Stability of Hybrid Organic-Inorganic Perovskite CH3NH3PbBr3 Nanocrystals under Co-Stresses of UV Light Illumination and Temperature
Abstract
1. Introduction
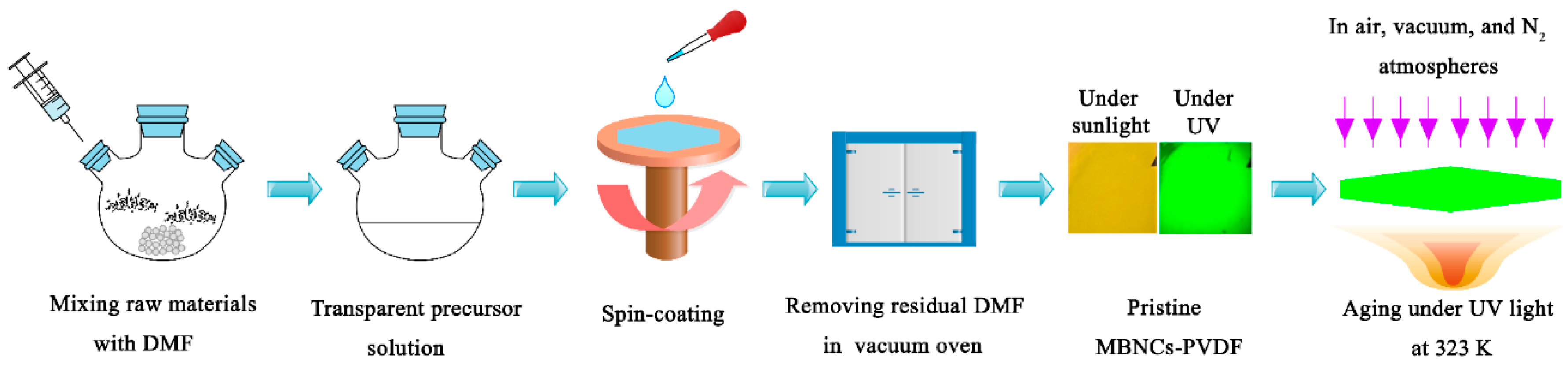
2. Materials and Methods
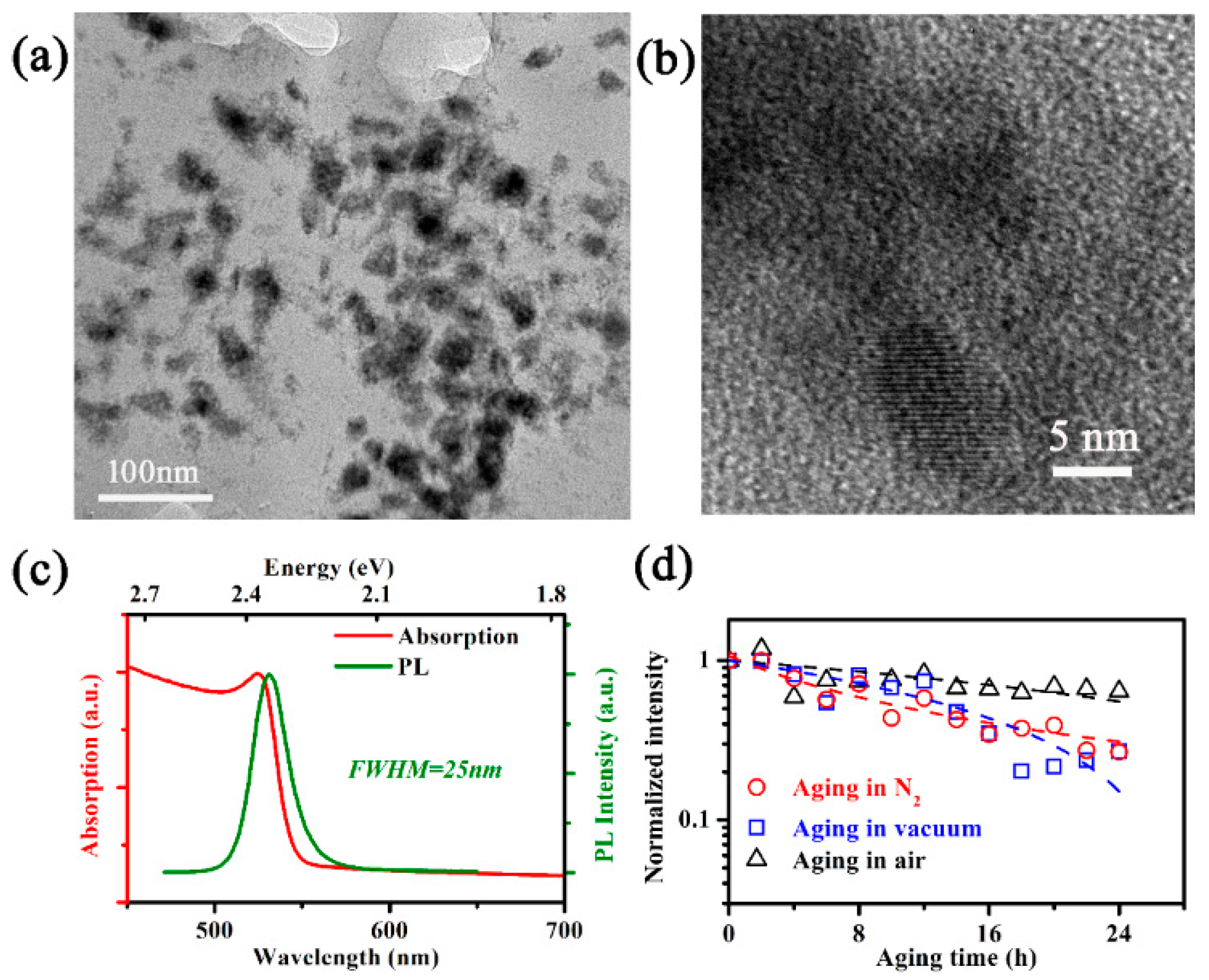
3. Results
3.1. PL Decay under Co-Stresses
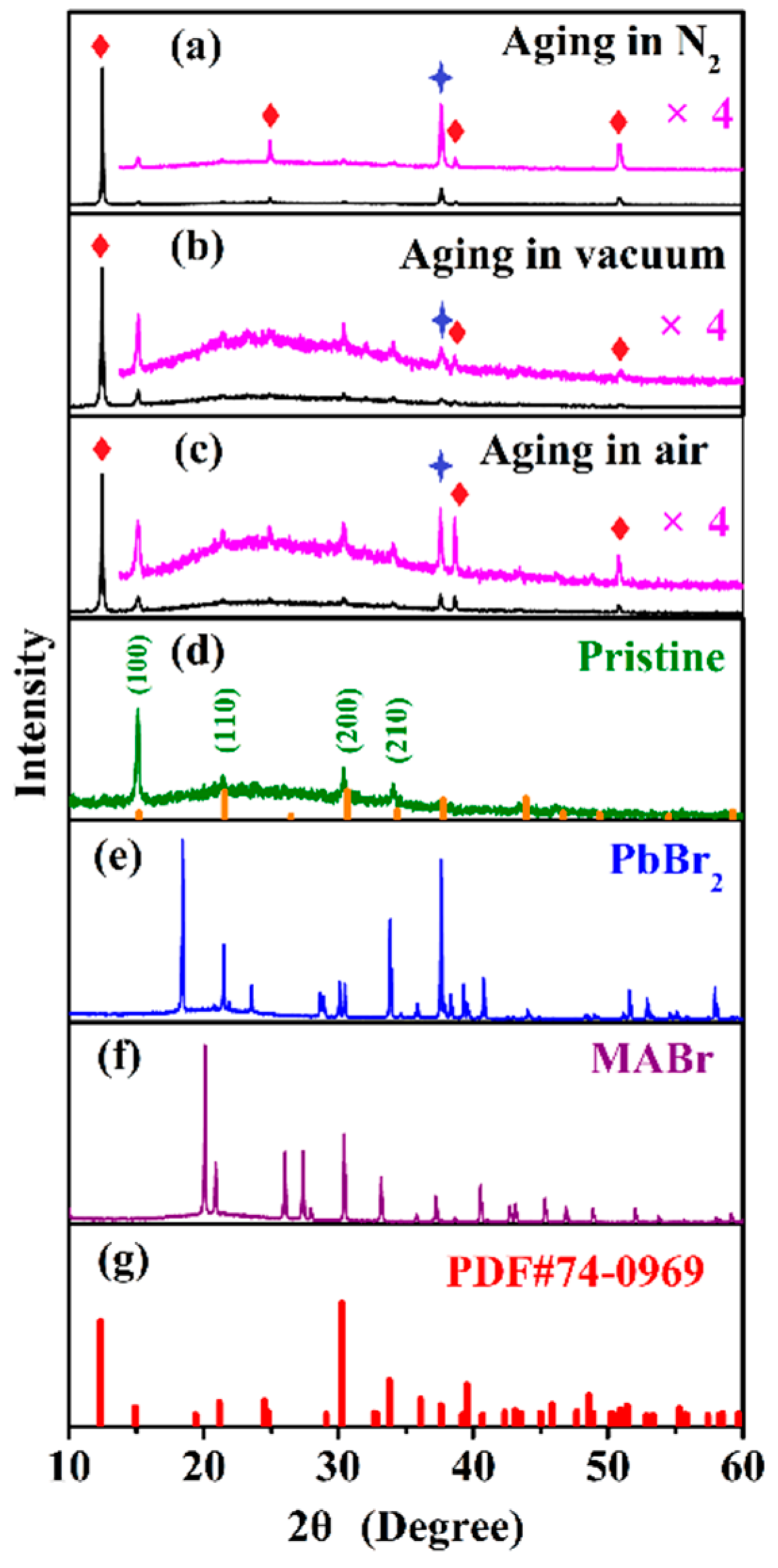
3.2. XRD Analysis
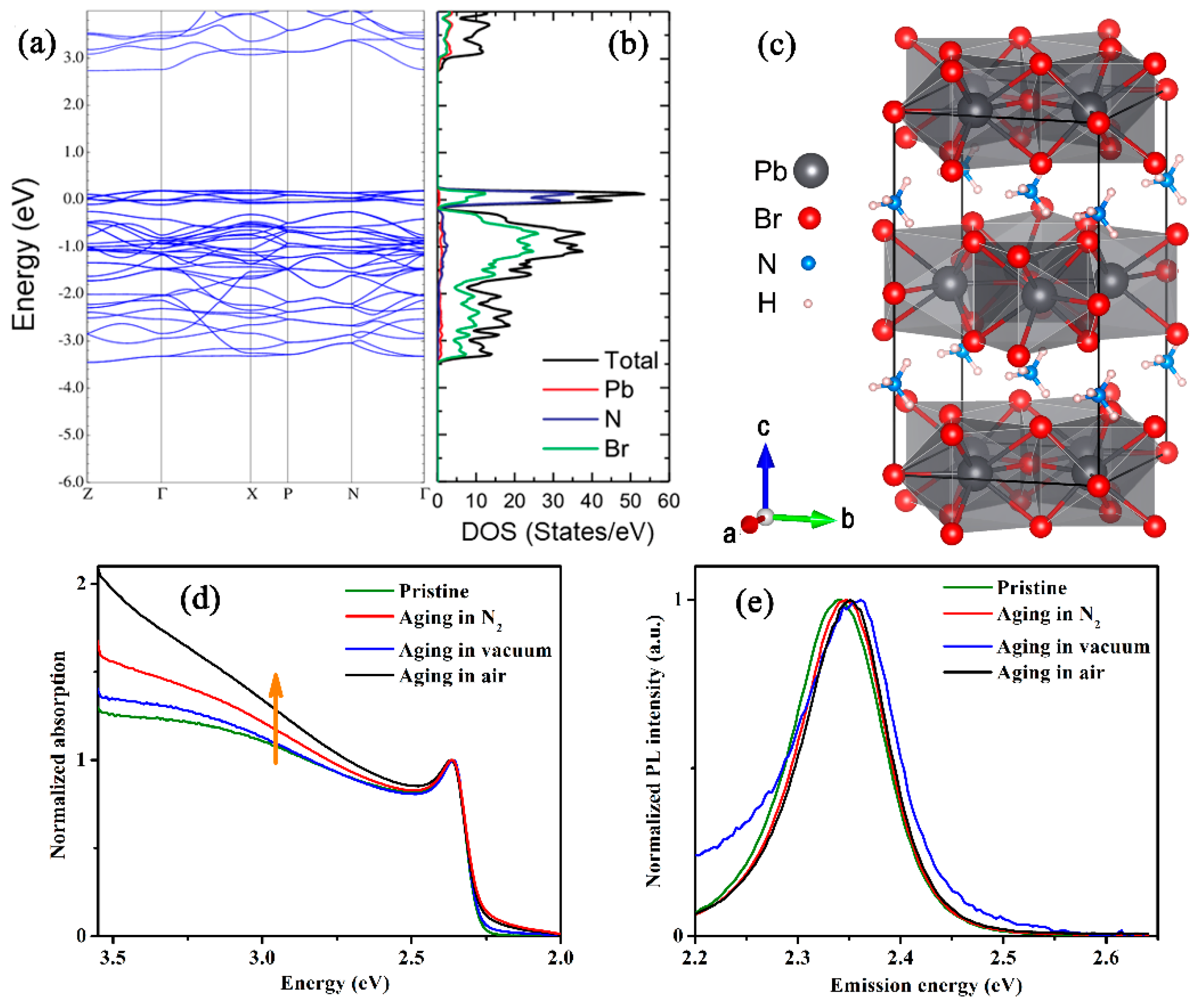
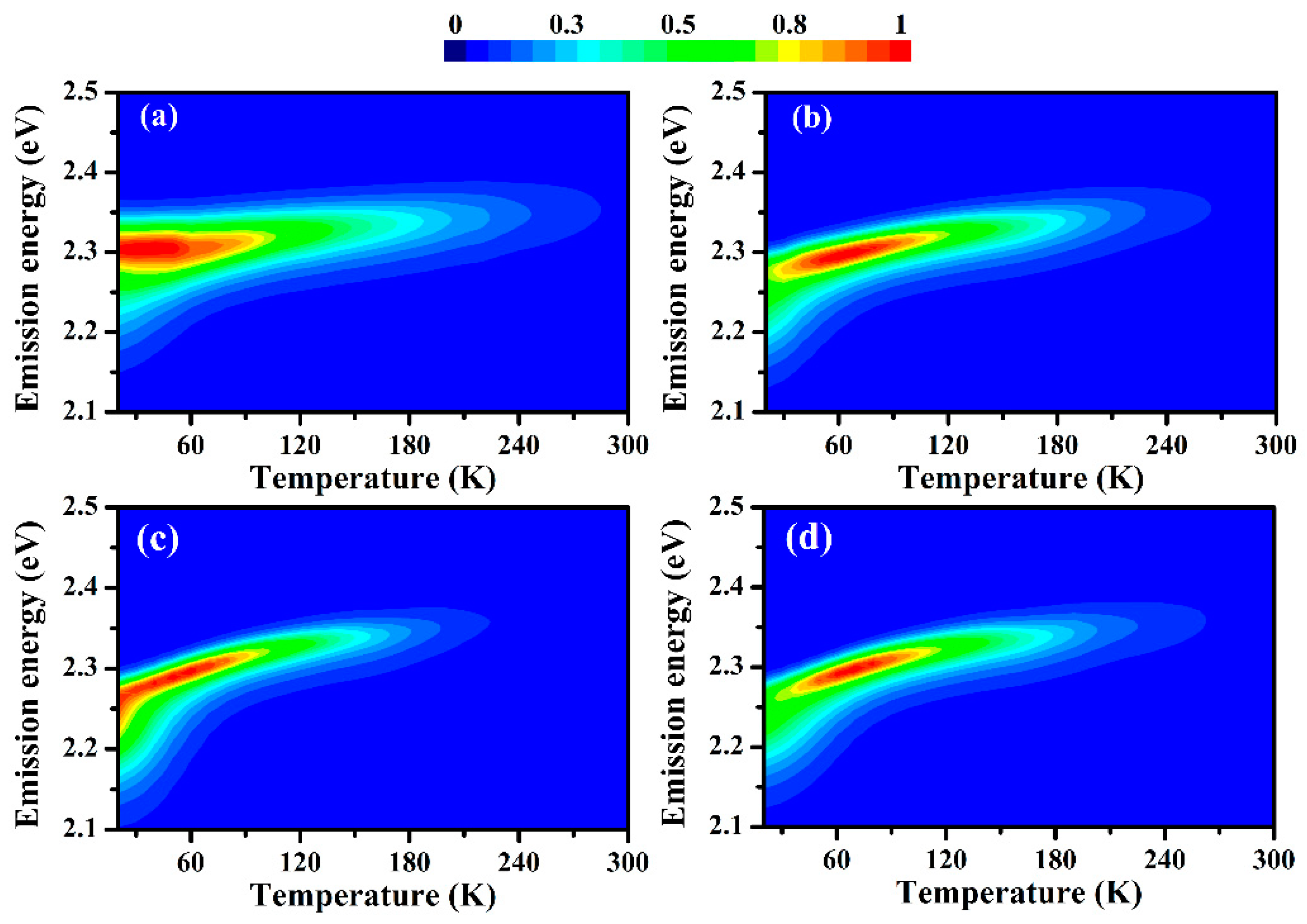
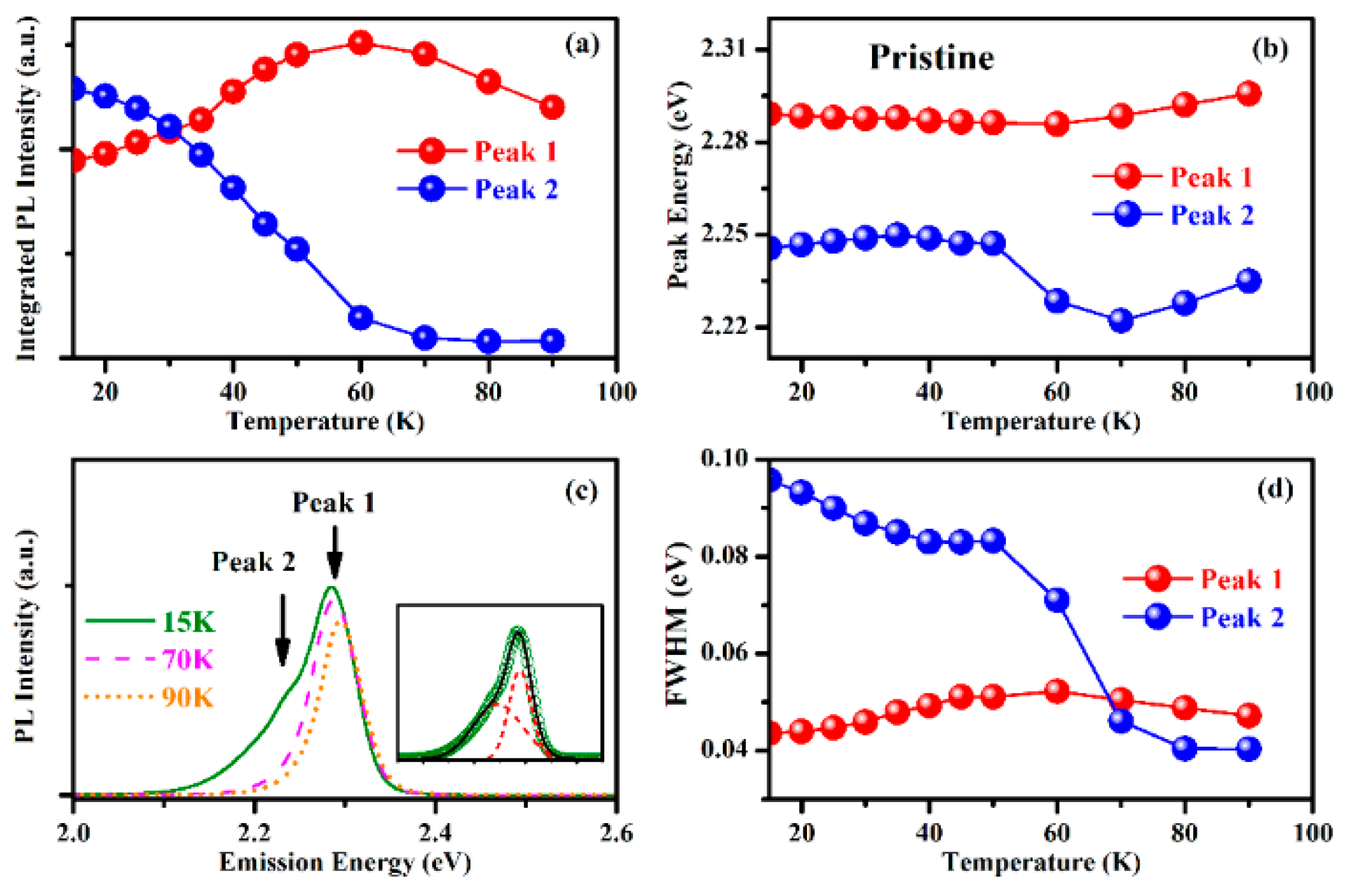
3.3. Temperature-Dependent PL Spectra
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and Potential Optoelectronic Applications of Lead Halide Perovskite Nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.C.; Bai, Z.L.; Lu, W.G.; Wang, Y.T.; Zou, B.S.; Zhong, H.Z. In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights. Adv. Mater. 2016, 28, 9163–9168. [Google Scholar] [CrossRef] [PubMed]
- Correa Baena, J.P.; Saliba, M.; Buonassisi, T.; Grätzel, M.; Abate, A.; Tress, W.; Hagfeldt, A. Promises and Challenges of Perovskite Solar Cells. Science 2017, 358, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; Rainò, G.; Kovalenko, M.V.; Manna, L. Genesis, Challenges and Opportunities for Colloidal Lead Halide Perovskite Nanocrystals. Nat. Mater. 2018, 17, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Li, G.R.; Tan, Z.K.; Di, D.W.; Lai, M.L.; Jiang, L.; Lim, J.H.; Friend, R.H.; Greenham, N.C. Efficient Light-emitting Diodes Based on Nanocrystalline Perovskite in a Dielectric Polymer Matrix. Nano Lett. 2015, 15, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Z.; Sher, C.W.; Lin, Y.; Lee, C.F.; Liang, S.J.; Lu, Y.J.; Huang, C.S.; Guo, W.J.; Kuo, H.C.; Chen, Z. Mini-LED and Micro-LED: Promising candidates for the next generation display technology. Appl. Sci. 2018, 8, 1557. [Google Scholar] [CrossRef]
- Gou, F.W.; Hsiang, E.L.; Tan, G.J.; Lan, Y.F.; Tsai, C.Y.; Wu, S.T. High performance color-converted micro-LED displays. J. Soc. Inf. Display 2019, 27, 199–206. [Google Scholar] [CrossRef]
- Han, H.V.; Lin, H.Y.; Lin, C.C.; Chong, W.C.; Li, J.R.; Chen, K.J.; Yu, P.; Chen, T.M.; Chen, H.M.; Lau, K.M. Resonant-Enhanced Full-Color Emission of Quantum-Qot-Micro LED Display Technology. Opt. Express 2015, 23, 32504–32515. [Google Scholar] [CrossRef]
- Wang, H.C.; Bao, Z.; Tsai, H.Y.; Tang, A.C.; Liu, R.S. Perovskite Quantum Dots and Their Application in Light-Emitting Diodes. Small 2017, 14, 1702433. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Shin, J.H.; Park, J.H.; Hong, S.K.; Park, S.H.; Lee, S.H.; Lee, J.H.; Kang, I.S.; Lee, K.J. Micro light-emitting diodes for display and flexible biomedical applications. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, H.; Kim, H.-S.; Liu, Y.; Yang, M.; Yue, N.; Ren, G.; Zhu, K.; Liu, S.; Park, N.-G.; et al. Multiple-Stage Structure Transformation of Organic-Inorganic Hybrid Perovskite CH3NH3PbI3. Phys. Rev. X 2016, 6, 031042. [Google Scholar] [CrossRef]
- Aristidou, N.; Eames, C.; Sanchez-Molina, I.; Bu, X.; Kosco, J.; Islam, M.S.; Haque, S.A. Fast Oxygen Diffusion and Iodide Defects Mediate Oxygen-Induced Degradation of Perovskite Solar Cells. Nat. Commun. 2017, 8, 15218. [Google Scholar] [CrossRef] [PubMed]
- Deretzis, I.; Smecca, E.; Mannino, G.; La Magna, A.; Miyasaka, T.; Alberti, A. Stability and Degradation in Hybrid Perovskites: Is the Glass Half-Empty or Half-Full? J. Phys. Chem. Lett. 2018, 9, 3000–3007. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.P.; Li, Y.Q.; Jin, T.Y.; Liu, Y.Q.; Bao, Q.Y.; O’Carroll, C.; Tang, J.X. In Situ Observation of Light Illumination-Induced Degradation in Organometal Mixed-Halide Perovskite Films. ACS Appl. Mater. Interfaces 2018, 10, 6737–6746. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, Z.; Kong, L.; Zhu, N.; Shan, A.; Li, L. Enhancing the Stability of CH3NH3PbBr3 Quantum Dots by Embedding in Silica Spheres Derived from Tetramethyl Orthosilicate in “Waterless” Toluene. J. Am. Chem. Soc. 2016, 138, 5749–5752. [Google Scholar] [CrossRef]
- Juarez-Perez, E.J.; Hawash, Z.; Raga, S.R.; Ono, L.K.; Qi, Y. Thermal Degradation of CH3NH3PbI3 Perovskite into NH3 and CH3I Gases Observed by Coupled Thermogravimetry–Mass Spectrometry Analysis. Energy Environ. Sci. 2016, 9, 3406–3410. [Google Scholar] [CrossRef]
- Ciccioli, A.; Latini, A. Thermodynamics and the Intrinsic Stability of Lead Halide Perovskites CH3NH3PbX3. J. Phys. Chem. Lett. 2018, 9, 3756–3765. [Google Scholar] [CrossRef]
- Zhong, Y.; Luna, C.A.M.; Hildner, R.; Li, C.; Huettner, S. In Situ Investigation of Light Soaking in Organolead Halide Perovskite Films. APL Mater. 2019, 7, 041114. [Google Scholar] [CrossRef]
- Alberti, A.; Deretzis, I.; Mannino, G.; Smecca, E.; Sanzaro, S.; Numata, Y.; Miyasaka, T.; La Magna, A. Revealing a Discontinuity in the Degradation Behavior of CH3NH3PbI3 during Thermal Operation. J. Phys. Chem. C 2017, 121, 13577–13585. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 1500477. [Google Scholar] [CrossRef]
- Manser, J.S.; Christians, J.A.; Kamat, P.V. Intriguing Optoelectronic Properties of Metal Halide Perovskites. Chem. Rev. 2016, 116, 12956–13008. [Google Scholar] [CrossRef]
- Blaha, P.; Schwarz, K.; Madsen, G.K.H.; Kvasnicka, D.; Luitz, L. WIEN2k, An. Augmented Plane Wave + Local Orbitals Program. for Calculating Crystal Properties; Karlheinz, S., Ed.; Technische Universität Wien: Wien, Austria, 2001. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wenger, B.; Nayak, P.K.; Wen, X.M.; Kesava, S.V.; Noel, N.K.; Snaith, H.J. Consolidation of the Optoelectronic Properties of CH3NH3PbBr3 Perovskite Single Crystals. Nat. Commun. 2017, 8, 590. [Google Scholar] [CrossRef]
- Woo, H.C.; Choi, J.W.; Shin, J.; Chin, S.H.; Ann, M.H.; Lee, C.L. Temperature-Dependent Photoluminescence of CH3NH3PbBr3 Perovskite Quantum Dots and Bulk Counterparts. J. Phys. Chem. Lett. 2018, 9, 4066–4074. [Google Scholar] [CrossRef]
- Polavarapu, L.; Nickel, B.; Feldmann, J.; Urban, A.S. Advances in Quantum-Confined Perovskite Nanocrystals for Optoelectronics. Adv. Energy Mater. 2017, 7, 1700267. [Google Scholar] [CrossRef]
- Bohn, B.J.; Tong, Y.; Gramlich, M.; Lai, M.L.; Döblinger, M.; Wang, K.; Hoye, R.L.Z.; Müller-Buschbaum, P.; Stranks, S.D.; Urban, A.S.; et al. Boosting Tunable Blue Luminescence of Halide Perovskite Nanoplatelets through Postsynthetic Surface Trap Repair. Nano Lett. 2018, 18, 5231–5238. [Google Scholar] [CrossRef]
- Powell, H.M.; Tasker, H.S. 25. The valency Angle of Bivalent Lead: The Crystal Structure of Ammonium, Rubidium, and Potassium Pentabromodiplumbites. J. Am. Chem. Soc. 1937, 0, 119–123. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. [Google Scholar] [CrossRef]
- Dursun, I.; De Bastiani, M.; Turedi, B.; Alamer, B.; Shkurenko, A.; Yin, J.; El-Zohry Ahmed, M.; Gereige, I.; AlSaggaf, A.; Mohammed Omar, F.; et al. CsPb2Br5 Single Crystals: Synthesis and Characterization. ChemSusChem 2017, 10, 3746–3749. [Google Scholar] [CrossRef]
- Wang, K.H.; Wu, L.; Li, L.; Yao, H.B.; Qian, H.S.; Yu, S.H. Large-Scale Synthesis of Highly Luminescent Perovskite-Related CsPb2Br5 Nanoplatelets and Their Fast Anion Exchange. Angew. Chem. Int. Ed. 2016, 55, 8328–8332. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X=Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Singh, S.; Cheng, L.; Panzer, F.; Narasimhan, K.L.; Graeser, A.; Gujar, T.P.; Köhler, A.; Thelakkat, M.; Huettner, S.; Kabra, D. Effect of Thermal and Structural Disorder on the Electronic Structure of Hybrid Perovskite Semiconductor CH3NH3PbI3. J. Phys. Chem. Lett. 2016, 7, 3014. [Google Scholar] [CrossRef]
- De Giorgi, M.L.; Perulli, A.; Yantara, N.; Boix, P.P.; Anni, M. Amplified Spontaneous Emission Properties of Solution Processed CsPbBr3 Perovskite Thin Films. J. Phys. Chem. C 2017, 121, 14772–14778. [Google Scholar] [CrossRef]
- Chun-Ren Ke, J.; Walton, A.S.; Lewis, D.J.; Tedstone, A.; O’Brien, P.; Thomas, A.G.; Flavell, W.R. In Situ Investigation of Degradation at Organometal Halide Perovskite Surfaces by X-ray Photoelectron Spectroscopy at Realistic Water Vapour Pressure. Chem. Commun. 2017, 53, 5231–5234. [Google Scholar] [CrossRef]
- Ruan, L.; Shen, W.; Wang, A.; Xiang, A.; Deng, Z. Alkyl-Thiol Ligand-Induced Shape- and Crystalline Phase-Controlled Synthesis of Stable Perovskite-Related CsPb2Br5 Nanocrystals at Room Temperature. J. Phys. Chem. Lett. 2017, 8, 3853–3860. [Google Scholar] [CrossRef]
- Palazon, F.; Dogan, S.; Marras, S.; Locardi, F.; Nelli, I.; Rastogi, P.; Ferretti, M.; Prato, M.; Krahne, R.; Manna, L. From CsPbBr3 Nano-Inks to Sintered CsPbBr3–CsPb2Br5 Films via Thermal Annealing: Implications on Optoelectronic Properties. J. Phys. Chem. C 2017, 121, 11956–11961. [Google Scholar] [CrossRef]
- Motti, S.G.; Gandini, M.; Barker, A.J.; Ball, J.M.; Srimath Kandada, A.R.; Petrozza, A. Photoinduced Emissive Trap States in Lead Halide Perovskite Semiconductors. ACS Energy Lett. 2016, 1, 726–730. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Mosconi, E.; De Angelis, F. Mechanism of Reversible Trap Passivation by Molecular Oxygen in Lead-Halide Perovskites. ACS Energy Lett. 2017, 2, 2794–2798. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, W.; Chen, N.; Xu, B.; Lu, Y.; Li, B.; Wu, T.; Cheng, Q.; Li, Y.; Chen, J.; Lin, Y.; et al. Stability of Hybrid Organic-Inorganic Perovskite CH3NH3PbBr3 Nanocrystals under Co-Stresses of UV Light Illumination and Temperature. Nanomaterials 2019, 9, 1158. https://doi.org/10.3390/nano9081158
Guo W, Chen N, Xu B, Lu Y, Li B, Wu T, Cheng Q, Li Y, Chen J, Lin Y, et al. Stability of Hybrid Organic-Inorganic Perovskite CH3NH3PbBr3 Nanocrystals under Co-Stresses of UV Light Illumination and Temperature. Nanomaterials. 2019; 9(8):1158. https://doi.org/10.3390/nano9081158
Chicago/Turabian StyleGuo, Weijie, Nan Chen, Binbin Xu, Yijun Lu, Bin Li, Tingzhu Wu, Qijin Cheng, Yang Li, Jin Chen, Yue Lin, and et al. 2019. "Stability of Hybrid Organic-Inorganic Perovskite CH3NH3PbBr3 Nanocrystals under Co-Stresses of UV Light Illumination and Temperature" Nanomaterials 9, no. 8: 1158. https://doi.org/10.3390/nano9081158
APA StyleGuo, W., Chen, N., Xu, B., Lu, Y., Li, B., Wu, T., Cheng, Q., Li, Y., Chen, J., Lin, Y., & Chen, Z. (2019). Stability of Hybrid Organic-Inorganic Perovskite CH3NH3PbBr3 Nanocrystals under Co-Stresses of UV Light Illumination and Temperature. Nanomaterials, 9(8), 1158. https://doi.org/10.3390/nano9081158





