New Perspectives on the Electronic and Geometric Structure of Au70S20(PPh3)12 Cluster: Superatomic-Network Core Protected by Novel Au12(µ3-S)10 Staple Motifs
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
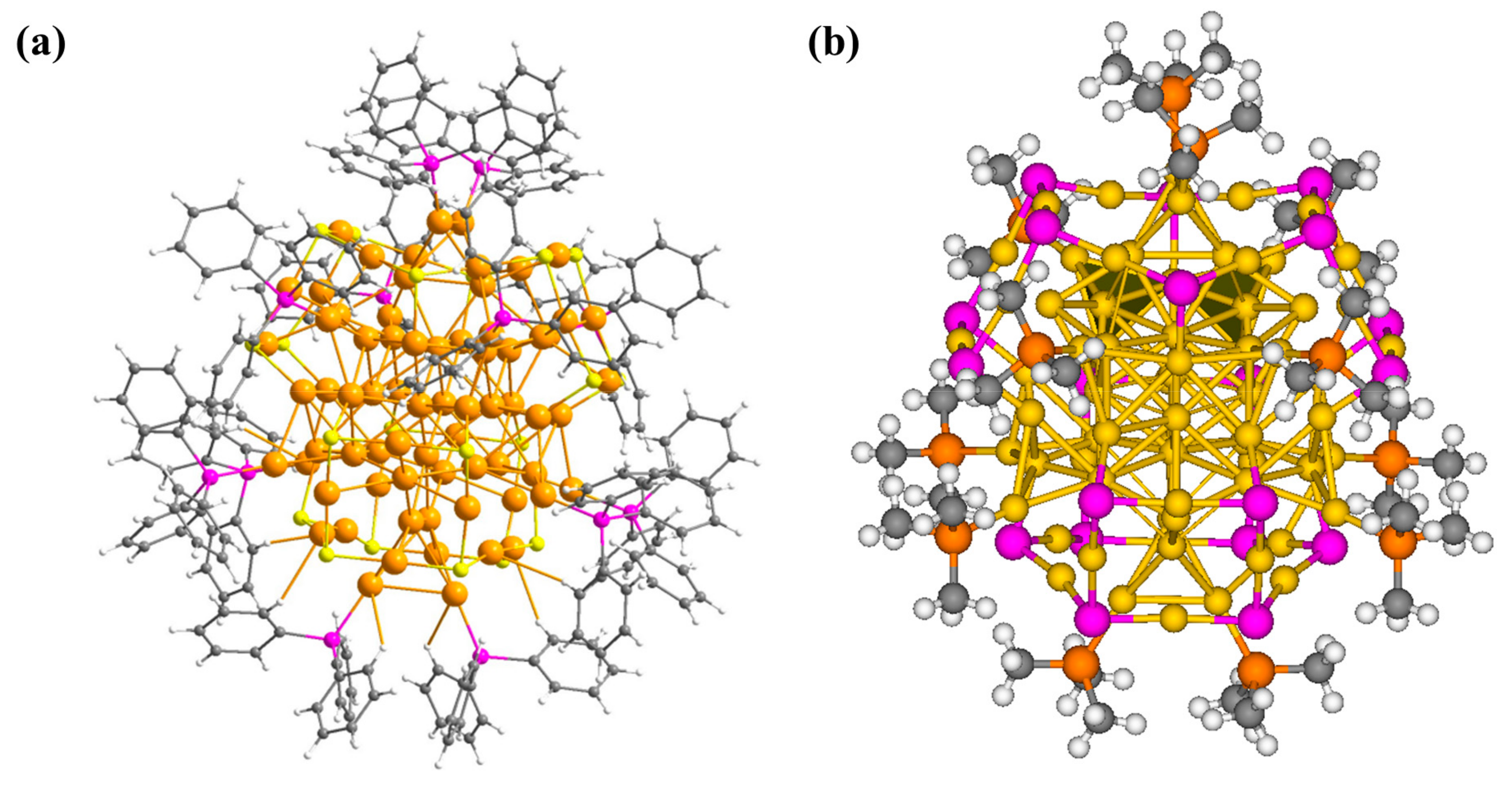
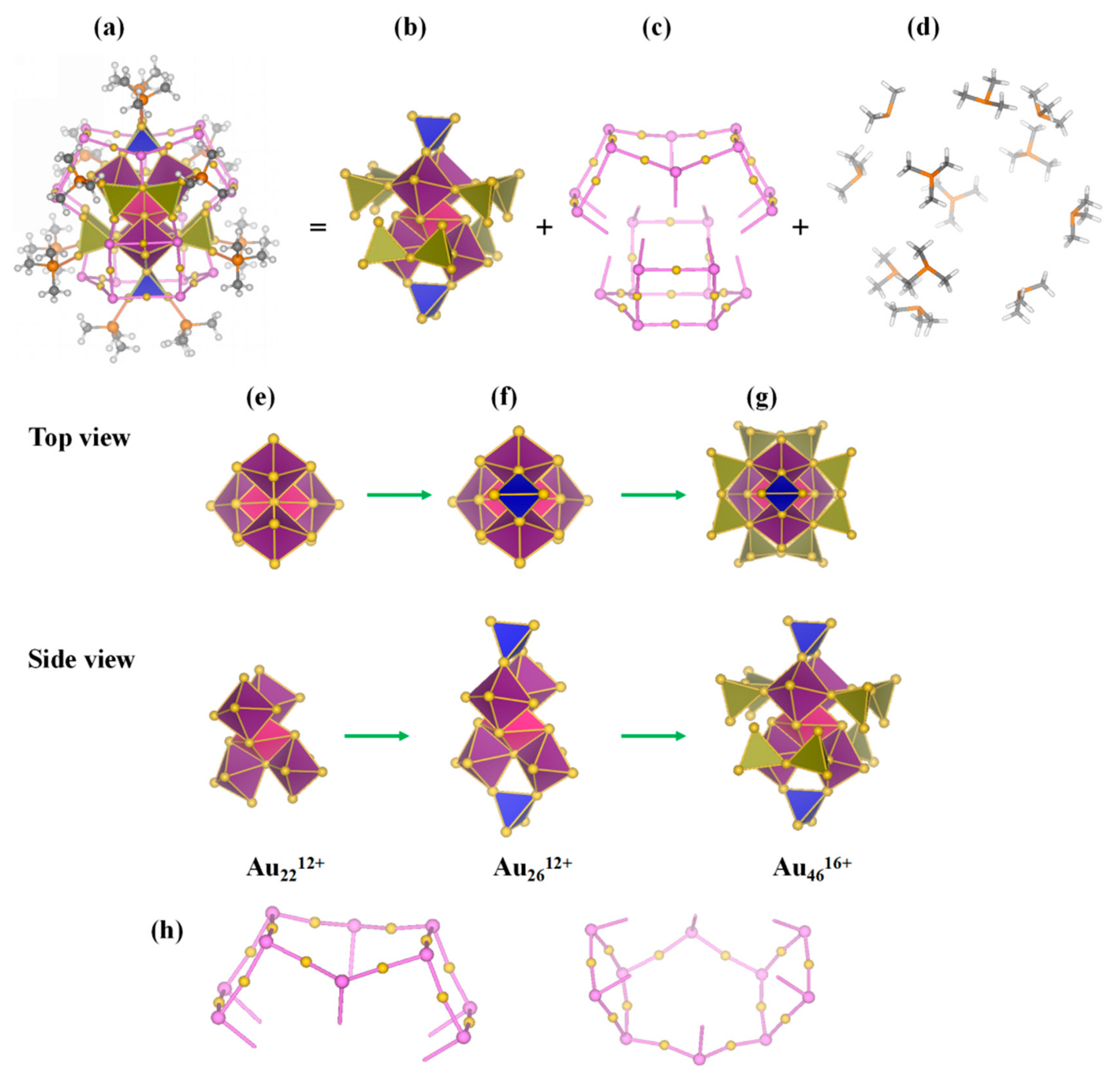
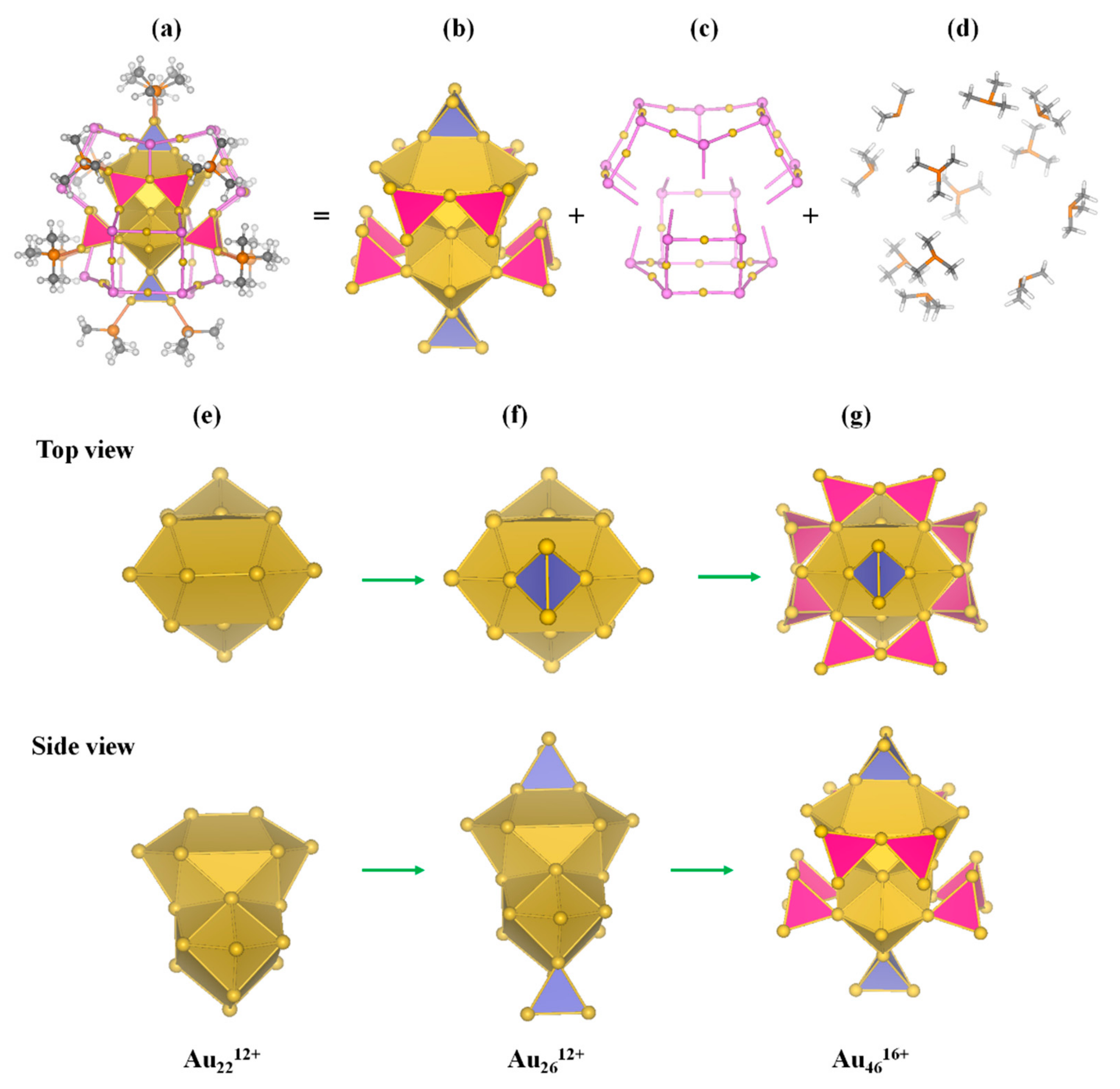
3.1. Geometric Structure
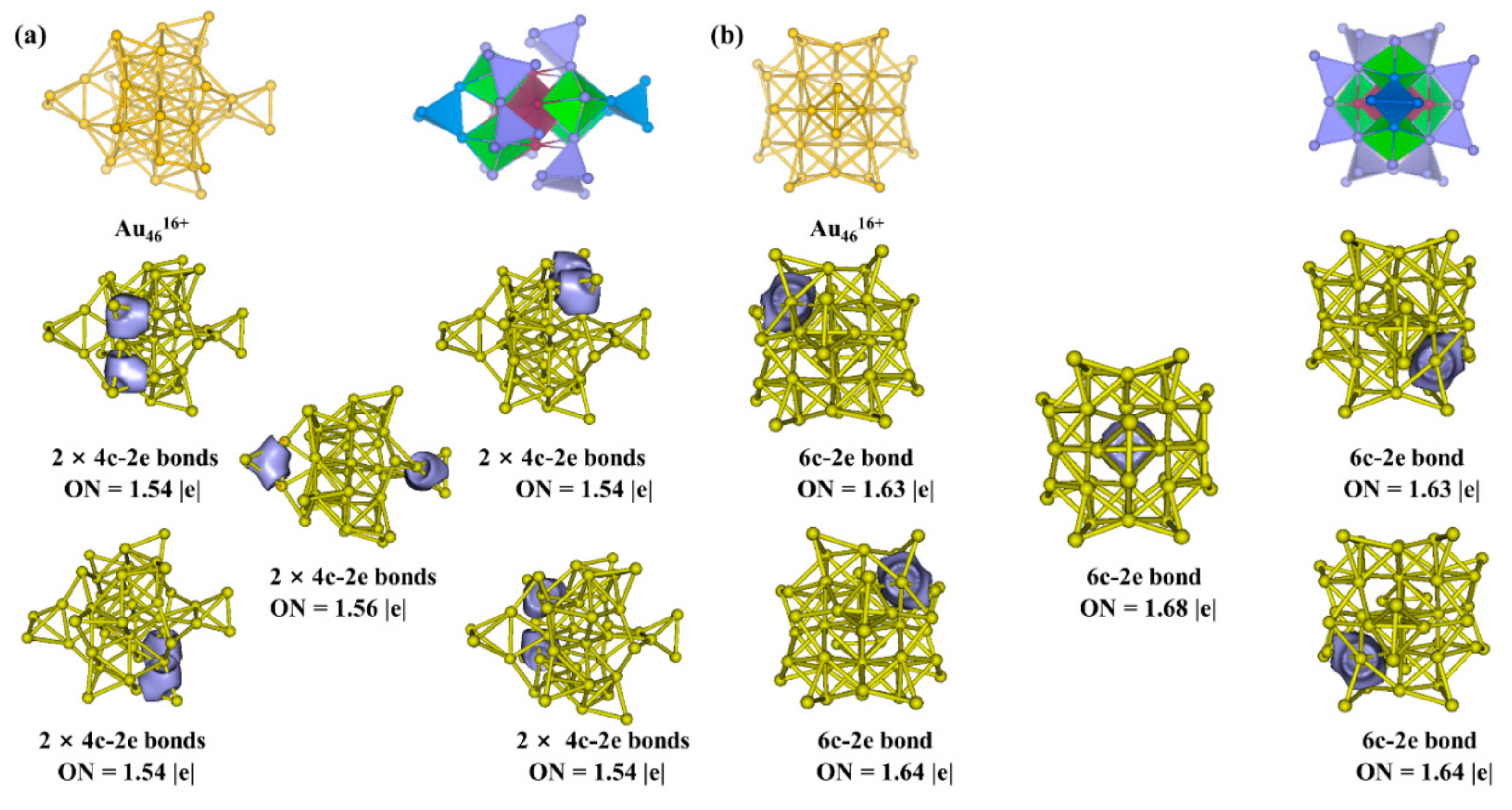
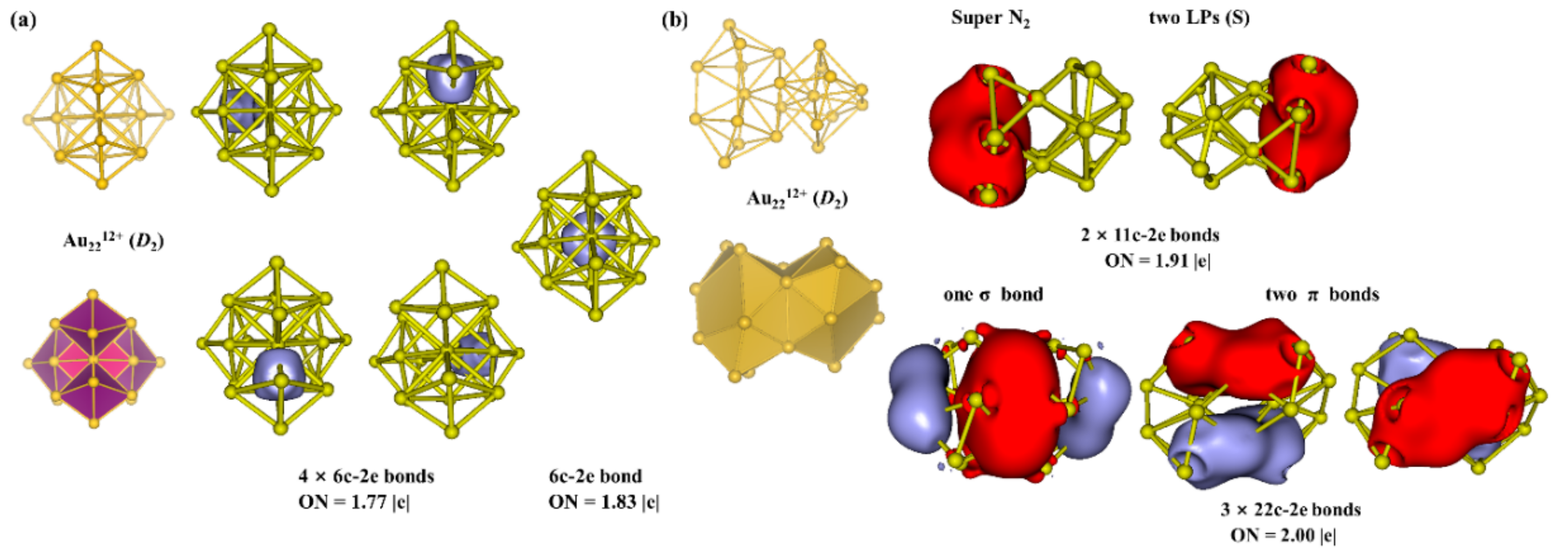
3.2. Chemical Bonding Analysis
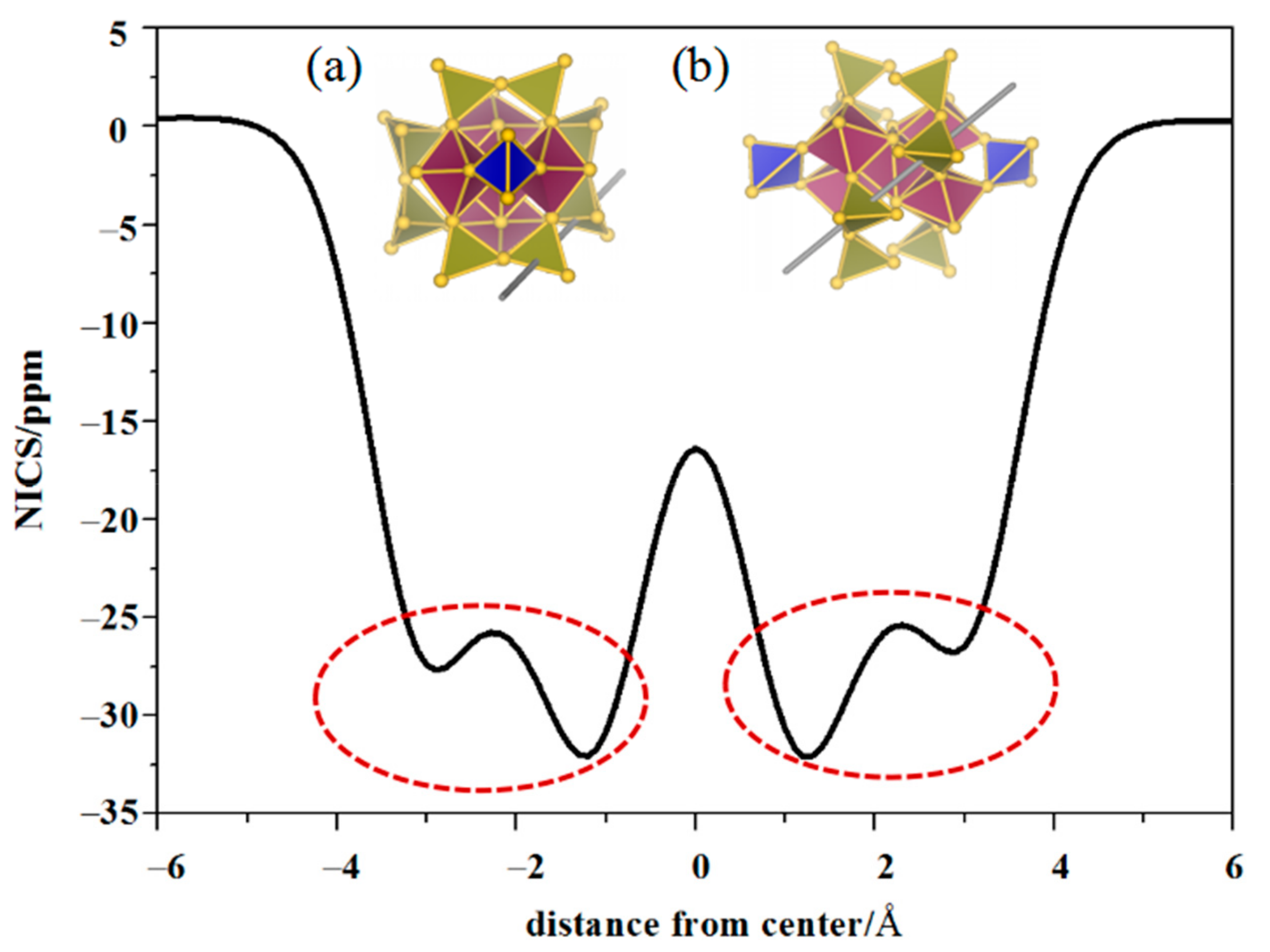
3.3. Aromatic Analysis
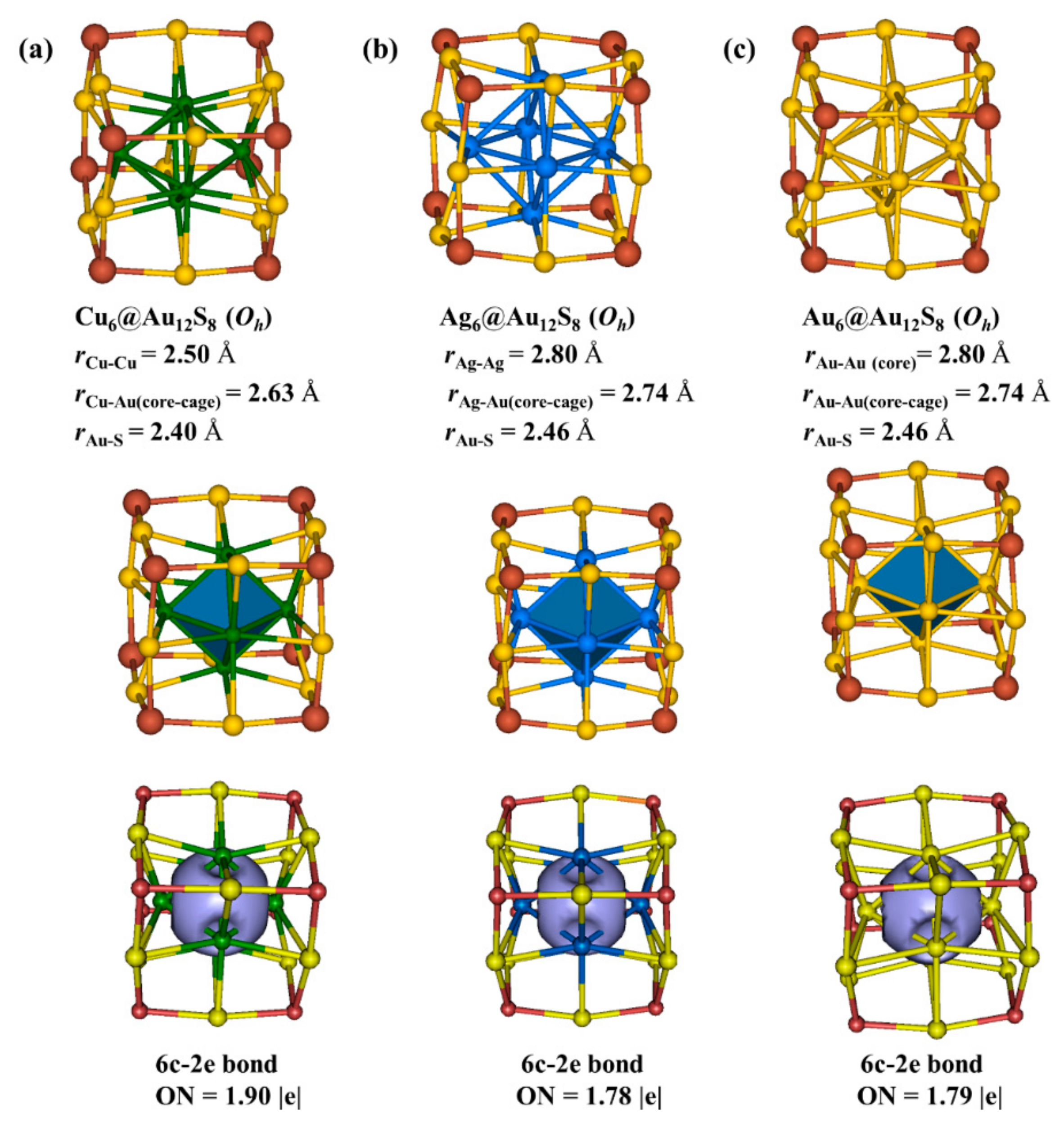
3.4. Cu6@Au12(μ3-S)8, Ag6@Au12(μ3-S)8, and Au6@Au12(μ3-S)8 Clusters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Bushnell, D.A.; Kornberg, R.D. Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science 2007, 318, 430–433. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Aikens, C.M.; Hollander, F.J.; Schatz, G.C.; Jin, R.C. Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties. J. Am. Chem. Soc. 2008, 130, 5883–5885. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.F.; Li, P.; Tang, Q.; Wan, X.K.; Nan, Z.A.; Jiang, D.E.; Wang, Q.M. Alkynyl-protected silver nanoclusters featuring an anticuboctahedral kernel. Nanoscale 2017, 9, 11405–11409. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Li, T.; Nobusada, K.; Zeng, C.; Rosi, N.L.; Jin, R.C. Nonsuperatomic [Au23(SC6H11) 16]− nanocluster featuring bipyramidal Au15 kernel and trimeric Au3(SR)4 motif. J. Am. Chem. Soc. 2013, 135, 18264–18267. [Google Scholar] [CrossRef] [PubMed]
- Dass, A.; Theivendran, S.; Nimmala, P.R.; Kumara, C.; Jupally, V.R.; Fortunelli, A.; Sementa, L.; Barcaro, G.; Zuo, X.B.; Noll, B.C. Au133(SPh-tBu)52 nanomolecules: X-ray crystallography, optical, electrochemical, and theoretical Analysis. J. Am. Chem. Soc. 2015, 137, 4610–4613. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Chen, M.; Wang, S.X.; Zhu, M.Z. Intramolecular metal exchange reaction promoted by thiol ligands. Nanomaterials 2018, 8, 1070. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Gao, Y.; Shao, N.; Zeng, X.C. Thiolate-protected Au20 (SR)16 cluster: Prolate Au8 core with new [Au3(SR)4] staple motif. J. Am. Chem. Soc. 2009, 131, 13619–13621. [Google Scholar] [CrossRef]
- Pei, Y.; Tang, J.; Tang, X.Q.; Huang, Y.Q.; Zeng, X.C. New structure model of Au22(SR)18: Bitetrahederon golden kernel enclosed by [Au6(SR)6] Au(I) complex. J. Phys. Chem. Lett. 2015, 6, 1390–1395. [Google Scholar] [CrossRef]
- Jiang, D.E.; Walter, M.; Akola, J. On the structure of a thiolated gold cluster: Au44(SR)282−. J. Phys. Chem. C 2010, 114, 15883–15889. [Google Scholar] [CrossRef]
- Malola, S.; Lehtovaara, L.; Knoppe, S.; Hu, K.J.; Palmer, R.E.; Bürgi, T.; Häkkinen, H. Au40(SR)24 cluster as a chiral dimer of 8-electron superatoms: Structure and optical properties. J. Am. Chem. Soc. 2012, 134, 19560–19563. [Google Scholar] [CrossRef]
- Tlahuice-Flores, A. Ligand effects on the optical and chiroptical properties of the thiolated Au18 cluster. Phys. Chem. Chem. Phys. 2016, 18, 27738–27744. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Castro, A.; Maturana, R.G. Understanding planar ligand-supported MAu5 and MAu6 cores. Theoretical survey of [MAu5(Mes)5] and [MAu6(Mes)6] (M = Cu, Ag, Au; Mes = 2, 4, 6-Me3C6H2) under the planar superatom model. J. Phys. Chem. C 2014, 118, 21185–21191. [Google Scholar] [CrossRef]
- Jin, R.C.; Zeng, C.J.; Zhou, M.Z.; Chen, Y.X. Atomically precise colloidal metal nanoclusters and nanoparticles: Fundamentals and opportunities. Chem. Rev. 2016, 116, 10346–10413. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.C.; Sementa, L.; Stener, M.; Gagnon, K.J.; Thanthirige, V.D.; Ramakrishna, G.; Fortunelli, A.; Dass, A. Au21S(SAdm)15: Crystal structure, mass spectrometry, optical spectroscopy, and first-principles theoretical analysis. J. Phys. Chem. C 2017, 121, 10865–10869. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wang, Y.; Edwards, A.J.; Yan, J.Z.; Zheng, N.F. High-yield synthesis and crystal structure of a green Au30 cluster co-capped by thiolate and sulfide. Chem. Commun. 2014, 50, 14325–14327. [Google Scholar] [CrossRef] [PubMed]
- Crasto, D.; Malola, S.; Brosofsky, G.; Dass, A.; Häkkinen, H. Single crystal XRD structure and theoretical analysis of the chiral Au30S(S-t-Bu)18 cluster. J. Am. Chem. Soc. 2014, 136, 5000–5005. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, T.; Li, G.; Nobusada, K.; Zeng, C.J.; Pang, G.; Rosi, N.L.; Jin, R.C. Observation of body-centered cubic gold nanocluster. Angew. Chem. Int. Ed. 2015, 54, 9826–9829. [Google Scholar] [CrossRef]
- Higaki, T.; Liu, C.; Zhou, M.; Luo, T.Y.; Rosi, N.L.; Jin, R.C. Tailoring the structure of 58-electron gold nanoclusters: Au103S2(S-Nap)41 and its implications. J. Am. Chem. Soc. 2017, 139, 9994–10001. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.M.; Cheng, L.J. Electronic and geometric structures of Au30 clusters: A network of 2e-superatom Au cores protected by tridentate protecting motifs with μ3-S. Nanoscale 2016, 8, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Kenzler, S.; Schrenk, C.; Schnepf, A. Au108S24(PPh3)16: A highly symmetric nanoscale gold cluster confirms the general concept of metalloid clusters. Angew. Chem. Int. Ed. 2017, 56, 393–396. [Google Scholar] [CrossRef]
- Kenzler, S.; Schrenk, C.; Frojd, A.R.; Hakkinen, H.; Clayborne, A.Z.; Schnepf, A. Au70S20(PPh3)12: An intermediate sized metalloid gold cluster stabilized by the Au4S4 ring motif and Au-PPh3 groups. Chem. Commun. 2018, 54, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Häkkinen, H.; Walter, M.; Grönbeck, H. Divide and protect: Capping gold nanoclusters with molecular gold-thiolate rings. J. Phys. Chem. B 2006, 110, 9927–9931. [Google Scholar] [CrossRef] [PubMed]
- Heaven, M.W.; Dass, A.; White, P.S.; Holt, K.M.; Murray, R.W. Crystal structure of the gold nanoparticle [N(C8H17)4] [Au25(SCH2CH2Ph)18]. J. Am. Chem. Soc. 2008, 130, 3754–3755. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Gao, Y.; Zeng, X.C. Structural prediction of thiolate-protected Au38: A face-fused bi-icosahedral Au core. J. Am. Chem. Soc. 2008, 130, 7830–7832. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Acevedo, O.; Akola, J.; Whetten, R.L.; Gronbeck, H.; Häkkinen, H. Structure and bonding in the ubiquitous icosahedral metallic gold cluster Au144(SR)60. J. Phys. Chem. C 2009, 113, 5035–5038. [Google Scholar] [CrossRef]
- Knoppe, S.; Wong, O.A.; Malola, S.; Häkkinen, H.; Bürgi, T.; Verbiest, T.; Ackerson, C.J. Chiral phase transfer and enantioenrichment of thiolate-protected Au102 clusters. J. Am. Chem. Soc. 2014, 136, 4129–4132. [Google Scholar] [CrossRef]
- Xu, W.W.; Gao, Y.; Zeng, X.C. Unraveling structures of protection ligands on gold nanoparticle Au68(SH)32. Sci. Adv. 2015, 1, e1400211. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Li, Y.; Gao, Y.; Zeng, X.C. Medium-sized Au40(SR)24 and Au52(SR)32 nanoclusters with distinct gold-kernel structures and spectroscopic features. Nanoscale 2016, 8, 1299–1304. [Google Scholar] [CrossRef]
- Xiong, L.; Peng, B.; Ma, Z.; Wang, P.; Pei, Y. A ten-electron (10e) thiolate-protected Au29(SR)19 cluster: Structure prediction and a ‘gold-atom insertion, thiolate-group elimination’ mechanism. Nanoscale 2017, 9, 2895–2902. [Google Scholar] [CrossRef]
- Xu, W.W.; Zhu, B.; Zeng, X.C.; Gao, Y. A grand unified model for liganded gold clusters. Nat. Commun. 2016, 7, 13574. [Google Scholar] [CrossRef]
- Xu, W.W.; Zeng, X.C.; Gao, Y. The structural isomerism in gold nanoclusters. Nanoscale 2018, 10, 9476–9483. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Wang, P.; Xiong, L.; Pei, Y. Thiolate-protected gold nanoclusters: Structural prediction and the understandings of electronic stability from first principles simulations. WIREs Comput. Mol. Sci. 2017, 7, e1315. [Google Scholar] [CrossRef]
- Nimmala, P.R.; Knoppe, S.; Jupally, V.R.; Delcamp, J.H.; Aikens, C.M.; Dass, A. Au36(SPh)24 nanomolecules: X-ray crystal structure, optical spectroscopy, electrochemistry, and theoretical analysis. J. Phys. Chem. B 2014, 118, 14157–14167. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.J.; Liu, C.; Chen, Y.X.; Rosi, N.L.; Jin, R.C. Gold-thiolate ring as a protecting motif in the Au20(SR)16 nanocluster and implications. J. Am. Chem. Soc. 2014, 136, 11922–11925. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Akola, J.; Lopez-Acevedo, O.; Jadzinsky, P.D.; Calero, G.; Ackerson, C.J.; Whetten, R.L.; Grönbeck, H.; Häkkinen, H. A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 9157–9162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.J.; Yuan, Y.; Zhang, X.Z.; Yang, J.L. Superatom networks in thiolate-protected gold nanoparticles. Angew. Chem. Int. Ed. 2013, 52, 9035–9039. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Lin, S.S.; Su, J.C.; Liu, C.Y. Structure prediction of Au44(SR)28: A chiral superatom cluster. J. Am. Chem. Soc. 2013, 135, 19060–19063. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision B 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef]
- Varetto, U. MOLEKEL, version 5.4.0.8; Swiss National Supercomputing Centre: Manno, Switzerland, 2009. Available online: http://ugovaretto.github.io/molekel/wiki/pmwiki.php/Main/HomePage.html (accessed on 1 August 2019).
- Jiang, D.E.; Walter, M.; Dai, S. Gold sulfide nanoclusters: A unique core-in-cage structure. Chem. Eur. J. 2010, 16, 4999–5003. [Google Scholar] [CrossRef]
- Pei, Y.; Shao, N.; Li, H.; Jiang, D.E.; Zeng, X.C. Hollow polyhedral structures in small gold-sulfide clusters. ACS Nano 2011, 5, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Cheng, L.J. Structural evolution of (Au2S) n (n = 1-8) clusters from first principles global optimization. RSC Adv. 2015, 5, 62543–62550. [Google Scholar] [CrossRef]
- Gerolf, M.; Joachim, S. Synthesis and crystal Structure of [Ph4As]4[Au12S8], a distorted cubane-like thioaurate(I). Angew. Chem. Int. Ed. Engl. 1984, 23, 715–716. [Google Scholar] [CrossRef]
- Xu, W.W.; Zeng, X.C.; Gao, Y. (Au3(μ3-S) (0e) elementary block: New insights into ligated gold clusters with μ3-sulfido motifs. Nanoscale 2017, 9, 8990–8996. [Google Scholar] [CrossRef] [PubMed]
- Knoppe, S.; Malola, S.; Lehtovaara, L.; Bürgi, T.; Häkkinen, H. Electronic structure and optical properties of the thiolate-protected Au28(SMe)20 Cluster. J. Phys. Chem. A 2013, 117, 10526–10533. [Google Scholar] [CrossRef]
- Pyykkö, P.; Mendizabal, F. Theory of d10-d10 closed-shell attraction. III. rings. Inorg. Chem. 1998, 37, 3018–3025. [Google Scholar] [CrossRef]
- Pekka, P.; Nino, R.; Fernando, M. Theory of the d10-d10 closed-shell attraction: 1. dimers near equilibrium. Chem. Eur. J. 1997, 3, 1451–1457. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.X.; Zhong, J.; Song, Y.B.; Zhang, J.; Sheng, H.T.; Pei, Y.; Zhu, M.Z. The structure and optical properties of the [Au18(SR)14] nanocluster. Angew. Chem. Int. Ed. 2015, 54, 3145–3149. [Google Scholar] [CrossRef]
- Das, A.; Liu, C.; Byun, H.Y.; Nobusada, K.; Zhao, S.; Rosi, N.; Jin, R.C. Structure determination of [Au18(SR)14]. Angew. Chem. 2015, 127, 3183–3187. [Google Scholar] [CrossRef]
- Stanger, A. Nucleus-independent chemical shifts (NICS): Distance dependence and revised criteria for aromaticity and antiaromaticity. J. Org. Chem. 2006, 71, 883–893. [Google Scholar] [CrossRef]
- Tian, Z.M.; Cheng, L.J. First principles study on the structural evolution and properties of (MCl)n (n = 1–12, M = Cu, Ag) clusters. RSC Adv. 2016, 6, 30311–30319. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, L. B142+: A magic number double-ring cluster. J. Chem. Phys. 2012, 137, 044308. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. Van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.; Xu, Y.; Cheng, L. New Perspectives on the Electronic and Geometric Structure of Au70S20(PPh3)12 Cluster: Superatomic-Network Core Protected by Novel Au12(µ3-S)10 Staple Motifs. Nanomaterials 2019, 9, 1132. https://doi.org/10.3390/nano9081132
Tian Z, Xu Y, Cheng L. New Perspectives on the Electronic and Geometric Structure of Au70S20(PPh3)12 Cluster: Superatomic-Network Core Protected by Novel Au12(µ3-S)10 Staple Motifs. Nanomaterials. 2019; 9(8):1132. https://doi.org/10.3390/nano9081132
Chicago/Turabian StyleTian, Zhimei, Yangyang Xu, and Longjiu Cheng. 2019. "New Perspectives on the Electronic and Geometric Structure of Au70S20(PPh3)12 Cluster: Superatomic-Network Core Protected by Novel Au12(µ3-S)10 Staple Motifs" Nanomaterials 9, no. 8: 1132. https://doi.org/10.3390/nano9081132
APA StyleTian, Z., Xu, Y., & Cheng, L. (2019). New Perspectives on the Electronic and Geometric Structure of Au70S20(PPh3)12 Cluster: Superatomic-Network Core Protected by Novel Au12(µ3-S)10 Staple Motifs. Nanomaterials, 9(8), 1132. https://doi.org/10.3390/nano9081132




