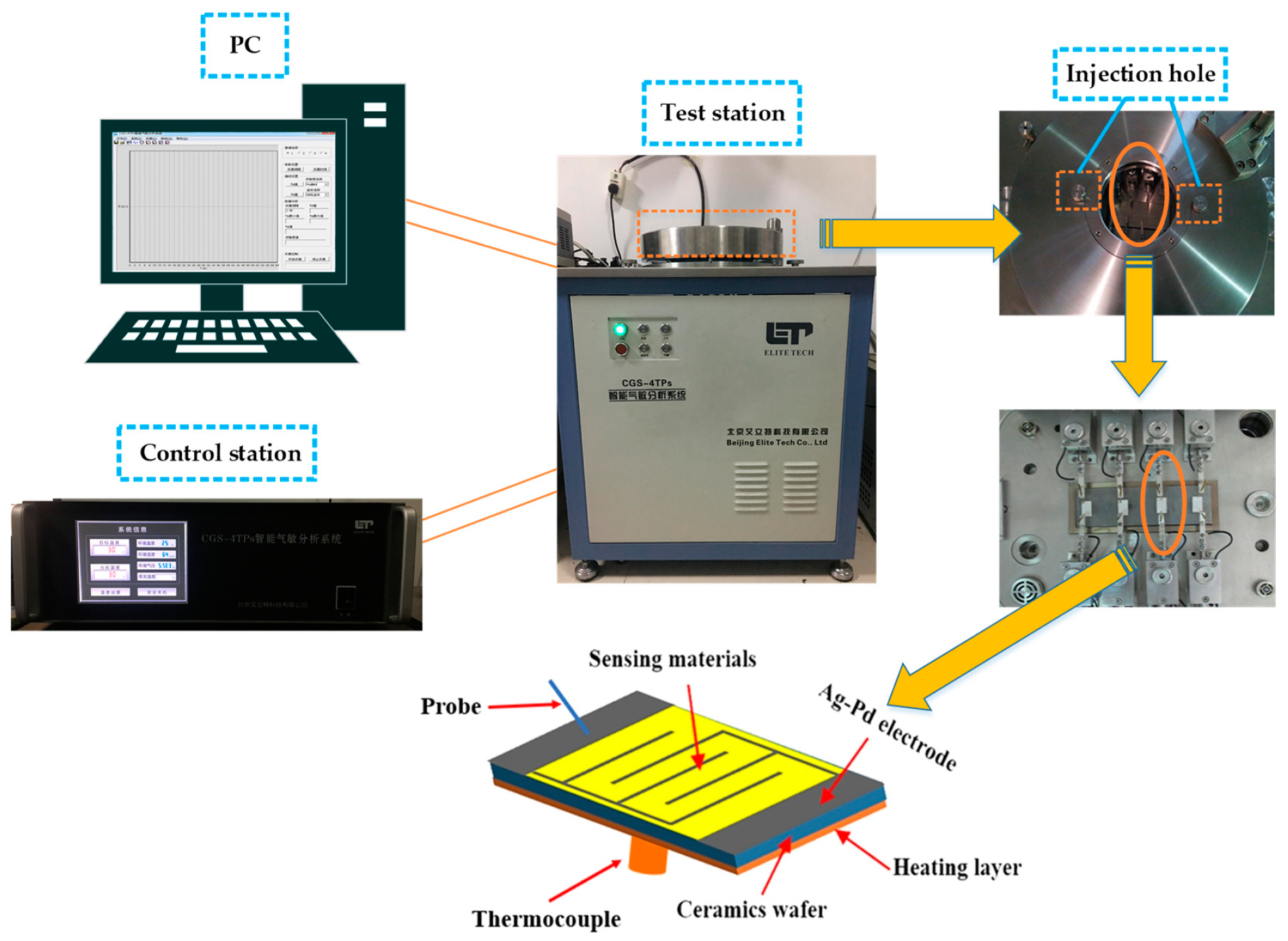
2.5. Gas sensing Measurements and Fabrication of Gas Sensors
3. Results and Discussion
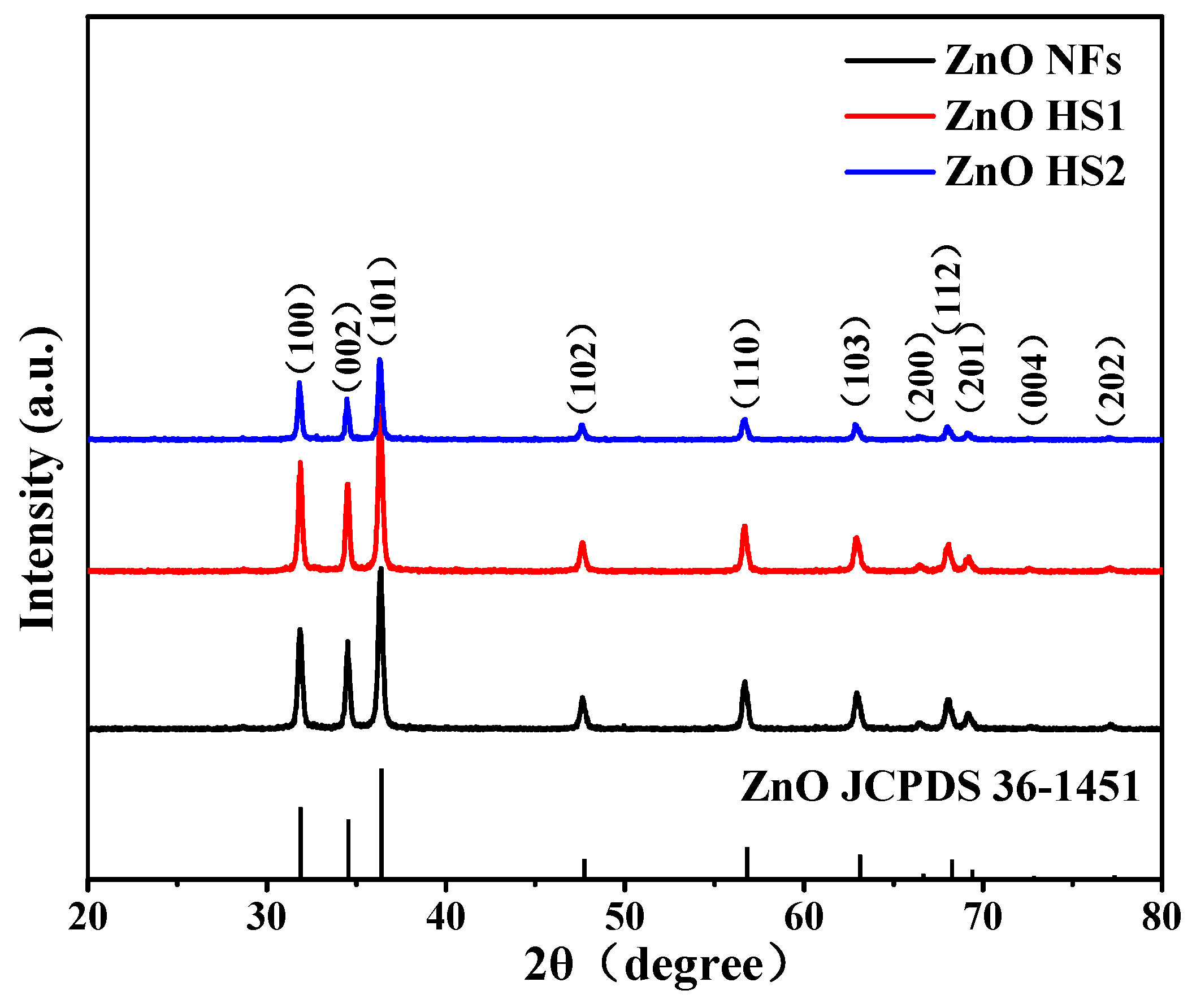
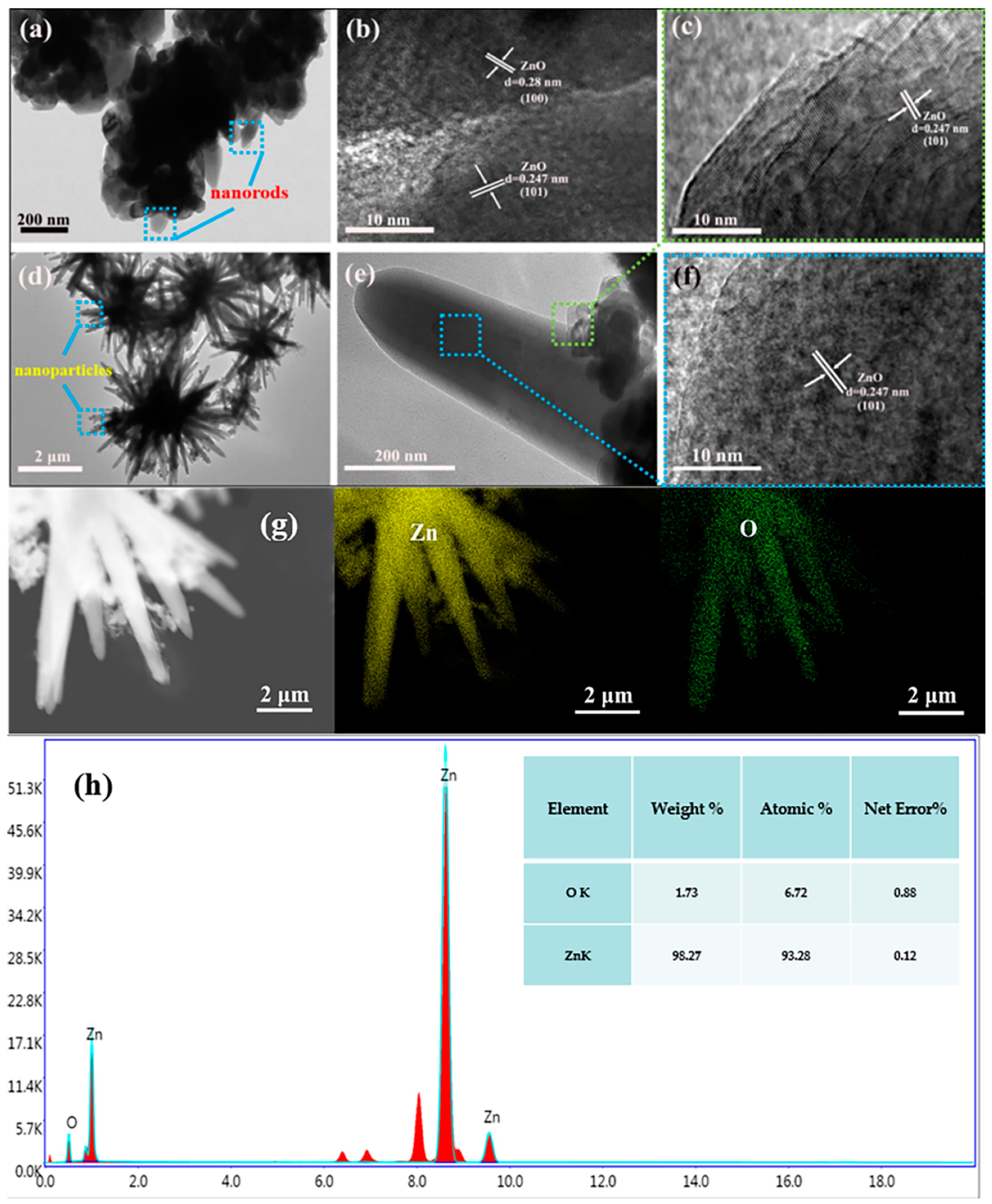
3.1. Materials Characterizations
3.2. Growth Mechanism
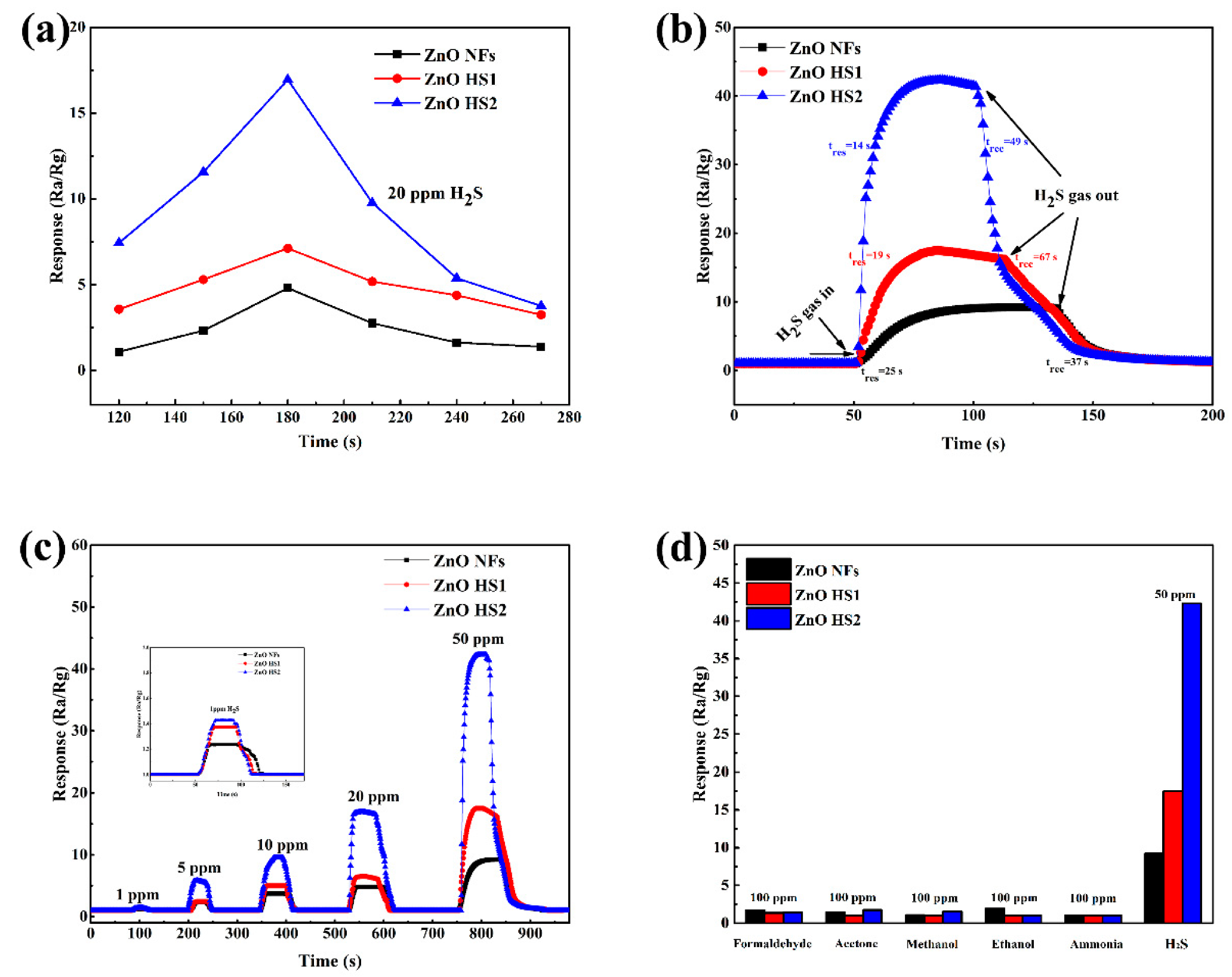
3.3. Gas Sensing Tests
3.4. Gas Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Abu-Hani, A.F.S.; Greish, Y.E.; Mahmoud, S.T.; Awwad, F.; Ayesh, A.I. Low-temperature and fast response H2S gas sensor using semiconducting chitosan film. Sens. Actuators B Chem. 2017, 253, 677–684. [Google Scholar] [CrossRef]
- Zhao, Y.; He, X.; Li, J.; Gao, X.; Jia, J. Porous CuO/SnO2 composite nanofibers fabricated by electrospinning and their H2S sensing properties. Sens. Actuators B Chem. 2012, 165, 82–87. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, X.; Wang, X.; Sun, J. Fe2O3 nanomaterials derived from Prussian blue with excellent H2S sensing properties. Sens. Actuators B Chem. 2019, 293, 136–143. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Sun, Y.P.; Yin, X.; Yin, G.C.; Wang, X.M.; Jia, F.C.; Liu, B. Effect of Surfactants on the Microstructures of Hierarchical SnO2 Blooming Nanoflowers and their Gas-Sensing Properties. Nanoscale Res. Lett. 2018, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Mani, G.K.; Elayaraja, R.; Durgadevi, E.; Navaneethan, M.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Hayakawa, Y. ZnO hierarchical 3D-flower like architectures and their gas sensing properties at room temperature. Appl. Surf. Sci. 2018, 449, 314–321. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Choi, K.S.; Chang, S.P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Yogendra, K.M.; Rainer, A. ZnO tetrapod materials for functional applications. Mater. Today 2018, 21, 631–651. [Google Scholar]
- Hou, L.; Zhang, C.; Li, L.; Du, C.; Li, X.; Kang, X.F.; Chen, W. CO gas sensors based on p-type CuO nanotubes and CuO nanocubes: Morphology and surface structure effects on the sensing performance. Talanta 2018, 188, 41–49. [Google Scholar] [CrossRef]
- Zhu, G.; Xu, H.; Xiao, Y.; Liu, Y.; Yuan, A.; Shen, X. Facile fabrication and enhanced sensing properties of hierarchically porous CuO architectures. ACS Appl. Mater. Interface 2012, 4, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Naberezhnyi, D.; Rumyantseva, M.; Filatova, D.; Batuk, M.; Hadermann, J.; Baranchikov, A.; Khmelevsky, N.; Aksenenko, A.; Konstantinova, E.; Gaskov, A. Effects of Ag Additive in Low Temperature CO Detection with In2O3 Based Gas Sensors. Nanomaterials 2018, 8, 801. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Jian, J.; Wu, R.; Li, J.; Sun, Y. Synthesis, electrochemical and gas sensing properties of In2O3 nanostructures with different morphologies. J. Alloys Compd. 2015, 645, 178–183. [Google Scholar] [CrossRef]
- Zhao, S.; Shen, Y.; Zhou, P.; Li, G.; Han, C.; Wei, D.; Zhong, X.; Zhang, Y.; Ao, Y. Influence of Synthesis Conditions on Microstructure and NO2 Sensing Properties of WO3 Porous Films Synthesized by Non-Hydrolytic Sol-Gel Method. Nanomaterials 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Ritu, M.; Vijay, K.T.; Torben, D.; Yogendra, K.M.; Lorenz, K. Cubic mesoporous Pd–WO3 loaded graphitic carbon nitride (g-CN) nanohybrids: Highly sensitive and temperature dependent VOC sensors. J. Mater. Chem. A 2018, 6, 10718–10730. [Google Scholar]
- Ekta, P.; Prashant, K.M.; Vijay, K.; Jasbir, S.; Rakesh, K.; Pramod, K.R.; Ritu, M.; Vijay, K.; Tomer, R.A.; Yogendra, K.M. Aero-gel based CeO2 nanoparticles: Synthesis, structural properties and detailed humidity sensing response. J. Mater. Chem. C 2019, 7, 5477–5487. [Google Scholar]
- Han, M.A.; Kim, H.-J.; Lee, H.C.; Park, J.-S.; Lee, H.-N. Effects of porosity and particle size on the gas sensing properties of SnO2 films. Appl. Surf. Sci. 2019, 481, 133–137. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Xu, Y.; Xu, R.; Lei, Y. Carrier Mobility-Dominated Gas Sensing: A Room-Temperature Gas-Sensing Mode for SnO2 Nanorod Array Sensors. ACS Appl. Mater. Interface 2018, 10, 13895–13902. [Google Scholar] [CrossRef] [PubMed]
- Modaberi, M.R.; Rooydell, R.; Brahma, S.; Akande, A.A.; Mwakikunga, B.W.; Liu, C.-P. Enhanced response and selectivity of H2S sensing through controlled Ni doping into ZnO nanorods by using single metal organic precursors. Sens. Actuators B Chem. 2018, 273, 1278–1290. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Cheng, Z.; Gao, L.; Zhang, Y.; Xu, J.; Zhao, H. Facile synthesis of reduced graphene oxide/hexagonal WO3 nanosheets composites with enhanced H2S sensing properties. Sens. Actuators B Chem. 2016, 230, 736–745. [Google Scholar] [CrossRef]
- Ao, D.; Li, Z.; Fu, Y.; Tang, Y.; Yan, S.; Zu, X. Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance. Nanomaterials 2019, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Jain, N.; Sharma, K.; Bhattacharya, S.; Roy, M.; Tyagi, A.K.; Gupta, S.K.; Yakhmi, J.V. Room-temperature H2S gas sensing at ppb level by single crystal In2O3 whiskers. Sens. Actuators B Chem. 2008, 133, 456–461. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Wang, N.; Yan, S.; Liu, W.; Fu, Y.Q.; Wang, Z. Hydrothermal synthesis of hierarchically flower-like CuO nanostructures with porous nanosheets for excellent H2S sensing. J. Alloys Compd. 2017, 725, 1136–1143. [Google Scholar] [CrossRef]
- Nakla, W.; Wisitsora-at, A.; Tuantranont, A.; Singjai, P.; Phanichphant, S.; Liewhiran, C. H2S sensor based on SnO2 nanostructured film prepared by high current heating. Sens. Actuators B Chem. 2014, 203, 565–578. [Google Scholar] [CrossRef]
- Sun, F.; Zhao, Z.; Qiao, X.; Tan, F.; Wang, W. Microwave synthesis and photocatalytic activities of ZnO bipods with different aspect ratios. Mater. Res. Bull. 2016, 74, 367–373. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, R.; Liu, S.; Song, J.; Chen, J.; Dong, B.; Song, H. NiO@ ZnO heterostructured nanotubes: Coelectrospinning fabrication, characterization, and highly enhanced gas sensing properties. Inorg. Chem. 2012, 51, 7733–7740. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Majhi, S.M.; Zhang, X.; Swager, T.M.; Salama, K.N. Recent progress and perspectives of gas sensors based on vertically oriented ZnO nanomaterials. Adv. Colloid Interface Sci. 2019, 270, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Tu, C.-H.; Lai, Y.-F.; Hsu, K.-Y.; Liu, C.-P. Growth and optical properties of a- and c-axis oriented Zn–ZnO nanocables fabricated via a facile one-step process. J. Alloys Compd. 2013, 554, 115–121. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhao, S.; Zhou, G.; Zhou, Y.; Qi, J. Synthesis of well-aligned ZnO nanowires by simple physical vapor deposition on c-oriented ZnO thin films without catalysts or additives. Appl. Phys. Lett. 2005, 86, 024108. [Google Scholar] [CrossRef]
- Zhao, G.; Xuan, J.; Liu, X.; Jia, F.; Sun, Y.; Sun, M.; Yin, G.; Liu, B. Low-Cost and High-Performance ZnO Nanoclusters Gas Sensor Based on New-Type FTO Electrode for the Low-Concentration H2S Gas Detection. Nanomaterials 2019, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, B.; Xu, S.; Lin, L.; Liu, S.; He, D. Synthesis of hierarchically structured ZnO nanomaterials via a supercritical assisted solvothermal process. Chem. Commun. 2014, 50, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, Y.; Zeng, W. Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl. Surf. Sci. 2018, 427, 281–287. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Yi, G.; Li, H.; Shi, C.; Sun, G.; Zhang, Z. Hydrothermally synthesized ZnO hierarchical structure for lower concentration methane sensing. Mater. Lett. 2019, 254, 242–245. [Google Scholar] [CrossRef]
- Diao, K.; Zhou, M.; Zhang, J.; Tang, Y.; Wang, S.; Cui, X. High response to H2S gas with facile synthesized hierarchical ZnO microstructures. Sens. Actuators B Chem. 2015, 219, 30–37. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, T.; Zhang, D.; Gong, X.; Xu, J. Hierarchically porous ZnO with high sensitivity and selectivity to H2S derived from biotemplates. Sens. Actuators B Chem. 2009, 136, 499–509. [Google Scholar] [CrossRef]
- Venkatachalam, S.; Hayashi, H.; Ebina, T.; Nakamura, T.; Wakui, Y.; Nanjo, H. Preparation and characterization of epitaxial growth of ZnO nanotip arrays by hydrothermal method. J. Colloid Interface Sci. 2013, 395, 64–67. [Google Scholar] [CrossRef]
- Son, D.-Y.; Bae, K.-H.; Kim, H.-S.; Park, N.-G. Effects of Seed Layer on Growth of ZnO Nanorod and Performance of Perovskite Solar Cell. J. Phys. Chem. C 2015, 119, 10321–10328. [Google Scholar] [CrossRef]
- Kanjwal, M.A.; Sheikh, F.A.; Barakat, N.A.M.; Li, X.; Kim, H.Y.; Chronakis, I.S. Zinc oxide’s hierarchical nanostructure and its photocatalytic properties. Appl. Surf. Sci. 2012, 258, 3695–3702. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Deng, Y. Hierarchical structured ZnO nanorods on ZnO nanofibers and their photoresponse to UV and visible lights. Sens. Actuators A Phys. 2010, 158, 176–182. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, Z.; Zhang, W.; Li, P.; Lian, K.; Hu, J. Synthesis of brush-like ZnO nanowires and their enhanced gas-sensing properties. J. Mater. Sci. 2015, 51, 1428–1436. [Google Scholar] [CrossRef]
- Zhai, H.-J.; Wu, W.-H.; Lu, F.; Wang, H.-S.; Wang, C. Effects of ammonia and cetyltrimethylammonium bromide (CTAB) on morphologies of ZnO nano- and micromaterials under solvothermal process. Mater. Chem. Phys. 2008, 112, 1024–1028. [Google Scholar] [CrossRef]
- Polsongkram, D.; Chamninok, P.; Pukird, S.; Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Effect of synthesis conditions on the growth of ZnO nanorods via hydrothermal method. Phys. B Condens. Matter 2008, 403, 3713–3717. [Google Scholar] [CrossRef]
- Zhang, C. High-quality oriented ZnO films grown by sol–gel process assisted with ZnO seed layer. J. Phys. Chem. Solids 2010, 71, 364–369. [Google Scholar] [CrossRef]
- Nimbalkar, A.R.; Patil, M.G. Synthesis of ZnO thin film by sol-gel spin coating technique for H2S gas sensing application. Phys. B Condens. Matter 2017, 527, 7–15. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Mortezaali, A.; Moradi, R. The correlation between the substrate temperature and morphological ZnO nanostructures for H2S gas sensors. Sens. Actuators A Phys. 2014, 206, 30–34. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; Ning, L.; Jia, J.; Li, X.; Cao, L. Electrospun Cu-doped ZnO nanofibers for H2S sensing. Sens. Actuators B Chem. 2011, 156, 588–592. [Google Scholar] [CrossRef]
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar]
- Liu, H.; Gong, S.P.; Hu, Y.X.; Liu, J.Q.; Zhou, D.X. Properties and mechanism study of SnO2 nanocrystals for H2S thick-film sensors. Sens. Actuators B Chem. 2009, 140, 190–195. [Google Scholar] [CrossRef]
- Kaur, M.; Kailasaganapathi, S.; Ramgir, N.; Datta, N.; Kumar, S.; Debnath, A.K.; Aswal, D.K.; Gupta, S.K. Gas dependent sensing mechanism in ZnO nanobelt sensor. Appl. Surf. Sci. 2017, 394, 258–266. [Google Scholar] [CrossRef]
- Wang, C.; Chu, X.; Wu, M. Detection of H2S down to ppb levels at room temperature using sensors based on ZnO nanorods. Sens. Actuators B Chem. 2006, 113, 320–323. [Google Scholar] [CrossRef]
- Dloczik, L.; Engelhardt, R.; Ernst, K.; Fiechter, S.; Sieber, I.; Könenkamp, R. Hexagonal nanotubes of ZnS by chemical conversion of monocrystalline ZnO columns. Appl. Phys. Lett. 2001, 78, 3687–3689. [Google Scholar] [CrossRef]
- Iversen, K.J.; Spencer, M.J.S. Effect of ZnO Nanostructure Morphology on the Sensing of H2S Gas. J. Phys. Chem. C 2013, 117, 26106–26118. [Google Scholar] [CrossRef]
- Tomer, V.K.; Malik, R.; Chaudhary, V.; Mishra, Y.K.; Kienle, L.; Ahuja, R.; Lin, L. Superior visible light photocatalysis and low-operating temperature VOCs sensor using cubic Ag(0)-MoS2 loaded g-CN 3D porous hybrid. Appl. Mater.Today 2019, 16, 193–203. [Google Scholar] [CrossRef]
- Usha, S.P.; Mishra, S.K.; Gupta, B.D. Fiber optic hydrogen sulfide gas sensors utilizing ZnO thin film/ZnO nanoparticles: A comparison of surface plasmon resonance and lossy mode resonance. Sens. Actuators B Chem. 2015, 218, 196–204. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B Chem. 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Touba, S.; Kimiagar, S. Enhancement of sensitivity and selectivity of α-Fe2O3 nanorod gas sensors by ZnO nanoparticles decoration. Mater. Sci. Semicond. Process. 2019, 102, 104603. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, H.I.; Hsu, C.S.; Huang, C.C.; Wu, J.S.; Chou, P.C.; Liu, W.C. Characteristics of ZnO nanorods-based ammonia gas sensors with a cross-linked configuration. Sens. Actuators B Chem. 2015, 221, 491–498. [Google Scholar] [CrossRef]
- Postica, V.; Gröttrup, J.; Adelung, R.; Lupan, O.; Mishra, A.K.; de Leeuw, N.H.; Ababii, N.; Carreira, J.F.C.; Rodrigues, J.; Sedrine, N.B.; et al. Multifunctional Materials: A Case Study of the Effects of Metal Doping on ZnO Tetrapods with Bismuth and Tin Oxides. Adv. Funct. Mater. 2017, 27, 1604676. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, X.; Gao, S.; Zhang, X.; Xu, Y.; Zhao, H.; Huo, L. Highly selective detection of saturated vapors of abused drugs by ZnO nanorod bundles gas sensor. Appl. Surf. Sci. 2019, 485, 266–273. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Bang, J.H.; Oum, W.; Kwon, Y.J.; Kim, J.H.; Choi, S.W.; Kim, S.S.; Kim, H.W. Selective H2S-sensing performance of Si nanowires through the formation of ZnO shells with Au functionalization. Sens. Actuators B Chem. 2019, 1–14. [Google Scholar] [CrossRef]


| Materials | H2S (ppm) | Response | Response Time | Recovery Time |
|---|---|---|---|---|
| ZnO NFs * | 50 | 9.223 | 25 s | 37 s |
| ZnO HS1 * | 50 | 17.506 | 19 s | 67 s |
| ZnO HS2 * | 50 | 42.298 | 14 s | 49 s |
| ZnO nanosheet [5] | 100 | 23 | 252 s | 3697 s |
| ZnO thin film [45] | 100 | 3.2 | 10 s | 198 s |
| ZnO nanorods [46] | 100 | 63 | 4 s | 60 s |
| ZnO nanostructures [47] | 20 | 80 | 35 s | 390 s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, C.; Sun, F.; Wang, X.; Huang, Z.; Keshvardoostchokami, M.; Kumar, P.; Liu, B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials 2019, 9, 1277. https://doi.org/10.3390/nano9091277
Fan C, Sun F, Wang X, Huang Z, Keshvardoostchokami M, Kumar P, Liu B. Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials. 2019; 9(9):1277. https://doi.org/10.3390/nano9091277
Chicago/Turabian StyleFan, Chao, Fazhe Sun, Xiaomei Wang, Zuzhen Huang, Mina Keshvardoostchokami, Parveen Kumar, and Bo Liu. 2019. "Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties" Nanomaterials 9, no. 9: 1277. https://doi.org/10.3390/nano9091277
APA StyleFan, C., Sun, F., Wang, X., Huang, Z., Keshvardoostchokami, M., Kumar, P., & Liu, B. (2019). Synthesis of ZnO Hierarchical Structures and Their Gas Sensing Properties. Nanomaterials, 9(9), 1277. https://doi.org/10.3390/nano9091277





