Preparation and Characterization of Nanocomposite Films Containing Nano-Aluminum Nitride and Cellulose Nanofibrils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Chemicals
2.2. Experimental Methods
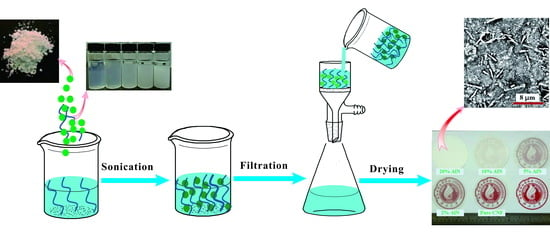
2.2.1. Preparation of CNFs
2.2.2. Preparation of CNF-AlN Composite Films
2.3. Analysis Methods
2.3.1. Transmission Electron Microscopy (TEM) Analysis
2.3.2. Scanning Electron Microscopy (SEM) Analysis
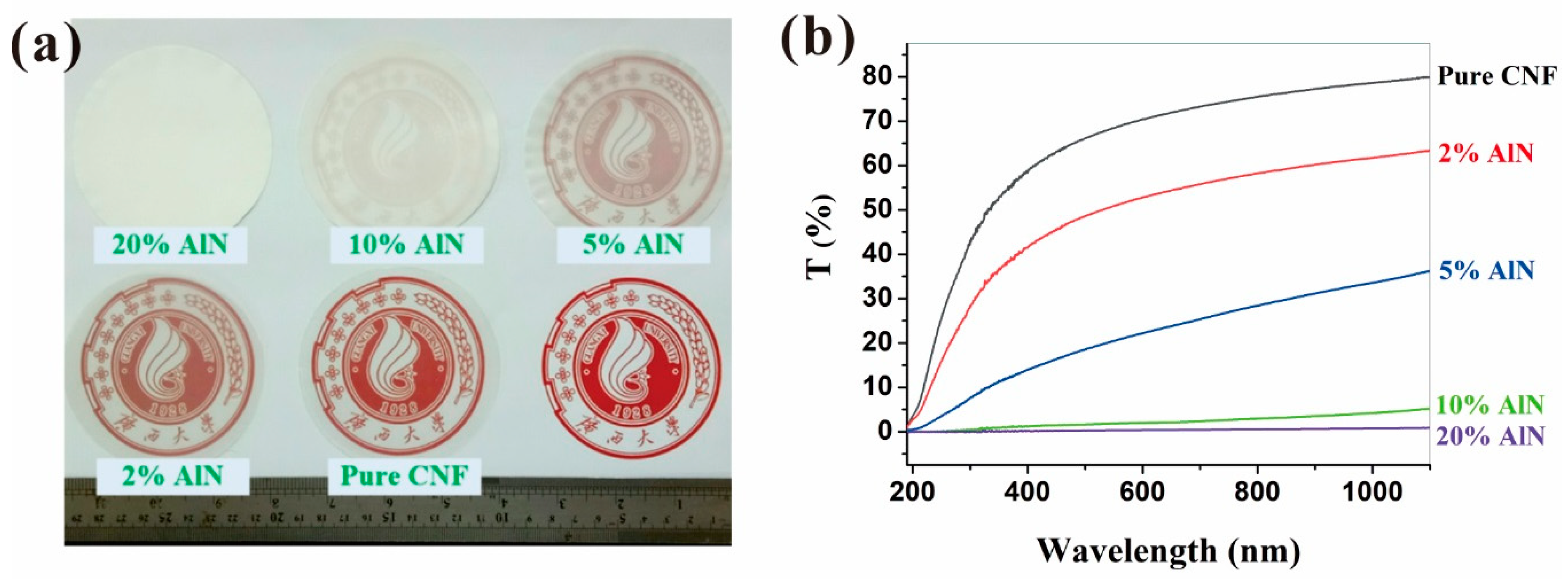
2.3.3. Ultraviolet-Visible (UV-Vis) Spectrum Analysis
2.3.4. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.3.5. X-Ray Diffraction (XRD) Analysis
2.3.6. Mechanical Properties Analysis
2.3.7. Thermogravimetric (TG) Analysis
3. Results and Discussion
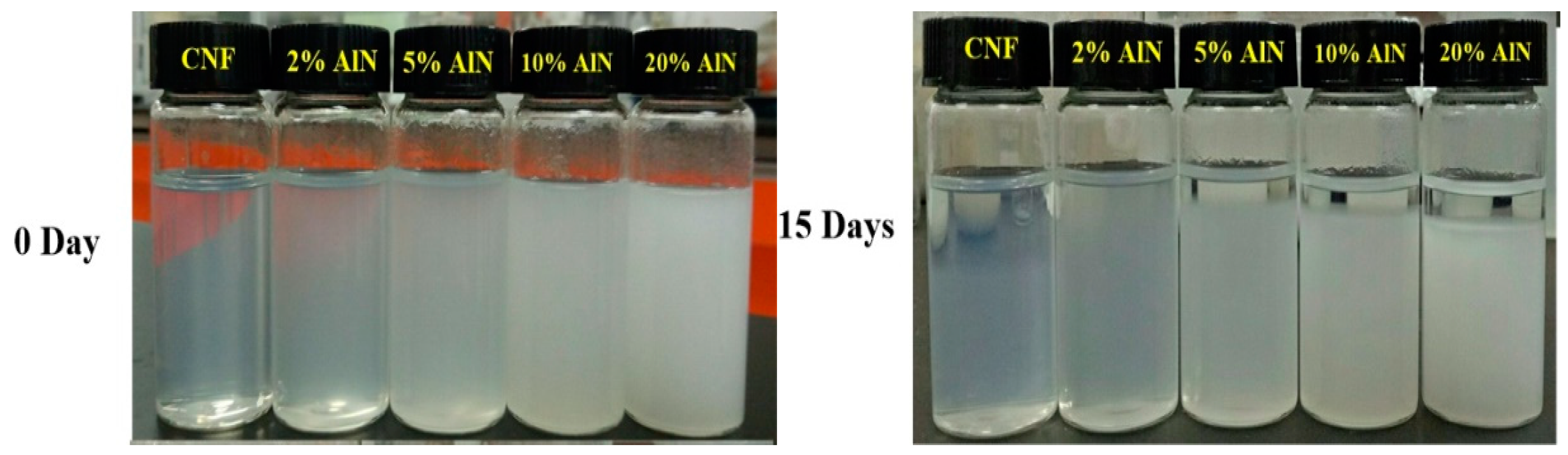
3.1. Characterization of CNFs and AlN Suspension
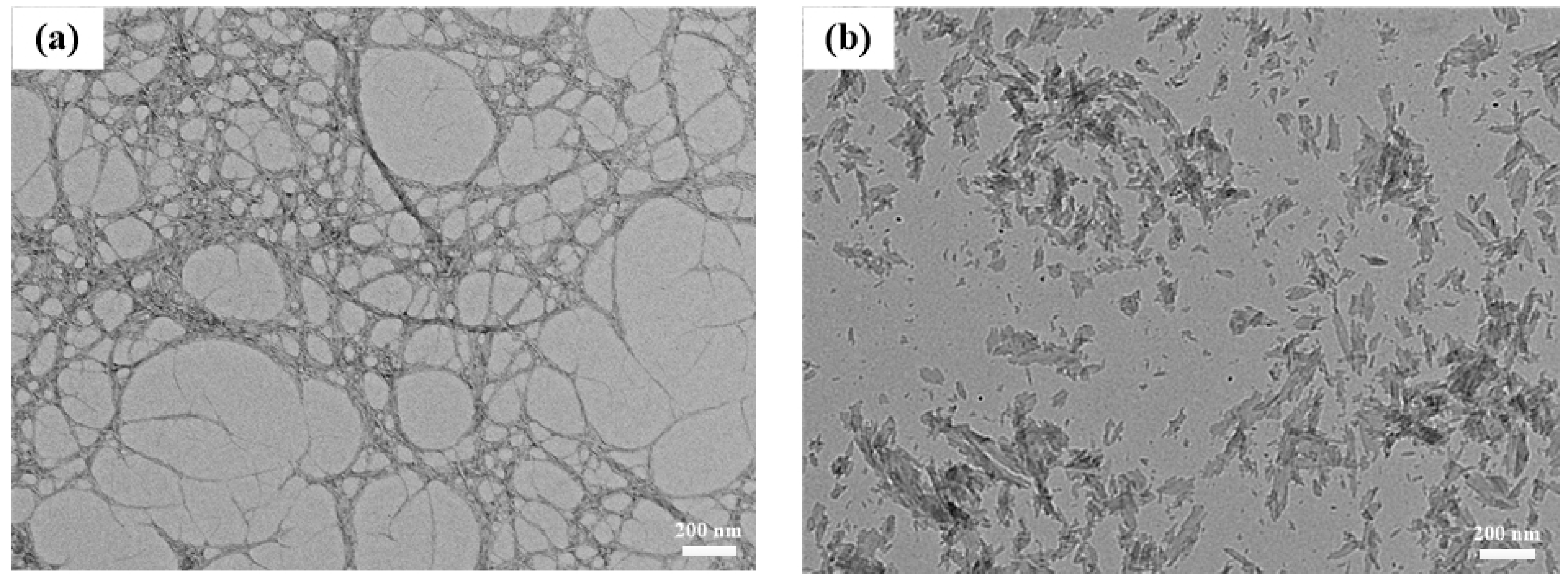
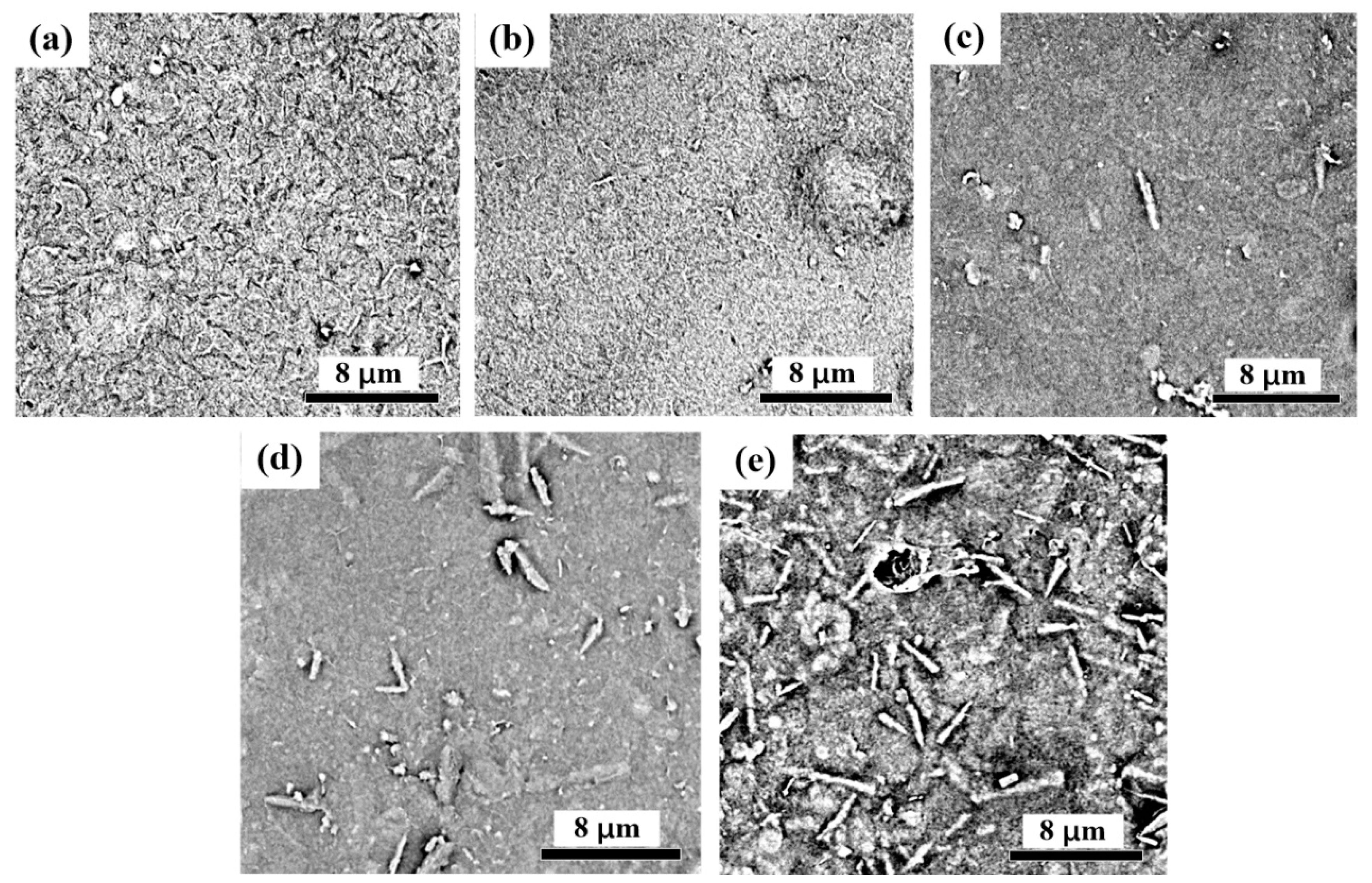
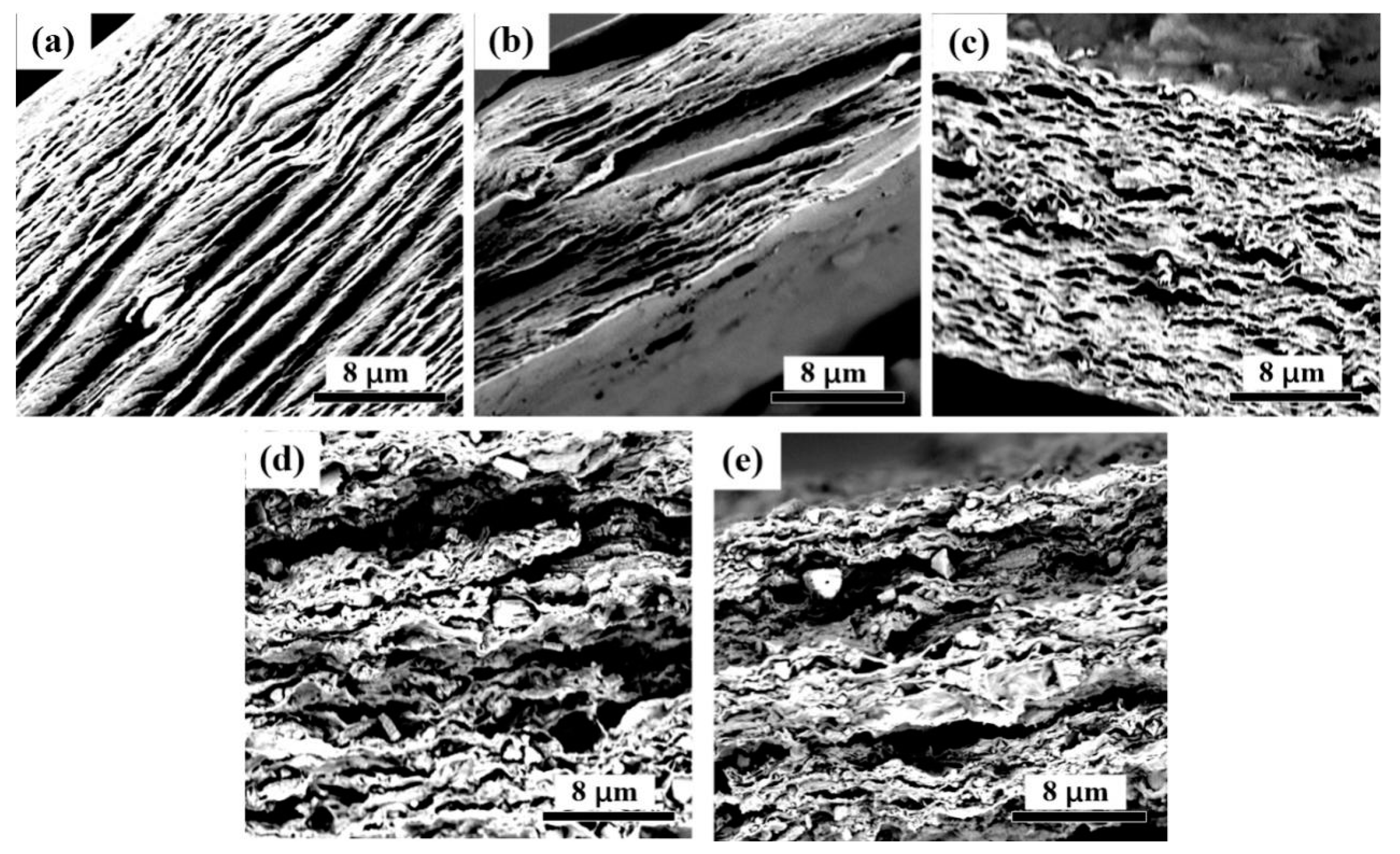
3.2. Microscopic Analysis of CNF-AlN Composites
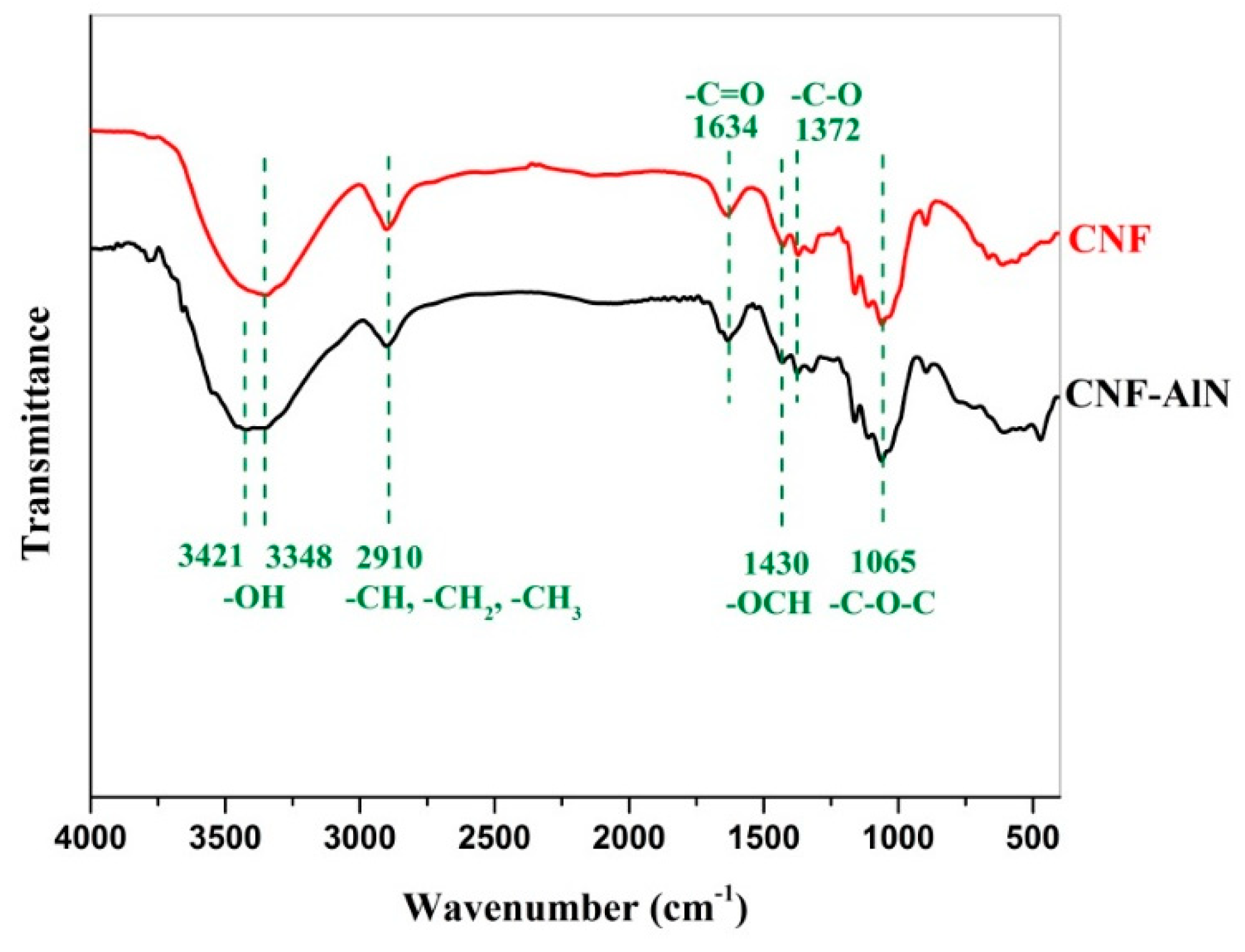
3.3. FTIR Analysis of CNF-AlN Composites
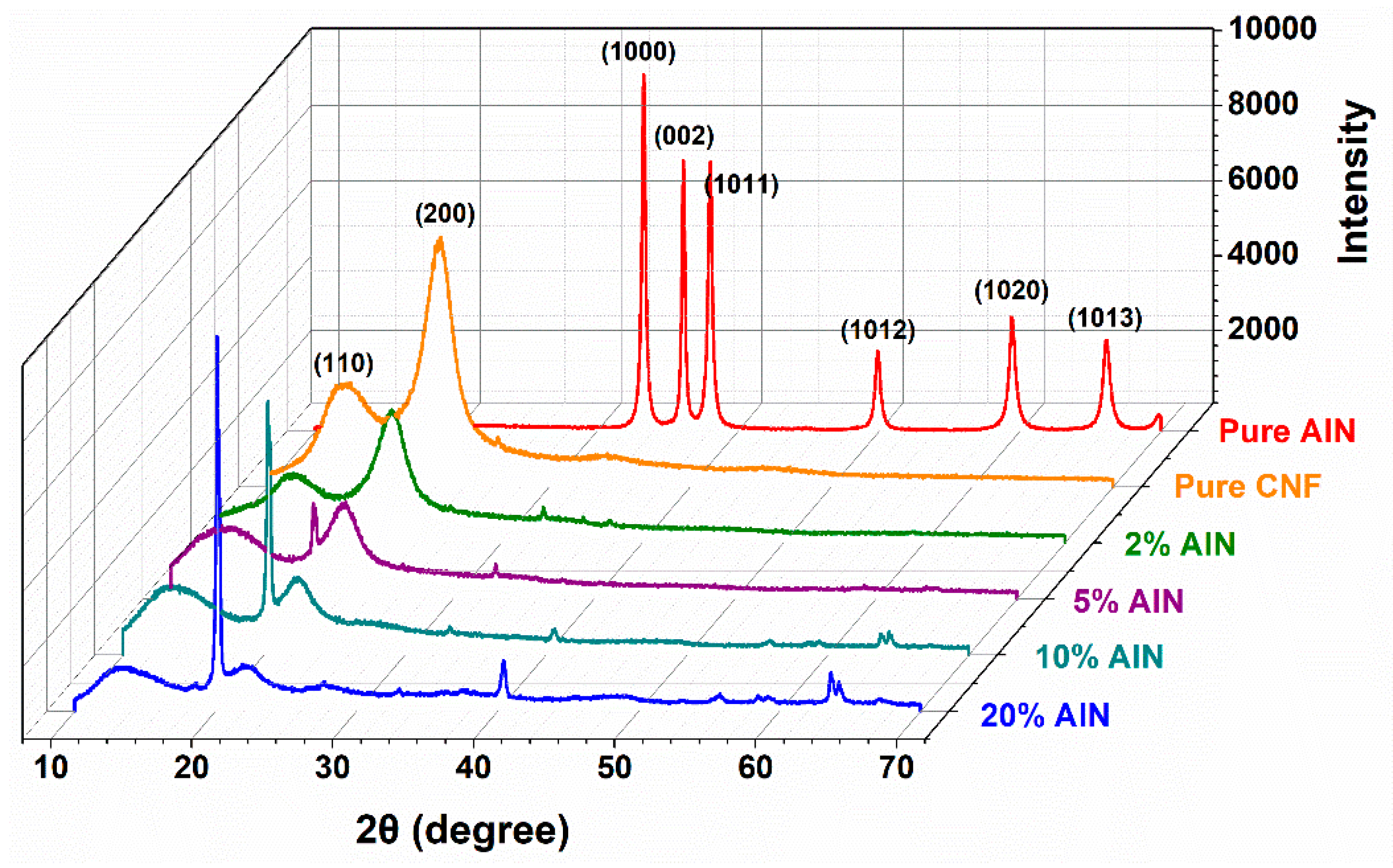
3.4. XRD Analysis of CNF-AlN Composites
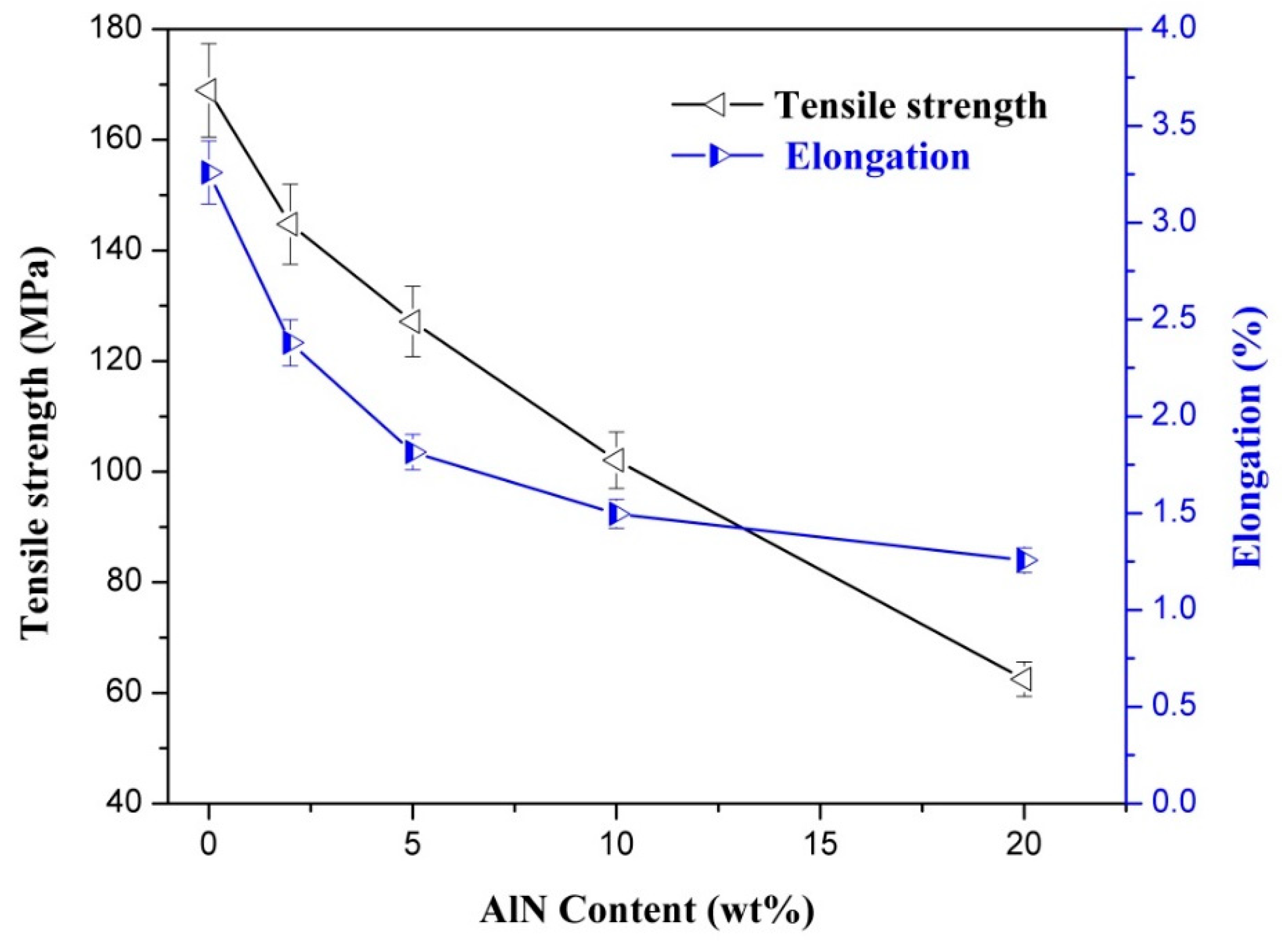
3.5. Mechanical Properties of CNF-AlN Composites
3.6. Thermal Stability of CNF-AlN Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xing, J.; Tao, P.; Wu, Z.; Xing, C.; Liao, X.; Nie, S. Nanocellulose-graphene composites- a promising nanomaterial for flexible supercapacitors. Carbohydr. Polym. 2019, 207, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Wang, S.P.; Fu, H.; Wang, Y.H.; Yu, K.F. Synthesis of au nanoparticles functionalized 1D α-MoO3 nanobelts and their gas sensing properties. Nano 2018, 13, 10. [Google Scholar] [CrossRef]
- Wu, K.; Lei, C.; Huang, R.; Yang, W.; Chai, S.; Geng, C.; Chen, F.; Fu, Q. Design and preparation of a unique segregated double network with excellent thermal conductive property. ACS Appl. Mater. Interfaces 2017, 9, 7637–7647. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected bn nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Dong, F.; Li, C.; Crittenden, J.; Zhang, T.; Lin, Q.; He, G.; Zhang, W.; Luo, J. Sulfadiazine destruction by chlorination in a pilot-scale water distribution system: Kinetics, pathway, and bacterial community structure. J. Hazard. Mater. 2019, 366, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; He, J.; Du, L.; Min, D.; Yong, Q. Structural characterization of the lignins from the green and yellow bamboo of bamboo culm (phyllostachys pubescens). J. Wood Chem. Technol. 2016, 36, 157–172. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, C.; Zhang, Q.; Zhang, K.; Zhang, Y.; Tao, P.; Wang, S. Enzymatic and cold alkaline pretreatments of sugarcane bagasse pulp to produce cellulose nanofibrils using a mechanical method. Ind. Crop. Prod. 2018, 124, 435–441. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, S.; Qin, C.; Wang, S. Removal of hexenuronic acid to reduce AOX formation in hot chlorine dioxide bleaching of bagasse pulp. Ind. Crop. Prod. 2019, 128, 338–345. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef]
- Lin, X.; Wu, Z.; Zhang, C.; Liu, S.; Nie, S. Enzymatic pulping of lignocellulosic biomass. Ind. Crop. Prod. 2018, 120, 16–24. [Google Scholar] [CrossRef]
- Nie, S.; Liu, X.; Wu, Z.; Zhan, L.; Yin, G.; Yao, S.; Song, H.; Wang, S. Kinetics study of oxidation of the lignin model compounds by chlorine dioxide. Chem. Eng. J. 2014, 241, 410–417. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Yuan, Y.; Wang, S.; Qin, C. Efficient extraction of bagasse hemicelluloses and characterization of solid remainder. Bioresour. Technol. 2015, 185, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Yan, D.; Zhang, C.; Nie, S. Enzyme-assisted mechanical production of cellulose nanofibrils: Thermal stability. Cellulose 2018, 25, 5049–5061. [Google Scholar] [CrossRef]
- Yao, S.; Nie, S.; Zhu, H.; Wang, S.; Song, X.; Qin, C. Extraction of hemicellulose by hot water to reduce adsorbable organic halogen formation in chlorine dioxide bleaching of bagasse pulp. Ind. Crop. Prod. 2017, 96, 178–185. [Google Scholar] [CrossRef]
- Nie, S.; Zhang, K.; Lin, X.; Yan, D.; Liang, H.; Wang, S. Enzymatic pretreatment for the improvements of dispersion and film properties of cellulose nanofibrils. Carbohydr. Polym. 2018, 181, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Wu, Z.; Xing, C.; Zhang, Q.; Wei, Z.; Nie, S. Effect of enzymatic treatment on the thermal stability of cellulose nanofibrils. Cellulose 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Sha, J.; Zhou, J.; Qin, C.; Wang, S. The influence of mechanical refining treatments on the rheosedimentation properties of bleached softwood pulp suspensions. Cellulose 2018, 25, 3609–3618. [Google Scholar] [CrossRef]
- Tao, P.; Zhang, Y.; Wu, Z.; Liao, X.; Nie, S. Enzymatic pretreatment for cellulose nanofibrils isolation from bagasse pulp: Transition of cellulose crystal structure. Carbohydr. Polym. 2019, 214, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Sun, J.; Yao, Y.; Sun, R.; Xu, J.B.; Wong, C.P. A combination of boron nitride nanotubes and cellulose nanofibers for the preparation of a nanocomposite with high thermal conductivity. ACS Nano 2017, 11, 5167–5178. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Fang, Z.; Xu, J.; Cao, F.; Wan, J.; Preston, C.; Yang, B.; Hu, L. Highly thermally conductive papers with percolative layered boron nitride nanosheets. ACS Nano 2014, 8, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zeng, X.; Wang, F.; Sun, R.; Xu, J.-b.; Wong, C.-P. Significant enhancement of thermal conductivity in bioinspired freestanding boron nitride papers filled with graphene oxide. Chem. Mater. 2016, 28, 1049–1057. [Google Scholar] [CrossRef]
- Fu, L.; Wang, T.; Yu, J.; Dai, W.; Sun, H.; Liu, Z.; Sun, R.; Jiang, N.; Yu, A.; Lin, C.-T. An ultrathin high-performance heat spreader fabricated with hydroxylated boron nitride nanosheets. 2D Mater. 2017, 4, 025047. [Google Scholar] [CrossRef]
- Wu, K.; Fang, J.; Ma, J.; Huang, R.; Chai, S.; Chen, F.; Fu, Q. Achieving a collapsible, strong, and highly thermally conductive film based on oriented functionalized boron nitride nanosheets and cellulose nanofiber. ACS Appl. Mater. Interfaces 2017, 9, 30035–30045. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Chen, G.; Ye, H.; Yan, G.; Li, G.; Guo, J.; Sun, S. Photoluminescence of hexagonal aln nanowires with native defect and oxygen impurity. Acta Photonica Sin. 2008, 37, 1599–1602. [Google Scholar]
- Zhou, J.; DeMiguel-Ramos, M.; Garcia-Gancedo, L.; Iborra, E.; Olivares, J.; Jin, H.; Luo, J.K.; Elhady, A.S.; Dong, S.R.; Wang, D.M.; et al. Characterisation of aluminium nitride films and surface acoustic wave devices for microfluidic applications. Sens. Actuators B Chem. 2014, 202, 984–992. [Google Scholar] [CrossRef]
- Zhang, K.; Tao, P.; Zhang, Y.; Liao, X.; Nie, S. Highly thermal conductivity of CNF/ALN hybrid films for thermal management of flexible energy storage devices. Carbohydr Polym 2019, 213, 228–235. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, S.; Rials, T.G.; Lee, S.H. Physical and mechanical properties of polyvinyl alcohol and polypropylene composite materials reinforced with fibril aggregates isolated from regenerated cellulose fibers. Cellulose 2007, 14, 593–602. [Google Scholar] [CrossRef]
- Cheng, Q.; Wang, S.; Rials, T.G. Poly(vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos. Part A 2009, 40, 218–224. [Google Scholar] [CrossRef]
- Nie, S.; Wang, S.; Qin, C.; Yao, S.; Ebonka, J.F.; Song, X.; Li, K. Removal of hexenuronic acid by xylanase to reduce adsorbable organic halides formation in chlorine dioxide bleaching of bagasse pulp. Bioresour. Technol. 2015, 196, 413–417. [Google Scholar] [CrossRef]
- Swain, S.K.; Dash, S.; Behera, C.; Kisku, S.K.; Behera, L. Cellulose nanobiocomposites with reinforcement of boron nitride: Study of thermal, oxygen barrier and chemical resistant properties. Carbohydr. Polym. 2013, 95, 728–732. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Liu, P.; Guo, X.; Nan, F.; Duan, Y.; Zhang, J. Modifying mechanical, optical properties and thermal processability of iridescent cellulose nanocrystal films using ionic liquid. ACS Appl. Mater. Interfaces 2017, 9, 3085–3092. [Google Scholar] [CrossRef]
- Jacquet, N.; Quiévy, N.; Vanderghem, C.; Janas, S.; Blecker, C.; Wathelet, B.; Devaux, J.; Paquot, M. Influence of steam explosion on the thermal stability of cellulose fibres. Polym. Degrad. Stab. 2011, 96, 1582–1588. [Google Scholar] [CrossRef]
- Gedler, G.; Antunes, M.; Realinho, V.; Velasco, J.I. Thermal stability of polycarbonate-graphene nanocomposite foams. Polym. Degrad. Stab. 2012, 97, 1297–1304. [Google Scholar] [CrossRef]
- Azubuike, C.P.; Rodríguez, H.; Okhamafe, A.O.; Rogers, R.D. Physicochemical properties of maize cob cellulose powders reconstituted from ionic liquid solution. Cellulose 2011, 19, 425–433. [Google Scholar] [CrossRef]
- Peng, Y.; Gardner, D.J.; Han, Y.; Kiziltas, A.; Cai, Z.; Tshabalala, M.A. Influence of drying method on the material properties of nanocellulose I: Thermostability and crystallinity. Cellulose 2013, 20, 2379–2392. [Google Scholar] [CrossRef]
- Yang, C.T.; Hsiang, H.I.; Huang, T.S.; Huang, P.C.; Han, Y.K. Thermal conductivity and dielectric properties of PEDOT:PSS-ALN filler reinforced water-soluble polymer composites. Ceram. Int. 2017, 43, 710–716. [Google Scholar] [CrossRef]
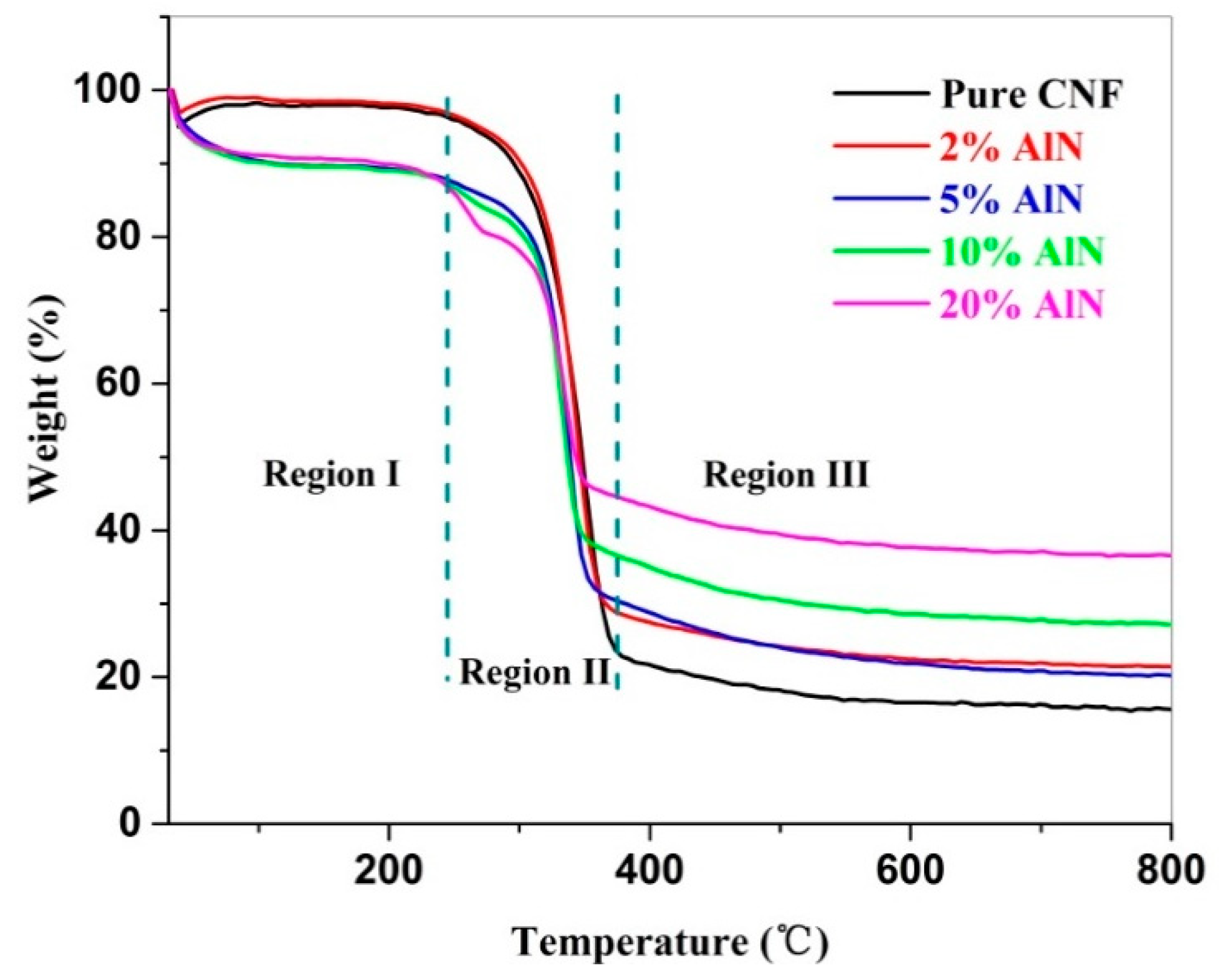
| Sample | Mass Residue (wt.%) | ||
|---|---|---|---|
| Region I | Region II | Region III | |
| Pure CNFs | 97.6 ± 0.2 | 25.5 ± 0.5 | 15.4 ± 0.4 |
| CNF-2%AlN | 98.3 ± 0.1 | 32.8 ± 0.3 | 21.4 ± 0.2 |
| CNF-5%AlN | 89.5 ± 0.1 | 33.5 ± 0.8 | 20.0 ± 0.6 |
| CNF-10%AlN | 89.4 ± 0.4 | 39.9 ± 0.2 | 27.0 ± 0.2 |
| CNF-20%AlN | 90.5 ± 0.4 | 47.5 ± 0.7 | 36.6 ± 0.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Zhang, Y.; Wang, L.; Wu, Q.; Wang, S. Preparation and Characterization of Nanocomposite Films Containing Nano-Aluminum Nitride and Cellulose Nanofibrils. Nanomaterials 2019, 9, 1121. https://doi.org/10.3390/nano9081121
Nie S, Zhang Y, Wang L, Wu Q, Wang S. Preparation and Characterization of Nanocomposite Films Containing Nano-Aluminum Nitride and Cellulose Nanofibrils. Nanomaterials. 2019; 9(8):1121. https://doi.org/10.3390/nano9081121
Chicago/Turabian StyleNie, Shuangxi, Yuehua Zhang, Linmao Wang, Qin Wu, and Shuangfei Wang. 2019. "Preparation and Characterization of Nanocomposite Films Containing Nano-Aluminum Nitride and Cellulose Nanofibrils" Nanomaterials 9, no. 8: 1121. https://doi.org/10.3390/nano9081121
APA StyleNie, S., Zhang, Y., Wang, L., Wu, Q., & Wang, S. (2019). Preparation and Characterization of Nanocomposite Films Containing Nano-Aluminum Nitride and Cellulose Nanofibrils. Nanomaterials, 9(8), 1121. https://doi.org/10.3390/nano9081121