L-Aspartic Acid Capped CdS Quantum Dots as a High Performance Fluorescence Assay for Sliver Ions (I) Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of L-Asp@CdS QDs
2.3. Detection of Ag+ by L-Asp@CdS QDs
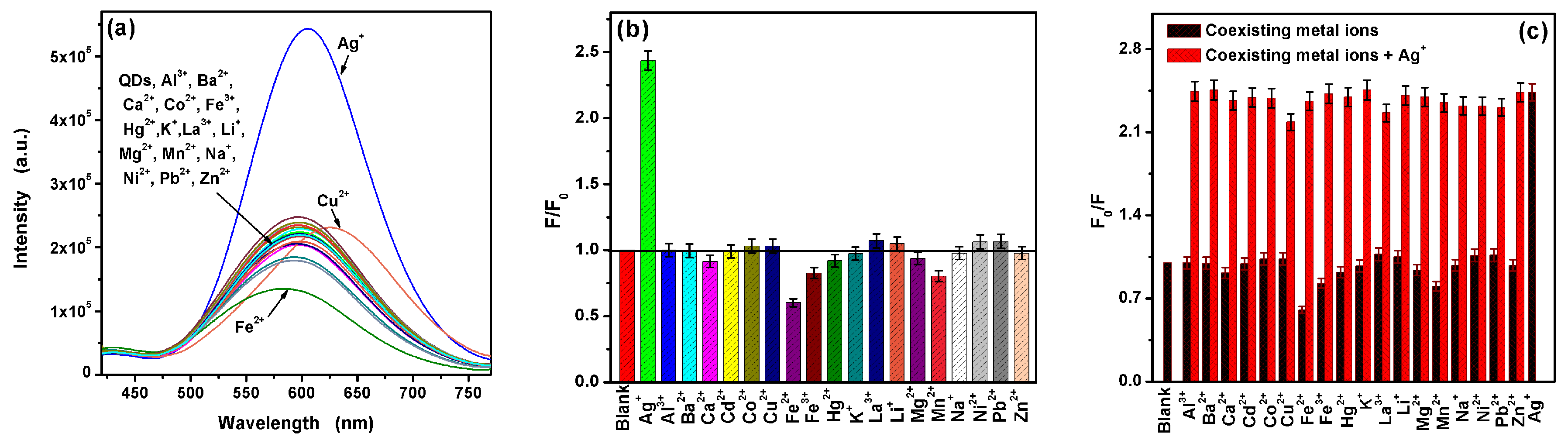
2.4. Interference Studies
3. Results and Discussion
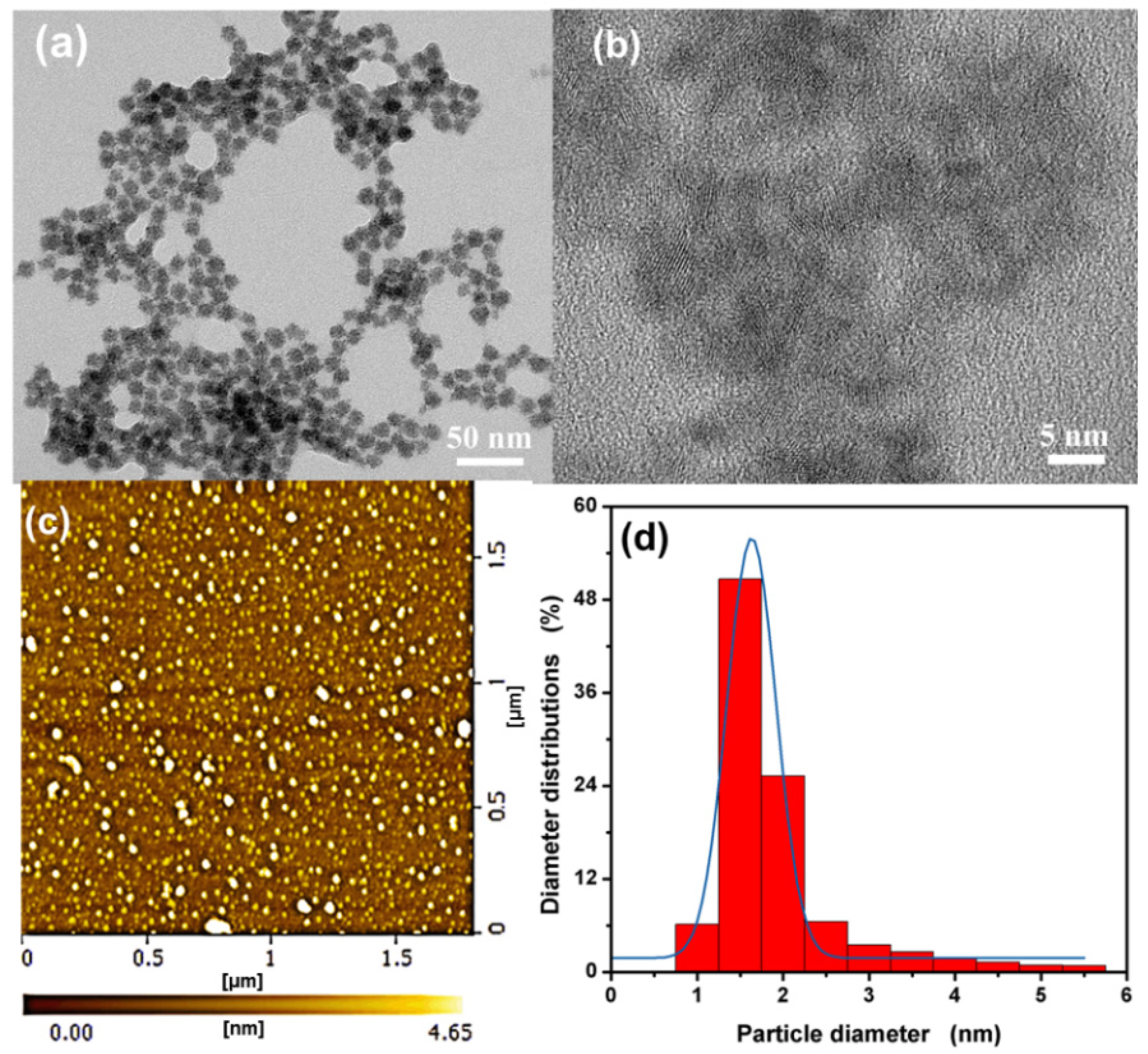
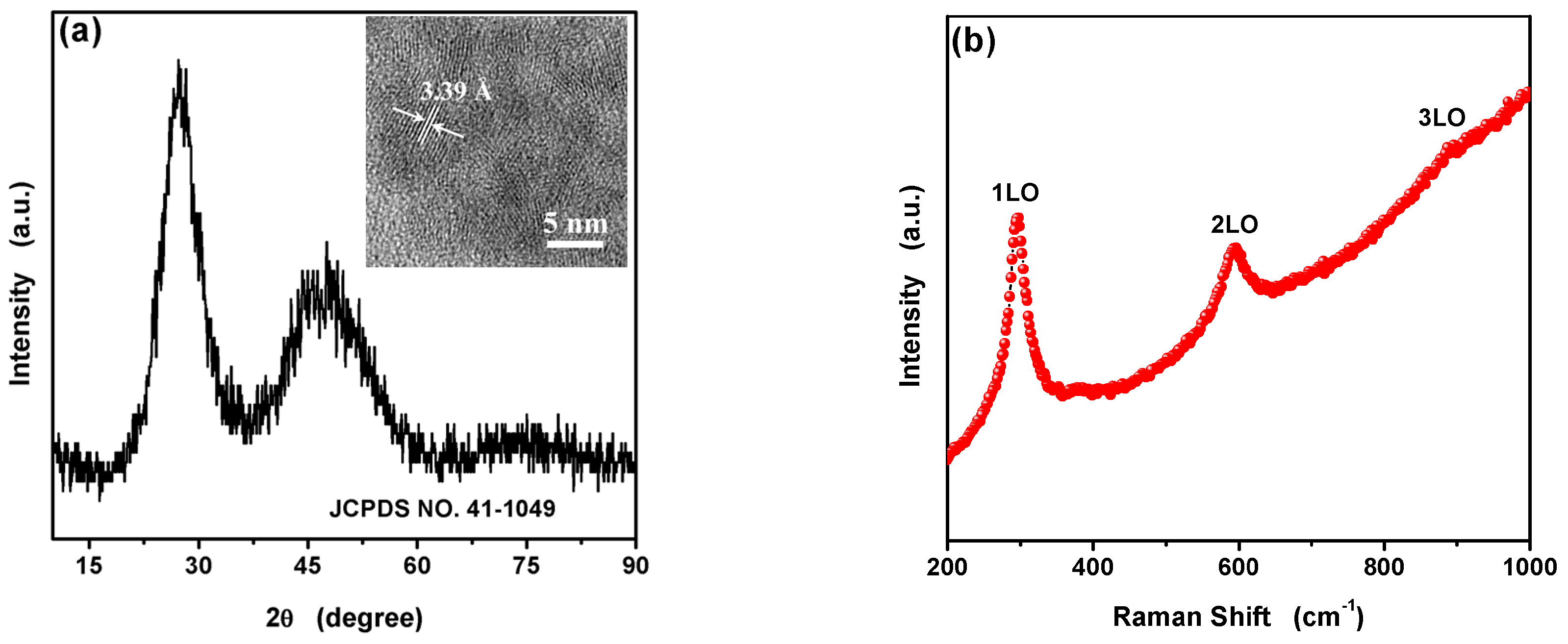
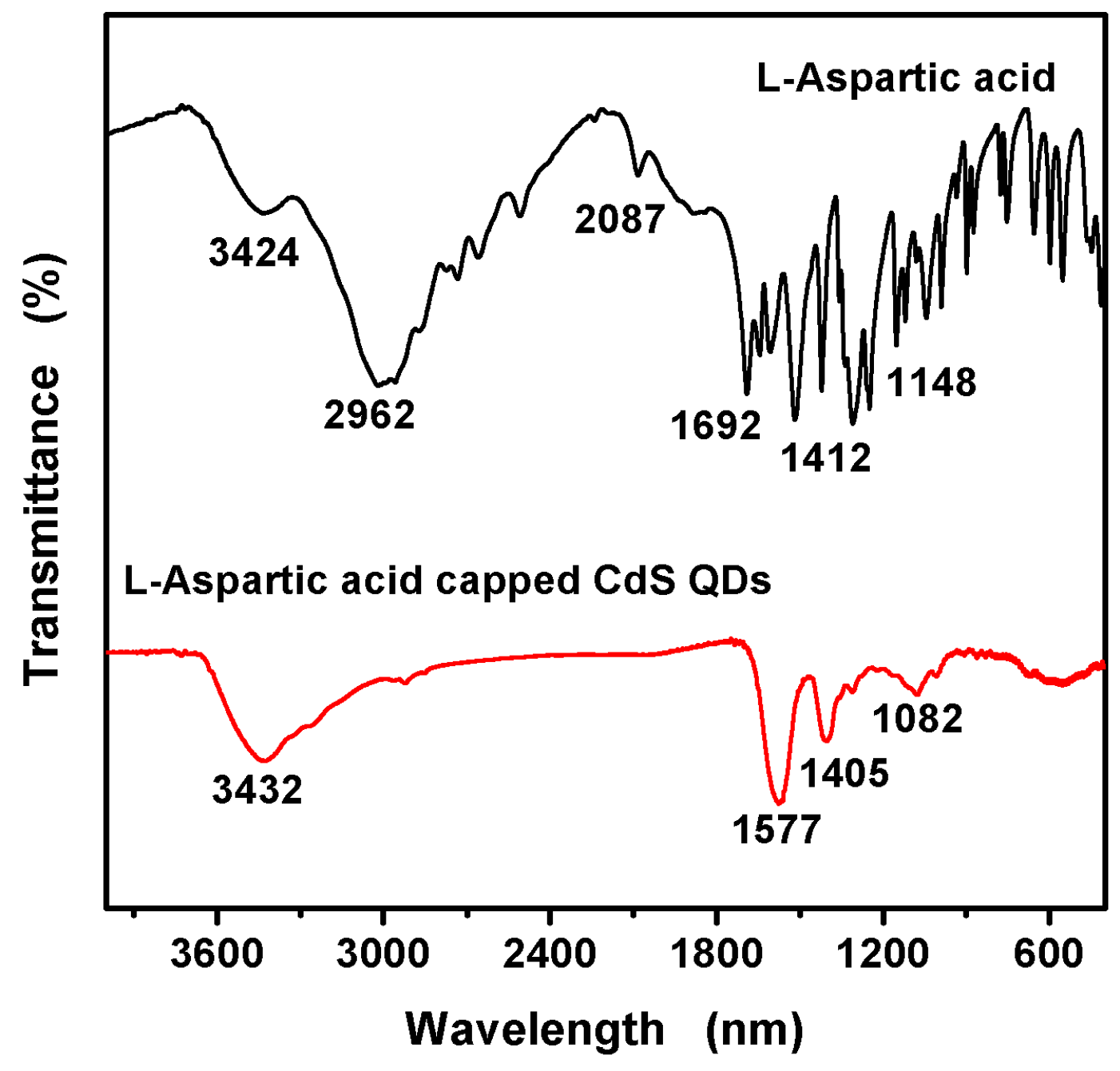
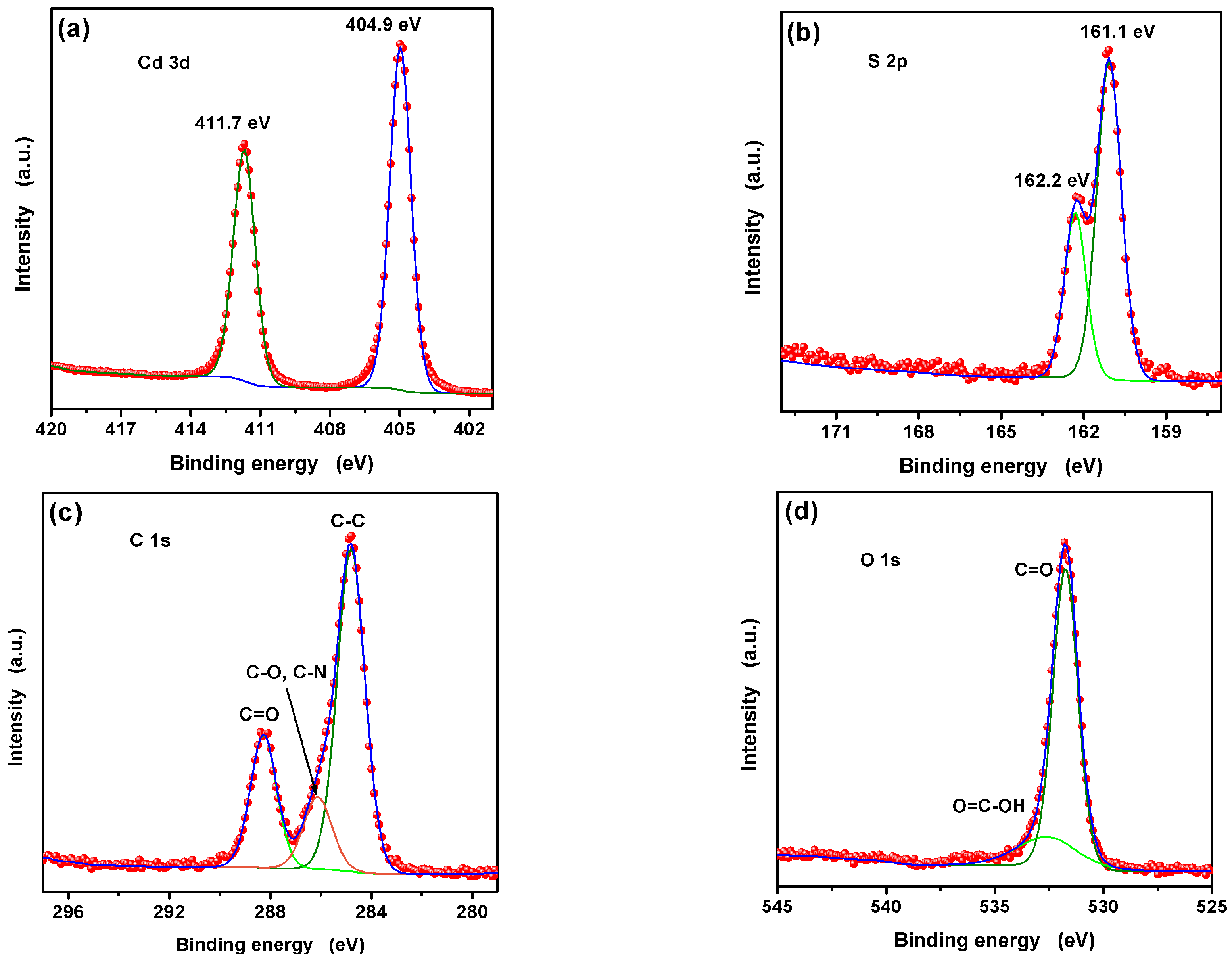
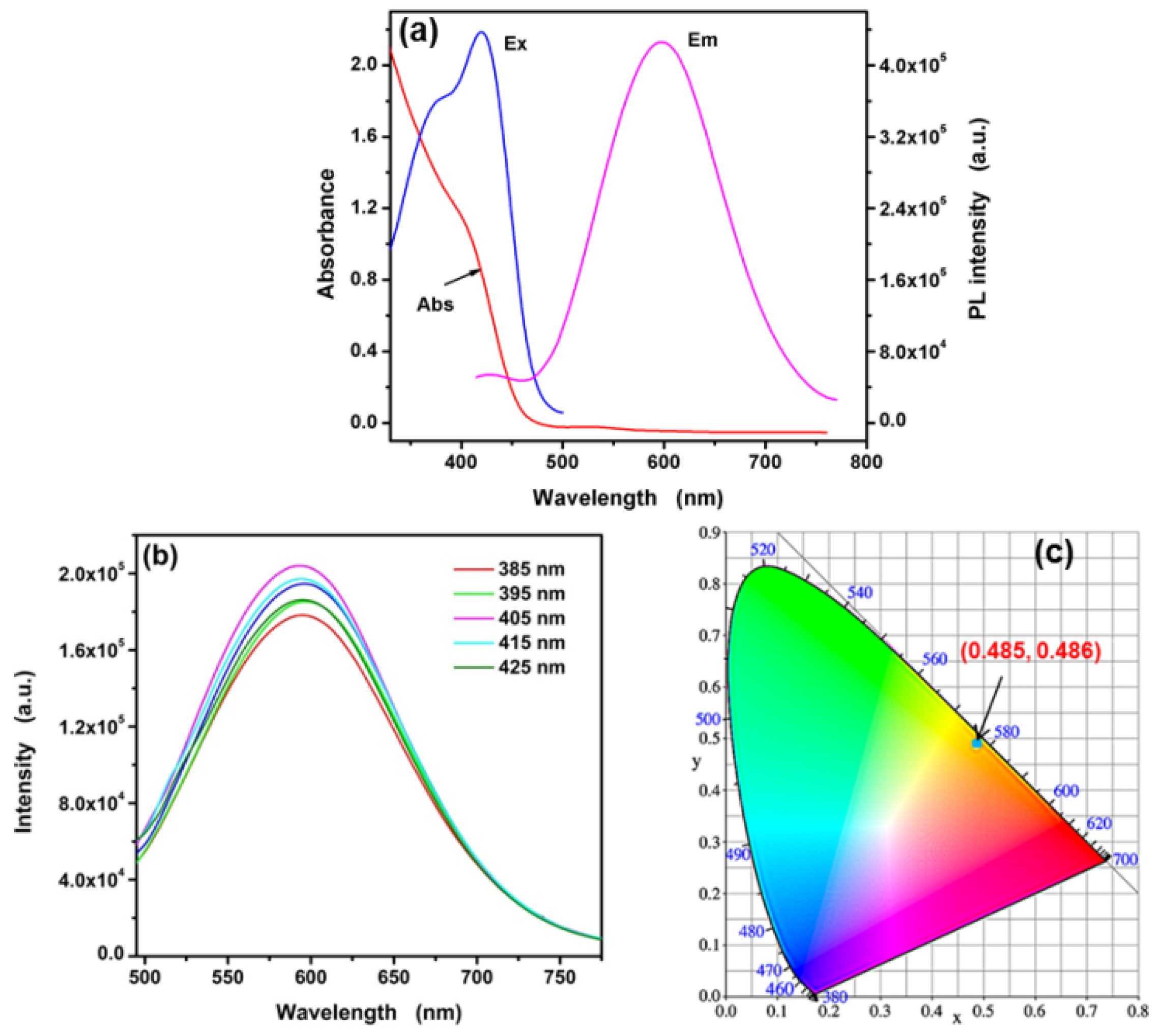
3.1. Characterization of the L-Asp@CdS QDs
3.2. Optical Properties of Synthesized L-Asp@CdS QDs
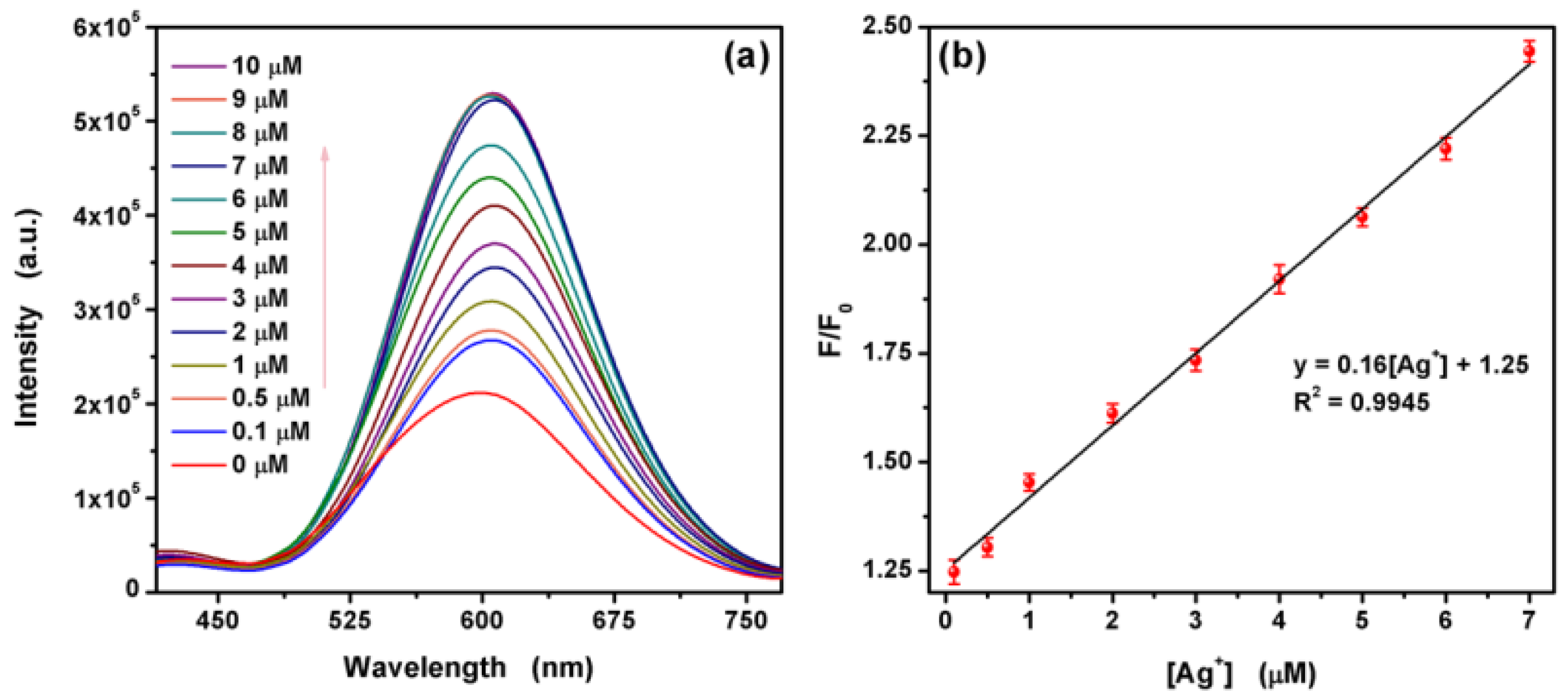
3.3. Ag+ Detection Based on the L-Asp@CdS QDs
3.4. Ag+ Detection on Real Water Sample
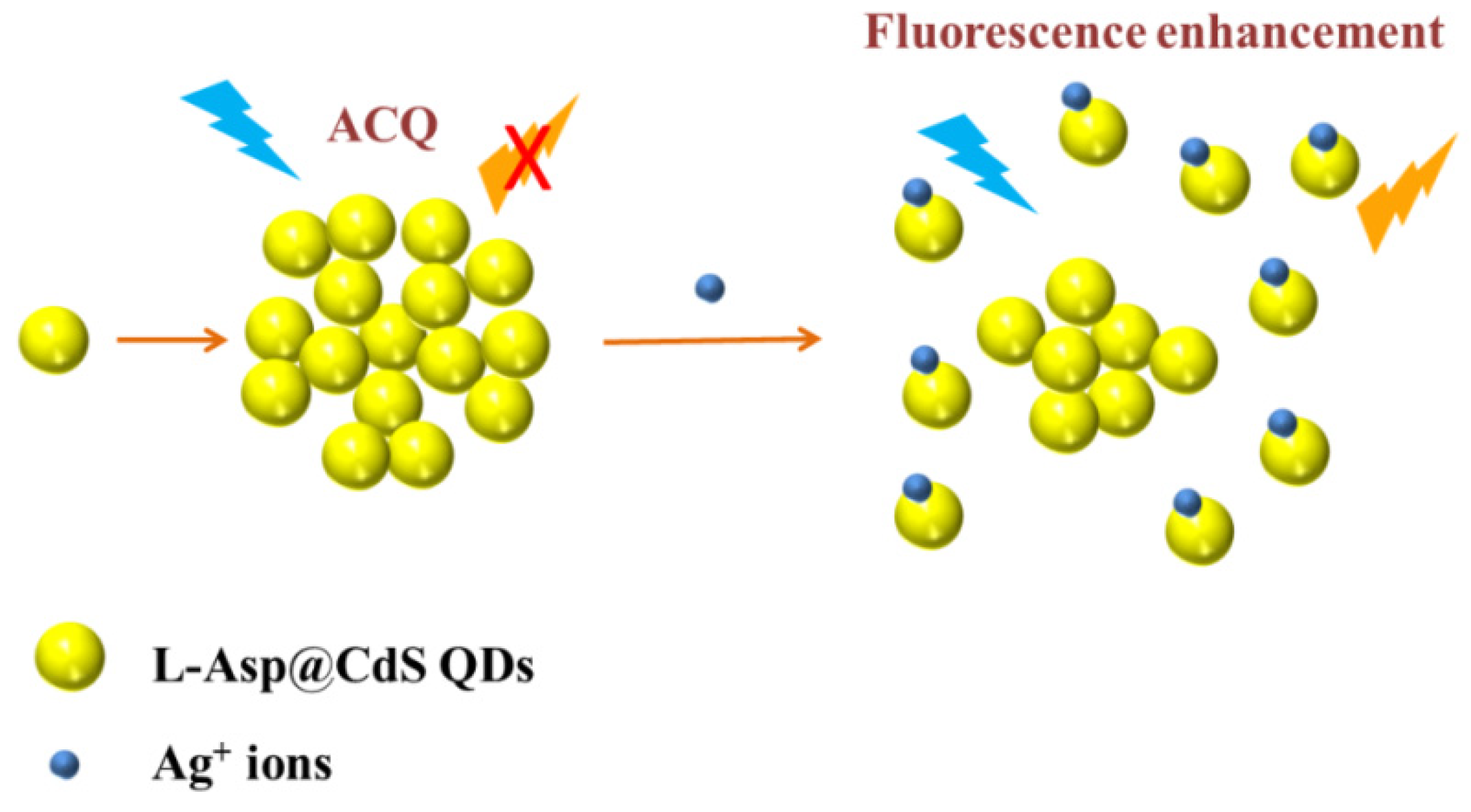
3.5. Mechanism of Ag+ Detection Based on the L-Asp@CdS QDs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sousa, J.C.L.; Vivas, M.G.; Ferrari, J.L.; Schiavon, M.A. Synthesis and optical properties of water-soluble CdTe:Zn2+ quantum dots prepared by the one-pot approach. J. Braz. Chem. Soc. 2018, 29, 2496–2501. [Google Scholar] [CrossRef]
- Hameau, S.; Guldner, Y.; Verzelen, O.; Ferreira, R.; Bastard, G.; Zeman, J.; Lemaitre, A.; Gerard, J.M. Strong electron-phonon coupling regime in quantum dots: Evidence for everlasting resonant polarons. Phys. Rev. Lett. 1999, 83, 4152–4155. [Google Scholar] [CrossRef]
- Chen, J.L.; Zhu, C.Q. Functionalized cadmium sulfide quantum dots as fluorescence probe for silver ion determination. Anal. Chim. Acta 2005, 546, 147–153. [Google Scholar] [CrossRef]
- Ramalingam, G.; Saravanan, K.V.; Vizhi, T.K.; Rajkumar, M.; Baskar, K. Synthesis of water-soluble and bio-taggable CdSe@ZnS quantum dots. RSC Adv. 2018, 8, 8516–8527. [Google Scholar] [CrossRef]
- Muthalif, M.P.A.; Sunesh, C.D.; Choe, Y. Enhanced light absorption and charge recombination control in quantum dot sensitized solar cells using tin doped cadmium sulfide quantum dots. J. Colloid Interface Sci. 2019, 534, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Huang, Y.; Yu, H.C.; Su, Y.K. Ultrathin PVK charge control layer for advanced manipulation of efficient giant CdSe@ZnS/ZnS quantum dot light-emitting diodes. Org. Electron. 2018, 63, 349–354. [Google Scholar] [CrossRef]
- Zou, W.S.; Deng, M.Y.; Wang, Y.Q.; Zhao, X.L.; Li, W.H.; Huang, X.H. Alginate capped and manganese doped ZnS quantum dots as a phosphorescent probe for time-resolved detection of copper(II). Microchim. Acta 2019, 186, 1. [Google Scholar] [CrossRef]
- Mohamed, N.B.N.; Brahim, N.; Mrad, R.; Haouari, M.; Ban Chaabane, R.; Negrerie, M. Use of MPA-capped CdS quantum dots for sensitive detection and quantification of Co2+ ions in aqueous solution. Anal. Chim. Acta 2018, 1028, 50–58. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Maxwell, D.J.; Gao, X.H.; Bailey, R.E.; Han, M.Y.; Ni, S.M. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Xiao, X.C.; Zou, T.; Yang, Y.; Xing, X.X.; Zhao, R.Z.; Wang, Z.D.; Wang, Y.D. Citric acid capped CdS quantum dots for fluorescence detection of copper ions (II) in aqueous solution. Nanomaterials 2019, 9, 32. [Google Scholar] [CrossRef]
- Xu, X.; Long, Y.W.; Pan, S.; Liu, H.; Yang, J.D.; Hu, X.L. S-doped carbon dots capped ZnCdTe quantum dots for ratiometric fluorescence sensing of guanine. Sens. Actuators B Chem. 2019, 279, 44–52. [Google Scholar] [CrossRef]
- Ngamdee, K.; Ngeontae, W. Circular dichroism glucose biosensor based on chiral cadmium sulfide quantum dots. Sens. Actuators B Chem. 2018, 274, 402–411. [Google Scholar] [CrossRef]
- Pourghbadi, Z.; Mirahmadpour, P.; Zare, H. Fluorescent biosensor for the selective determination of dopamine by TGA-capped CdTe quantum dots in human plasma samples. Opt. Mater. 2018, 84, 757–762. [Google Scholar] [CrossRef]
- Sianglam, P.; Kulchat, S.; Tuntulani, T.; Ngeontae, W. A circular dichroism sensor for selective detection of Cd2+ and S2− based on the in-situ generation of chiral CdS quantum dots. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 183, 408–416. [Google Scholar] [CrossRef]
- Park, J.; Choi, W.; Jang, K.; Na, S. High-sensitivity detection of silver ions using oligonucleotide-immobilized oscillator. Biosens. Bioelectron. 2013, 41, 471–476. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, H.Q. Long-term effects of silver nanoparticles on the abundance and activity of soil microbiome. J. Environ. Sci. 2018, 69, 3–4. [Google Scholar] [CrossRef]
- Tang, H.Q.; Feng, H.J.; Zhang, J.H.; Zhao, J. A study on antibacterial properties of Ag+-implanted pyrolytic carbon. Surf. Coat. Technol. 2007, 201, 5633–5636. [Google Scholar] [CrossRef]
- Chun, K.Y.; Oh, Y.; Rho, J.; Ahu, J.H.; Kim, Y.J.; Choi, H.R.; Baik, S. Highly conductive, printable and stretchable composite films of carbon nanotubes and silver. Nat. Nanotechnol. 2010, 5, 853–857. [Google Scholar] [CrossRef]
- Feng, D.Q.; Liu, G.; Zheng, W.; Liu, J.; Chen, T.; Li, D. A highly selective and sensitive on-off sensor for silver ions and cysteine by light scattering technique of DNA-functionalized gold nanoparticles. Chem. Commun. 2011, 47, 8557–8559. [Google Scholar] [CrossRef]
- Haghnazari, N.; Alizadeh, A.; Karami, C.; Hamidi, Z. Simple optical determination of silver ion in aqueous solutions using benzo crown-ether modified gold nanoparticles. Microchim. Acta 2013, 180, 287–294. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization’s Guidelines for Drinking-water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; p. 564. [Google Scholar]
- Frankowski, M.; Ziola-Frankowsha, A.; Siepak, J. New method for speciation analysis of aluminium fluoride complexes by HPLC–FAAS hyphenated technique. Talanta 2010, 80, 2120–2126. [Google Scholar] [CrossRef]
- Katarina, P.K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Synthesis of a chitosan-based chelating resin and its application to the selective concentration and ultratrace determination of silver in environmental water samples. Anal. Chim. Acta 2006, 558, 246–253. [Google Scholar] [CrossRef]
- Dasbasi, T.; Sacmaci, S.; Sahan, S.; Kartal, S.; Ulgen, A. Synthesis, characterization and application of a new chelating resin for on-line separation, preconcentration and determination of Ag(I) by flame atomic absorption spectrometry. Talanta 2013, 103, 1–7. [Google Scholar] [CrossRef]
- Thambidurai, M.; Muthukumarasamy, N.; Agilan, S.; Arul, N.S.; Murugan, N.; Balasundaraprabhu, R. Structural and optical characterization of Ni-doped CdS quantum dots. J. Mater. Sci. 2011, 46, 3200–3206. [Google Scholar] [CrossRef]
- Solanki, R.G.; Rajaram, P.; Bajpai, P.K. Growth, characterization and estimation of lattice strain and size in CdS nanoparticles: X-ray peak profile analysis. Indian J. Phys. 2018, 92, 595–603. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Kamali, M. Synthesis and characterization of aspartic acid-capped CdS/ZnS quantum dots in reverse micelles and its application to Hg(II) determination. J. Lumin. 2015, 167, 51–58. [Google Scholar] [CrossRef]
- Heidari-Chaleshtori, M.; Nezamzadeh-Ejhieh, A. Clinoptilolitenano-particles modified with aspartic acid for removal of Cu(II) from aqueous solutions: Isotherms and kinetic aspects. New J. Chem. 2015, 39, 9396–9406. [Google Scholar] [CrossRef]
- Mahapatra, N.; Panja, S.; Mandal, A.; Haider, M. A single source-precursor route for the one-pot synthesis of highly luminescent CdS quantum dots as ultra-sensitive and selective photoluminescence sensor for Co2+ and Ni2+ ions. J. Mater. Chem. C 2015, 2, 7373–7384. [Google Scholar] [CrossRef]
- Krishnakumar, V.; Ranjith, R.; Jayaprakash, J.; Boobas, S.; Venkatesan, J. Enhancement of photocatalytic degradation of methylene blue under visible light using transparent Mg-doped CdS–PVA nanocomposite films. J. Mater. Sci. Mater. Electron. 2017, 28, 13990–13999. [Google Scholar] [CrossRef]
- Gopannagari, M.; Kumar, D.P.; Park, H.; Kim, E.H.; Bhavani, P.; Reddy, D.A. Influence of surface-functionalized multi-walled carbon nanotubes on CdS nanohybrids for effective photocatalytic hydrogen production. Appl. Catal. B Environ. 2018, 236, 294–303. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, X.C.; Xing, X.X.; Wang, Z.Z.; Zou, T.; Wang, Z.D.; Zhao, R.Z.; Wang, Y.D. Rhodamine B assisted graphene quantum dots fluorescent sensor system for sensitive recognition of mercury ions. J. Lumin. 2019, 207, 273–281. [Google Scholar] [CrossRef]
- Wang, R.Y.; Fan, H.L.; Jing, W.; Ni, G.S.; Qu, S.J. Amino-functionalized graphene quantum dots prepared using high-softening point asphalt and their application in Fe3+ detection. Appl. Surf. Sci. 2019, 467, 446–455. [Google Scholar] [CrossRef]
- Sun, W.L.; Yao, J.L.; Yao, T.M.; Shi, S. Label-free fluorescent DNA sensor for the detection of silver ions based on molecular light switch Ru complex and unmodified quantum dots. Analyst 2013, 138, 421–424. [Google Scholar] [CrossRef]
- Zhao, X.E.; Lei, C.H.; Gao, Y.; Gao, H.; Zhu, S.H.; Yang, X.; Yao, J.M.; Wang, H. A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens. Actuators B Chem. 2017, 253, 239–246. [Google Scholar] [CrossRef]
- Bian, S.Y.; Shen, C.; Qian, Y.T.; Liu, J.Y.; Xi, F.N.; Dong, X.P. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuators B Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhu, W.J.; Fang, M.; Xu, Y.; Li, C. Turn-on fluorescence probe for high sensitive and selective detection of Ag+ by L-glutathione capped CdTe quantum dots in aqueous medium. J. Lumin. 2016, 180, 14–19. [Google Scholar] [CrossRef]
- Mahapatra, N.; Mandal, A.; Panja, S.; Haider, M. Emergence of the selective ultra-sensing of Ag(I): Thiolactic acid as efficient capping agent for cadmium chalcogenide quantum dots in modulating photoluminescence and metal reception. Sens. Actuators B Chem. 2017, 240, 543–552. [Google Scholar] [CrossRef]
- Khantaw, T.; Boonmee, C.; Tuntulani, T.; Ngeontae, W. Selective turn-on fluorescence sensor for Ag+ using cysteamine capped CdS quantumdots: Determination of free Ag+ in silver nanoparticles solution. Talanta 2013, 115, 849–856. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polym. Science 1994, 265, 765–768. [Google Scholar] [CrossRef]
- Nie, H.; Hu, K.; Cai, Y.J.; Peng, Q.; Zhao, Z.J.; Hu, R.R.; Chen, J.W.; Su, S.J.; Qin, A.J.; Tang, B.Z. Tetraphenylfuran: Aggregation-induced emission or aggregation caused quenching? Mater. Chem. Front. 2017, 1, 1125–1129. [Google Scholar] [CrossRef]
| Fluorescent Probe | Detection Mode | Linear Range (μM) | Detection Limit (μM) | Sensitivity (μM−1) | Ref. |
|---|---|---|---|---|---|
| CdTe QDs | a F.Q. | 2–10 | 0.1 | Not given | [34] |
| GQDs | Ratiometric | 0–115.2 | 0.25 | 0.01649 | [35] |
| S-GQDs | a F.Q. | 0.1–130 | 0.03 | 0.0053 | [36] |
| GSH@CdTe QDs | b F.E. | 0.02–0.2 | 0.0013 | 0.4626 | [37] |
| TLA-CdTe QDs | a F.Q. | 0.05–10/10–100 | 0.05 | 0.1151/0.055 | [38] |
| Cys-CdS QDs | b F.E. | 0.1–1.5 | 0.068 | 74.71 | [39] |
| L-Asp@CdS QDs | b F.E. | 0.1–7 | 0.039 | 0.16 | This work |
| Samples | Spiked (μM) | Found (μM) | Recovery (%, n = 5) | Relative Standard Deviation (RSD) (%, n = 5) |
|---|---|---|---|---|
| Drinking water | 0.500 | 0.493 | 98.6 | 3.6 |
| 1.000 | 1.121 | 112 | 2.1 | |
| 3.000 | 3.168 | 105 | 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Xiao, X.; Yang, Y.; Zou, T.; Xing, X.; Zhao, R.; Wang, Z.; Wang, Y. L-Aspartic Acid Capped CdS Quantum Dots as a High Performance Fluorescence Assay for Sliver Ions (I) Detection. Nanomaterials 2019, 9, 1165. https://doi.org/10.3390/nano9081165
Wang Z, Xiao X, Yang Y, Zou T, Xing X, Zhao R, Wang Z, Wang Y. L-Aspartic Acid Capped CdS Quantum Dots as a High Performance Fluorescence Assay for Sliver Ions (I) Detection. Nanomaterials. 2019; 9(8):1165. https://doi.org/10.3390/nano9081165
Chicago/Turabian StyleWang, Zhezhe, Xuechun Xiao, Yue Yang, Tong Zou, Xinxin Xing, Rongjun Zhao, Zidong Wang, and Yude Wang. 2019. "L-Aspartic Acid Capped CdS Quantum Dots as a High Performance Fluorescence Assay for Sliver Ions (I) Detection" Nanomaterials 9, no. 8: 1165. https://doi.org/10.3390/nano9081165
APA StyleWang, Z., Xiao, X., Yang, Y., Zou, T., Xing, X., Zhao, R., Wang, Z., & Wang, Y. (2019). L-Aspartic Acid Capped CdS Quantum Dots as a High Performance Fluorescence Assay for Sliver Ions (I) Detection. Nanomaterials, 9(8), 1165. https://doi.org/10.3390/nano9081165





