Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems
Abstract
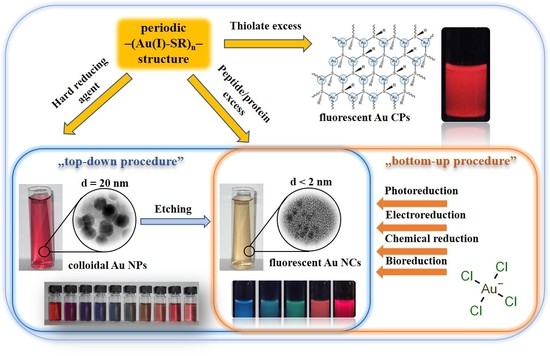
:1. Introduction
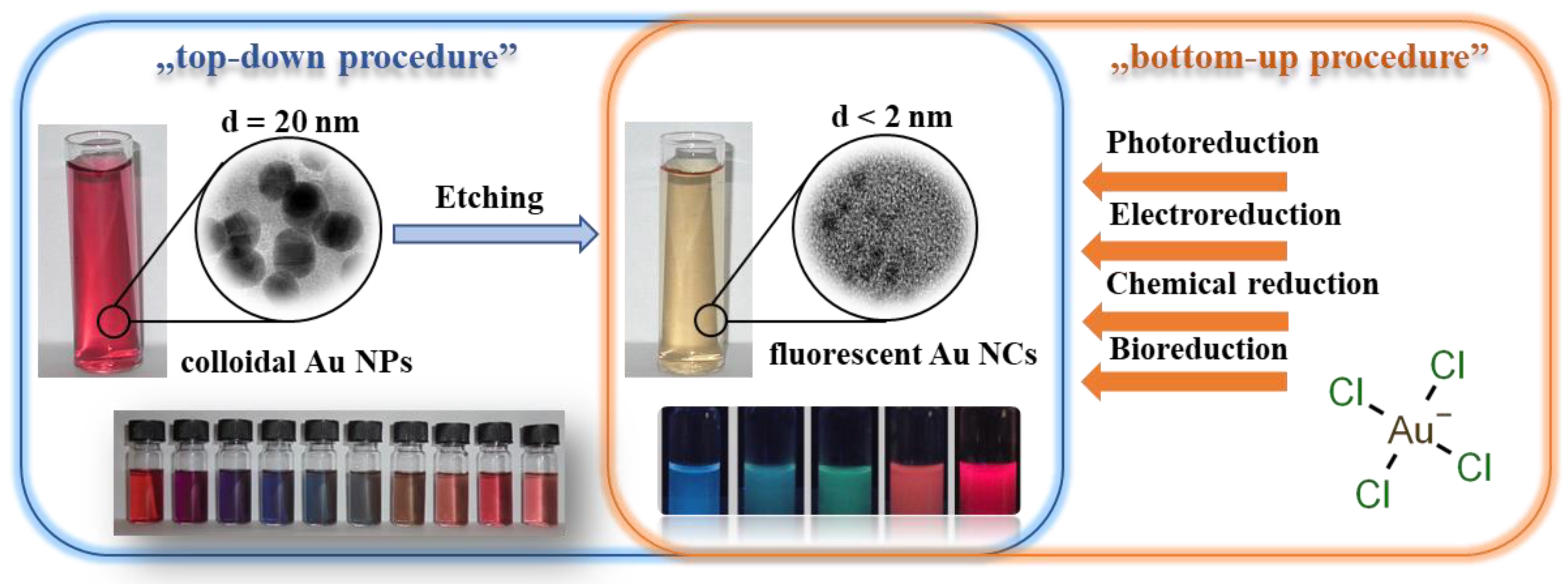
2. Preparation of Amino Acid-Reduced Colloidal Au NPs Having Plasmonic Property
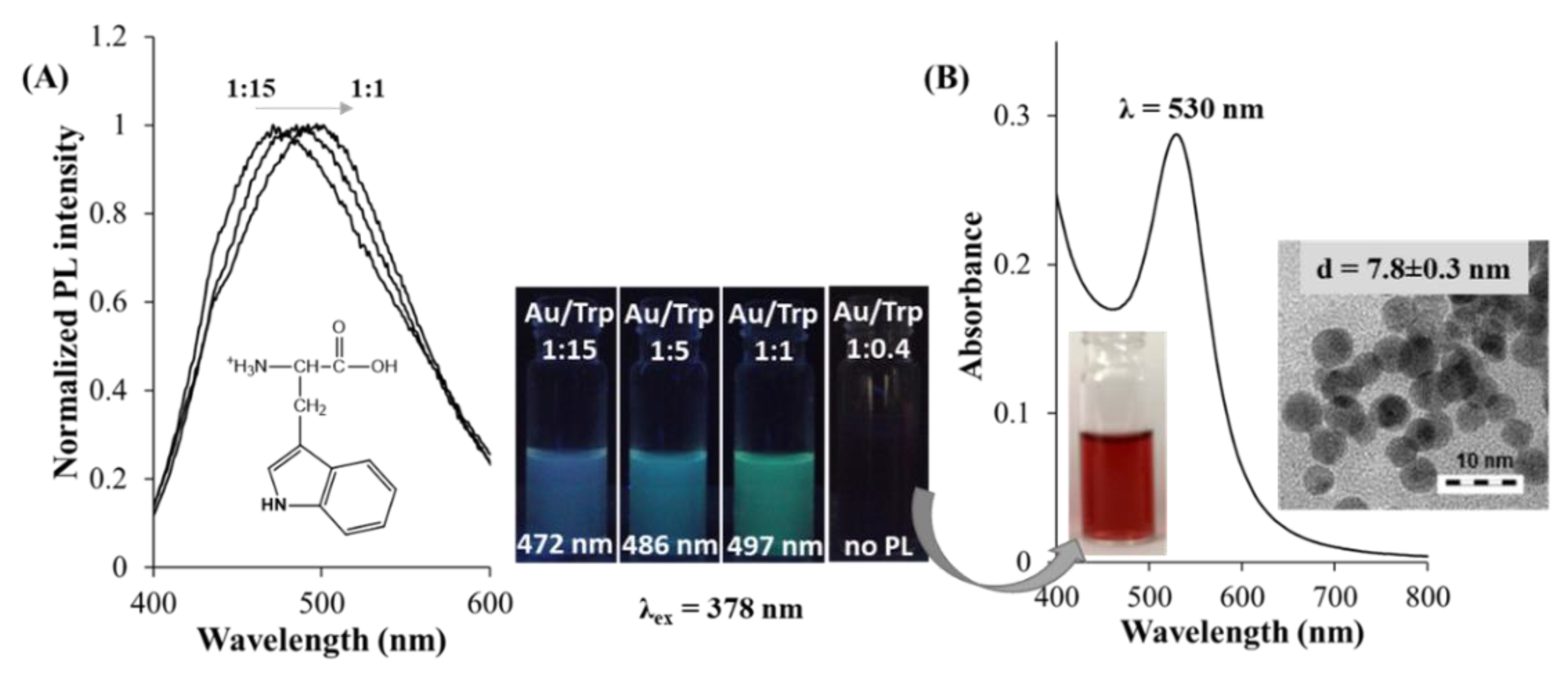
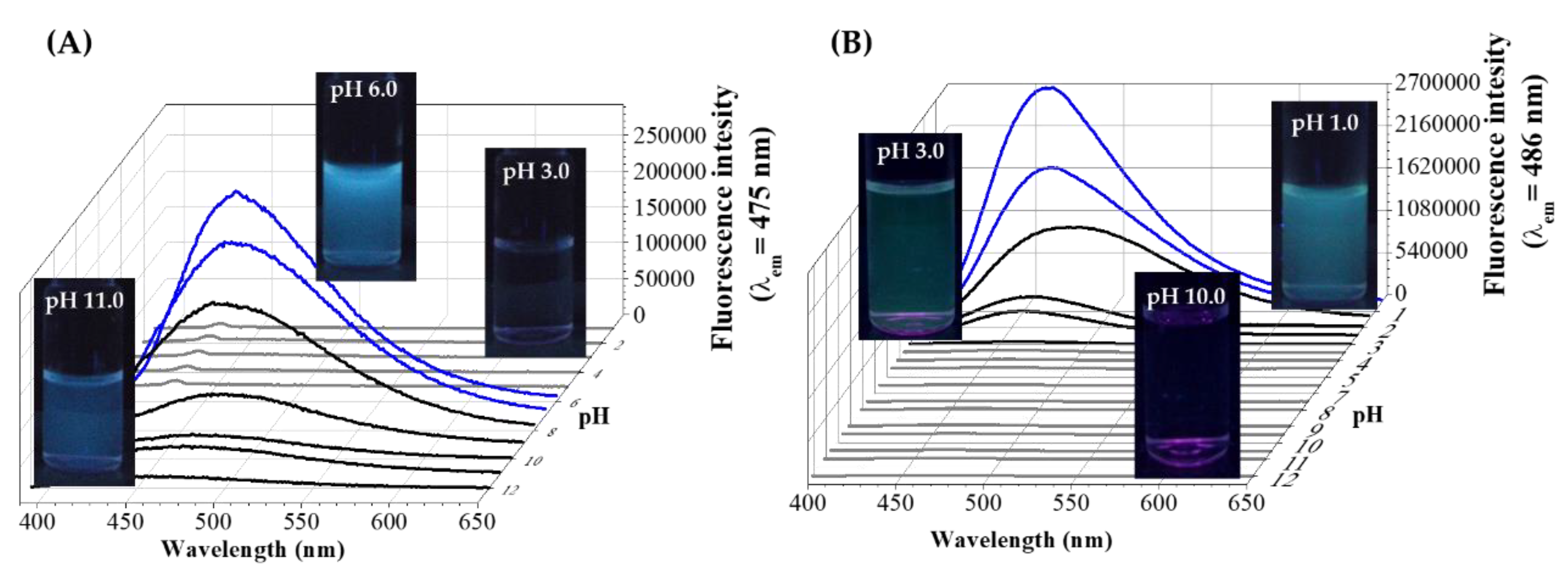
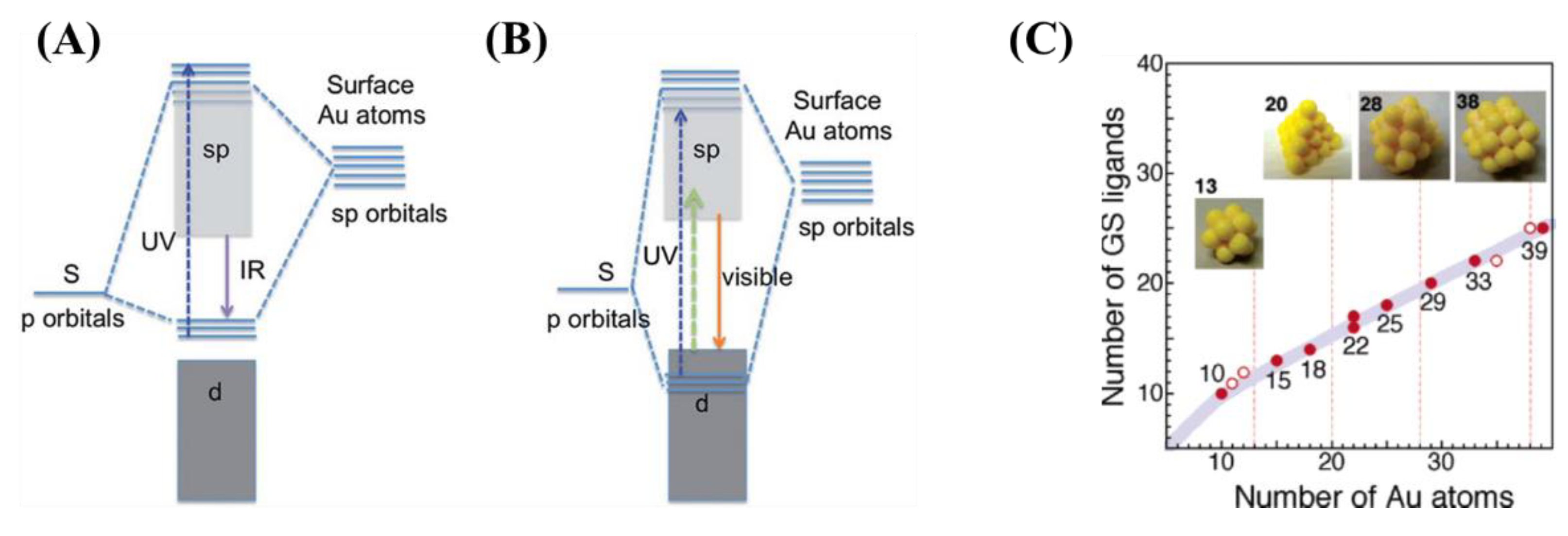
3. Synthetic Routes of Amino Acid-Reduced Fluorescent Au NCs
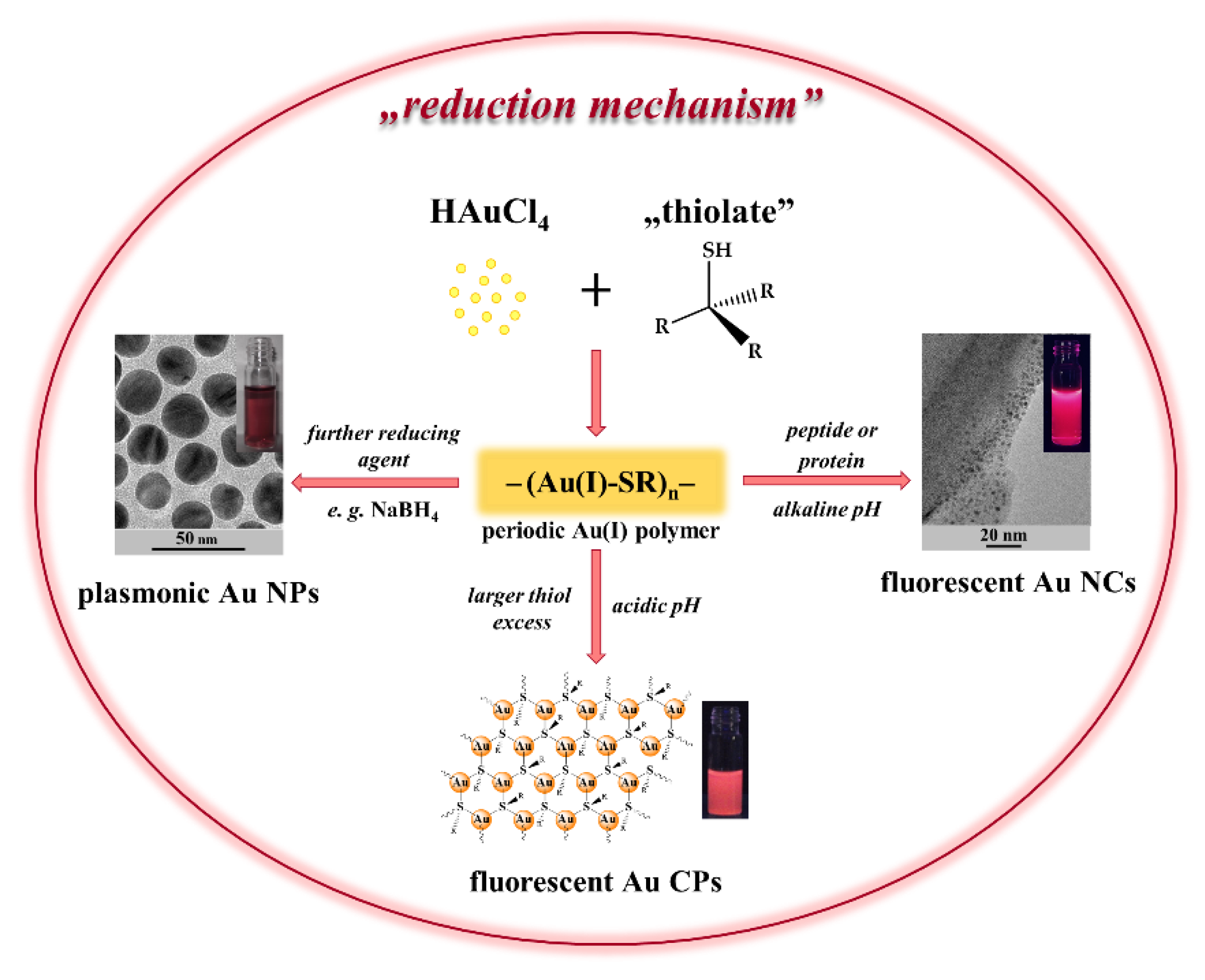
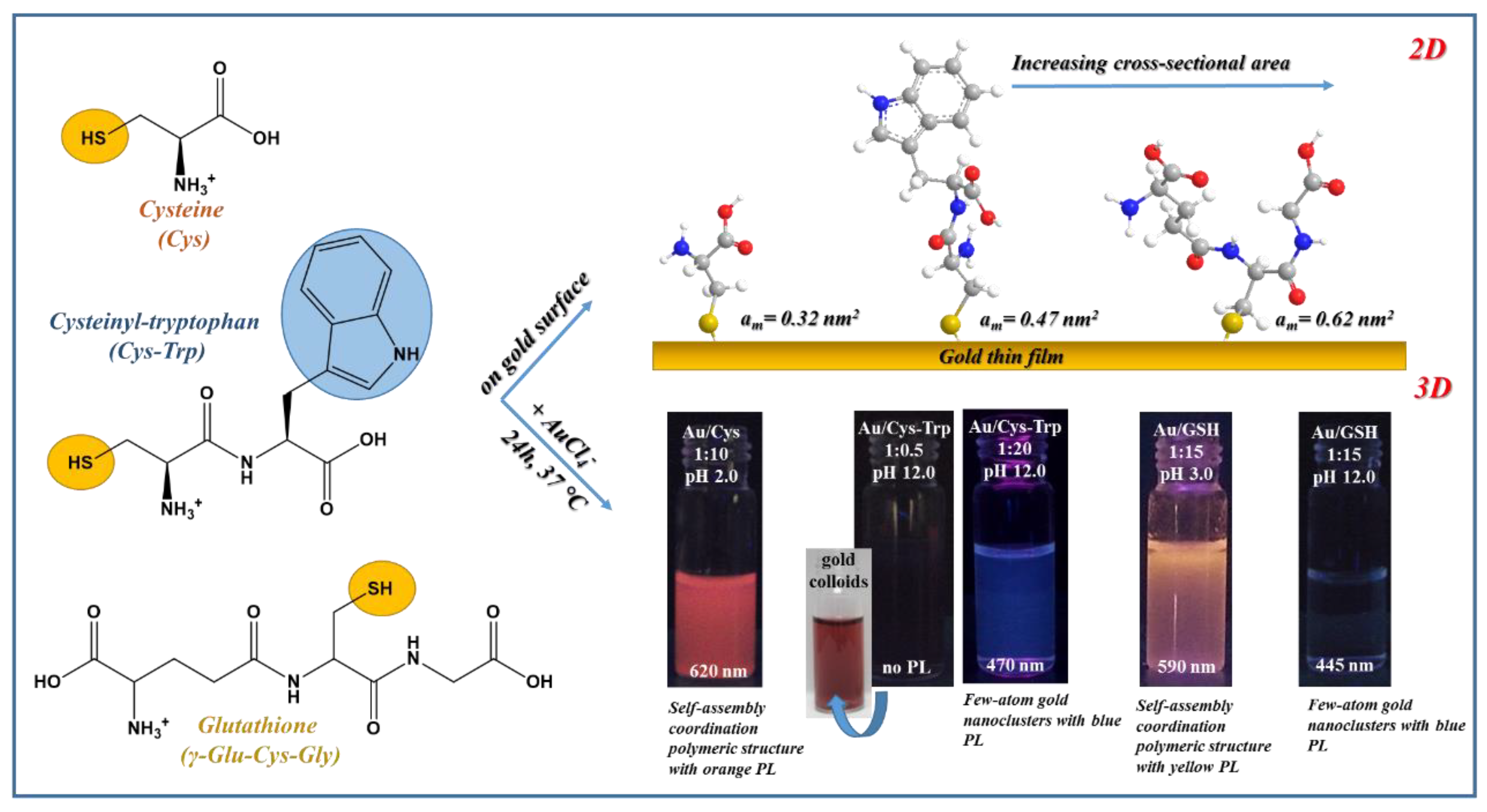
4. Fabrication Protocols of Thiolate-Protected Au Nanohybrid Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Zaitsev, S.Y.; Solovyeva, D.O. Supramolecular nanostructures based on bacterial reaction center proteins and quantum dots. Adv. Colloid Interface Sci. 2015, 218, 34–47. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef]
- Faraday, M. The Bakerian Lecture: Experimental Relations of Gold (and Other Metals) to Light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet-visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef] [Green Version]
- Mafuné, F.; Kohno, J.Y.; Takeda, Y.; Kondow, T. Full physical preparation of size-selected gold nanoparticles in solution: Laser ablation and laser-induced size control. J. Phys. Chem. B 2002, 106, 7575–7577. [Google Scholar] [CrossRef]
- Freitas de Freitas, L.; Varca, G.; dos Santos Batista, J.; Benévolo Lugão, A. An Overview of the Synthesis of Gold Nanoparticles Using Radiation Technologies. Nanomaterials 2018, 8, 939. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.K.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanoparticle Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase Liquid–Liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Kuzmann, E.; Csapó, E.; Stichleutner, S.; Garg, V.K.; de Oliveira, A.C.; da Silva, S.W.; Sing, L.H.; Pati, S.S.; Guimaraes, E.M.; Lengyel, A.; et al. Fine structure of gold nanoparticles stabilized by buthyldithiol: Species identified by Mössbauer spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 260–266. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55. [Google Scholar] [CrossRef]
- Majzik, A.; Patakfalvi, R.; Hornok, V.; Dékány, I. Growing and stability of gold nanoparticles and their functionalization by cysteine. Gold Bull. 2009, 42, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Csapó, E.; Oszkó, A.; Varga, E.; Juhász, Á.; Buzás, N.; Kőrösi, L.; Majzik, A.; Dékány, I. Synthesis and characterization of Ag/Au alloy and core(Ag)–shell(Au) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 281–287. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Q.; Gao, Z. Simple one-step synthesis of gold nanoparticles with controlled size using cationic Gemini surfactants as ligands: Effect of the variations in concentrations and tail lengths. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 201–210. [Google Scholar] [CrossRef]
- Bali, K.; Sáfrán, G.; Pécz, B.; Mészáros, R. Preparation of Gold Nanocomposites with Tunable Charge and Hydrophobicity via the Application of Polymer/Surfactant Complexation. ACS Omega 2017, 2, 8709–8716. [Google Scholar] [CrossRef]
- Polavarapu, L.; Xu, Q.H. A single-step synthesis of gold nanochains using an amino acid as a capping agent and characterization of their optical properties. Nanotechnology 2008, 19, 075601. [Google Scholar] [CrossRef]
- Slocik, J.M.; Stone, M.O.; Naik, R.R. Synthesis of gold nanoparticles using multifunctional peptides. Small 2005, 1, 1048–1052. [Google Scholar] [CrossRef]
- Francois, T.; Onani, M.; Madiehe, A.; Meyer, M. Aqueous soluble gold nanoparticle synthesis using polyethyleneimine and reduced glutathione. Int. J. Mater. Res. 2014, 105, 1025–1037. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Microbial production of gold nanoparticles. Gold Bull. 2006, 39, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Sharma, N.; Pinnaka, A.K.; Raje, M.; FNU, A.; Bhattacharyya, M.S.; Choudhury, A.R. Exploitation of marine bacteria for production of gold nanoparticles. Microb. Cell Fact. 2012, 11, 1. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Ma, X.; Tian, B.; Li, T.; Yu, J.; Dai, S.; Weng, Y.; Hua, Y. Biosynthesis of gold nanoparticles by the extreme bacterium Deinococcus radiodurans and an evaluation of their antibacterial properties. Int. J. Nanomed. 2016, 11, 5931–5944. [Google Scholar] [CrossRef]
- Chen, L.Y.Y.; Wang, C.W.W.; Yuan, Z.; Chang, H.T.T. Fluorescent gold nanoclusters: Recent advances in sensing and imaging. Anal. Chem. 2015, 87, 216–229. [Google Scholar] [CrossRef]
- Jin, R.; Zeng, C.; Zhou, M.; Chen, Y. Atomically Precise Colloidal Metal Nanoclusters and Nanoparticles: Fundamentals and Opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.R. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef]
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent Gold Nanoclusters: Synthesis and Recent Biological Application. J. Nanomater. 2015, 2015, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Chen, S.; Wu, A. Green chemistry synthesis of gold nanoparticles using lactic acid as a reducing agent. Micro Nano Lett. 2010, 5, 270. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Mehta, S. Preparation of gold nanoparticles using tea: A green chemistry experiment. J. Chem. Educ. 2012, 89, 1316–1318. [Google Scholar] [CrossRef]
- Sujitha, M.V.; Kannan, S. Green synthesis of gold nanoparticles using Citrus fruits (Citrus limon, Citrus reticulata and Citrus sinensis) aqueous extract and its characterization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 15–23. [Google Scholar] [CrossRef]
- Antoine, R.; Bertorelle, F.; Broyer, M.; Compagnon, I.; Dugourd, P.; Kulesza, A.; Mitrič, R.; Bonačić-Koutecký, V. Gas-phase synthesis and intense visible absorption of tryptophangold cations. Angew. Chem. Int. Ed. 2009, 48, 7829–7832. [Google Scholar] [CrossRef]
- Le Guével, X.; Daum, N.; Schneider, M. Synthesis and characterization of human transferrin-stabilized gold nanoclusters. Nanotechnology 2011, 22, 275103. [Google Scholar] [CrossRef]
- Liu, C.L.; Wu, H.T.; Hsiao, Y.H.; Lai, C.W.; Shih, C.W.; Peng, Y.K.; Tang, K.C.; Chang, H.W.; Chien, Y.C.; Hsiao, J.K.; et al. Insulin-directed synthesis of fluorescent gold nanoclusters: Preservation of insulin bioactivity and versatility in cell imaging. Angew. Chem. Int. Ed. 2011, 50, 7056–7060. [Google Scholar] [CrossRef]
- Duan, H.; Nie, S. Etching Colloidal Gold Nanocrystals with Hyperbranched and Multivalent Polymers: A New Route to Fluorescent and Water-Soluble Atomic Clusters. J. Am. Chem. Soc. 2007, 129, 2412–2413. [Google Scholar] [CrossRef]
- Sun, X.; Dong, S.; Wang, E. One-step preparation and characterization of poly(propyleneimine) dendrimer-protected silver nanoclusters. Macromolecules 2004, 37, 7105–7108. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Ge, W.; Li, Q.; Wang, X. Cytidine-directed rapid synthesis of water-soluble and highly yellow fluorescent bimetallic AuAg nanoclusters. Langmuir 2014, 30, 10910–10917. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, X. Cytidine-stabilized gold nanocluster as a fluorescence turn-on and turn-off probe for dual functional detection of Ag+ and Hg2+. Anal. Chim. Acta 2015, 870, 1–7. [Google Scholar] [CrossRef]
- Ahn, J.K.; Kim, H.Y.; Baek, S.; Park, H.G. A new s-adenosylhomocysteine hydrolase-linked method for adenosine detection based on DNA-templated fluorescent Cu/Ag nanoclusters. Biosens. Bioelectron. 2017, 93, 330–334. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, A.; Dékány, I. Nucleotide-directed syntheses of gold nanohybrid systems with structure-dependent optical features: Selective fluorescence sensing of Fe3+ ions. Colloids Surf. B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef]
- Nafady, A.; Afridi, H.I.; Sara, S.; Shah, A.; Niaz, A. Direct synthesis and stabilization of Bi-sized cysteine-derived gold nanoparticles: Reduction catalyst for methylene blue. J. Iran. Chem. Soc. 2011, 8, S34–S43. [Google Scholar] [CrossRef]
- Lavenn, C.; Okhrimenko, L.; Guillou, N.; Monge, M.; Ledoux, G.; Dujardin, C.; Chiriac, R.; Fateeva, A.; Demessence, A. A luminescent double helical gold(I)–thiophenolate coordination polymer obtained by hydrothermal synthesis or by thermal solid-state amorphous-to-crystalline isomerization. J. Mater. Chem. C 2015, 3, 4115–4125. [Google Scholar] [CrossRef]
- Deák, A.; Jobbágy, C.; Marsi, G.; Molnár, M.; Szakács, Z.; Baranyai, P. Anion-, Solvent-, Temperature-, and Mechano-Responsive Photoluminescence in Gold(I) Diphosphine-Based Dimers. Chem. A Eur. J. 2015, 21, 11495–11508. [Google Scholar] [CrossRef]
- Aljuhani, M.A.; Bootharaju, M.S.; Sinatra, L.; Basset, J.M.; Mohammed, O.F.; Bakr, O.M. Synthesis and Optical Properties of a Dithiolate/Phosphine-Protected Au28 Nanocluster. J. Phys. Chem. C 2017, 121, 10681–10685. [Google Scholar] [CrossRef]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T. Kinetic stabilization of growing gold clusters by passivation with thiolates. J. Phys. Chem. B 2006, 110, 12218–12221. [Google Scholar] [CrossRef]
- Le Guével, X.; Spies, C.; Daum, N.; Jung, G.; Schneider, M. Highly fluorescent silver nanoclusters stabilized by glutathione: A promising fluorescent label for bioimaging. Nano Res. 2012, 5, 379–387. [Google Scholar] [CrossRef]
- Bayse, C.A.; Ming, J.L.; Miller, K.M.; McCollough, S.M.; Pike, R.D. Photoluminescence of silver(I) and gold(I) cyanide 1D coordination polymers. Inorg. Chim. Acta 2011, 375, 47–52. [Google Scholar] [CrossRef]
- Luo, Z.; Yuan, X.; Yu, Y.; Zhang, Q.; Leong, D.T.; Lee, J.Y.; Xie, J. From Aggregation-Induced Emission of Au(I)–Thiolate Complexes to Ultrabright Au(0)@Au(I)–Thiolate Core–Shell Nanoclusters. J. Am. Chem. Soc. 2012, 134, 16662–16670. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824–828. [Google Scholar] [CrossRef]
- Feng, Y.; Lin, X.; Wang, Y.; Wang, Y.; Hua, J. Diversity of Aurum bioreduction by Rhodobacter capsulatus. Mater. Lett. 2008, 62, 4299–4302. [Google Scholar] [CrossRef]
- Nune, S.K.; Chanda, N.; Shukla, R.; Katti, K.; Kulkarni, R.R.; Thilakavathy, S.; Mekapothula, S.; Kannan, R.; Katti, K.V. Green nanotechnology from tea: Phytochemicals in tea as building blocks for production of biocompatible gold nanoparticles. J. Mater. Chem. 2009, 19, 2912–2920. [Google Scholar] [CrossRef]
- Kasthuri, J.; Veerapandian, S.; Rajendiran, N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf. B Biointerfaces 2009, 68, 55–60. [Google Scholar] [CrossRef]
- Vemula, P.K.; Aslam, U.; Mallia, V.A.; John, G. In situ synthesis of gold nanoparticles using molecular gels and liquid crystals from vitamin-C amphiphiles. Chem. Mater. 2007, 19, 138–140. [Google Scholar] [CrossRef]
- Lee, J.; Ryu, J.; Choi, W. Preparation of Gold and Platinum Nanoparticles Using Visible Light Activated Fe III -complex. Chem. Lett. 2007, 36, 176–177. [Google Scholar] [CrossRef]
- Nasr, G.; Guerlin, A.; Dumur, F.; Baudron, S.A.; Dumas, E.; Miomandre, F.; Clavier, G.; Sliwa, M.; Mayer, C.R. Dithiolate-appended iridium(III) complex with dual functions of reducing and capping agent for the design of small-sized gold nanoparticles. J. Am. Chem. Soc. 2011, 133, 6501–6504. [Google Scholar] [CrossRef]
- Kasthuri, J.; Rajendiran, N. Functionalization of silver and gold nanoparticles using amino acid conjugated bile salts with tunable longitudinal plasmon resonance. Colloids Surf. B Biointerfaces 2009, 73, 387–393. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Controlled synthesis of dendritic gold nanostructures assisted by supramolecular complexes of surfactant with cyclodextrin. Langmuir 2010, 26, 7582–7589. [Google Scholar] [CrossRef]
- Hussain, I.; Brust, M.; Papworth, A.J.; Cooper, A.I. Preparation of Acrylate-Stabilized Gold and Silver Hydrosols and Gold−Polymer Composite Films. Langmuir 2003, 19, 4831–4835. [Google Scholar] [CrossRef]
- Sardar, R.; Park, J.W.; Shumaker-Parry, J.S. Polymer-induced synthesis of stable gold and silver nanoparticles and subsequent ligand exchange in water. Langmuir 2007, 23, 11883–11889. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Heywood, B.R.; Mann, S. Influence of Membrane Composition on the Intravesicular Precipitation of Nanophase Gold Particles. J. Colloid Interface Sci. 1993, 161, 66–71. [Google Scholar] [CrossRef]
- Wangoo, N.; Kaur, S.; Bajaj, M.; Jain, D.V.S.; Sharma, R.K. One pot, rapid and efficient synthesis of water dispersible gold nanoparticles using alpha-amino acids. Nanotechnology 2014, 25, 435608. [Google Scholar] [CrossRef]
- Mandal, S.; Selvakannan, P.R.; Phadtare, S.; Pasricha, R.; Sastry, M. Synthesis of a stable gold hydrosol by the reduction of chloroaurate ions by the amino acid, aspartic acid. Proc. Indian Acad. Sci. Chem. Sci. 2002, 114, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Selvakannan, P.R.; Mandal, S.; Phadtare, S.; Pasricha, R.; Sastry, M. Capping of gold nanoparticles by the amino acid lysine renders them water-dispersible. Langmuir 2003, 19, 3545–3549. [Google Scholar] [CrossRef]
- Selvakannan, P.R.; Mandal, S.; Phadtare, S.; Gole, A.; Pasricha, R.; Adyanthaya, S.D.; Sastry, M. Water-dispersible tryptophan-protected gold nanoparticles prepared by the spontaneous reduction of aqueous chloroaurate ions by the amino acid. J. Colloid Interface Sci. 2004, 269, 97–102. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Booth, J.M.; Agrawal, S.; Coloe, P.; Kar, G. Gold nanoparticle formation during bromoaurate reduction by amino acids. Langmuir 2005, 21, 5949–5956. [Google Scholar] [CrossRef]
- Newman, J.D.S.S.D.S.; Blanchard, G.J.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef]
- Wangoo, N.; Bhasin, K.K.; Mehta, S.K.; Suri, C.R. Synthesis and capping of water-dispersed gold nanoparticles by an amino acid: Bioconjugation and binding studies. J. Colloid Interface Sci. 2008, 323, 247–254. [Google Scholar] [CrossRef]
- Liu, Z.; Zu, Y.; Fu, Y.; Meng, R.; Guo, S.; Xing, Z.; Tan, S. Hydrothermal synthesis of histidine-functionalized single-crystalline gold nanoparticles and their pH-dependent UV absorption characteristic. Colloids Surf. B Biointerfaces 2010, 76, 311–316. [Google Scholar] [CrossRef]
- Cai, H.; Yao, P. Gold nanoparticles with different amino acid surfaces: Serum albumin adsorption, intracellular uptake and cytotoxicity. Colloids Surf. B Biointerfaces 2014, 123, 900–906. [Google Scholar] [CrossRef]
- Courrol, L.C.; de Matos, R.A. Synthesis of Gold Nanoparticles Using Amino Acids by Light Irradiation. In Catalytic Application of Nano-Gold Catalysts; InTech: Bergharen, The Netherlands, 2016; Volume i, p. 13. [Google Scholar]
- Maruyama, T.; Fujimoto, Y.; Maekawa, T. Synthesis of gold nanoparticles using various amino acids. J. Colloid Interface Sci. 2014, 447, 254–257. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Kele, Z.; Baranyai, P.; Deák, A.; Juhász, Á.; Janovák, L.; Dékány, I. Influence of pH and aurate/amino acid ratios on the tuneable optical features of gold nanoparticles and nanoclusters. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 601–608. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, E. Metal nanoclusters: New fluorescent probes for sensors and bioimaging. Nano Today 2014, 9, 132–157. [Google Scholar] [CrossRef]
- Khandelwal, P.; Poddar, P. Fluorescent metal quantum clusters: An updated overview of the synthesis, properties, and biological applications. J. Mater. Chem. B 2017, 5, 9055–9084. [Google Scholar] [CrossRef]
- Shang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar] [CrossRef]
- Chevrier, D.M.; Chatt, A.; Zhang, P. Properties and applications of protein-stabilized fluorescent gold nanoclusters: Short review. J. Nanophotonics 2012, 6, 064504. [Google Scholar] [CrossRef]
- Ungor, D.; Horváth, K.; Dékány, I.; Csapó, E. Red-emitting gold nanoclusters for rapid fluorescence sensing of tryptophan metabolites. Sens. Actuators B Chem. 2019, 288, 728–733. [Google Scholar] [CrossRef] [Green Version]
- Tsunoyama, H.; Tsukuda, T. Magic numbers of gold clusters stabilized by PVP. J. Am. Chem. Soc. 2009, 131, 18216–18217. [Google Scholar] [CrossRef]
- Li, Z.; Liu, R.; Xing, G.; Wang, T.; Liu, S. A novel fluorometric and colorimetric sensor for iodide determination using DNA-templated gold/silver nanoclusters. Biosens. Bioelectron. 2017, 96, 44–48. [Google Scholar] [CrossRef]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High Quantum Yield Blue Emission from Water-Soluble Au 8 Nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef]
- Zheng, J.; Nicovich, P.R.; Dickson, R.M. Highly Fluorescent Noble-Metal Quantum Dots. Annu. Rev. Phys. Chem. 2007, 58, 409–431. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhou, C.; Yu, M.; Liu, J. Different sized luminescent gold nanoparticles. Nanoscale 2012, 4, 4073. [Google Scholar] [CrossRef]
- Yang, X.; Shi, M.; Zhou, R.; Chen, X.; Chen, H. Blending of HAuCl4 and histidine in aqueous solution: A simple approach to the Au10 cluster. Nanoscale 2011, 3, 2596–2601. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, F.G.; Liu, P.; Gu, N.; Chen, Z. Enhanced fluorescence of gold nanoclusters composed of HAuCl4 and histidine by glutathione: Glutathione detection and selective cancer cell imaging. Small 2014, 10, 5170–5177. [Google Scholar] [CrossRef]
- Liu, X.; Yu, X.; Luo, X. Ultrasensitive iodide detection based on the resonance light scattering of histidine-stabilized gold nanoclusters. Microchim. Acta 2014, 181, 1379–1384. [Google Scholar] [CrossRef]
- Schmidt, L.A.; Kirk, L.; Appleman, W.K. The Appaern Dissociation costants of Arginine and of Lysine and the Apparent Heats of Ionization of Certain Amino Acids. J. Biol. Chem. 1930, 88, 285–293. [Google Scholar]
- Zhang, Y.; Hu, Q.; Paau, M.C.; Xie, S.; Gao, P.; Chan, W.; Choi, M.M.F. Probing histidine-stabilized gold nanoclusters product by high-performance liquid chromatography and mass spectrometry. J. Phys. Chem. C 2013, 117, 18697–18708. [Google Scholar] [CrossRef]
- Guo, Y.; Long, T.; Lin, M.; Liu, Z.; Huang, C.; Zhao, X. Histidine-mediated synthesis of chiral fluorescence gold nanoclusters: Insight into the origin of nanoscale chirality. RSC Adv. 2015, 5, 61449–61454. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Zhuo, Y.; Feng, Y.; Zhu, S. Novel synthesis of gold nanoclusters templated with L-tyrosine for selective analyzing tyrosinase. Anal. Chim. Acta 2014, 840, 87–92. [Google Scholar] [CrossRef]
- Mu, X.; Qi, L.; Qiao, J.; Ma, H. One-pot synthesis of tyrosine-stabilized fluorescent gold nanoclusters and their application as turn-on sensors for Al3+ ions and turn-off sensors for Fe3+ ions. Anal. Methods 2014, 6, 6445–6451. [Google Scholar] [CrossRef]
- Mu, X.; Qi, L.; Dong, P.; Qiao, J.; Hou, J.; Nie, Z.; Ma, H. Facile one-pot synthesis of L-proline-stabilized fluorescent gold nanoclusters and its application as sensing probes for serum iron. Biosens. Bioelectron. 2013, 49, 249–255. [Google Scholar] [CrossRef]
- Zheng, S.; Yin, H.; Li, Y.; Bi, F.; Gan, F. One–step synthesis of L-tryptophan-stabilized dual-emission fluorescent gold nanoclusters and its application for Fe3+ sensing. Sens. Actuators B Chem. 2017, 242, 469–475. [Google Scholar] [CrossRef]
- Deng, H.H.; Zhang, L.N.; He, S.B.; Liu, A.L.; Li, G.W.; Lin, X.H.; Xia, X.H.; Chen, W. Methionine-directed fabrication of gold nanoclusters with yellow fluorescent emission for Cu2+ sensing. Biosens. Bioelectron. 2015, 65, 397–403. [Google Scholar] [CrossRef]
- Csapó, E.; Ungor, D.; Juhász, Á.; Tóth, G.K.; Dékány, I. Gold nanohybrid systems with tunable fluorescent feature: Interaction of cysteine and cysteine-containing peptides with gold in two- and three-dimensional systems. Colloids Surf. A Physicochem. Eng. Asp. 2016, 511, 264–271. [Google Scholar] [CrossRef]
- Söptei, B.; Mihály, J.; Szigyártó, I.C.; Wacha, A.; Németh, C.; Bertóti, I.; May, Z.; Baranyai, P.; Sajó, I.E.; Bóta, A. The supramolecular chemistry of gold and L-cysteine: Formation of photoluminescent, orange-emitting assemblies with multilayer structure. Colloids Surf. A Physicochem. Eng. Asp. 2015, 470, 8–14. [Google Scholar] [CrossRef]
- Jin, R. Quantum sized, thiolate-protected gold nanoclusters. Nanoscale 2010, 2, 343–362. [Google Scholar] [CrossRef]
- Nasaruddin, R.R.; Chen, T.; Yan, N.; Xie, J. Roles of thiolate ligands in the synthesis, properties and catalytic application of gold nanoclusters. Coord. Chem. Rev. 2018, 368, 60–79. [Google Scholar] [CrossRef]
- Xavier, P.L.; Chaudhari, K.; Baksi, A.; Pradeep, T. Protein-protected luminescent noble metal quantum clusters: An emerging trend in atomic cluster nanoscience. Nano Rev. 2012, 3, 14767. [Google Scholar] [CrossRef]
- Yee, C.K.; Jordan, R.; Ulman, A.; White, H.; King, A.; Rafailovich, M.; Sokolov, J. Novel One-Phase Synthesis of Thiol-Functionalized Gold, Palladium, and Iridium Nanoparticles Using Superhydride. Langmuir 1999, 15, 3486–3491. [Google Scholar] [CrossRef]
- Frenkel, A.I.; Nemzer, S.; Pister, I.; Soussan, L.; Harris, T.; Sun, Y.; Rafailovich, M.H. Size-controlled synthesis and characterization of thiol-stabilized gold nanoparticles. J. Chem. Phys. 2005, 123, 184701. [Google Scholar] [CrossRef] [Green Version]
- Perala, S.R.K.; Kumar, S. On the Mechanism of Metal Nanoparticle Synthesis in the Brust–Schiffrin Method. Langmuir 2013, 29, 9863–9873. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, L.; Zhang, R.; Wang, X.; Guo, C.; Sun, P.; Xue, G. Investigation on the Mechanism of the Synthesis of Gold(I) Thiolate Complexes by NMR. J. Phys. Chem. C 2014, 118, 10434–10440. [Google Scholar] [CrossRef]
- Briñas, R.P.; Hu, M.; Qian, L.; Lymar, E.S.; Hainfeld, J.F. Gold Nanoparticle Size Controlled by Polymeric Au(I) Thiolate Precursor Size. J. Am. Chem. Soc. 2008, 130, 975–982. [Google Scholar] [CrossRef]
- Schaaff, T.G.; Whetten, R.L. Giant Gold−Glutathione Cluster Compounds: Intense Optical Activity in Metal-Based Transitions. J. Phys. Chem. B 2000, 104, 2630–2641. [Google Scholar] [CrossRef]
- Negishi, Y.; Takasugi, Y.; Sato, S.; Yao, H.; Kimura, K.; Tsukuda, T.; Characterization, S.; Tsukuda, T. Magic-Numbered Aun Clusters Protected by Glutathione Monolayers (n = 18, 21, 25, 28, 32, 39): Isolation and Spectroscopic Characterization. J. Am. Chem. Soc. 2004, 126, 6518–6519. [Google Scholar] [CrossRef]
- Negishi, Y.; Nobusada, K.; Tsukuda, T. Glutathione-Protected Gold Clusters Revisited: Bridging the Gap between Gold(I)−Thiolate Complexes and Thiolate-Protected Gold Nanocrystals. J. Am. Chem. Soc. 2005, 127, 5261–5270. [Google Scholar] [CrossRef]
- Bachman, R.E.; Bodolosky-Bettis, S.A.; Glennon, S.C.; Sirchio, S.A. Formation of a Novel Luminescent Form of Gold(I) Phenylthiolate via Self-Assembly and Decomposition of Isonitrilegold(I) Phenylthiolate Complexes. J. Am. Chem. Soc. 2000, 122, 7146–7147. [Google Scholar] [CrossRef]
- Odriozola, I.; Loinaz, I.; Pomposo, J.A.; Grande, H.J. Gold–glutathione supramolecular hydrogels. J. Mater. Chem. 2007, 17, 4843. [Google Scholar] [CrossRef]
- Nie, H.; Li, M.; Hao, Y.; Wang, X.; Zhang, S.X.A. Time-resolved monitoring of dynamic self-assembly of Au(I)-thiolate coordination polymers. Chem. Sci. 2013, 4, 1852. [Google Scholar] [CrossRef]
- Royappa, A.T.; Tran, C.M.; Papoular, R.J.; Khan, M.; Marbella, L.E.; Millstone, J.E.; Gembicky, M.; Chen, B.; Shepard, W.; Elkaim, E. Copper(I) and gold(I) thiolate precursors to bimetallic nanoparticles. Polyhedron 2018, 155, 359–365. [Google Scholar] [CrossRef]
- Cappellari, P.S.; Buceta, D.; Morales, G.M.; Barbero, C.A.; Sergio Moreno, M.; Giovanetti, L.J.; Ramallo-López, J.M.; Requejo, F.G.; Craievich, A.F.; Planes, G.A. Synthesis of ultra-small cysteine-capped gold nanoparticles by pH switching of the Au(I)–cysteine polymer. J. Colloid Interface Sci. 2015, 441, 17–24. [Google Scholar] [CrossRef]
- Habeeb Muhammed, M.A.; Verma, P.K.; Pal, S.K.; Retnakumari, A.; Koyakutty, M.; Nair, S.; Pradeep, T. Luminescent Quantum Clusters of Gold in Bulk by Albumin-Induced Core Etching of Nanoparticles: Metal Ion Sensing, Metal-Enhanced Luminescence, and Biolabeling. Chem. A Eur. J. 2010, 16, 10103–10112. [Google Scholar] [CrossRef]
- Hemmateenejad, B.; Shakerizadeh-shirazi, F.; Samari, F. BSA-modified gold nanoclusters for sensing of folic acid. Sens. Actuators B Chem. 2014, 199, 42–46. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Z.; Li, Y.; Yu, J.; Liu, G.; Liu, R.; Yue, Z. BSA-AuNCs based enhanced photoelectrochemical biosensors and its potential use in multichannel detections. J. Photochem. Photobiol. A Chem. 2017, 342, 15–24. [Google Scholar] [CrossRef]
- Gui, R.; Jin, H. Aqueous synthesis of human serum albumin-stabilized fluorescent Au/Ag core/shell nanocrystals for highly sensitive and selective sensing of copper(II). Analyst 2013, 138, 7197. [Google Scholar] [CrossRef]
- Yu, Y.; New, S.Y.; Xie, J.; Su, X.; Tan, Y.N. Protein-based fluorescent metal nanoclusters for small molecular drug screening. Chem. Commun. 2014, 50, 13805–13808. [Google Scholar] [CrossRef]
- Wei, H.; Wang, Z.; Yang, L.; Tian, S.; Hou, C.; Lu, Y. Lysozyme-stabilized gold fluorescent cluster: Synthesis and application as Hg2+ sensor. Analyst 2010, 135, 1406. [Google Scholar] [CrossRef]
- Chan, P.H.; Wong, S.Y.; Lin, S.H.; Chen, Y.C. Lysozyme-encapsulated gold nanocluster-based affinity mass spectrometry for pathogenic bacteria. Rapid Commun. Mass Spectrom. 2013, 27, 2143–2148. [Google Scholar] [CrossRef]
- Lu, D.; Liu, L.; Li, F.; Shuang, S.; Li, Y.; Choi, M.M.F.; Dong, C. Lysozyme-stabilized gold nanoclusters as a novel fluorescence probe for cyanide recognition. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 121, 77–80. [Google Scholar] [CrossRef]
- Hornok, V.; Csapó, E.; Varga, N.; Ungor, D.; Sebők, D.; Janovák, L.; Laczkó, G.; Dékány, I. Controlled syntheses and structural characterization of plasmonic and red-emitting gold/lysozyme nanohybrid dispersions. Colloid Polym. Sci. 2016, 294, 49–58. [Google Scholar] [CrossRef]
- Russell, B.A.A.; Jachimska, B.; Komorek, P.; Mulheran, P.A.A.; Chen, Y. Lysozyme encapsulated gold nanoclusters: Effects of cluster synthesis on natural protein characteristics. Phys. Chem. Chem. Phys. 2017, 19, 7228–7235. [Google Scholar] [CrossRef]
- Kawasaki, H.; Yoshimura, K.; Hamahuchi, K.; Arakawa, R. Trypsin-Stabilized Fluorescent Gold Nanocluster for Sensitive and Selective Hg2+ Detection. Anal. Sci. 2011, 27, 591. [Google Scholar] [CrossRef]
- Kawasaki, H.; Hamaguchi, K.; Osaka, I.; Arakawa, R. pH-Dependent Synthesis of Pepsin-Mediated Gold Nanoclusters with Blue Green and Red Fluorescent Emission. Adv. Funct. Mater. 2011, 21, 3508–3515. [Google Scholar] [CrossRef]
- Zhu, M.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R. Correlating the Crystal Structure of A Thiol-Protected Au25 Cluster and Optical Properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, M.; Chen, X.; Wang, M.; Chen, H. Atomically Monodispersed and Fluorescent Sub-Nanometer Gold Clusters Created by Biomolecule-Assisted Etching of Nanometer-Sized Gold Particles and Rods. Chem. A Eur. J. 2009, 15, 4944–4951. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-Directed Synthesis of Highly Fluorescent Gold Nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Chaudhari, K.; Xavier, P.L.; Pradeep, T. Understanding the Evolution of Luminescent Gold Quantum Clusters in Protein Templates. ACS Nano 2011, 5, 8816–8827. [Google Scholar] [CrossRef]
| Amino Acid | cAuCl4 (mM) | AuCl4¯:Amino Acid Ratio | T (°C) | Product | λex (nm) | λem (nm) QY (%) | Ref. |
|---|---|---|---|---|---|---|---|
| His | 2.50 | 1:30 | 25 | Au10 NCs | 386 | 490 (8.78%) | [82] |
| His | 2.50 | 1:30 | 25 | Au10–Au14 NCs | 370 | 475 (no inf.) | [86] |
| His | 2.50 | 1:30 | 25 | Au NCs * | 386 | 475 (no inf.) | [83] |
| His | 2.50 | 1:30 | 25 | Au NCs * | 365 | 450 (4.60%) | [84] |
| His | 2.50 | 1:45 | 25 | Au NCs * | 386 | 498 (8.96%) | [87] |
| His | 1.00 | 1:30 | 37 | Au(I)-His CP | 378 | 475 (3.60%) | [71] |
| Tyr | 2.50 | 1:1.8 | 37 | Au NCs * | 385 | 470 (2.50%) | [88] |
| Tyr | 0.07 | 1:0.76 | 100 | Au10 NCs | 383 | 498 (1.68%) | [89] |
| Pro | 2.40 | 1:830 | 100 | Au7 NCs | 365 | 440 (2.94%) | [90] |
| Trp | 0.43 | 1:2.7 | 100 | Au8 NCs | 365 | 450 (no inf.) | [91] |
| Trp | 0.50 | 1:1 | 37 | Au3–Au6 NCs | 378 | 497 (1.10%) | [71] |
| 0.50 | 1:5 | 37 | Au3–Au6 NCs | 378 | 486 (1.30%) | [71] | |
| 0.50 | 1:15 | 37 | Au3–Au6 NCs | 378 | 472 (1.70%) | [71] | |
| Met | 4.06 | 1:20 | 37 | Au NCs * | 420 | 530 (2.80%) | [92] |
| Cys | 1.00 | 1:10 | 37 | Au(I)-Cys CP | 395 | 620 (no inf.) | [93] |
| Cys | 5.00 | 1:10 | 25 | Au(I)-Cys CP | 365 | 630 (1.10%) | [94] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ungor, D.; Dékány, I.; Csapó, E. Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems. Nanomaterials 2019, 9, 1229. https://doi.org/10.3390/nano9091229
Ungor D, Dékány I, Csapó E. Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems. Nanomaterials. 2019; 9(9):1229. https://doi.org/10.3390/nano9091229
Chicago/Turabian StyleUngor, Ditta, Imre Dékány, and Edit Csapó. 2019. "Reduction of Tetrachloroaurate(III) Ions With Bioligands: Role of the Thiol and Amine Functional Groups on the Structure and Optical Features of Gold Nanohybrid Systems" Nanomaterials 9, no. 9: 1229. https://doi.org/10.3390/nano9091229