Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Propylene Glycol Monopalmitate
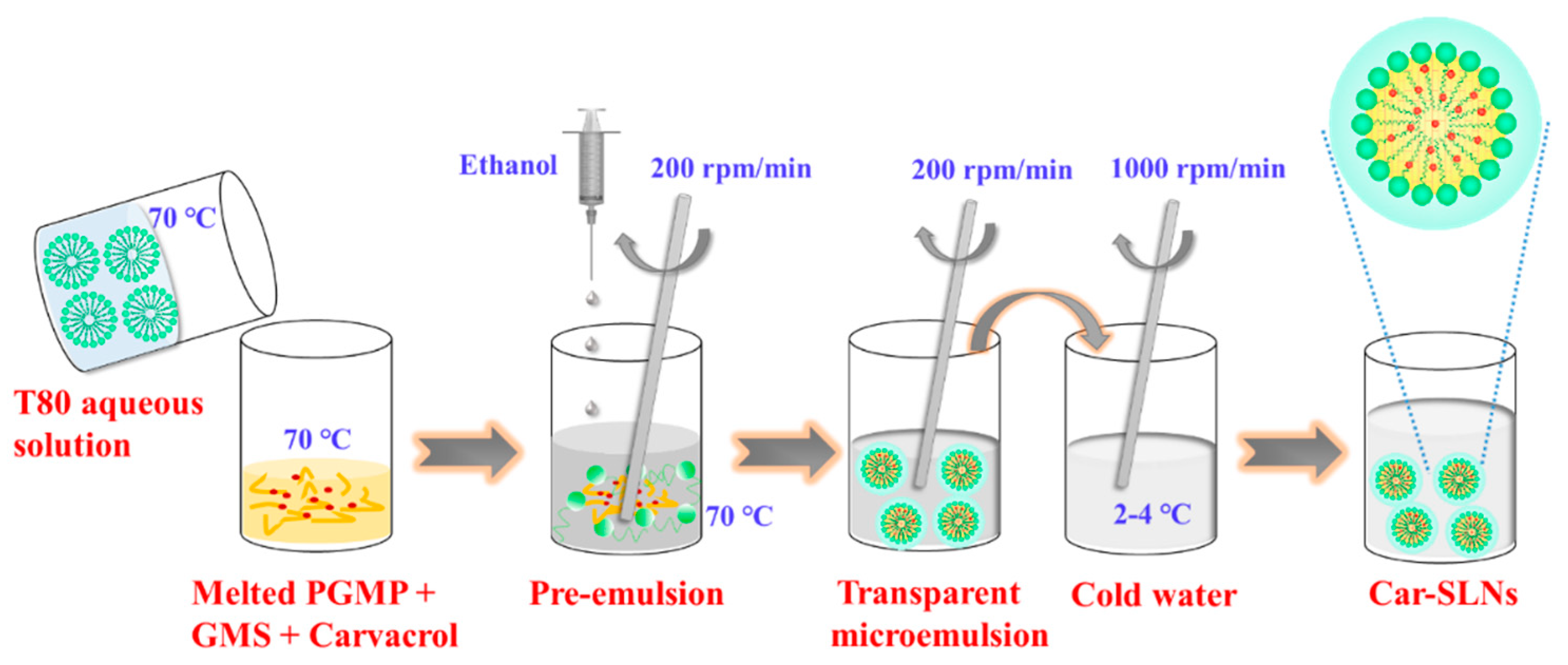
2.3. Preparation of SLN Dispersions
2.4. Determination of Z-Average Mean Diameter, Polydispersity Index (PDI), and Zeta-Potential
2.5. Preparation and Characterization of Carvacrol-Loaded SLNs
2.6. Determination of Entrapment Efficiency of Car-SLNs
2.7. Chemical Stability of Carvacrol Loaded in SLNs
2.8. Fluorescence Study of Car-SLN Dispersions
2.9. Release Kinetics of Carvacrol from the SLNs
2.10. Transmission Electron Microscopy
2.11. Antimicrobial Activity of Car-SLNs
2.12. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of PGMP
3.2. Effect of the Mass Ratio of PGMP and GMS on SLN Formation
3.3. Properties of SLNs Encapsulating Carvacrol
3.4. Chemical Stability of Carvacrol in SLNs
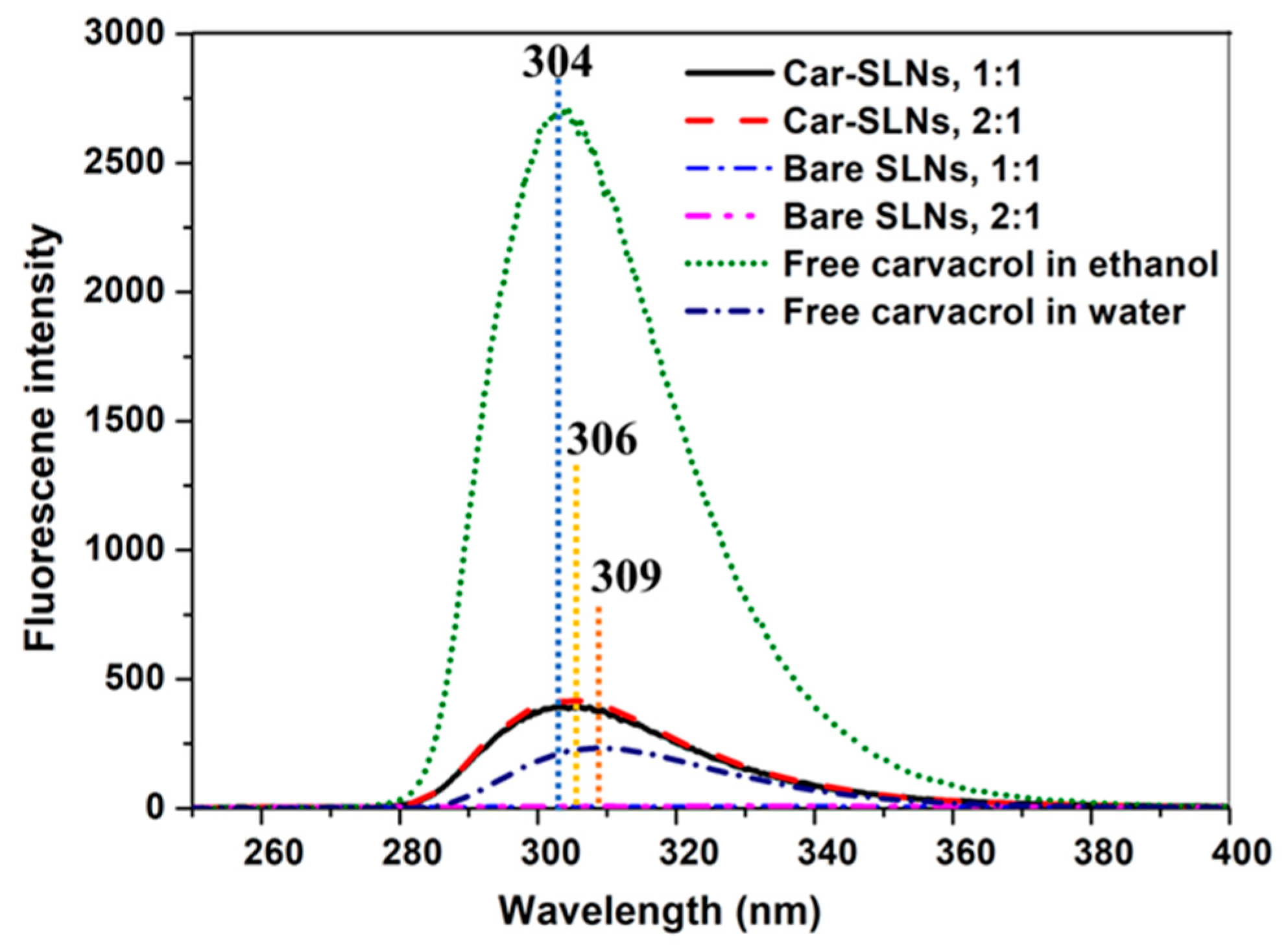
3.5. Interactions between Lipids and Carvacrol Studied with Fluorescence Spectroscopy
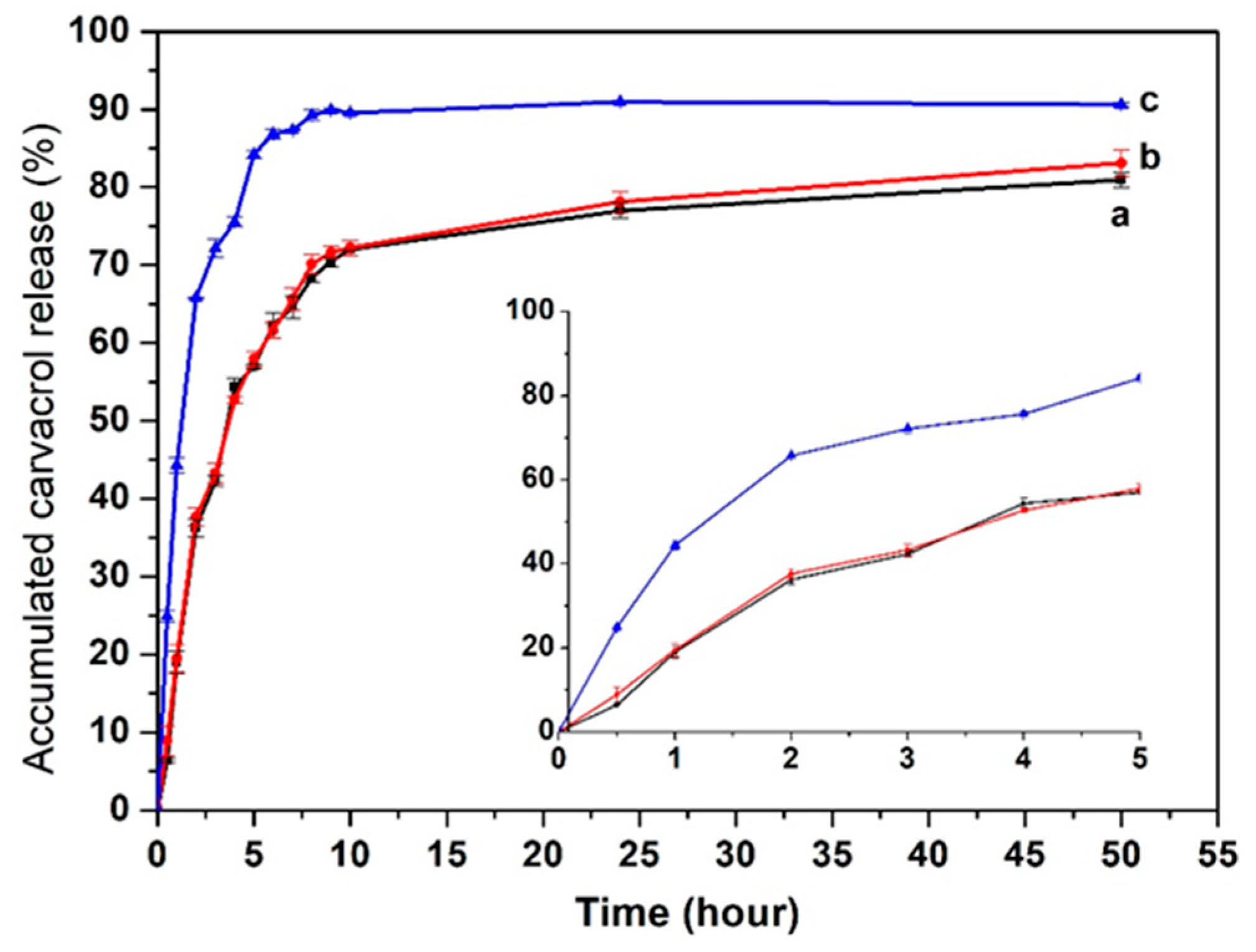
3.6. Release Profile of Carvacrol in SLNs
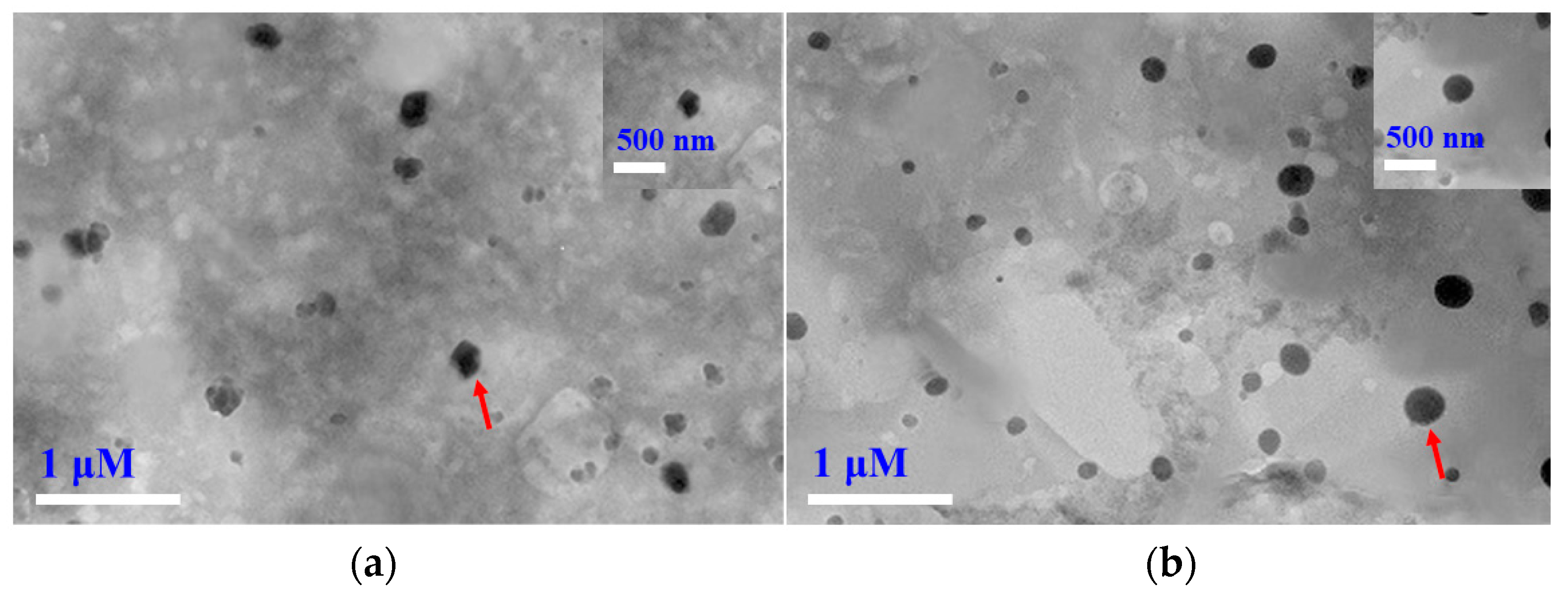
3.7. Morphology of Carvacrol-Loaded SLNs
3.8. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S.; Randhawa, J.K. High melting lipid based approach for drug delivery: Solid lipid nanoparticles. Mater. Sci. Eng. C 2013, 33, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Decker, E.A.; McClements, D.J.; Kristbergsson, K.; Helgason, T.; Awad, T. Solid lipid nanoparticles as delivery systems for bioactive food components. Food Biophys. 2008, 3, 146–154. [Google Scholar] [CrossRef]
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146. [Google Scholar] [CrossRef]
- Aditya, N.P.; Ko, S. Solid lipid nanoparticles (SLNs): Delivery vehicles for food bioactives. RSC Adv. 2015, 5, 30902–30911. [Google Scholar] [CrossRef]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, 131–155. [Google Scholar] [CrossRef]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Huang, S.; He, J.; Han, L.; Zhang, W.; Zhong, Q. 1-Laurin-3-palmitin as a novel matrix of solid lipid particles: Higher loading capacity of thymol and better Stability of dispersions than those of glyceryl monostearate and glyceryl tripalmitate. Nanomaterials 2019, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.C.; Marangoni, A.G. Advances in the application of food emulsifier α-gel phases: Saturated monoglycerides, polyglycerol fatty acid esters, and their derivatives. J. Colloid Interface Sci. 2016, 483, 394–403. [Google Scholar] [CrossRef]
- Kuhrt, N.H.; Broxholm, R.A.; Blum, W.P. Conjoined crystals. I. composition and physical properties. J. Am. Oil Chem. Soc. 1963, 40, 725–730. [Google Scholar] [CrossRef]
- Kuhrt, N.H.; Broxholm, R.A. Conjoined crystals. II. applications. J. Am. Oil Chem. Soc. 1963, 40, 730–733. [Google Scholar] [CrossRef]
- Silva, F.V.; Guimares, A.G.; Silva, E.R.S.; Sousa-Neto, B.P.; Machado, F.D.F.; Quintans-Júnior, L.J.; Arcanjo, D.D.R.; Oliveira, F.A.; Oliveira, R.C.M. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J. Med. Food 2012, 15, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Rawat, D.S. Synthesis and antioxidant activity of thymol and carvacrol based Schiff bases. Bioorg. Med. Chem. Lett. 2013, 23, 641–645. [Google Scholar] [CrossRef]
- Kamimura, J.A.; Santos, E.H.; Hill, L.E.; Gomes, C.L. Antimicrobial and antioxidant activities of carvacrol microencapsulated in hydroxypropyl-beta-cyclodextrin. LWT-Food Sci. Technol. 2014, 57, 701–709. [Google Scholar] [CrossRef]
- Veldhuizen, E.J.A.; Tjeerdsma-van Bokhoven, J.L.M.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Liolios, C.C.; Gortzi, O.; Lalas, S.; Tsaknis, J.; Chinou, I. Liposomal incorporation of carvacrol and thymol isolated from the essential oil of Origanum dictamnus L. and in vitro antimicrobial activity. Food Chem. 2009, 112, 77–83. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Amodio, M.L.; Colelli, G. Carvacrol-loaded chitosan nanoparticles maintain quality of fresh-cut carrots. Innov. Food Sci. Emerg. 2017, 41, 56–63. [Google Scholar] [CrossRef]
- Santos, E.H.; Kamimura, J.A.; Hill, L.E.; Gomes, C.L. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT-Food Sci. Technol. 2015, 60, 583–592. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physicochemical properties and antimicrobial efficacy of carvacrol nanoemulsions formed by spontaneous emulsification. J. Agric. Food Chem. 2013, 61, 8906–8913. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Y.; Wang, Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT-Food Sci. Technol. 2012, 48, 283–290. [Google Scholar] [CrossRef]
- Lutton, E.S.; Stewart, C.B.; Martin, J.B. Clarification of propylene glycol monoester polymorphism. J. Am. Oil Chem. Soc. 1972, 49, 186–187. [Google Scholar] [CrossRef]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Xue, J.; Michael Davidson, P.M.; Zhong, Q. Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. Int. J. Food Microbiol. 2015, 210, 1–8. [Google Scholar] [CrossRef]
- Sjöblom, J.; Lindberg, R.; Friberg, S.E. Microemulsions—Phase equilibria characterization, structures, applications and chemical reactions. Adv. Colloid Interface Sci. 1996, 65, 125–287. [Google Scholar] [CrossRef]
- Nayak, A.P.; Tiyaboonchai, W.; Patankar, S.; Madhusudhan, B.; Souto, E.B. Curcuminoids-loaded lipid nanoparticles: Novel approach towards malaria treatment. Colloids Surf. B 2010, 81, 263–273. [Google Scholar] [CrossRef]
- Matsaridou, I.; Barmpalexis, P.; Salis, A.; Nikolakakis, I. The influence of surfactant HLB and oil/surfactant ratio on the formation and properties of self-emulsifying pellets and microemulsion reconstitution. AAPS PharmSciTech 2012, 13, 1319–1330. [Google Scholar] [CrossRef]
- Taylor, P. Ostwald ripening in emulsions. Colloid Surface. A 1995, 99, 175–185. [Google Scholar] [CrossRef]
- Chen, H.; Davidson, P.M.; Zhong, Q. Impacts of sample preparation methods on solubility and antilisterial characteristics of essential oil components in milk. Appl. Environ. Microbiol. 2014, 80, 907–916. [Google Scholar] [CrossRef]
- Xu, R.; Wu, C.; Xu, H. Particle size and zeta potential of carbon black in liquid media. Carbon 2007, 45, 2806–2809. [Google Scholar] [CrossRef]
- Chalier, P.; Arfa, A.B.; Preziosi-Belloy, L.; Gontard, N. Carvacrol losses from soy protein coated papers as a function of drying conditions. J. Appl. Polym. Sci. 2007, 106, 611–620. [Google Scholar] [CrossRef]
- Noack, A.; Hause, G.; Mäder, K. Physicochemical characterization of curcuminoid-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 423, 440–451. [Google Scholar] [CrossRef]
- Jasim, F.; Ali, F. A novel and rapid method for the spectrofluorometric determination of curcumin in curcumin spices and flavors. Microchem. J. 1992, 46, 209–214. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Wang, C.C. Cationic solid lipid nanoparticles with primary and quaternary amines for release of saquinavir and biocompatibility with endothelia. Colloids Surf. B 2013, 101, 101–105. [Google Scholar] [CrossRef]
- Kakkar, V.; Singh, S.; Singla, D.; Kaur, I.P. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011, 55, 495–503. [Google Scholar] [CrossRef]
- Lobovkina, T.; Jacobson, G.B.; Gonzalez-Gonzalez, E.; Hickerson, R.P.; Leake, D.; Kaspar, R.L.; Contag, C.H.; Zare, R.N. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano 2011, 5, 9977–9983. [Google Scholar] [CrossRef]
- Muchow, M.; Maincent, P.; Müller, R.H. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev. Ind. Pharm. 2008, 34, 1394–1405. [Google Scholar] [CrossRef]
- Sparsø, F.V. Propylene Glycol Fatty Acid Esters. In Emulsifiers in Food Technology, 2nd ed.; Norn, V., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2015; pp. 231–250. [Google Scholar]
- Arishima, T.; Sato, K. Polymorphism of POP and SOS III. Solvent crystallization of β2 and β1 polymorphs. J. Am. Oil Chem. Soc. 1989, 66, 1614–1617. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.X.; Avena-Bustillos, R.J.; Berrios Jde, J.; Sambo, P.; McHugh, T.H. Optimization of antimicrobial and physical properties of alginate coatings containing carvacrol and methyl cinnamate for strawberry application. J. Agric. Food Chem. 2014, 62, 984–990. [Google Scholar] [CrossRef]
- Keawchaoon, L.; Yoksan, R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B 2011, 84, 163–171. [Google Scholar] [CrossRef]
- Fett, W.F. Naturally occurring biofilms on alfalfa and other types of sprouts. J. Food Prot. 2000, 63, 625–632. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef]
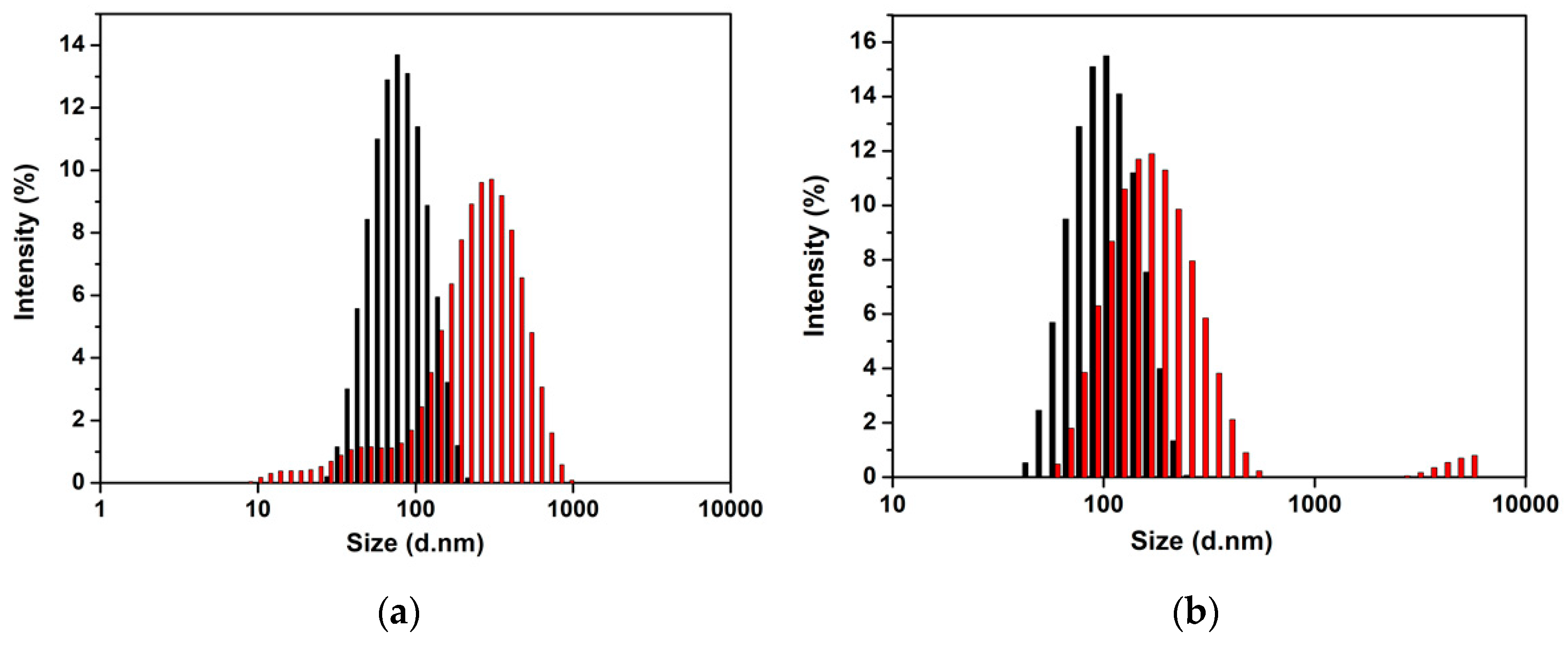
| PGMP:GMS | Z-Average (nm) | PDI | Zeta-Potential (mV) | Visual Stability |
|---|---|---|---|---|
| 1:0 | 14.9 ± 0.4 | 0.133 ± 0.017 | 5.6 ± 0.9 | Precipitation after 3 days |
| 2:1 | 18.6 ± 0.2 | 0.178 ± 0.008 | −11.0 ± 0.1 | Stable for 1 month |
| 1:1 | 25.3 ± 0.7 | 0.296 ± 0.003 | −13.7 ± 0.8 | Stable for 1 month |
| 1:2 | ND 1 | ND | ND | Precipitation after 1 day |
| 0:1 | ND | ND | ND | Precipitation after 1 day |
| PGMP:GMS | Carvacrol Loading (% Mass of Lipids) | Z-Average (nm) | PDI | Zeta-Potential (mV) | EE (%) | |||
|---|---|---|---|---|---|---|---|---|
| 0 Day | 30 Days | 0 Day | 30 Days | 0 Day | 30 Days | 0 Days | ||
| 2:1 | 0 | 18.6 ± 0.2 d | 32.9 ± 0.5 d | 0.178 ± 0.008 a | 0.292 ± 0.027 b | −11.0 ± 0.1 ab | −14.1 ± 1.6 c | |
| 20 | 39.8 ± 0.6 c | 149.4 ± 1.8 c | 0.184 ± 0.010 a | 0.221 ± 0.018 c | −9.73 ± 0.1 b | −18.5 ± 0.7 b | 98.0 ± 0.1 c | |
| 30 | 68.9 ± 0.1 b | 159.8 ± 2.5 b | 0.182 ± 0.012 a | 0.313 ± 0.002 a,b | −12.6 ± 1.7 a | −21.2 ± 1.6 a,b | 98.3 ± 0.1 a | |
| 40 | 95.4 ± 0.7 a | 165.8 ± 3.4 a | 0.182 ± 0.008 a | 0.355 ± 0.051 a | −11. 9± 1.4 a,b | −23.2 ± 2.9 a | 98.2 ± 0.2 a,b | |
| 1:1 | 0 | 25.3 ± 0.7 d | 55.1 ± 0.2 d | 0.296 ± 0.003 a | 0.278 ± 0.003 a | −13.7 ± 0.8 b | −15.3 ± 1.3 c | |
| 20 | 56.1 ± 0.3 c | 98.7 ± 0.9 c | 0.211 ± 0.010 c | 0.198 ± 0.002 c | −17.1 ± 0.6 a | −17.4 ± 1.1 b | 99.2 ± 0.1 b | |
| 30 | 89.3 ± 1.2 b | 155.2 ± 2.7 b | 0.224 ± 0.008 c | 0.233 ± 0.004 b | −17.9 ± 1.4 a | −18.6 ± 1.2 a,b | 99.4 ± 0.1 a | |
| 40 | 115.8 ± 0.8 a | 193.5 ± 2.5 a | 0.239 ± 0.005 b | 0.228 ± 0.016 b | −16.6 ± 0.6 a | −19.9 ± 0.5 a | 98.1 ± 0.1 c | |
| PGMP:GMS | Carvacrol Loading (% Mass of Lipids) | Residual Carvacrol (%) 2 | |
|---|---|---|---|
| 7 day | 30 days | ||
| 2:1 | 20 | 91.6 ± 3.9 b | 90.3 ± 3.1 ab |
| 30 | 92.7 ± 2.1 ab | 86.5 ± 2.2 bc | |
| 40 | 91.6 ± 3.0 b | 83.7 ± 3.9 cd | |
| 1:1 | 20 | 95.7 ± 3.1 a | 92.0 ± 2.8 a |
| 30 | 95.9 ± 0.7 a | 92.6 ± 1.3 a | |
| 40 | 95.0 ± 2.0 a | 87.1 ± 1.2 bc | |
| Free carvacrol 1 | 84.1 ± 1.1 c | 80.0 ± 1.8 d | |
| PGMP:GMS | MIC (mg/mL) | MBC (mg/mL) | ||
|---|---|---|---|---|
| E. coli O157: H7 | S. aureus | E. coli O157: H7 | S. aureus | |
| 2:1, bare SLNs | 0.300 | 0.300 | 0.350 | 0.350 |
| 2:1, Car-SLNs | 0.150 | 0.100 | 0.200 | 0.125 |
| 1:1, bare SLNs | 0.250 | 0.250 | 0.300 | 0.300 |
| 1:1, Car-SLNs | 0.125 | 0.100 | 0.125 | 0.100 |
| Free carvacrol | 0.250 | 0.125 | 0.250 | 0.125 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Huang, S.; Sun, X.; Han, L.; Chang, C.; Zhang, W.; Zhong, Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials 2019, 9, 1162. https://doi.org/10.3390/nano9081162
He J, Huang S, Sun X, Han L, Chang C, Zhang W, Zhong Q. Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials. 2019; 9(8):1162. https://doi.org/10.3390/nano9081162
Chicago/Turabian StyleHe, Junbo, Shuangshuang Huang, Xiaotao Sun, Lijuan Han, Chao Chang, Weinong Zhang, and Qixin Zhong. 2019. "Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity" Nanomaterials 9, no. 8: 1162. https://doi.org/10.3390/nano9081162
APA StyleHe, J., Huang, S., Sun, X., Han, L., Chang, C., Zhang, W., & Zhong, Q. (2019). Carvacrol Loaded Solid Lipid Nanoparticles of Propylene Glycol Monopalmitate and Glyceryl Monostearate: Preparation, Characterization, and Synergistic Antimicrobial Activity. Nanomaterials, 9(8), 1162. https://doi.org/10.3390/nano9081162