Thin Functional Polymer Films by Electropolymerization
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of the Electrochromic Devices Based on 1D Polymer Films for Educational Chemistry Purposes
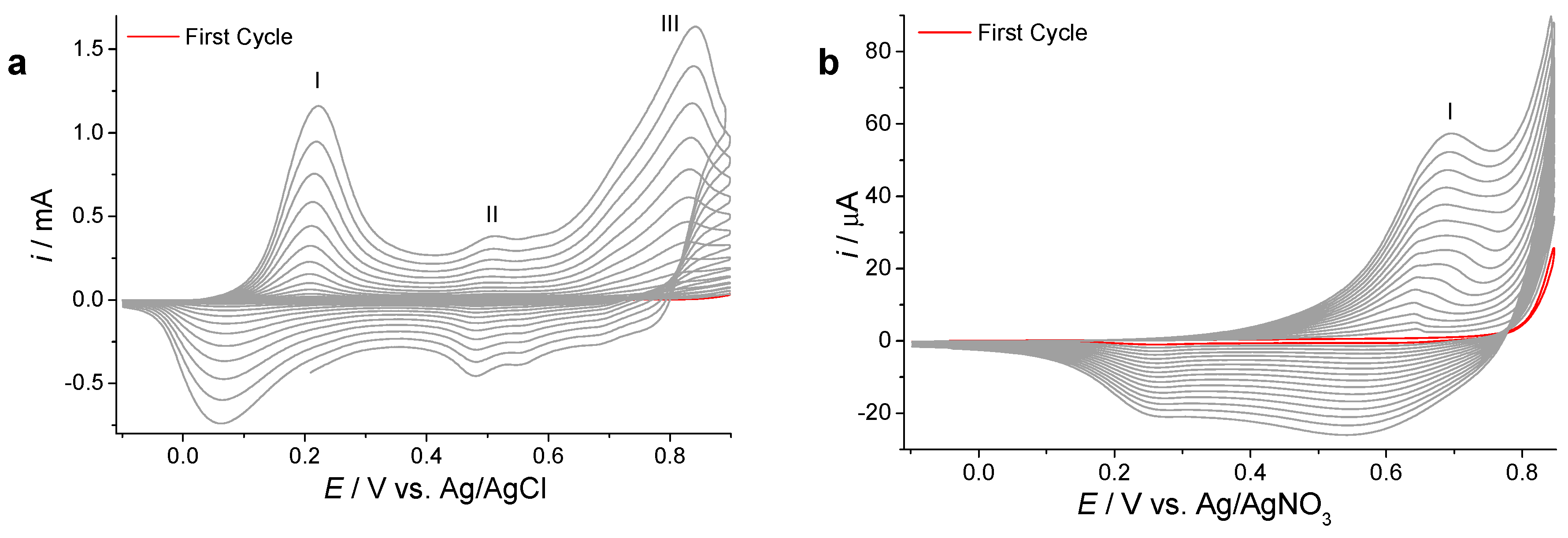
2.2. Electrochemical Synthesis and Response to Nitrobenzene (NB) of Microporous 3D Polymer Films as Current Topic in Materials Chemistry
3. Results and Discussion
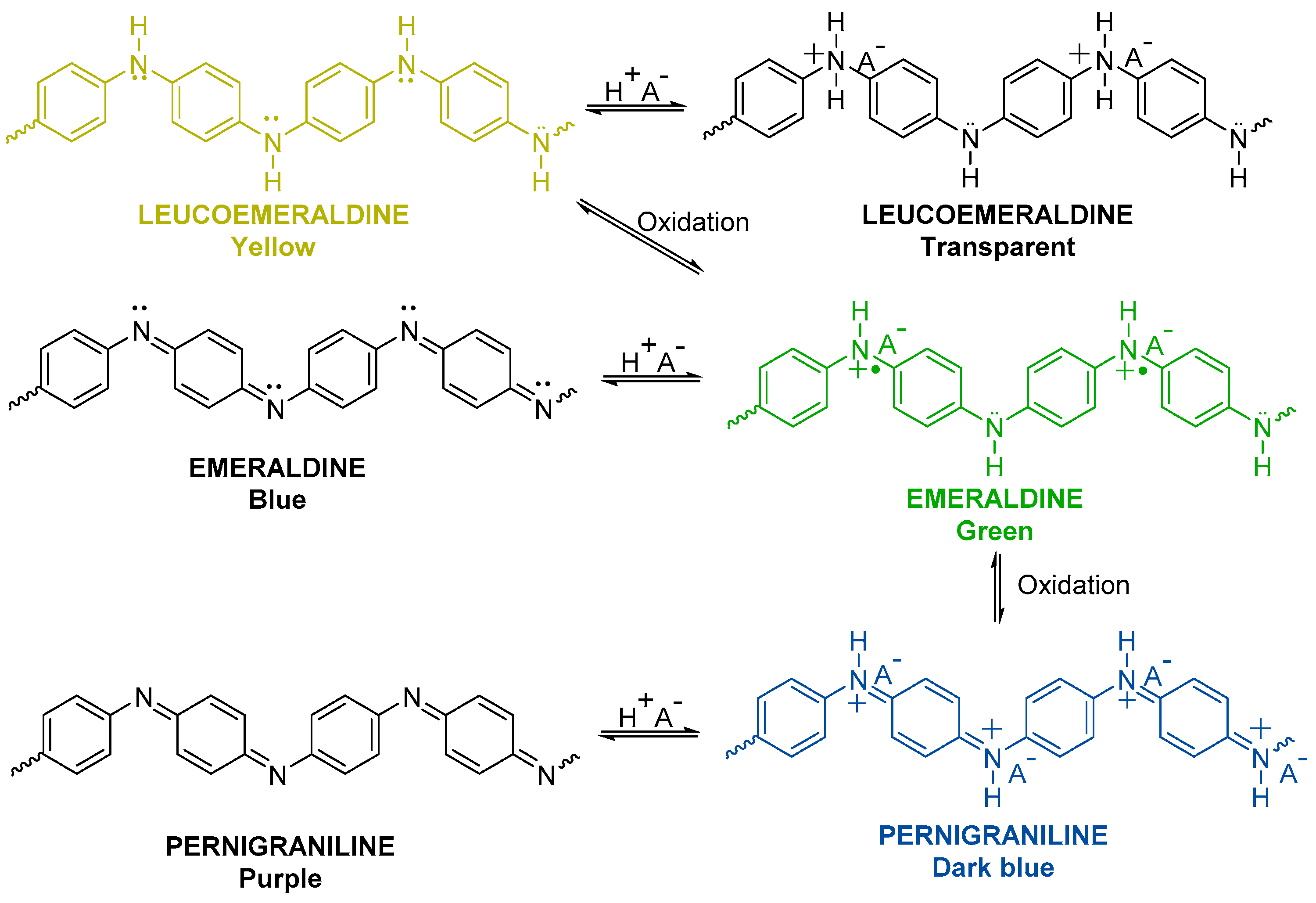
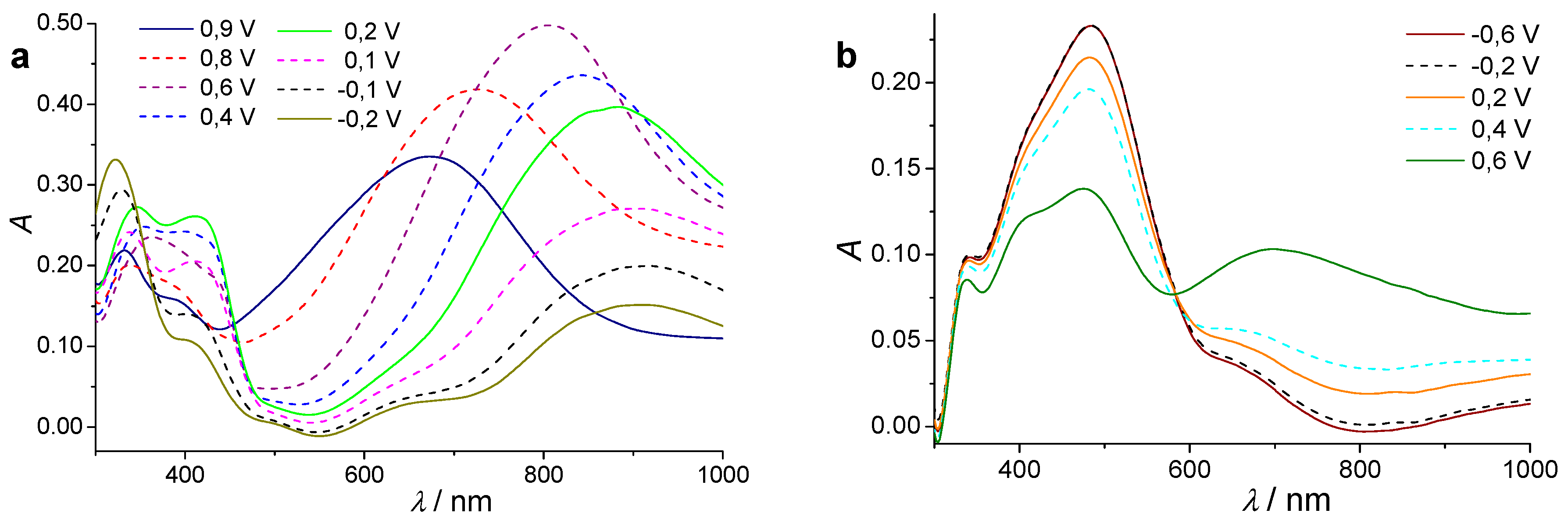
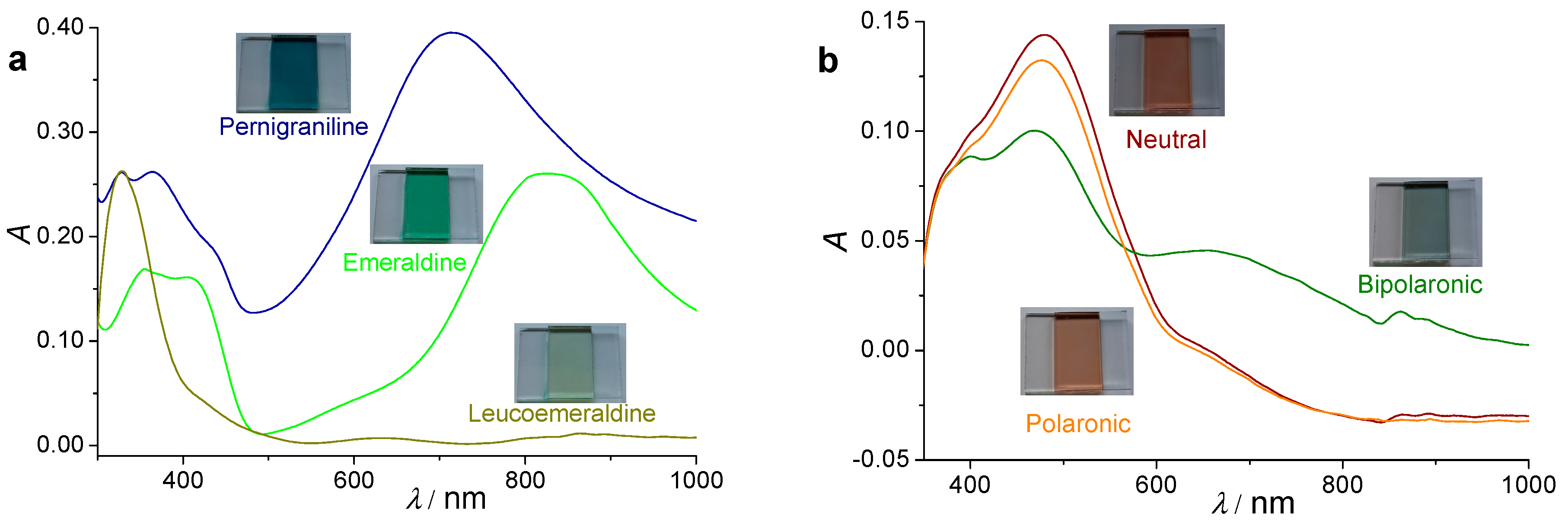
3.1. Electrochemical Polymerization of Bifunctional Monomers for a Simple Demonstration of Electrochromic Effects
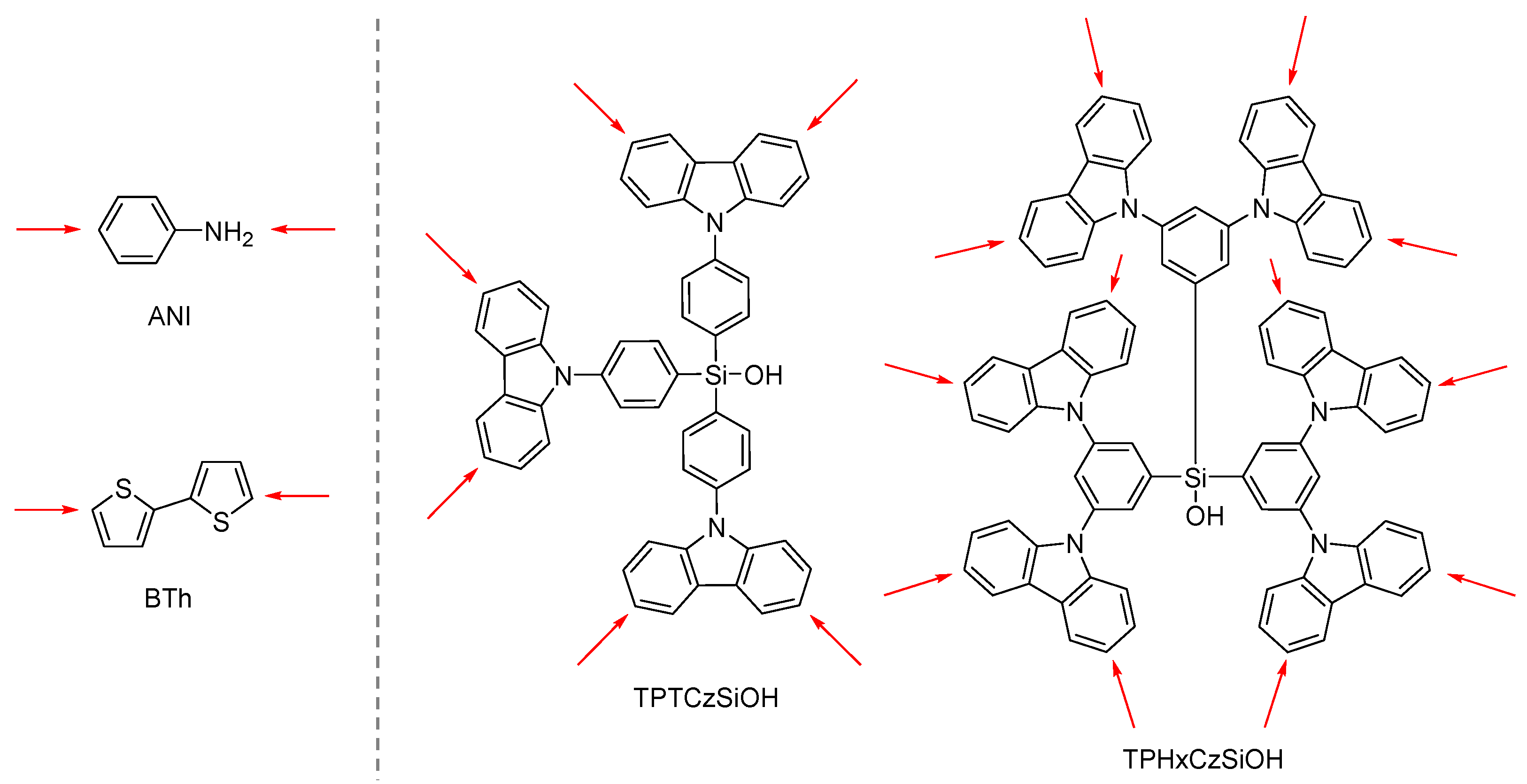
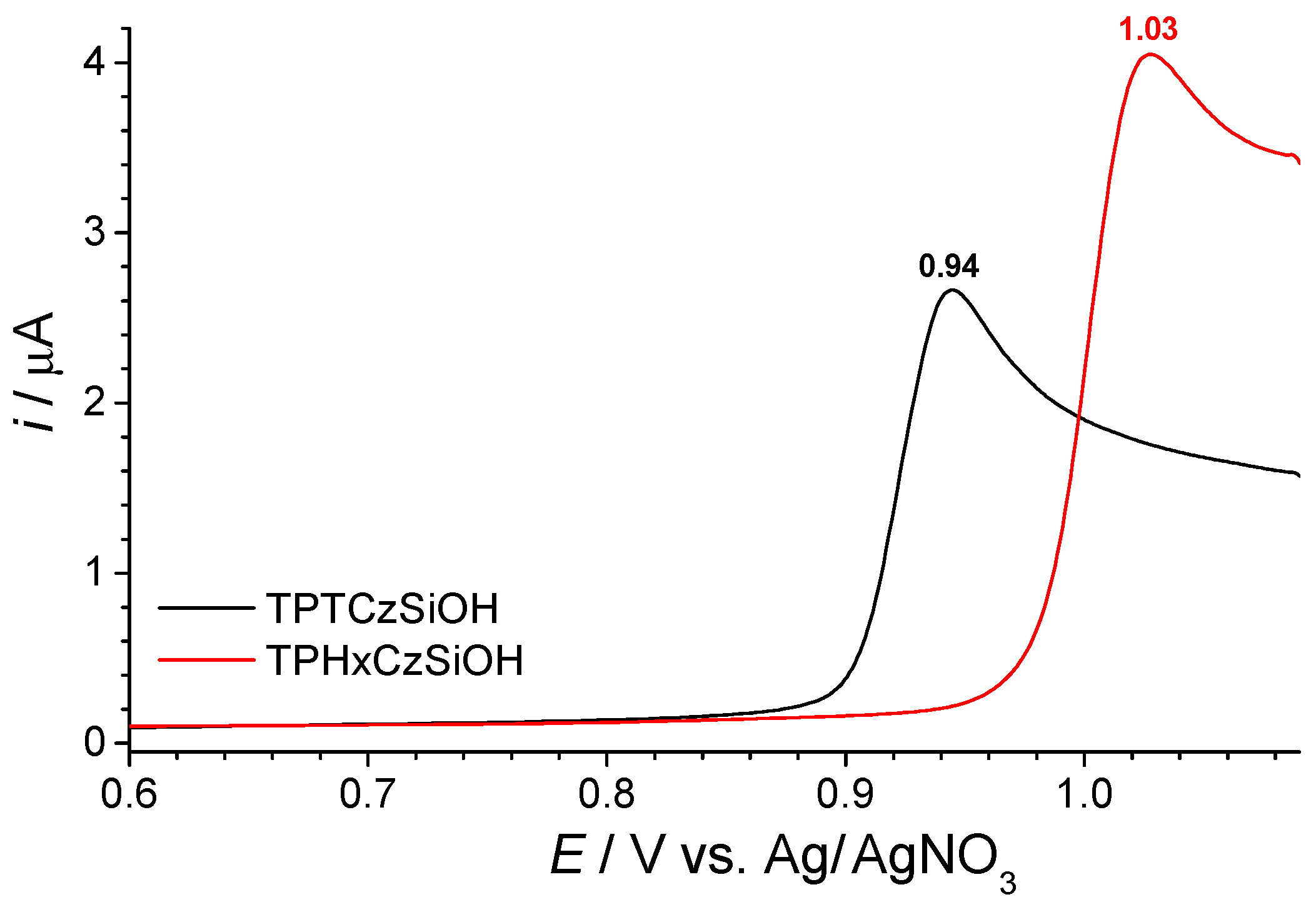
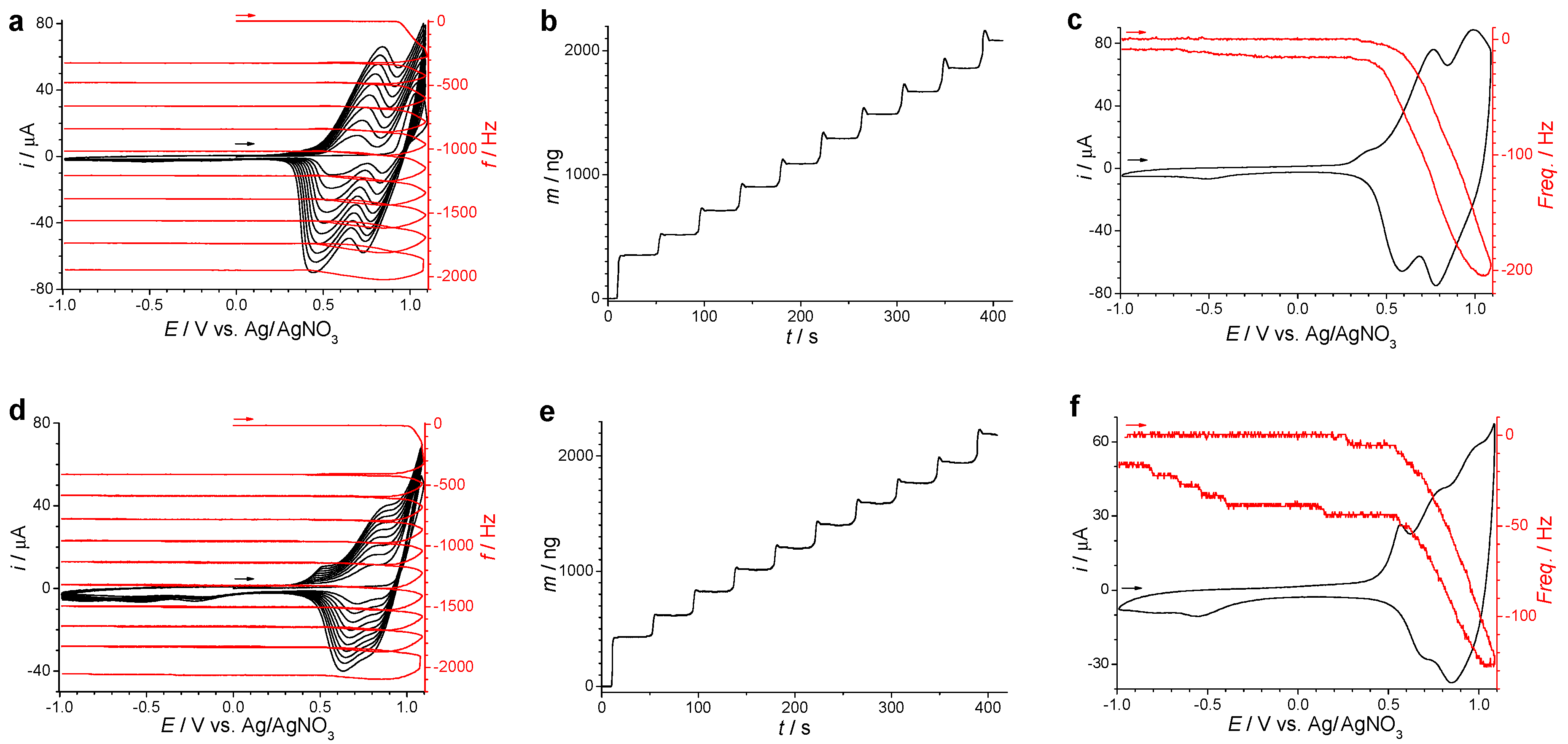
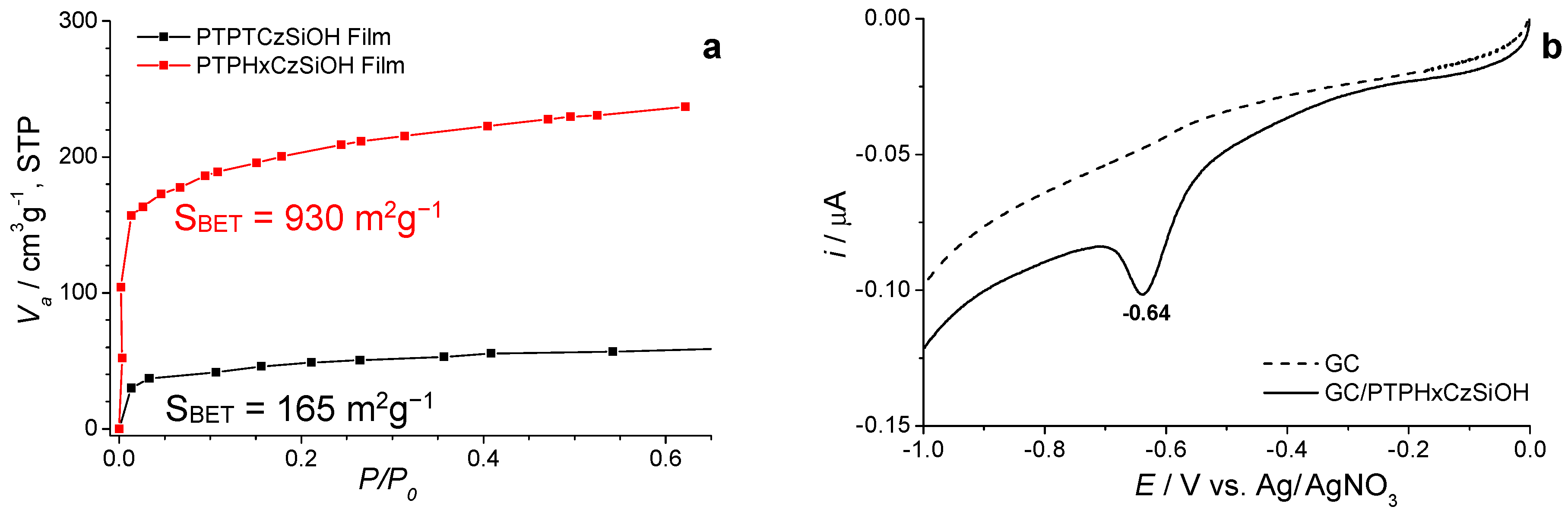
3.2. Electrochemical Polymerization of Multifunctional Monomers as a Current Topic for the Electrodeposition of Functional Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH) x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Bidan, G. Electropolymerized Films of π-Conjugated Polymers. A Tool for Surface Functionalization: A Brief Historical Evolution and Recent Trends. In Electropolymerization: Concepts, Materials and Applications; Cosnier, S., Karyakin, A., Eds.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Palma-Cando, A.; Frontana-Uribe, B.; Varela-Guerrero, V. Relationship Between Charge Transfer Diffusion Coefficients and Doping Level for Electrogenerated Thin PEDOT Films on ITO. Bionatura 2019. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers-Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Bunting, R.K.; Swarat, K.; Yan, D.; Finello, D. Synthesis and Characterization of a Conducting Polymer: An Electrochemical Experiment for General Chemistry. J. Chem. Educ. 1997, 74, 421. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincón, M.E.; Gutierrez-Granados, S.; Chahma, M.h.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting Polymers in the Fields of Energy, Environmental Remediation, and Chemical–Chiral Sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.C.; Euler, W.B.; Force, R.R. The Modern Student Laboratory: Polyaniline-A Conducting Polymer: Electrochemical Synthesis and Electrochromic Properties. J. Chem. Educ. 1994, 71, A94. [Google Scholar] [CrossRef]
- Schmidt, D.J.; Pridgen, E.M.; Hammond, P.T.; Love, J.C. Layer-by-Layer Assembly of a pH-Responsive and Electrochromic Thin Film. J. Chem. Educ. 2010, 87, 208–211. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Puente-Caballero, R.; Torres-Perez, J.; Bustos, D.; Carmona-Orbezo, A.; Sevilla, F.B. An Inexpensive Device for Studying Electrochromism. J. Chem. Educ. 2012, 89, 1205–1207. [Google Scholar] [CrossRef]
- Kerszulis, J.A.; Amb, C.M.; Dyer, A.L.; Reynolds, J.R. Follow the Yellow Brick Road: Structural Optimization of Vibrant Yellow-to-Transmissive Electrochromic Conjugated Polymers. Macromolecules 2014, 47, 5462–5469. [Google Scholar] [CrossRef]
- Argun, A.A.; Aubert, P.-H.; Thompson, B.C.; Schwendeman, I.; Gaupp, C.L.; Hwang, J.; Pinto, N.J.; Tanner, D.B.; MacDiarmid, A.G.; Reynolds, J.R. Multicolored Electrochromism in Polymers: Structures and Devices. Chem. Mater. 2004, 16, 4401–4412. [Google Scholar] [CrossRef]
- Reeves, B.D.; Thompson, B.C.; Abboud, K.A.; Smart, B.E.; Reynolds, J.R. Dual Cathodically and Anodically Coloring Electrochromic Polymer Based on a Spiro Bipropylenedioxythiophene [(Poly(spiroBiProDOT)]. Adv. Mater. 2002, 14, 717–719. [Google Scholar] [CrossRef]
- Kumar, A.; Welsh, D.M.; Morvant, M.C.; Piroux, F.; Abboud, K.A.; Reynolds, J.R. Conducting Poly (3,4-alkylenedioxythiophene) Derivatives as Fast Electrochromics with High-Contrast Ratios. Chem. Mater. 1998, 10, 896–902. [Google Scholar] [CrossRef]
- Rendón-Enríquez, I.N.; Scherf, U.; Tausch, W.M. Elektrochrome Fenster. Chem. Shule 2016, 65, 34–39. [Google Scholar]
- Rendón-Enríquez, I.N.; Tausch, M.W.; Scherf, U. Elektrochrome Fenster mit Leitenden Polymeren. Chem. Unserer Zeit 2016, 50, 400–405. [Google Scholar] [CrossRef]
- Inzelt, G. Recent Advances in the Field of Conducting Polymers. J. Solid State Electrochem. 2017, 21, 1965–1975. [Google Scholar] [CrossRef]
- Palma-Cando, A.U.; Frontana-Uribe, B.A.; Maldonado, J.L.; Hernández, M.R. Control of Thickness of PEDOT Electrodeposits on Glass/ITO Electrodes from Organic Solutions and its Use as Anode in Organic Solar Cells. Procedia Chem. 2014, 12, 92–99. [Google Scholar] [CrossRef]
- Piron, F.; Leriche, P.; Grosu, I.; Roncali, J. Electropolymerizable 3Dπ-Conjugated Architectures with Ethylenedioxythiophene (EDOT) End-Groups as Precursors of Electroactive Conjugated Networks. J. Mater. Chem. 2010, 20, 10260–10268. [Google Scholar] [CrossRef][Green Version]
- Palma-Cando, A.; Scherf, U. Electrochemically Generated Thin Films of Microporous Polymer Networks: Synthesis, Properties, and Applications. Macromol. Chem. Phys. 2016, 217, 827–841. [Google Scholar] [CrossRef]
- Suresh, V.M.; Scherf, U. Electrochemically Generated Conjugated Microporous Polymer Network Thin Films for Chemical Sensor Applications. Macromol. Chem. Phys. 2018, 219, 1800207. [Google Scholar] [CrossRef]
- Bildirir, H.; Gregoriou, V.G.; Avgeropoulos, A.; Scherf, U.; Chochos, C.L. Porous Organic Polymers as Emerging New Materials for Organic Photovoltaic Applications: Current Status and Future Challenges. Mater. Horiz. 2017, 4, 546–556. [Google Scholar] [CrossRef]
- Bildirir, H.; Osken, I.; Ozturk, T.; Thomas, A. Reversible Doping of a Dithienothiophene-Based Conjugated Microporous Polymer. Chem. Eur. J. 2015, 21, 9306–9311. [Google Scholar] [CrossRef]
- Gu, C.; Chen, Y.; Zhang, Z.; Xue, S.; Sun, S.; Zhang, K.; Zhong, C.; Zhang, H.; Pan, Y.; Lv, Y.; et al. Electrochemical Route to Fabricate Film-Like Conjugated Microporous Polymers and Application for Organic Electronics. Adv. Mater. 2013, 25, 3443–3448. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Scherf, U. Electrogenerated Thin Films of Microporous Polymer Networks with Remarkably Increased Electrochemical Response to Nitroaromatic Analytes. ACS Appl. Mater. Interfaces 2015, 7, 11127–11133. [Google Scholar] [CrossRef]
- Gu, C.; Huang, N.; Chen, Y.; Qin, L.; Xu, H.; Zhang, S.; Li, F.; Ma, Y.; Jiang, D. π-Conjugated Microporous Polymer Films: Designed Synthesis, Conducting Properties, and Photoenergy Conversions. Angew. Chem. 2015, 127, 13798–13802. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Brunklaus, G.; Scherf, U. Thiophene-Based Microporous Polymer Networks via Chemical or Electrochemical Oxidative Coupling. Macromolecules 2015, 48, 6816–6824. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Woitassek, D.; Brunklaus, G.; Scherf, U. Luminescent Tetraphenylethene-Cored, Carbazole- and Thiophene-Based Microporous Polymer Films for the Chemosensing of Nitroaromatic Analytes. Mater. Chem. Front. 2017, 1, 1118–1124. [Google Scholar] [CrossRef]
- Palma-Cando, A.; Preis, E.; Scherf, U. Silicon- or Carbon-Cored Multifunctional Carbazolyl Monomers for the Electrochemical Generation of Microporous Polymer Films. Macromolecules 2016, 49, 8041–8047. [Google Scholar] [CrossRef]
- Räupke, A.; Palma-Cando, A.; Shkura, E.; Teckhausen, P.; Polywka, A.; Görrn, P.; Scherf, U.; Riedl, T. Highly Sensitive Gas-Phase Explosive Detection by Luminescent Microporous Polymer Networks. Sci. Rep. 2016, 6, 29118. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, H.; Hu, W. Electrochemical Polymerization for Two-Dimensional Conjugated Polymers. J. Mater. Chem. C 2018, 6, 10672–10686. [Google Scholar] [CrossRef]
- Molapo, M.K.; Ndangili, M.P.; Ajayi, F.R.; Mbambisa, G.; Milu, M.S.; Njomo, N.; Masikini, M.; Baker, P.; Iwuoha, I.E. Electronics of Conjugated Polymers (I): Polyaniline. Int. J. Electrochem. Sci. 2012, 7, 11859–11875. [Google Scholar]
- Genies, E.M.; Lapkowski, M. Spectroelectrochemical Study of Polyaniline versus Potential in the Equilibrium State. J. Electroanal. Chem. Interfacial Electrochem. 1987, 220, 67–82. [Google Scholar] [CrossRef]
- Geniès, E.M.; Lapkowski, M.; Penneau, J.F. Cyclic Voltammetry of Polyaniline: Interpretation of the Middle Peak. J. Electroanal. Chem. Interfacial Electrochem. 1988, 249, 97–107. [Google Scholar] [CrossRef]
- Zotti, G.; Cattarin, S.; Comisso, N. Electrodeposition of polythiophene, polypyrrole and polyaniline by the cyclic potential sweep method. J. Electroanal. Chem. Interfacial Electrochem. 1987, 235, 259–273. [Google Scholar] [CrossRef]
- Funt, B.L.; Lowen, S.V. Mechanistic Studies of the Electropolymerization of 2,2′-bithiophene and of Pyrrole to form Conducting PSolymers. Synth. Met. 1985, 11, 129–137. [Google Scholar] [CrossRef]
- Atwani, O.; Baristiran, C.; Erden, A.; Sonmez, G. A stable, low band gap electroactive polymer: Poly(4,7-dithien-2-yl-2,1,3-benzothiadiazole). Synth. Met. 2008, 158, 83–89. [Google Scholar] [CrossRef]
- Heinze, J. Electrochemistry IV. In Topics in Current Chemistry; Steckhan, E., Ed.; Springer: Berlin, Germany, 1990. [Google Scholar]
- Inzelt, G. Role of polymeric properties in the electrochemical behaviour of redox polymer-modified electrodes. Electrochim. Acta 1989, 34, 83–91. [Google Scholar] [CrossRef]
- Wallace, G.G.; Spinks, G.M.; Kane-Maguire, L.A.P.; Teasdale, P.R. Conductive Electroactive Polymers, 3rd ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Huang, W.S.; MacDiarmid, A.G. Optical properties of polyaniline. Polymer 1993, 34, 1833–1845. [Google Scholar] [CrossRef]
- Monk, P.M.A.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Ding, Y.; Invernale, M.A.; Mamangun, D.M.D.; Kumar, A.; Sotzing, G.A. A Simple, Low Waste and Versatile Procedure to make Polymer Electrochromic Devices. J. Mater. Chem. 2011, 21, 11873–11878. [Google Scholar] [CrossRef]
- Ambrose, J.F.; Nelson, R.F. Anodic Oxidation Pathways of Carbazoles: I. Carbazole and N-Substituted Derivatives. J. Electrochem. Soc. 1968, 115, 1159–1164. [Google Scholar] [CrossRef]
- Yu, T.; Wu, X.; Lv, Y.; Liu, L.; Du, L.; Zhou, J.; Xie, Z.; Ma, Y. An Electrochemically Deposited Film as an Interface Layer to Improve the Performance of Polymer Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 4117–4120. [Google Scholar] [CrossRef]
- Mothika, V.S.; Räupke, A.; Brinkmann, K.O.; Riedl, T.; Brunklaus, G.; Scherf, U. Nanometer-Thick Conjugated Microporous Polymer Films for Selective and Sensitive Vapor-Phase TNT Detection. ACS Appl. Nano Mater. 2018, 1, 6483–6492. [Google Scholar] [CrossRef]
- Salinas, G.; Del-Oso, J.-A.; Espinoza-Montero, P.-J.; Heinze, J.; Frontana-Uribe, B.A. Electrochemical Polymerization, Characterization and in-situ Conductivity Studies of Poly-3,4-ortho-xylendioxythiophene (PXDOT). Synth. Met. 2018, 245, 135–143. [Google Scholar] [CrossRef]
- Inzelt, G. Electrochemical Quartz Crystal Microbalance. In Electrochemical Dictionary, 1st ed.; Bard, A.J., Inzelt, G., Scholz, F., Eds.; Springer: Berlin, Germany, 2008; p. 723. [Google Scholar]
- Sauerbrey, G. Use of Quartz Vibration for Weighing Thin Films on a Microbalance. J. Phys. 1959, 155, 206–212. [Google Scholar]
- Weidlich, C.; Mangold, K.M.; Jüttner, K. EQCM Study of the Ion Exchange Behaviour of Polypyrrole with Different Counterions in Different Electrolytes. Electrochim. Acta 2005, 50, 1547–1552. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Chen, Q.; Luo, M.; Hammershøj, P.; Zhou, D.; Han, Y.; Laursen, B.W.; Yan, C.-G.; Han, B.-H. Microporous Polycarbazole with High Specific Surface Area for Gas Storage and Separation. J. Am. Chem. Soc. 2012, 134, 6084–6087. [Google Scholar] [CrossRef]
- Laviron, E.; Meunier-Prest, R.; Vallat, A.; Roullier, L.; Lacasse, R. The Reduction Mechanism of Aromatic Nitro Compounds in Aqueous Medium: Part II. The Reduction of 4-nitropyridine Between H0 = −6 and pH 9.6. J. Electroanal. Chem. 1992, 341, 227–255. [Google Scholar] [CrossRef]
- Bai, S.; Hu, Q.; Zeng, Q.; Wang, M.; Wang, L. Variations in Surface Morphologies, Properties, and Electrochemical Responses to Nitro-Analyte by Controlled Electropolymerization of Thiophene Derivatives. ACS Appl. Mater. Interfaces 2018, 10, 11319–11327. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Cando, A.; Rendón-Enríquez, I.; Tausch, M.; Scherf, U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials 2019, 9, 1125. https://doi.org/10.3390/nano9081125
Palma-Cando A, Rendón-Enríquez I, Tausch M, Scherf U. Thin Functional Polymer Films by Electropolymerization. Nanomaterials. 2019; 9(8):1125. https://doi.org/10.3390/nano9081125
Chicago/Turabian StylePalma-Cando, Alex, Ibeth Rendón-Enríquez, Michael Tausch, and Ullrich Scherf. 2019. "Thin Functional Polymer Films by Electropolymerization" Nanomaterials 9, no. 8: 1125. https://doi.org/10.3390/nano9081125
APA StylePalma-Cando, A., Rendón-Enríquez, I., Tausch, M., & Scherf, U. (2019). Thin Functional Polymer Films by Electropolymerization. Nanomaterials, 9(8), 1125. https://doi.org/10.3390/nano9081125





