Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Characterization Methods
2.3. Evaluating Catalytic Activity
3. Results
3.1. Characterization of the Supports
3.1.1. Structure and Texture Properties of the Supports
3.1.2. Identifying the Existential States of Mg Species
3.2. Characterization of the Catalysts
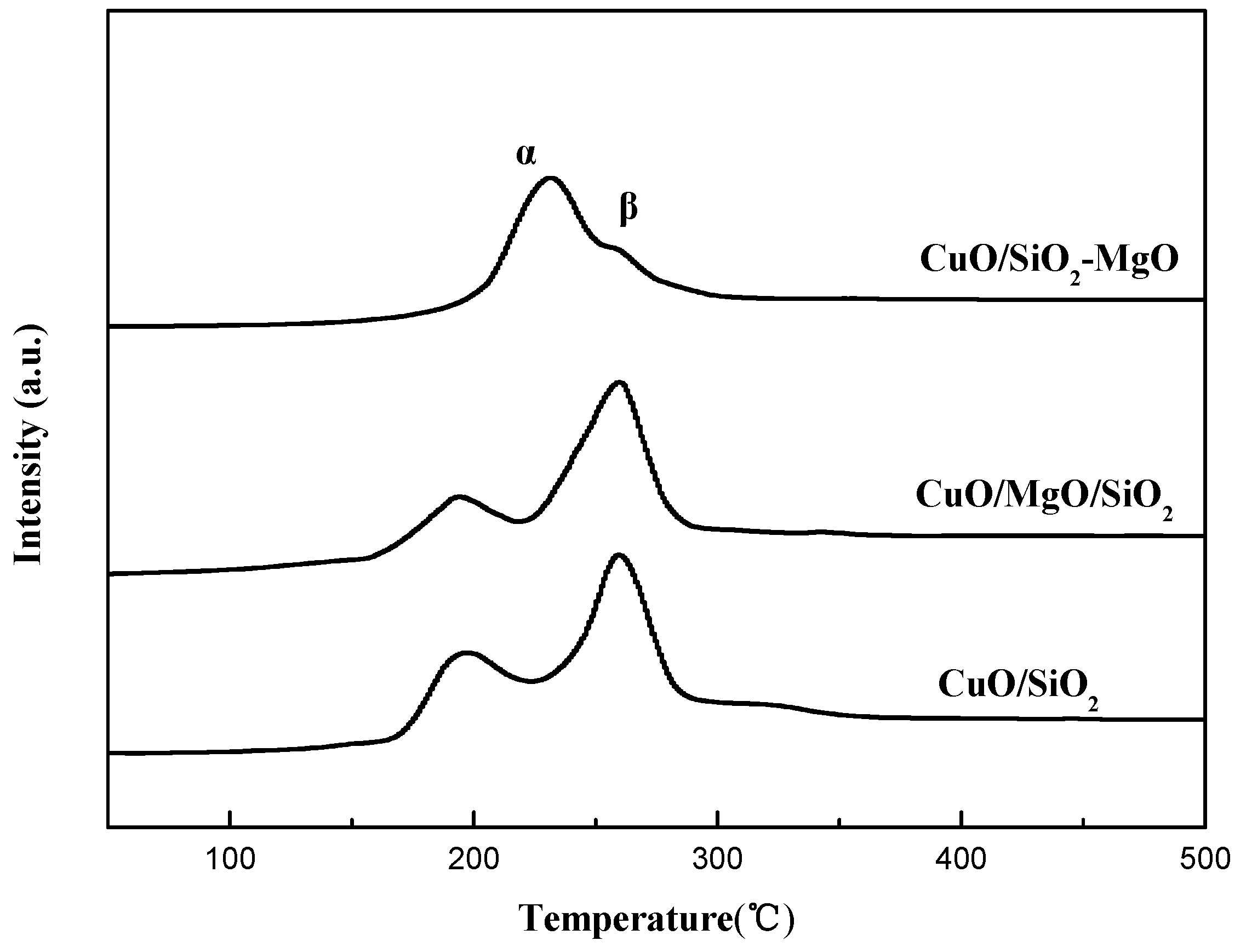
3.2.1. Structure and Texture Properties of the Catalysts
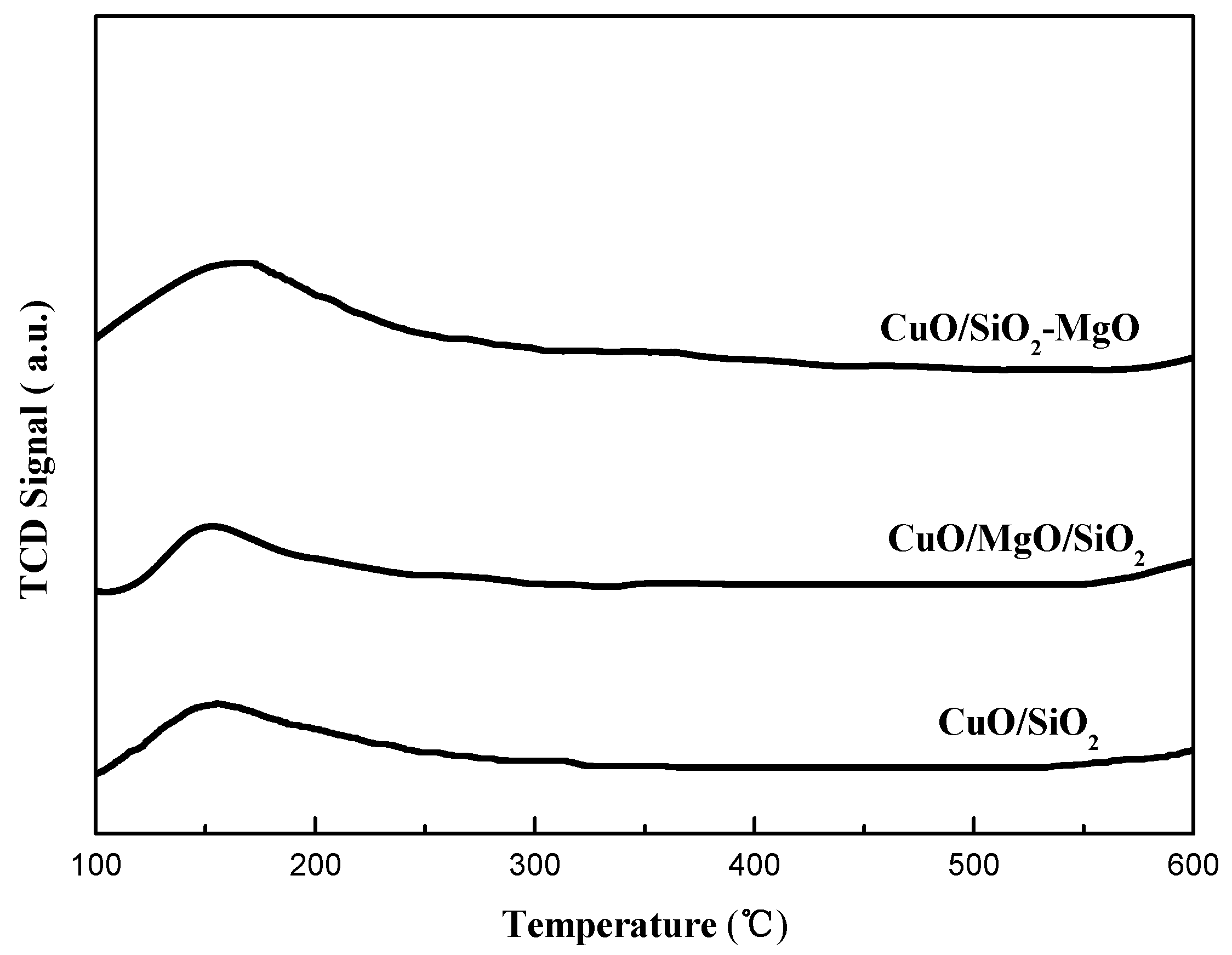
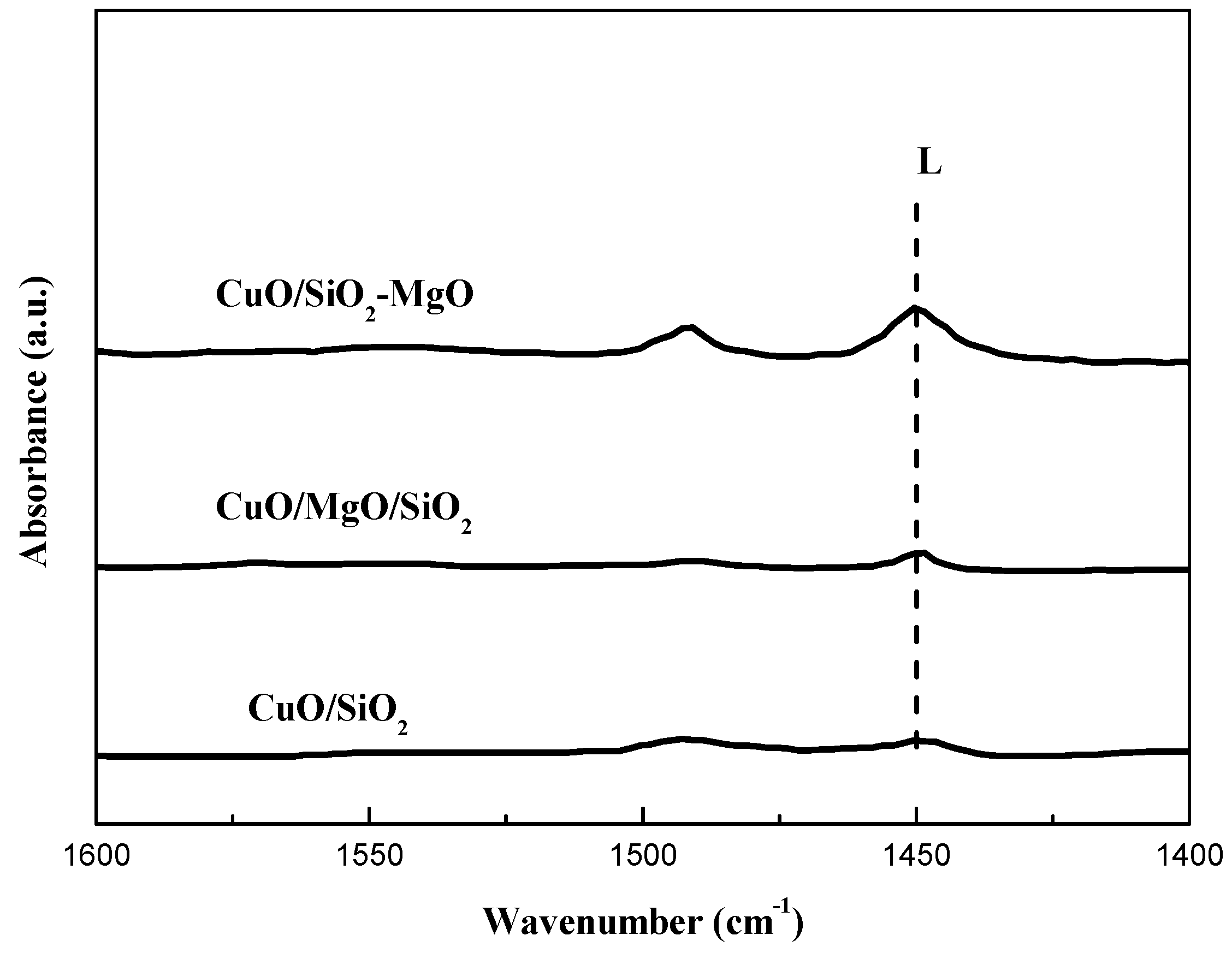
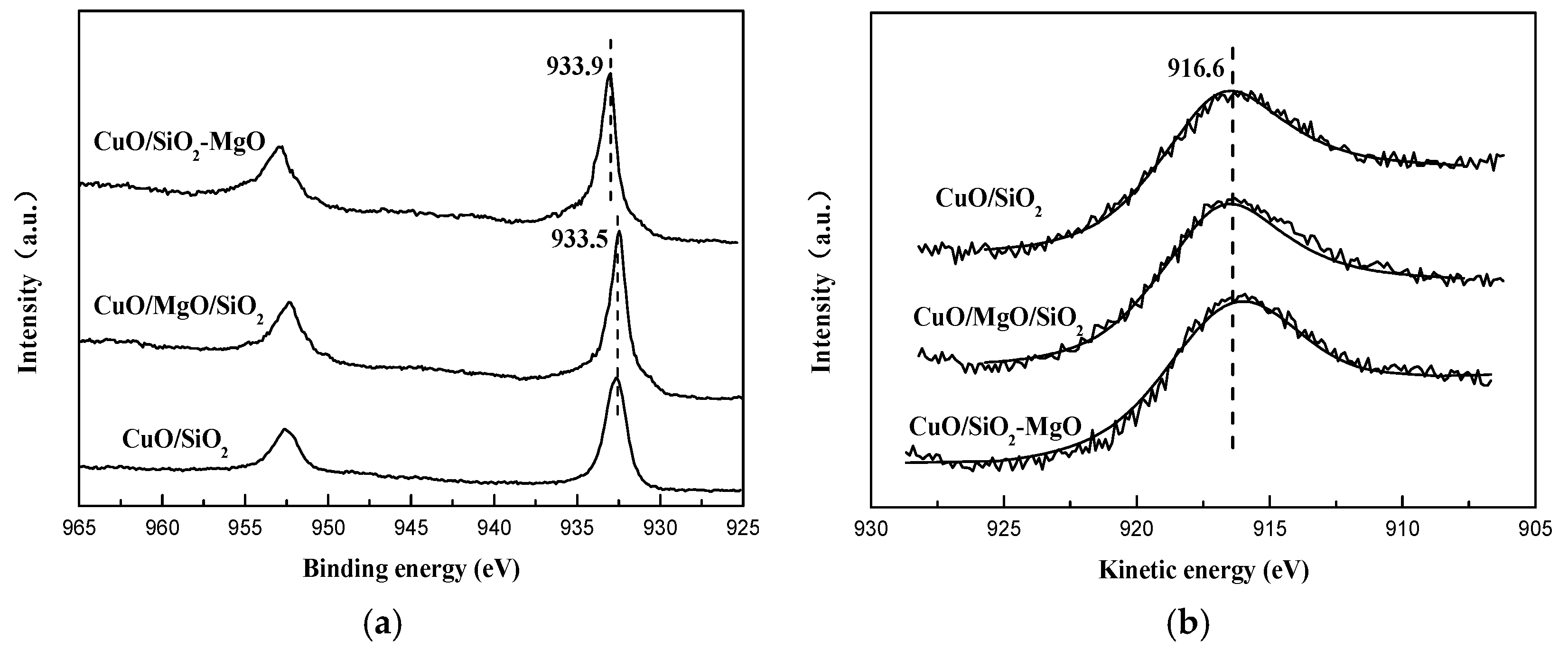
3.2.2. Surface Properties of the Catalysts
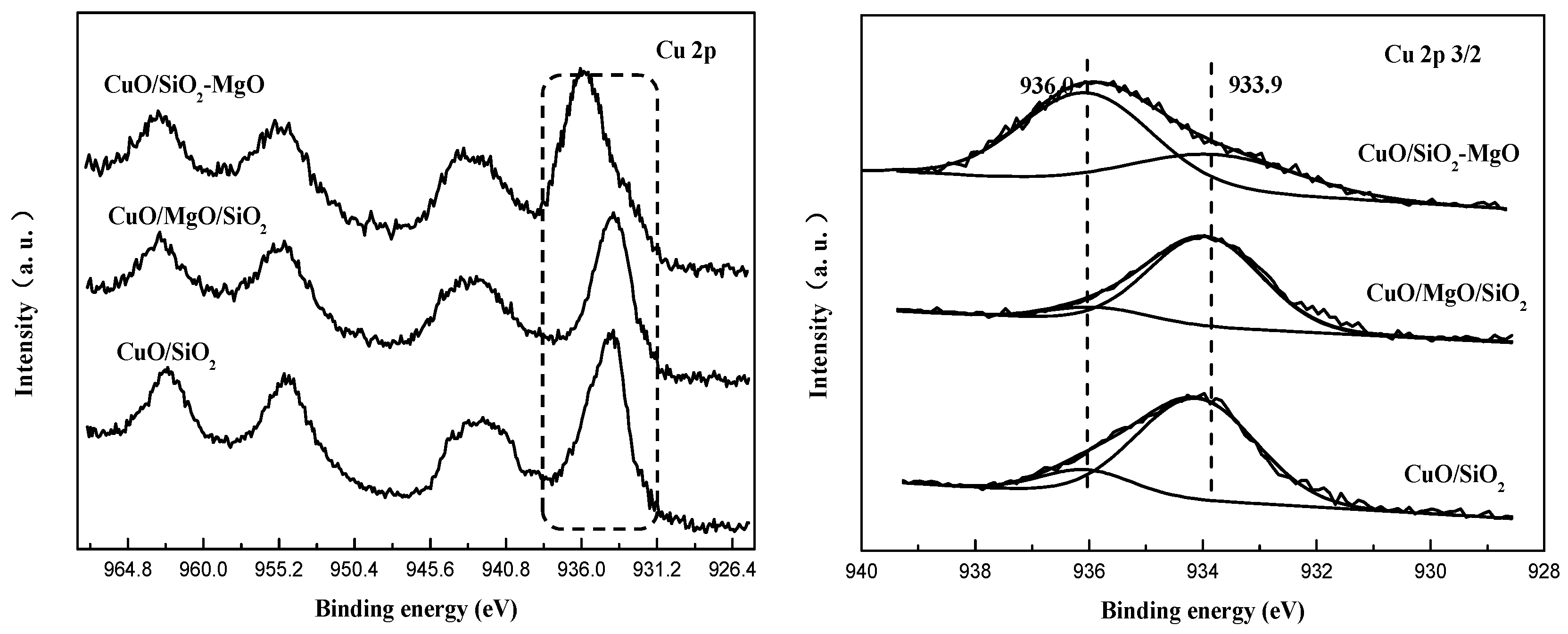
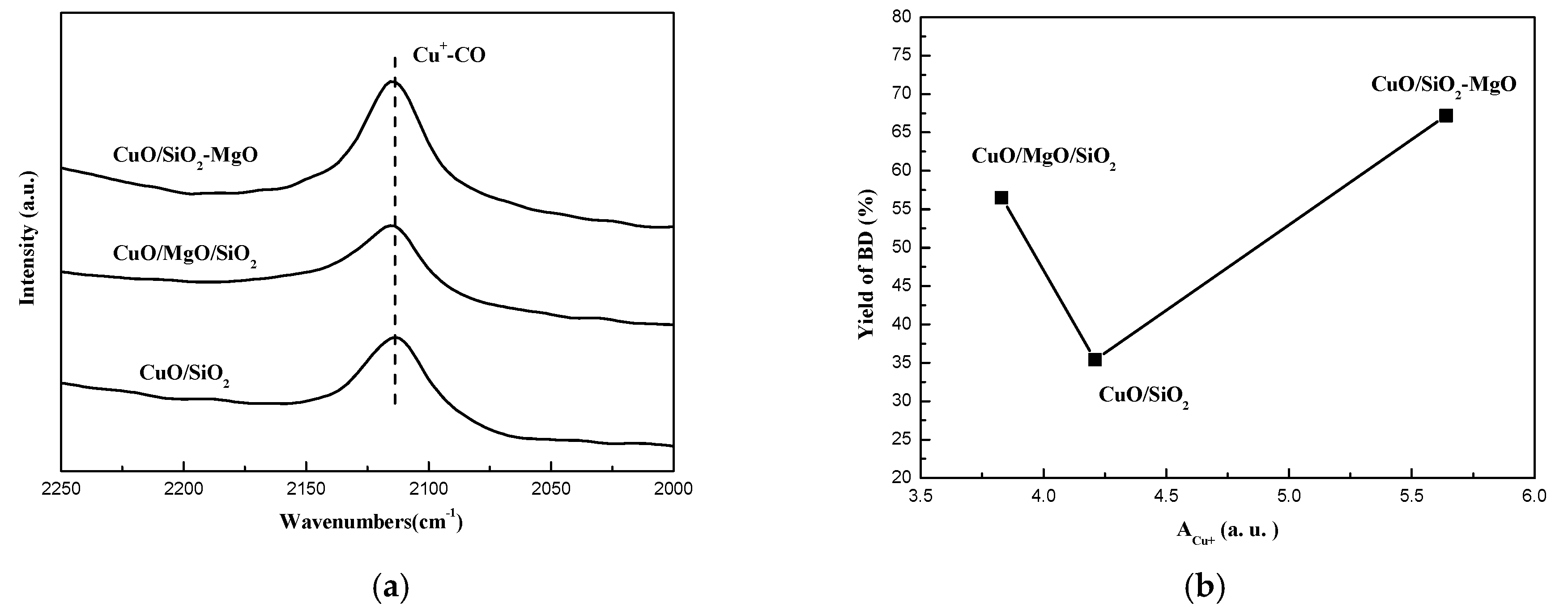
3.2.3. Identifying the Cu-Support Interaction
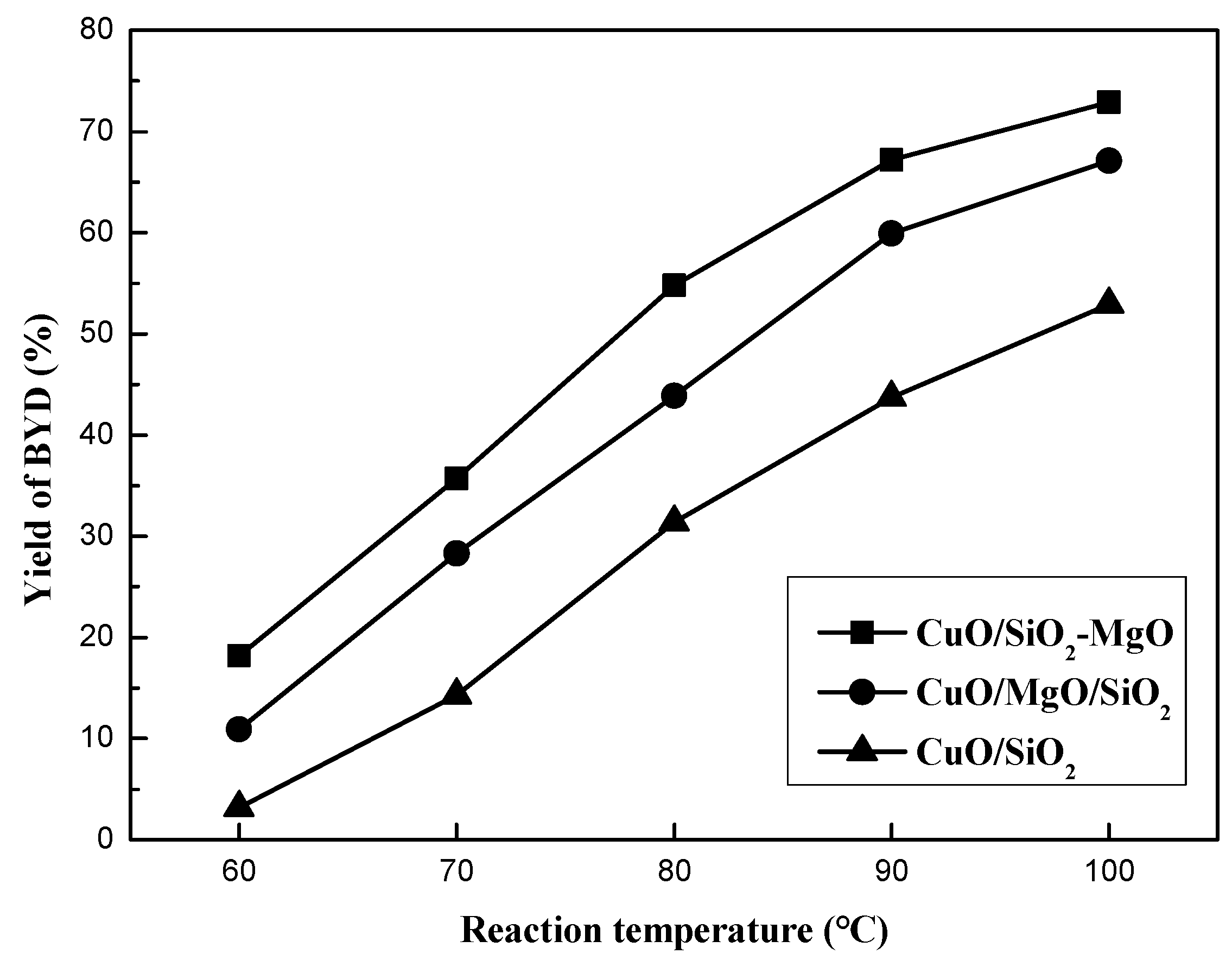
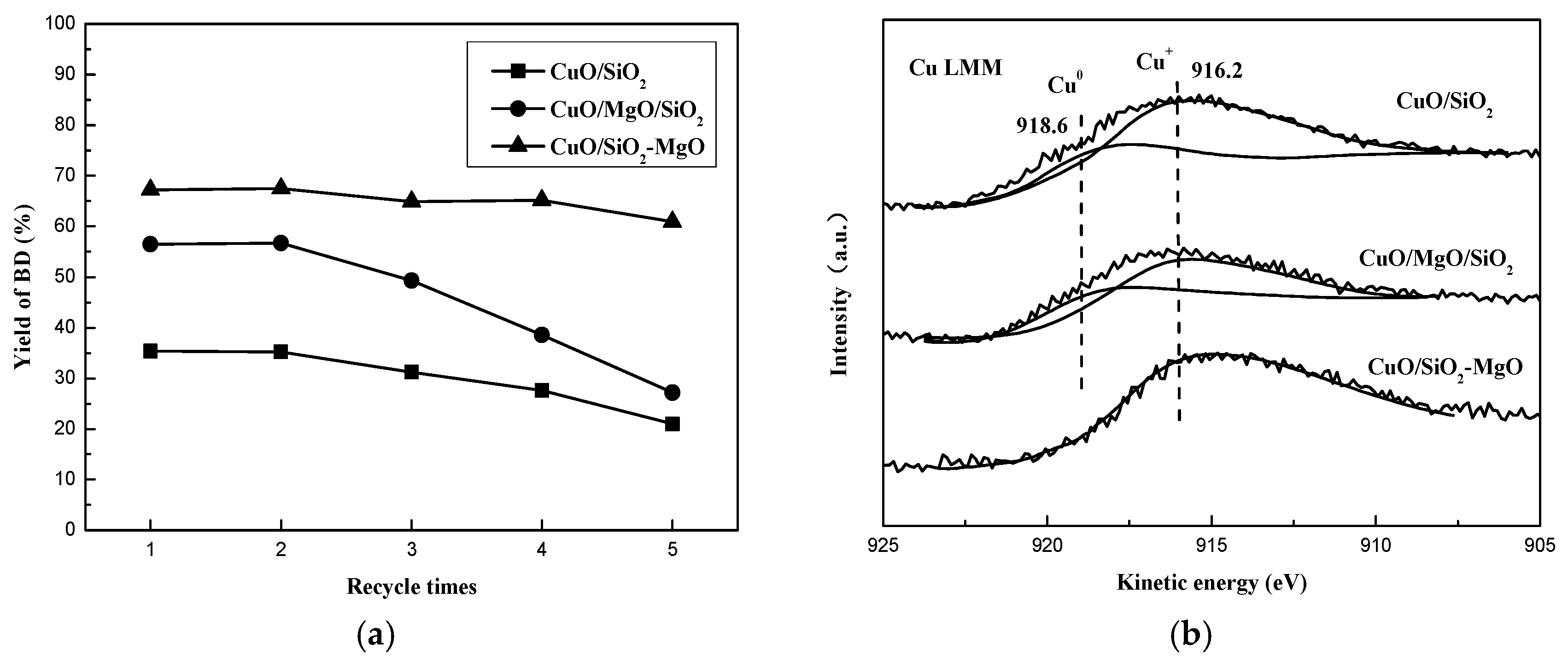
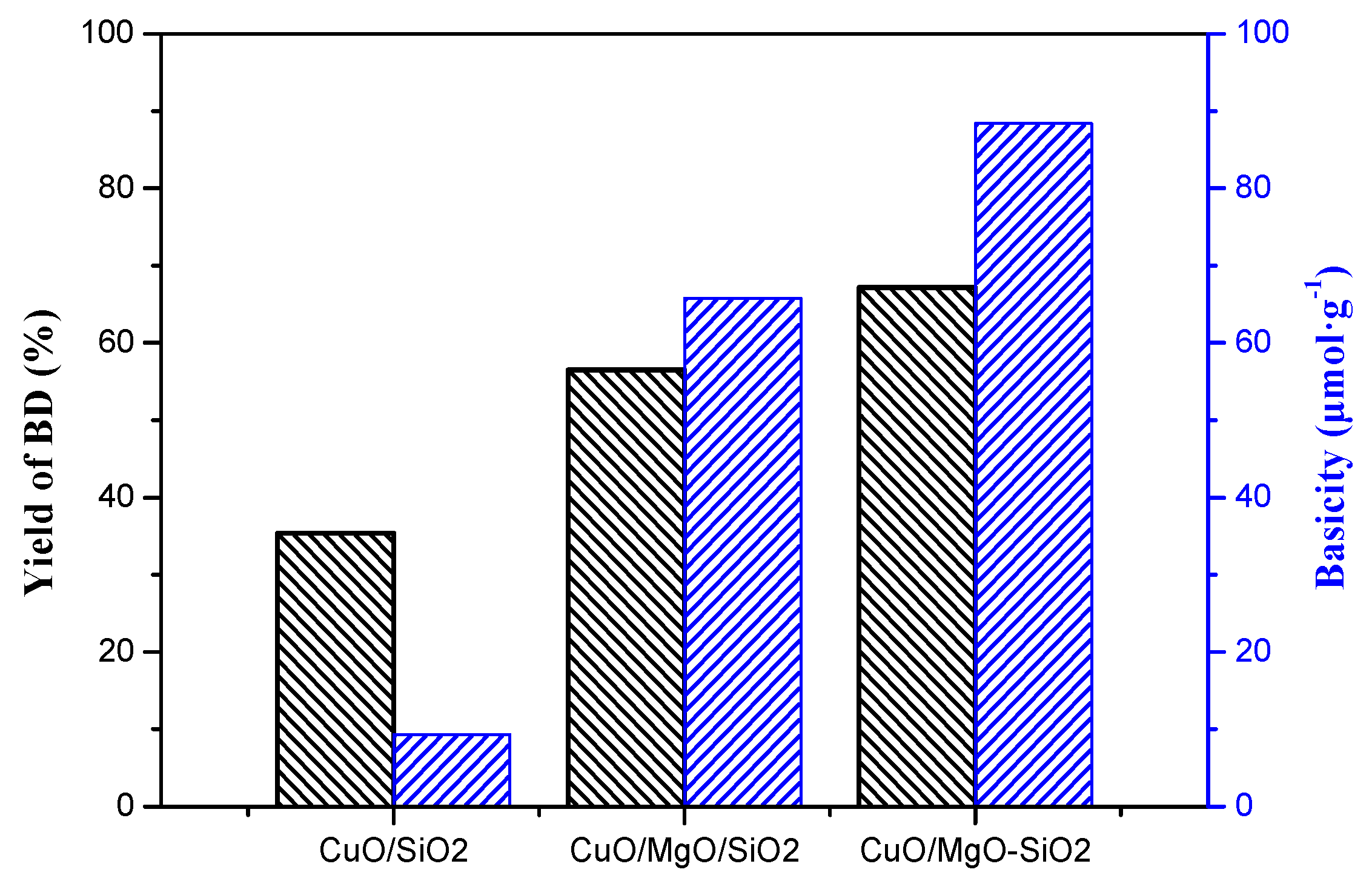
3.3. Catalytic Performance
4. Discussion
5. Conclusions
- (1)
- The strong interaction derived from the formation of Si-O-Mg structures in SiO2-MgO support conduced to altering the dispersion and the reduction behavior of the copper species. The highly dispersed and stablized Cu+ species formed on the SiO2-MgO support are responsible for the superior catalytic stability.
- (2)
- The incorporation of Mg species also altered the basic property on the surface of the catalysts. In this catalytic system, the medium-strong basic sites play a key role in ≡C-H activation due to their deprotonation ability, showing a strongly synergistic effect with Cu+ species, resulting in a dramatical improvement in ethynylation activity.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanielyan, S.; Schmidt, S.; Marin, N.; Alvez, G.; Augustine, R. Selective hydrogenation of 2-butyne-1,4-diol to 1,4-butanediol over particulate Raney® nickel catalysts. Top. Catal. 2010, 53, 1145–1149. [Google Scholar] [CrossRef]
- Nadgeri, J.M.; Telkar, M.M.; Rode, C.V. Hydrogenation activity and selectivity behavior of supported palladium nanoparticles. Catal. Commun. 2008, 9, 441–446. [Google Scholar] [CrossRef]
- Trotuş, I.T.; Zimmermann, T.; Schuüth, F. Catalytic reactions of acetylene: A feedstock for the chemical industry revisited. Chem. Rev. 2014, 114, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Y.; Ikushima, Y.; Arai, M. Hydrogenation of 2-butyne-1,4-diol in supercritical carbon dioxide promoted by stainless steel reactor wall. Catal. Today 2004, 93–95, 439–443. [Google Scholar] [CrossRef]
- Li, C.; Zhang, M.M.; Di, X.; Yin, D.D.; Li, W.Z.; Liang, C.H. One-step synthesis of Pt@ZIF-8 catalyst for the selective hydrogenation of 1,4-butynediol to 1,4-butenediol. Chin. J. Catal. 2016, 37, 1555–1561. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, H.T.; Gao, C.G.; Zhao, Y.X. Heterogeneous CaO-ZrO2 acid–base bifunctional catalysts for vapor-phase selective dehydration of 1,4-butanediol to 3-buten-1-ol. Appl. Catal. A Gen. 2013, 466, 233–239. [Google Scholar] [CrossRef]
- Li, X.; Cui, Y.Y.; Yang, X.L.; Dai, W.L.; Fan, K.N. Highly efficient and stable Au/Mn2O3 catalyst for oxidative cyclization of 1,4-butanediol to γ-butyrolactone. Appl. Catal. A Gen. 2013, 458, 63–70. [Google Scholar] [CrossRef]
- Jun, X.; Guo, B.H. Poly(butylene succinate) and its copolymers: Research, development and industrialization. J. Biotechnol. 2010, 5, 1149–1163. [Google Scholar]
- Li, H.T.; Zhao, Y.X.; Gao, C.G.; Wang, Y.Z.; Sun, Z.J.; Liang, X.Y. Study on deactivation of Ni/Al2O3 catalyst for liquid phase hydrogenation of crude 1,4-butanediol aqueous solution. Chem. Eng. J. 2012, 181/182, 501–507. [Google Scholar] [CrossRef]
- Wang, Z.P.; Ban, L.J.; Meng, P.F.; Li, H.T.; Zhao, Y.X. Ethynylation of formaldehyde over binary Cu-based catalysts: Study on synergistic effect between Cu+ species and acid/base sites. Nanomaterials 2019, 9, 1038. [Google Scholar] [CrossRef]
- Wang, Z.P.; Niu, Z.Z.; Ban, L.J.; Hao, Q.A.; Zhang, H.X.; Li, H.T.; Zhao, Y.X. Formaldehyde ethynylation reaction over Cu2O supported on TiO2 with different phases. Chem. J. Chin. Univ. 2019, 40, 334–341. [Google Scholar]
- Wang, Z.P.; Niu, Z.Z.; Hao, Q.A.; Ban, L.J.; Li, H.T.; Zhao, Y.X.; Jiang, Z. Enhancing the ethynylation performance of CuO-Bi2O3 nanocatalysts by tuning Cu-Bi interactions and phase structures. Catalysts 2019, 9, 35. [Google Scholar] [CrossRef]
- Li, H.T.; Ban, L.J.; Wang, Z.P.; Meng, P.F.; Zhang, Y.; Wu, R.F.; Zhao, Y.X. Regulation of Cu species in CuO/SiO2 and its structural evolution in ethynylation reaction. Nanomaterials 2019, 9, 842. [Google Scholar] [CrossRef]
- Bukur, D.B.; Okabe, K.; Rosynek, M.P.; Li, C.P.; Wang, D.J.; Rao, K.R.P.M.; Huffman, G.P. Activation studies with a precipitated iron catalyst for Fischer-Tropsch synthesis. J. Catal. 1995, 155, 353–365. [Google Scholar] [CrossRef]
- Gao, F.F.; Wang, H.; Qing, M.; Yang, Y.; Li, Y.W. Controlling the phase transformations and performance of iron-based catalysts in the Fischer-Tropsch synthesis. Chin. J. Catal. 2013, 34, 1312–1325. [Google Scholar] [CrossRef]
- Luo, M.; Li, H.T.; Ma, Z.Q.; Wang, J.J.; Zhao, Y.X. Researches on activation process of CuO-Bi2O3/SiO2-MgO catalyst in formaldehyde ethynylation reaction. Ind. Catal. (China) 2014, 22, 363–368. [Google Scholar]
- Haas, T.; Jaeger, B.; Weber, R.; Mitchell, S.F.; King, C.F. New diol process: 1,3-propanediol and 1,4-butanediol. Appl. Catal. A Gen. 2005, 280, 83–88. [Google Scholar] [CrossRef]
- Zak, D.J. Butynediol Production. U.S. Patent 4,085,151, 18 April 1978. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol. U.S. Patent 4,584,418, 22 April 1986. [Google Scholar]
- Reppe, W.; Steinhofer, A.; Spaenig, H. Production of Alkinols. U.S. Patent 2,300,969, 3 November 1942. [Google Scholar]
- Fremont, J.M. Preparation of Butynediol Using Bismuth Modified Spheroidal Malachite. U.S. Patent 4,127,734, 28 November 1978. [Google Scholar]
- Kirchner, R.J. Ethynylation of Formaldehyde. U.S. Patent 3,560,576, 2 February 1971. [Google Scholar]
- Bonrath, W.; Bernd, E.; Reinhard, K.; Schneider, M. Ethynylation Process. U.S. Patent 6,949,685, 27 September 2005. [Google Scholar]
- Hort, E.V.; Piscataway, N.J. Ethynylation Catalyst and Method of Production Alkynols by Low Pressure Reactions. U.S. Patent 3,920,759, 18 November 1975. [Google Scholar]
- Zheng, Y.; Sun, Z.J.; Wang, Y.Z.; Li, H.T.; Wang, S.A.; Luo, M.; Zhao, J.L.; Zhao, Y.X. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance. J. Mol. Catal. (China) 2012, 26, 233–238. [Google Scholar]
- Di Cosimo, J.I.; Díez, V.K.; Ferretti, C.; Apesteguía, C.R. Basic catalysis on MgO: Generation, characterization and catalytic properties of active sites. Catalysis 2014, 26, 1–28. [Google Scholar]
- Coluccia, S.; Marchese, L. Surface sites of microcrystals: Coordination and reactivity. Catal. Today 1998, 41, 229–238. [Google Scholar] [CrossRef]
- Yu, H.M.; Wang, X.X.; Shu, Z.; Fujii, M.; Song, C.S. Al2O3 and CeO2-promoted MgO sorbents for CO2 capture at moderate temperatures. Front. Chem. Sci. Eng. 2018, 12, 83–93. [Google Scholar] [CrossRef]
- Huang, X.X.; Men, Y.; Wang, J.G.; An, W.; Wang, Y.Q. Highly active and selective binary MgO–SiO2 catalysts for the production of 1,3-butadiene from ethanol. Catal. Sci. Technol. 2017, 7, 168–180. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal. Sci. Technol. 2015, 5, 2869–2879. [Google Scholar] [CrossRef]
- Angelici, C.; Velthoen, M.E.Z.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Effect of Preparation Method and CuO Promotion in the Conversion of Ethanol into 1,3-Butadiene over SiO2–MgO Catalysts. ChemSusChem 2014, 7, 2505–2515. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhu, Y.L.; Ding, G.Q.; Zhu, S.H.; Zheng, H.Y.; Li, Y.W. Highly selective synthesis of ethylene glycol and ethanol via hydrogenation of dimethyl oxalate on Cu catalysts: Influence of support. Appl. Catal. A Gen. 2013, 468, 296–304. [Google Scholar] [CrossRef]
- Wehrli, J.T.; Wainwright, M.S.; Trimm, D.L.; Cant, N.W. Hydrogenolysis of diethyl oxalate over copper-based catalysts. Appl. Catal. A Gen. 1992, 86, 101–114. [Google Scholar]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B Environ. 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Li, S.X.; Men, Y.; Wang, J.G.; Wang, X.F.; Ji, F.; Chai, S.S.; Song, Q.L. Morphological control of inverted MgO-SiO2 composite catalysts for effcient conversion of ethanol to 1,3-butadiene. Appl. Catal. A Gen. 2019, 577, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.W.; Dai, Y.J.; Liu, J.; Xu, Y.B. Hydration evolution of MgO-SiO2 slurries in the presence of sodium metasilicate. Ceram. Int. 2018, 44, 6626–6633. [Google Scholar] [CrossRef]
- Martini, F.; Tonelli, M.; Geppi, M.; Ridi, F.; Borsacchi, S.; Calucci, L. Hydration of MgO/SiO2 and Portland cement mixtures: A structural investigation of the hydrated phases by means of X-ray diffraction and solid state NMR spectroscopy. Cem. Concr. Res. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Fujita, S.; Segawa, S.; Kawashima, K.; Nie, X.; Erata, T.; Arai, M. One-pot room-temperature synthesis of Mg containing MCM-41 mesoporous silica for aldol reactions. J. Mater. Sci. Technol. 2018, 34, 2521–2528. [Google Scholar] [CrossRef]
- Díaz, G.; Pérez-Hernández, R.; Gómez-Cortés, A.; Benaissa, M.; Mariscal, R.; Fierro, J.L.G. CuO-SiO2 Sol-Gel catalysts: Characterization and catalytic properties for NO reduction. J. Catal. 1999, 187, 1–14. [Google Scholar] [CrossRef]
- Toupance, T.; Kermarec, M.; Lambert, J.-F.; Louis, C. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption. J. Phys. Chem. B 2002, 106, 2277–2286. [Google Scholar] [CrossRef]
- Karmakar, B.; De, G.; Ganguli, D. Dense silica microspheres from organic and inorganic acid hydrolysis of TEOS. J. Non-Cryst. Solids 2000, 272, 119–126. [Google Scholar] [CrossRef]
- Coluccia, S.; Tench, A.J.; Segall, R.L. Surface structure and surface states in magnesium oxide powders. J. Chem. Soc. Faraday Trans. 1979, 75, 1769–1779. [Google Scholar] [CrossRef]
- Coluccia, S.; Barton, A.; Tench, A.J. Reactivity of low-coordination sites on the surface of magnesium oxide. J. Chem. Soc. Faraday Trans. 1981, 77, 2203–2207. [Google Scholar] [CrossRef]
- Kvisle, S.; Aguero, A.; Sneeden, R.P.A. Transformation of ethanol into 1,3-butadiene over magnesium oxide/silica catalysts. Appl. Catal. 1988, 43, 117–131. [Google Scholar] [CrossRef]
- Scotti, N.; Dangate, M.; Gervasini, A.; Evangelisti, C.; Ravasio, N.; Zaccheria, F. Unraveling the role of low coordination sites in a Cu metal nanoparticles: A step toward the selective synthesis of second generation biofuels. ACS Catal. 2014, 4, 2818–2826. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.L.; Ding, G.Q.; Zheng, H.Y.; Li, Y.W. Modification of the supported Cu/SiO2 catalyst by alkaline earth metals in the selective conversion of 1,4-butanediol to γ-butyrolactione. Appl. Catal. A Gen. 2012, 443–444, 191–201. [Google Scholar] [CrossRef]
- Chakraborty, B.; Viswanathan, B. Surface acidity of MCM-41 by in situ IR studies of pyridine adsorption. Catal. Today 1999, 49, 253–260. [Google Scholar] [CrossRef]
- Thomas, C.L. Chemistry of cracking catalysts. Ind. Eng. Chem. 1949, 41, 2564–2573. [Google Scholar] [CrossRef]
- Angelici, C.; Meirer, F.; Eerden, A.M.J.; Schaink, H.L.; Goryachev, A.; Hofmann, J.P.; Hensen, E.J.M.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Ex situ and operando studies on the role of copper in Cu-promoted SiO2-MgO catalysts for the Lebedev ethanol-to butadinene process. ACS Catal. 2015, 5, 6005–6015. [Google Scholar] [CrossRef]
- Meng, X.J.; Yuan, L.J.; Guo, H.Q.; Hou, B.; Chen, C.B.; Sun, D.K.; Wang, J.G.; Li, D.B. Carbonylation of methanol to methyl acetate over Cu/TiO2-SiO2 catalysts: Influence of copper precursors. Mol. Catal. 2018, 456, 1–9. [Google Scholar] [CrossRef]
- Shang, H.H.; Zhang, X.M.; Xu, J.; Han, Y.F. Effects of preparation methods on the activity of CuO/CeO2 catalysts for CO oxidation. Front. Chem. Sci. Eng. 2017, 11, 603–612. [Google Scholar] [CrossRef]
- Wang, Z.L.; Liu, Q.S.; Yu, J.F.; Wu, T.H.; Wang, G.J. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and sol–gel methods. Appl. Catal. A Gen. 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Li, B.T.; Luo, X.; Huang, J.; Wang, X.J.; Liang, Z.X. One-pot synthesis of ordered mesoporous Cu-KIT-6 and its improved catalytic behavior for the epoxidation of styrene: Effects of the pH value of the initial gel. Chin. J. Catal. 2017, 38, 518–528. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.Y.; Cui, Y.Y.; Yang, X.L.; Dai, W.L.; Fan, K.N. Enhanced catalytic performance for SiO2-TiO2 binary oxide supported Cu-based catalyst in the hydrogenation of dimethyloxalate. Appl. Catal. A Gen. 2013, 458, 82–89. [Google Scholar] [CrossRef]
- Hornes, A.; Bera, P.; Camara, A.L.; Gamarra, D.; Munuera, G.; Martinez-Arias, A. CO-TPR-DRIFTS-MS in situ study of CuO/Ce1−xTbxO2−y (x = 0, 0.2 and 0.5) catalysts: Support effects on redox properties and CO oxidation catalysis. J. Catal. 2009, 268, 367–375. [Google Scholar] [CrossRef]
- Manzoli, M.; Monte, R.D.; Boccuzzi, F.; Coluccia, S.; Kašpar, J. CO oxidation over CuOx-CeO2-ZrO2 catalysts: Transient behaviour and role of copper clusters in contact with ceria. Appl. Catal. B Environ. 2005, 61, 192–205. [Google Scholar] [CrossRef]
- Boccuzzi, F.; Ghiotti, G.; Chiorino, A. Surface reactions of CO on a metal-semiconductor system: Cu/ZnO. Surf. Sci. 1985, 162, 361–367. [Google Scholar] [CrossRef]
- Scarano, D.; Bordiga, S.; Lamberti, C.; Spoto, G.; Ricchiardi, G.; Zecchina, A.; Otero Areán, C. FTIR study of the interaction of CO with pure and silica-supported copper(I) oxide. Surf. Sci. 1998, 411, 272–285. [Google Scholar] [CrossRef]
- Guo, X.L.; Zhou, R.X. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Xue, J.J.; Wang, X.Q.; Qi, G.S.; Wang, J.; Shen, M.Q.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Li, C.J. The development of catalytic nucleophilic additions of terminal alkynes in water. Acc. Chem. Res. 2010, 43, 581–590. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing oxide reducibility: The role of metal/oxide interfaces in the formation of oxygen vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef]
- Tedeschi, R.J.; Casey, A.W.; Clark, G.S., Jr.; Huckel, R.W.; Kindley, L.M.; Russell, J.R. Base-catalyzed reaction of acetylene and vinylacetylenes with carbonyl compounds in liquid ammonia under pressure. J. Org. Chem. 1963, 28, 1740–1743. [Google Scholar] [CrossRef]
- Takita, R.; Fukuta, Y.; Tsuji, R.; Ohshima, T.; Shibasaki, M. A new entry in catalytic alkynylation of aldehydes and ketones: Dual activation of soft nucleophiles and hard electrophiles by Indium(III) catalyst. Org. Lett. 2005, 7, 1363–1366. [Google Scholar] [CrossRef]
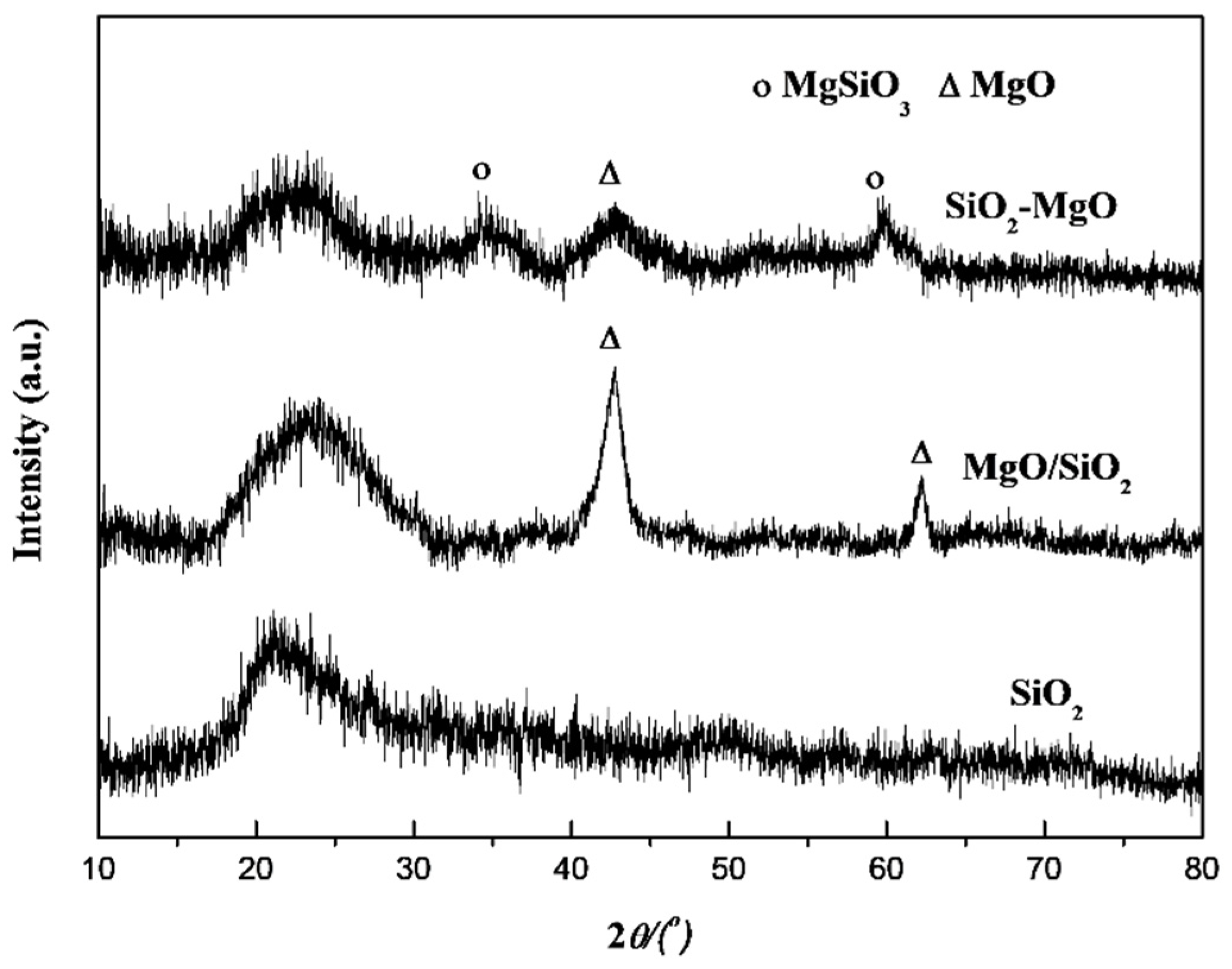
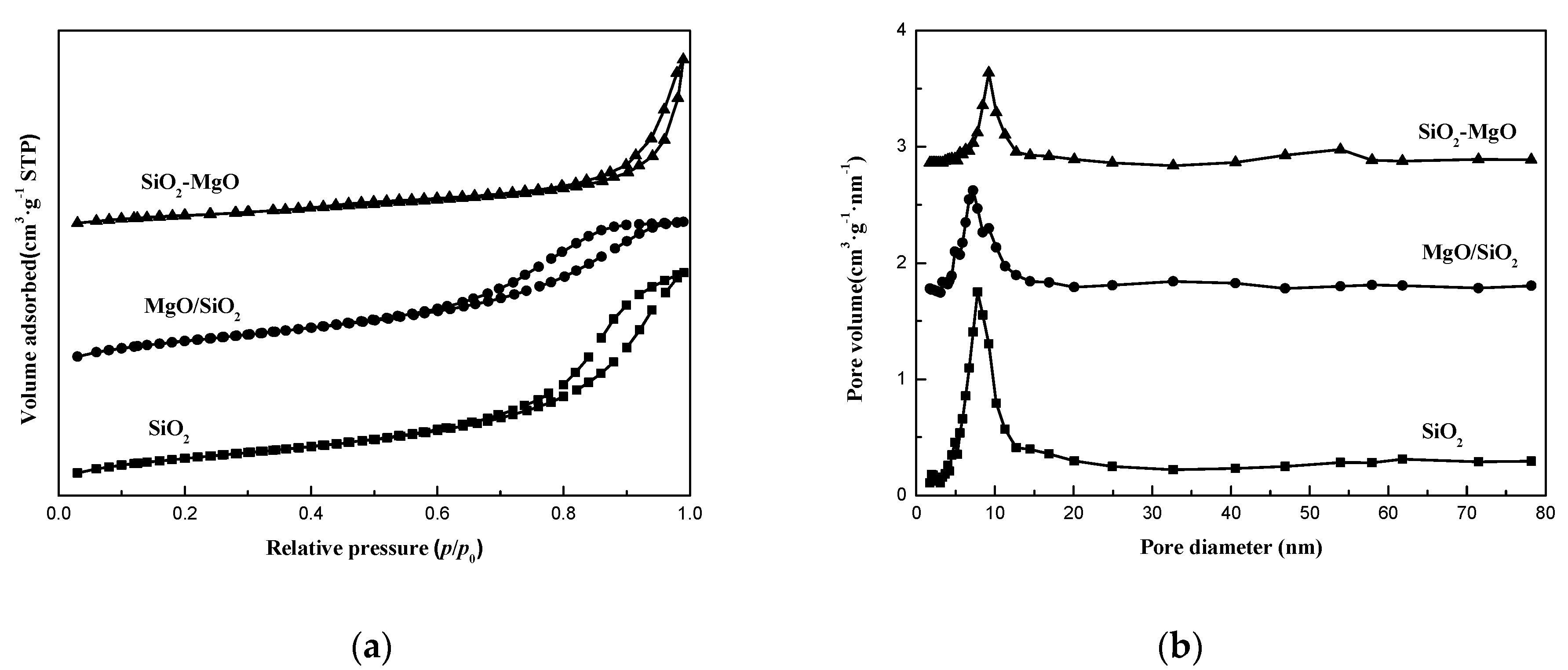
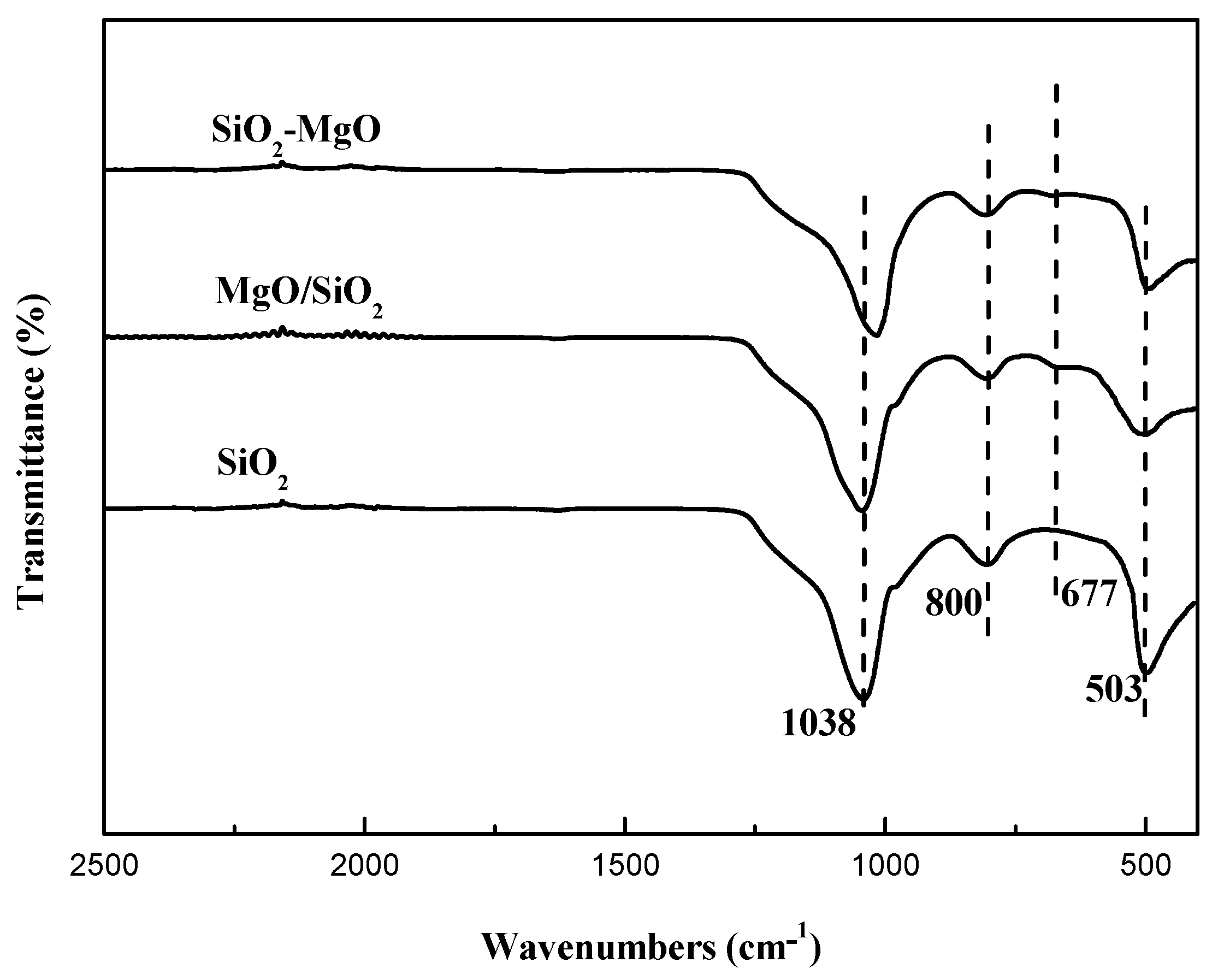
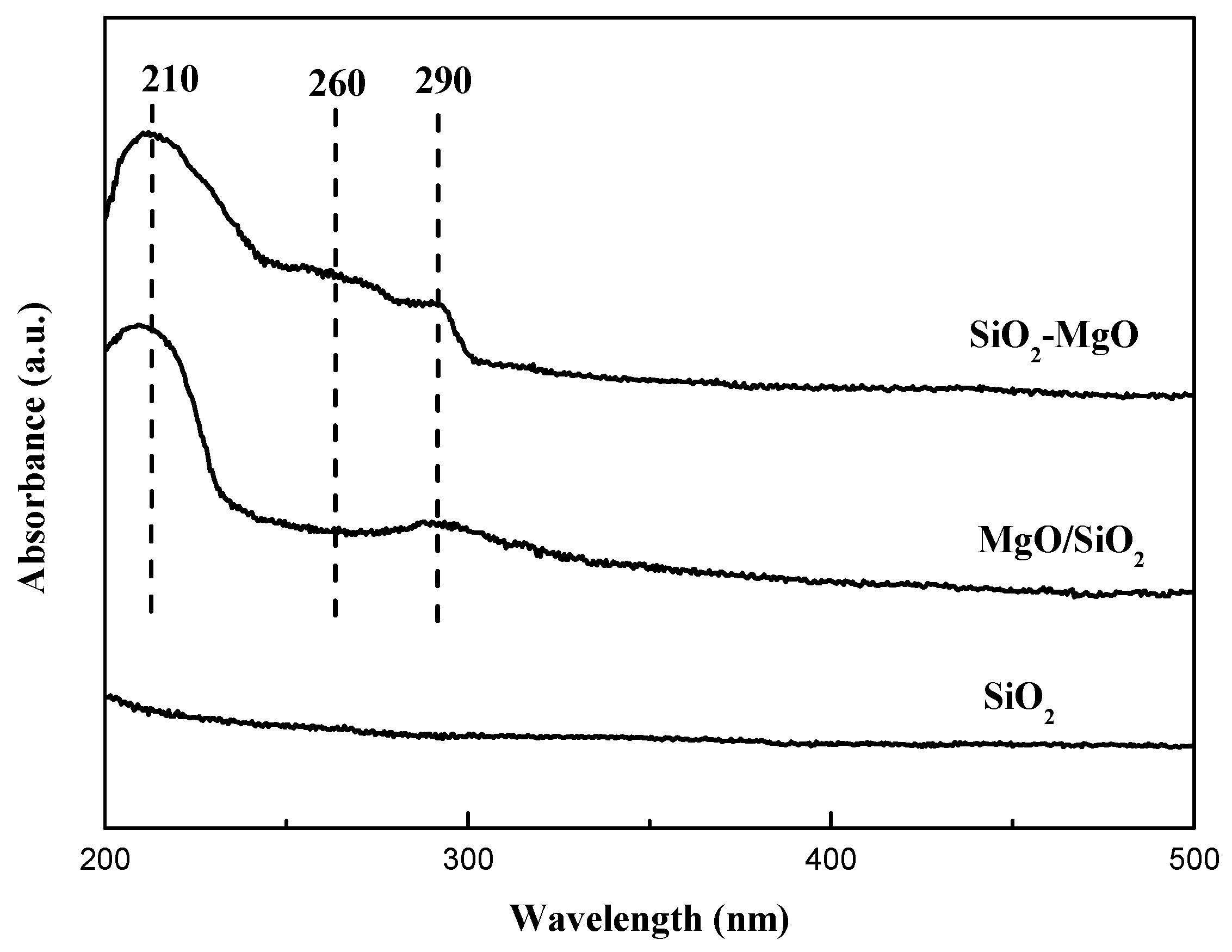
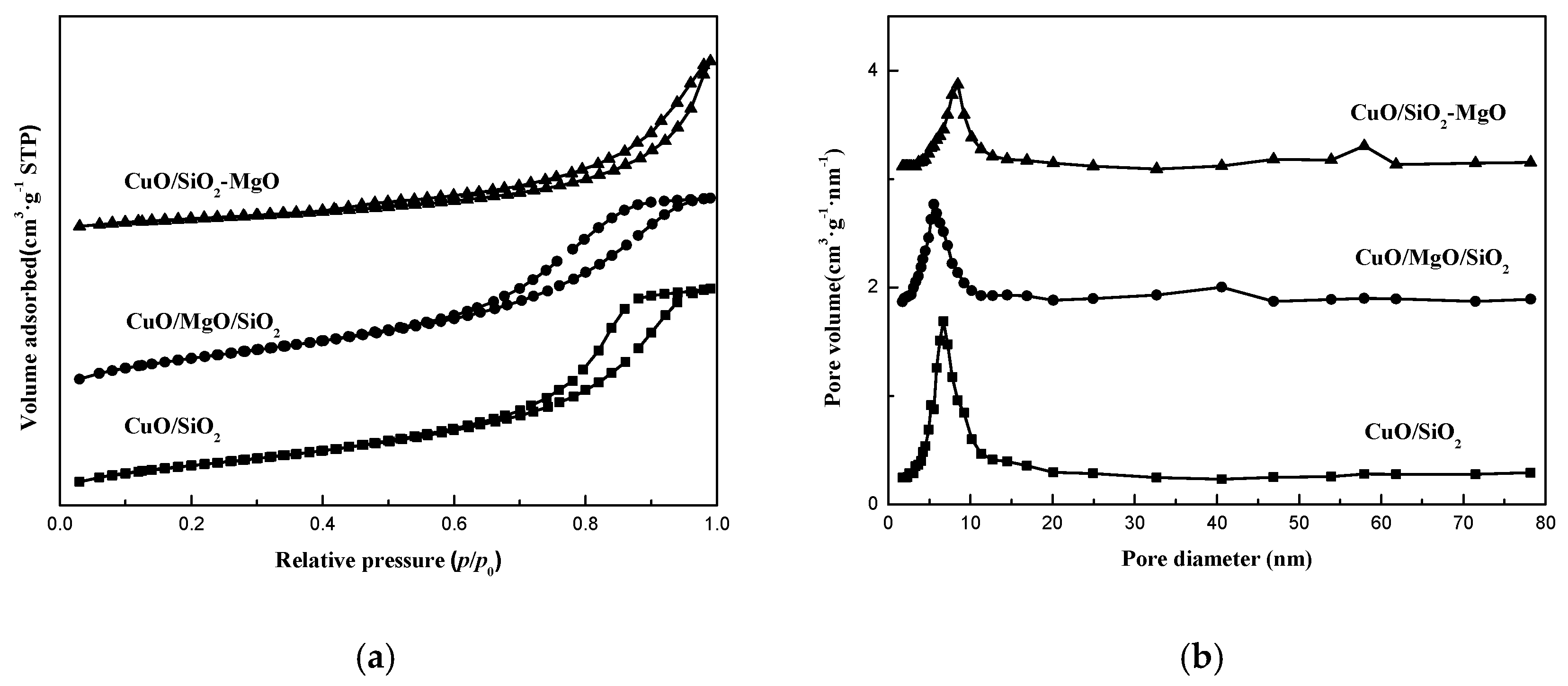
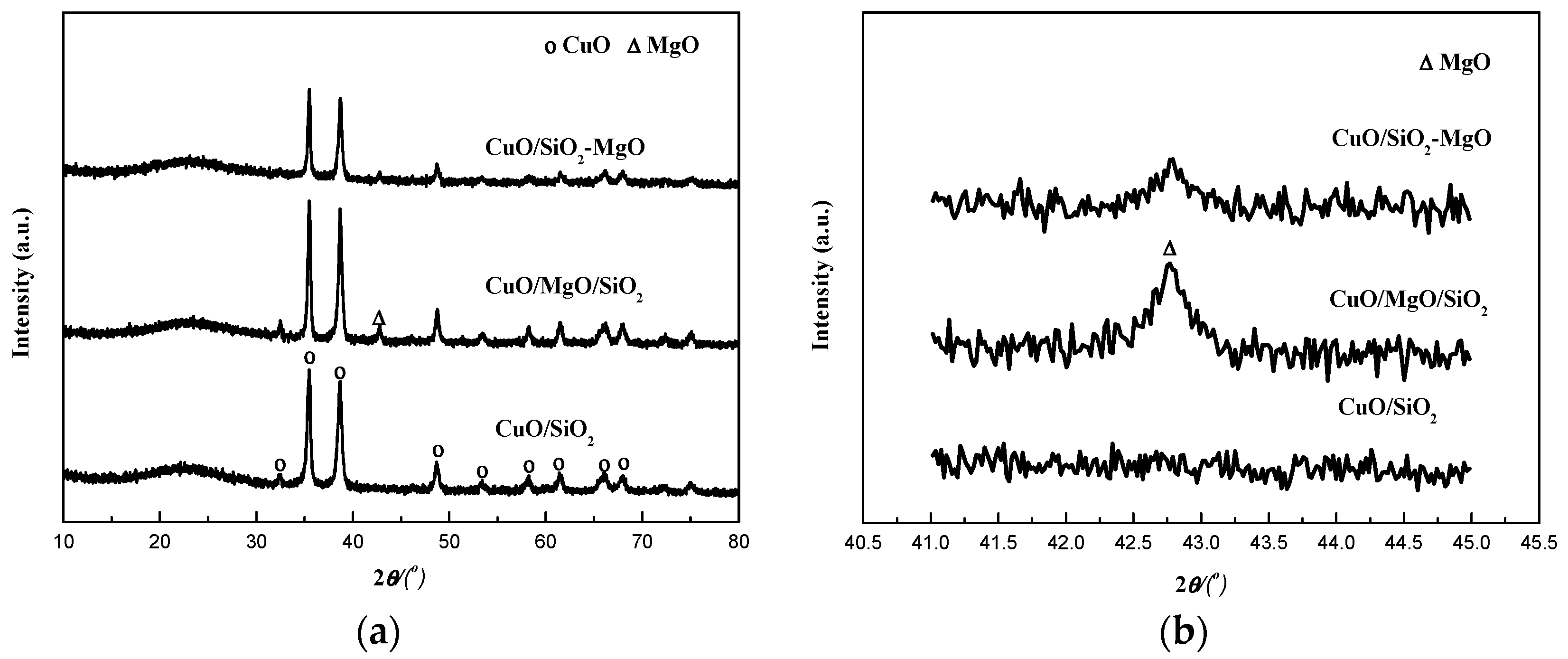
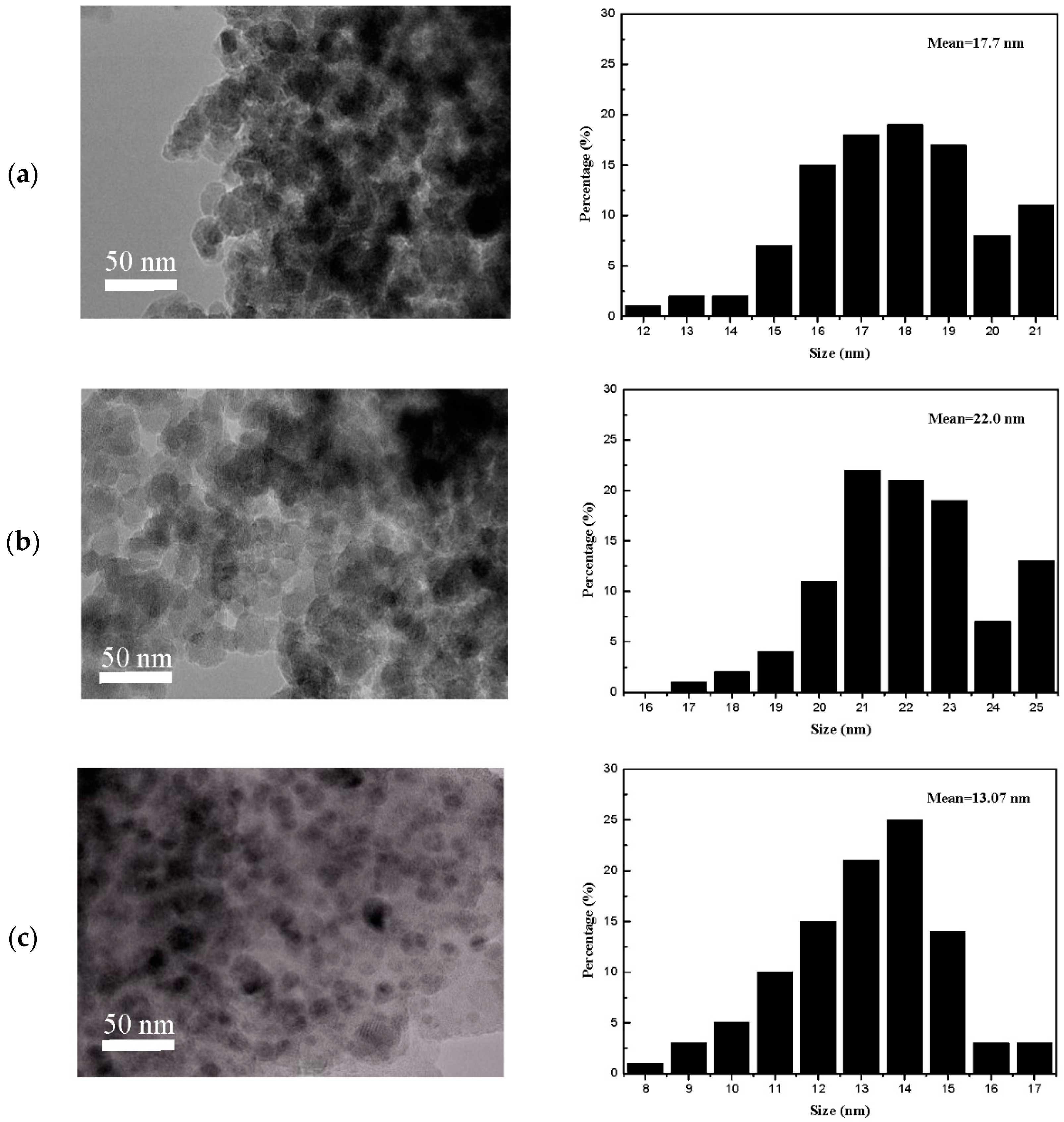
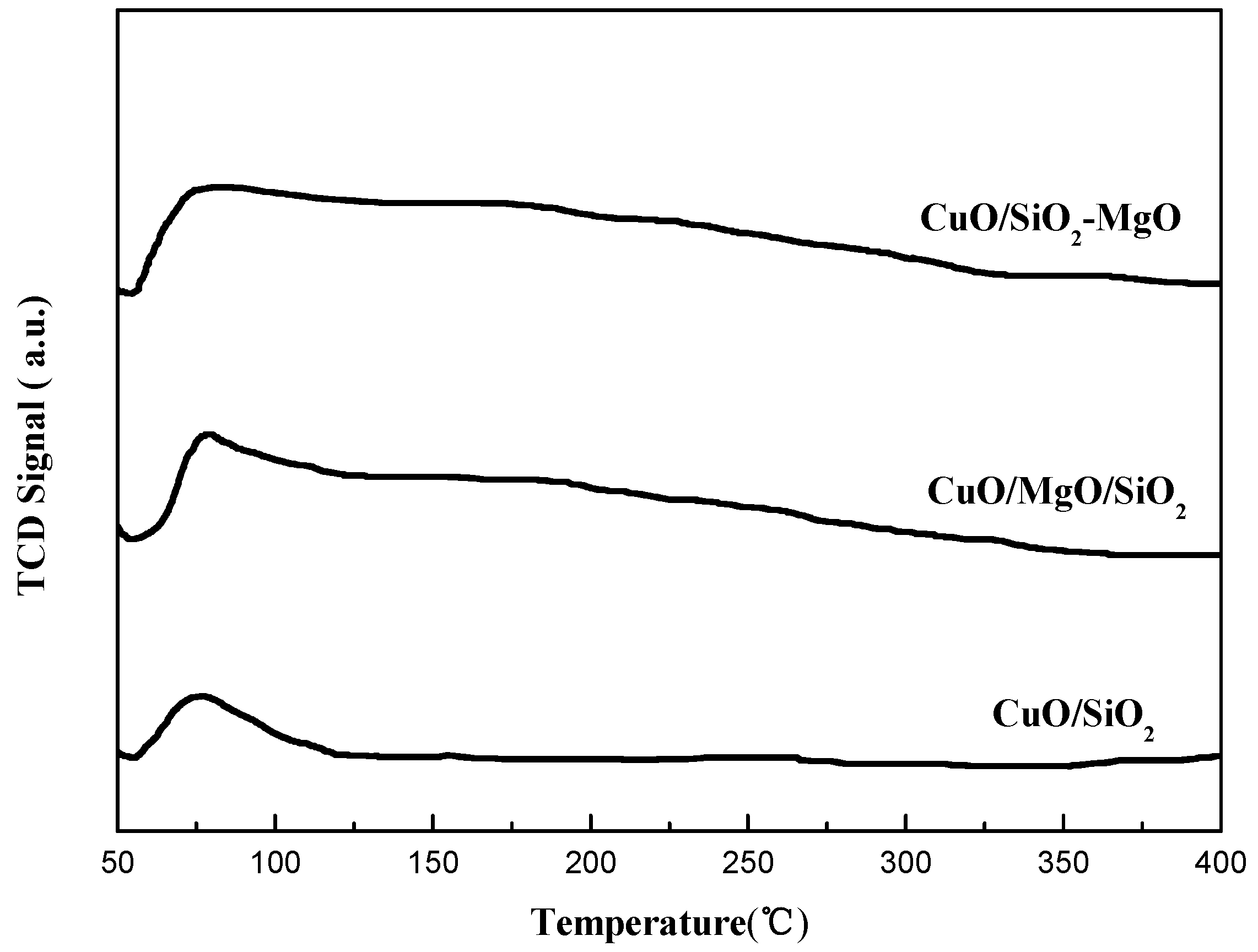

| Samples | Cu Content (wt.%) a | SBET (m2·g−1) b | Vp (cm3·g−1) b | dCuO (nm) c | Cu+/(Cu0+Cu1+) (%) d | ACu+ e (a.u.) |
|---|---|---|---|---|---|---|
| SiO2 | - | 1031.7 | 2.31 | - | - | - |
| MgO/SiO2 | - | 898.5 | 2.11 | - | - | - |
| SiO2-MgO | - | 753.6 | 1.97 | - | - | - |
| CuO/SiO2 | 16.7 | 642.7 | 1.01 | 18.1 | 82.1 | 4.2 |
| CuO/MgO/SiO2 | 17.5 | 499.2 | 0.92 | 21.3 | 81.7 | 3.8 |
| CuO/SiO2-MgO | 17.3 | 385.8 | 0.88 | 14.7 | 100 | 5.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ban, L.; Meng, P.; Li, H.; Zhao, Y. Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species. Nanomaterials 2019, 9, 1137. https://doi.org/10.3390/nano9081137
Wang Z, Ban L, Meng P, Li H, Zhao Y. Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species. Nanomaterials. 2019; 9(8):1137. https://doi.org/10.3390/nano9081137
Chicago/Turabian StyleWang, Zhipeng, Lijun Ban, Pingfan Meng, Haitao Li, and Yongxiang Zhao. 2019. "Ethynylation of Formaldehyde over CuO/SiO2 Catalysts Modified by Mg Species: Effects of the Existential States of Mg Species" Nanomaterials 9, no. 8: 1137. https://doi.org/10.3390/nano9081137





